Abstract
Glufosfamide is an alkylating agent consisting of iphosphoramide mustard conjugated to glucose that is currently included in clinical studies of pancreatic cancer. We studied the effects of glufosfamide, in combination with gemcitabine, on in vitro and in vivo models of pancreatic cancer. In proliferation assays, glufosfamide and gemcitabine inhibited the growth of MiaPaCa-2, H766t, and PANC-1 cells, but the combination of the two agents provided greater effects. Apoptosis of MiaPaCa-2 cells, measured by fluorescence-activated cell sorting, was enhanced by the combination of the two drugs, compared to single-agent treatment. Glufosfamide alone inhibited the growth of red fluorescent protein-expressing MiaPaCa-2 tumors in an orthotopic nude mouse model in a dose-dependent manner. Combining glufosfamide (30 mg/kg) with gemcitabine resulted in enhanced inhibition of tumor growth and significantly prolonged survival. Immunohistochemistry of excised tumors revealed that both glufosfamide and gemcitabine increased levels of apoptosis (measured by terminal deoxynucleotidyl transferase-mediated nick end labeling staining) and reduced proliferation (measured by proliferating cell nuclear antigen staining). No effects on microvessel density were observed. These results support the use of the alkylating agent glufosfamide and the DNA synthesis inhibitor gemcitabine, rather than the use of either agent alone, to provide greater benefits and demonstrate that this combination treatment should be useful in the clinical treatment of pancreatic carcinoma.
Keywords: Glufosfamide, gemcitabine, pancreatic cancer, orthotopic model, alkylating agent
Introduction
Adenocarcinoma of the ductal pancreas is characterized by extensive local invasion and early metastasis. In most patients, the disease, when detected, is usually already well developed locally or has already metastasized [1,2]. Thus, the 5-year survival rate of these patients is only 3% to 4% [3], with a median survival after diagnosis of approximately 6 months [4]. Gemcitabine, a deoxycytidine analogue, has a moderately extended median survival for patients with advanced pancreatic cancer [5,6] and is currently the standard of care as first-line therapy. Gemcitabine induces apoptosis of human pancreatic cancer cells and can inhibit tumor growth and progression [7]. In addition, intracellular phosphorylation of gemcitabine produces diphosphate and triphosphate molecular forms capable of acting as fraudulent bases in DNA and also capable of inhibiting DNA synthesis-dependent ribonucleotide reductase [8], together producing a strong cytotoxic effect. A recent study has suggested that gemcitabine may be more effective as adjuvant therapy after complete surgical resection of pancreatic cancer and may increase survival by as much as 6 months in these patients receiving surgery [9]. Even so, the moderate therapeutic advantage of gemcitabine therapy over best supportive care, either as primary care or in the adjuvant setting, indicates that there is a need for new treatment strategies for pancreatic cancer. Among the promising agents currently under investigation for first-line and secondline therapies for the treatment of pancreatic carcinoma is glufosfamide, a glucose-coupled iphosphoramide mustard with alkylating properties. Glufosfamide was developed to avoid the need for the activation of ifosfamide by P450 in the liver, thus reducing toxicities associated with systemic exposure to multiple metabolites of ifosfamide, including acrolein [10]. The glucose moiety may allow enhanced uptake by tumor cells because of upregulated sodium transporters [11]. One potential glucose transport mechanism has been implicated [12], although others have not been ruled out. In tumor cells, glufosfamide is cleaved by glucosidases to liberate the cytostatic agent iphosphoramide mustard [13]. Glufosfamide exhibits lower myelotoxicity and increased antitumor activity in preclinical in vitro and in vivo studies [14]. Here we present data that specifically address the likelihood that glufosfamide and gemcitabine may have benefits as combination therapy. Data on this particular combination treatment have not been previously reported, and the results support the use of these two agents to provide at least additive effects on models of human pancreatic cancer.
Methods
Cell Lines and Reagents
MiaPaCa-2, AsPC-1, H766t, and PANC-1 cell lines were obtained from the American Type Culture Collection (Rockville, MD). All cell lines were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, sodium pyruvate, nonessential amino acids, l-glutamine, vitamins, and antibiotics. Cells were maintained in a humidified incubator containing 10% CO2 at 37°C. All chemical reagents were purchased from Sigma Chemical Co. (St. Louis, MO), unless otherwise specified. Gemcitabine was purchased from Eli Lilly Co. (Indianapolis, IN), reconstituted in sterile phosphate-buffered saline (PBS), and stored at room temperature for in vivo studies or in aliquots at 20°C for in vitro studies. Glufosfamide was provided directly by Threshold Pharmaceuticals, Inc. (Redwood City, CA), and freshly reconstituted in PBS for each study. Anti-proliferating cell nuclear antigen (PCNA) and anti-CD31 monoclonal antibodies were obtained from Dakocytomation Corp. (Carpinteria, CA).
Cell Proliferation Assay
Cells were collected from exponentially growing cultures. Cell numbers were determined by direct counting with a hemocytometer, and cell viability was determined by trypan blue exclusion. For cell growth curves, 1 x 106 cells were plated in triplicate for each dose level. After 24 hours, the culture medium was removed and replaced with a fresh medium mixed with 1 µg/ml gemcitabine, 10 µg/ml glufosfamide, or both. These concentrations were selected based on preliminary studies and represent approximately 50% inhibitory concentrations. Cells were counted with a hemocytometer every 24 hours for 3 days. Each experiment was conducted on three separate occasions.
Determination of DNA Fragmentation By Fluorescence-Activated Cell Sorting (FACS) Analysis
Cells (1 x 106) were incubated with vehicle, gemcitabine, glufosfamide, or a combination of both agents at 10 µg/ml for 24 hours. The cells were then collected by gentle trypsinization, washed with PBS, and pelleted by centrifugation. Cells were resuspended in PBS containing 50 µg/ml propidium iodide (PI), 0.1% Triton X-100, and 0.1% sodium citrate. The samples were then stored at 4°C for 16 hours and vortexmixed before FACS analysis. The relative percentage of cells in the sub-G1 region was then quantitated and used as an estimate of cells undergoing apoptosis. FACS channels were set based on reference analysis and maintained constant during the experiments.
Orthotopic Mouse Model of Pancreatic Cancer
Orthotopic tumors were generated using MiaPaCa-2 cells that had been transfected to stably express red fluorescent protein (RFP), as previously described [15]. Tumor stocks were made by subcutaneously injecting MiaPaCa-2-RFP cells, at a concentration of 5 x 106 cells per 200 µl, into the flanks of nude mice. Tumor tissues were resected aseptically, and any grossly necrotic or suspected necrotic or non-RFP tumor tissues were removed. The remaining healthy tumor tissues were subsequently cut into small fragments of approximately 1 mm3. Recipient mice were anesthetized with isoflurane, and the surgical area was sterilized using iodine and alcohol. An incision approximately 1.5 cm long was made on the left upper abdomen of nude mice using a pair of surgical scissors. The pancreas was exposed, and then two pieces of MiaPaCa-2-RFP tumor fragments (in mm3) were transplanted to the mouse pancreas with 8-0 surgical sutures (nylon) after the capsule of the transplantation site had been stripped. The abdomen was closed with 6-0 surgical sutures (silk). All procedures of the operation described above were performed with a x7 magnification microscope (Olympus, Center Valley, PA) under high-efficiency particulate-arresting filter laminar flow hoods.
During the course of the study, the primary tumor size for each animal was followed on a weekly basis. Primary tumor sizes were estimated by measuring perpendicular minor dimension (W) and major dimension (L) using sliding calipers. Approximate tumor volume was calculated by the formula: W2L x 1/2. At the end of the study, the primary tumor burden was verified, and metastasis was evaluated by whole-body imaging as previously described [15,16]. Briefly, the mice were placed in a fluorescent light box equipped with a fiber-optic light source of 490 nm (Lightools Research, Encinitas, CA). Selective excitation of RFP was produced through a D425/60 bandpass filter and a 470 DCXR dichroic mirror. Emitted fluorescence was collected through a longpass filter GG475 (Chroma Technology, Brattleboro, VT) on a Hamamatsu C5810 3 chip-cooled color charge-coupled device camera (Hamamatsu Photonics, Bridgewater, NJ). Images were processed for contrast and brightness, and analyzed with the use of Image Pro Plus 3.1 software (Media Cybernetics, Silver Spring, MD). High-resolution images of 1024 x 724 pixels were captured directly on an IBM PC (IBM, Armonk, NY) or continuously through video output on a highresolution Sony VCR (model SLV-R1000; Sony, Tokyo, Japan).
To evaluate the efficacy of glufosfamide alone in this model and to determine the optimal dosage for combination experiments, an initial experiment, in which groups of six mice were randomized into one of several groups when tumors had reached 100 to 150 mm3, was conducted. Glufosfamide was administered intravenously, in doses of 3 to 100 mg/kg per day, for 14 days. Saline was administered to control mice. Based on the results of this study, doses of 10 and 30 mg/kg per day were selected for a second study. In the second study, groups of 10 mice were treated with glufosfamide alone, intravenously, daily for 14 days; with gemcitabine alone, intraperitoneally, at 300 mg/kg once a week for 3 weeks; or in combination. Based on the observations of animals in this study and in general pharmacology studies previously reported [17], this dose of gemcitabine, although higher than used in some studies, was considered safe. However, to be sure, we have conducted a study in which nude mice were injected once a week for 3 weeks and detected no changes in liver or renal enzymes. Saline-treated mice served as controls. As before, treatment was initiated when tumors had reached 100 to 150 mm3. The metastatic frequency of all groups was analyzed with Fisher's exact test. Differences in animal survival time between each treatment group and control were compared with log-rank analysis. Tumor sizes at 14 and 21 days were compared using Kruskal-Wallis test followed by log-rank analysis. All values were considered significant at P < .05.
Immunohistochemistry of Pancreatic Tumors
In a separate experiment, tumors were established in groups of five nude mice by an intrapancreatic injection of 1 x 106 MiaPaCa-2 cells after a midline incision and laparotomy under isoflurane anesthesia and aseptic conditions. When tumor size was approximately 150 mm3, treatment was initiated with 30 mg/kg per day of glufosfamide, intravenously, for 14 days; with 300 mg/kg per day of gemcitabine, intraperitoneally, once a week for 2 weeks (days 1 and 8 of treatment); or with both agents. PBS served as vehicle control. One day after the final glufosfamide dose, tumors were harvested and frozen until analyzed.
Frozen tissue sections were fixed, and 5-µm sections embedded in paraffin were prepared for terminal deoxynucleotidyl transferase-mediated nick end labeling (TUNEL) assay using a commercial kit (Promega, Madison, WI) according to the manufacturer's instructions. Background reactivity was determined by processing slides in the absence of terminal deoxynucleotidyl transferase (negative control). Nuclei were stained with PI (1 µg/ml) for 10 minutes. Fluorescent bleaching was minimized with an enhancing reagent (Prolong; Molecular Probes, Eugene, OR). Immunofluorescence microscopy was performed with a fluorescent microscope equipped with narrow bandpass excitation filters. Images were captured using a Nikon camera (Photometrics, Tucson, AZ). DNA fragmentation was detected by localized green fluorescence within the nucleus of apoptotic cells. For quantification of total TUNEL expression, the number of apoptotic events was counted in 10 random fields at x100 magnification.
For the detection of PCNA and CD31 by immunohistochemistry, paraffin-embedded tissues were mounted on positively charged Superfrost slides (Fisher Scientific, Houston, TX) and dried overnight. Sections were deparaffinized in xylene, treated with a graded series of alcohol (100%, 95%, and 80% ethanol/double-distilled H2O, vol/vol), and rehydrated in PBS (pH 7.5). To enhance antigen retrieval, sections were microwaved for 5 minutes. For detection of CD31, paraffin-embedded tissues were treated with pepsin (Biomeda, Foster City, CA) for 15 minutes at 37°C and washed with PBS. After exposure to anti-PCNA or anti-CD31 antibodies followed by washing with PBS, positive reactions were visualized by incubating the slides with stable 3,3-diaminobenzidine for 10 to 20 minutes. The sections were rinsed with distilled water, counterstained with Gill's hematoxylin for 30 seconds, and mounted with Universal Mount (Research Genetics, Carlsbad, CA). Control samples exposed to secondary antibody alone showed no specific staining. For quantification of microvessel density (MVD), 10 randomly selected fields at x100 magnification were captured for each tumor, and microvessels were quantified according to the method described previously [26,27]. For quantification of PCNA expression, the number of positive cells was quantified in 10 randomly selected fields at x100 magnification.
Results
Proliferation Assay
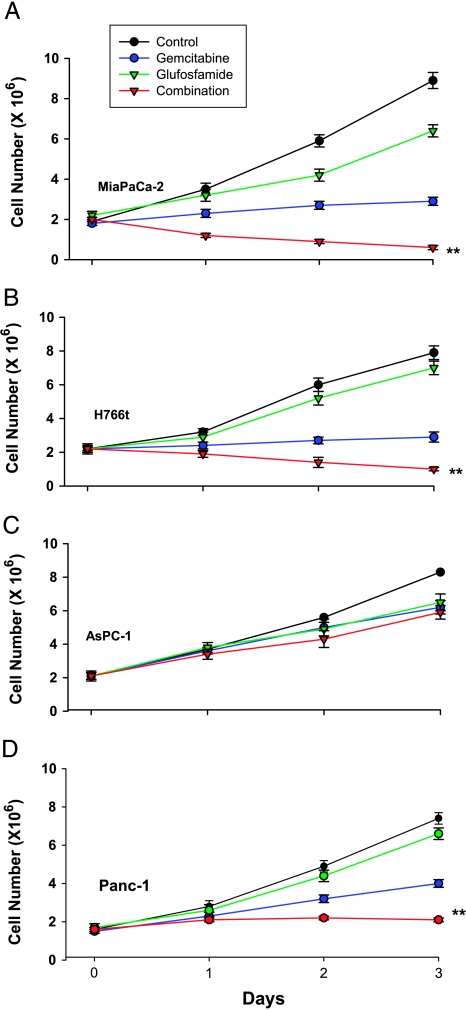
Based on preliminary experiments, a gemcitabine concentration of 1 µg/ml was selected to examine its growth-inhibitory effects. Three separate experiments, in which gemcitabine strongly inhibited the growth of MiaPaCa-2, H766t, and PANC-1 cells and exhibited moderate effects on the growth of AsPC-1 cells, were conducted (Figure 1). Glufosfamide at 10 µg/ml was less effective than gemcitabine. However, when glufosfamide was added to gemcitabine, additional inhibition of the cell growth of MiaPaCa-2, PANC-1, and H766t cells, but not of AsPC-1 cells, was observed.
Figure 1.
Effects of glufosfamide and gemcitabine on proliferation assays. MiaPaCa-2 (A), H766t (B), and AsPC-1 (C) pancreatic cells were grown in culture media for 3 days, as described in Methods. Glufosfamide and gemcitabine were added to the media at the indicated concentrations alone or together, and cell numbers were determined daily with a hemocytometer. Each experiment was conducted in triplicate. **P < .01 vs gemcitabine alone.
DNA Fragmentation
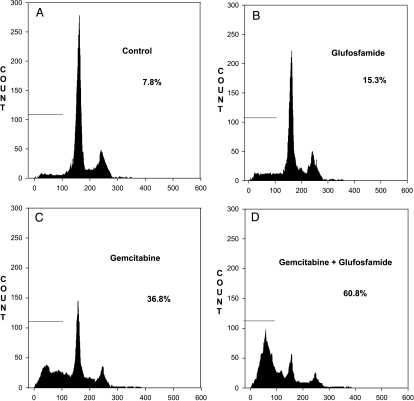
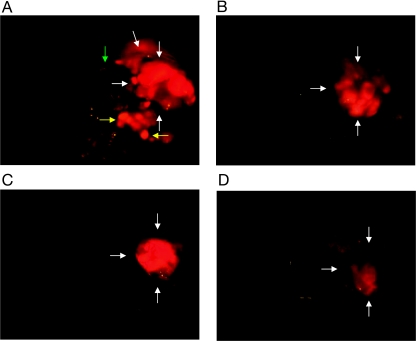
Because cytotoxicity assay indicated that glufosfamide and gemcitabine, either alone or in combination, were most effective against MiaPaCa-2 cells, FACS analysis of the DNA fragmentation of MiaPaCa-2 cells was conducted to determine whether apoptosis was induced by this combination. Treatment of MiaPaCa-2 cells with 10 µg/ml glufosfamide or 1 µg/ml gemcitabine resulted in induction of apoptosis as measured by an increase in apoptosis (sub-G1 fraction; Figure 2), although the effect of gemcitabine was greater. Treatment with both agents resulted in enhanced apoptosis that was slightly greater than additive.
Figure 2.
Analysis of DNA fragmentation by FACS. Cells (1 x 106) were incubated with vehicle (A), gemcitabine (B), glufosfamide (C), or the combination of both agents (D) at 10 µg/ml for 24 hours, with the addition of PI. DNA fragmentation (apoptosis) is indicated by events in Sub-G1 Phase.
Orthotopic Model of Pancreatic Cancer
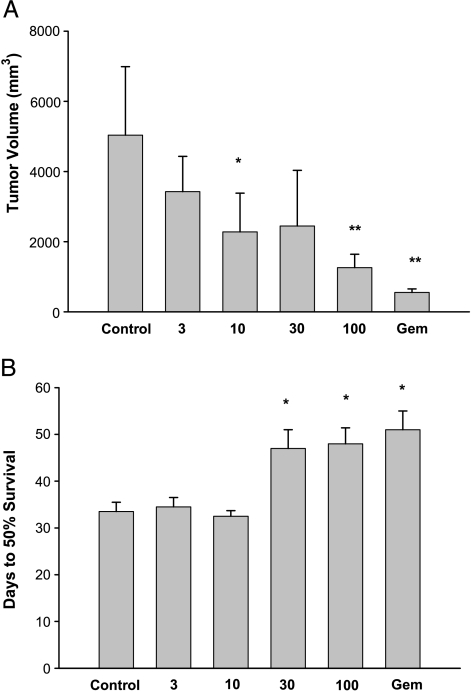
In a preliminary study, the activity of glufosfamide was evaluated in the MiaPaCa-2-RFP model to determine the best doses to be used in combination with gemcitabine. Figure 3A shows final tumor volumes recorded at necropsy, for glufosfamide at 3 to 100 mg/kg, intravenously, for 14 days, and gemcitabine at 300 mg/kg, intraperitoneally, once a week for 3 weeks. Treatment was initiated when tumors had reached 100 to 150 mm3. Glufosfamide treatment resulted in dose-related reductions in tumor volume; a dose of 100 mg/kg was similar in effect to gemcitabine. In addition, at doses of 30 mg/kg or higher, there was an significant increase in the time to 50% survival—an effect not significantly different from gemcitabine (Figure 3B).
Figure 3.
Final tumor volumes (A) and 50% survival (B) recorded in the MiaPaCa-2-RFP pancreatic cancer model. Glufosfamide was administered intravenously daily for 14 days. *P < .05 vs control.
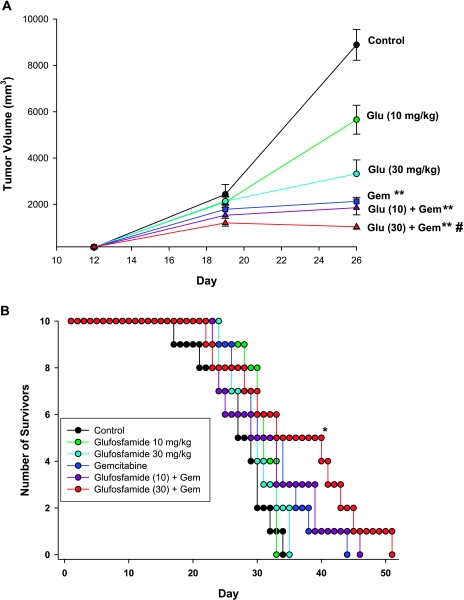
To evaluate combination therapy in the same model, doses of 10 and 30 mg/kg glufosfamide were chosen to be tested with and without 300 mg/kg gemcitabine. Figure 4A shows the weekly primary tumor volumes for each group. Because significant numbers of deaths were observed in three of the groups (vehicle, 10 mg/kg glufosfamide, and 30 mg/kg glufosfamide) after day 26, statistical evaluation was restricted to the initial 26-day period. On day 26, 14 days after initiation of treatment, glufosfamide at 10 mg/kg alone was without effect, whereas 30 mg/kg significantly reduced tumor size by approximately 50%. The effect of gemcitabine was somewhat greater than that of glufosfamide. However, combining 30 mg/kg glufosfamide with gemcitabine resulted in a significant increase above the level achieved by gemcitabine alone. Figure 5 shows images obtained from representative animals from the vehicle, gemcitabine, and combination groups. RFP expression was evident in the pancreas but was also seen to have spread to the abdominal lymph nodes of all control mice and to the diaphragm of a few mice (Table 1). Treatment with glufosfamide or gemcitabine reduced RFP expression in the primary tumor and also reduced the incidence of metastasis. The magnitude of the inhibition of metastatic incidence was similar among treatments, except for the 10-mg/kg-glufosfamide group, which was ineffective under the conditions of this study (Table 2). Figure 4B shows the survival curves for each group and indicates that only the combination of 30 mg/kg glufosfamide and gemcitabine significantly increased survival relative to control. Treatment with either agent alone or with both agents had no effect on body weights in this study (data not shown).
Figure 4.
Primary tumor volumes (A) and survival curves (B) recorded in the MiaPaCa-2-RFP pancreatic cancer model. Gemcitabine (intraperitoneally, once a week for 3 weeks) and glufosfamide (intravenously, daily for 14 days) each significantly reduced tumor volume measured on day 26, but the combination of 30 mg/kg and gemcitabine resulted in significantly greater reduction. The same combination of treatments also provided significant improvement in survival. *P < .05.
Figure 5.
Representative open-body images of individual mice from each treatment group. (A) Saline treatment. (B) Glufosfamide treatment. (C) Gemcitabine treatment. (D) Glufosfamide + gemcitabine. White arrows, primary tumor; yellow arrows, abdominal lymph node metastases; green arrow, diaphragmatic metastases.
Table 1.
Incidence of Metastasis to the Lymph Nodes and Diaphragm.
| Groups | Dose (mg/kg) | Available Tested Animals (n) | Total Metastatic Incidence (All Organs) | Abdominal Lymph Nodes | Diaphragm | ||
| Metastatic Incidence | P | Metastatic Incidence | P | ||||
| Control | - | 9 | 10 | 9 | - | 1 | - |
| Glufosfamide | 10 | 10 | 9 | 8 | .474 | 1 | 1.000 |
| Glufosfamide | 30 | 9 | 6 | 4 | .029 | 2 | 1.000 |
| Gemcitabine | 300 | 9 | 4 | 3 | .009 | 1 | 1.000 |
| Glufosfamide + gemcitabine | 10 + 300 | 8 | 1 | 1 | .000 | 0 | 1.000 |
| Glufosfamide + gemcitabine | 30 + 300 | 10 | 2 | 2 | .001 | 0 | .474 |
Table 2.
FACS Analysis of Pancreatic Cells Lines Treated with Gemcitabine and Glufosfamide.
| Groups | Cell Lines (% Cell Fragmentation) | |||
| MiaPaCa-2 | H766t | AsPC-1 | PANC-1 | |
| Control | 7.8 | 6.3 | 8.1 | 8.9 |
| Glufosfamide | 15.3 | 22.7 | 11.8 | 13.2 |
| Gemcitabine | 36.8 | 44.6 | 19.1 | 22.2 |
| Glufosfamide + gemcitabine | 60.8 | 78.2 | 34.3 | 41.9 |
Immunohistochemistry of Pancreatic Tumors
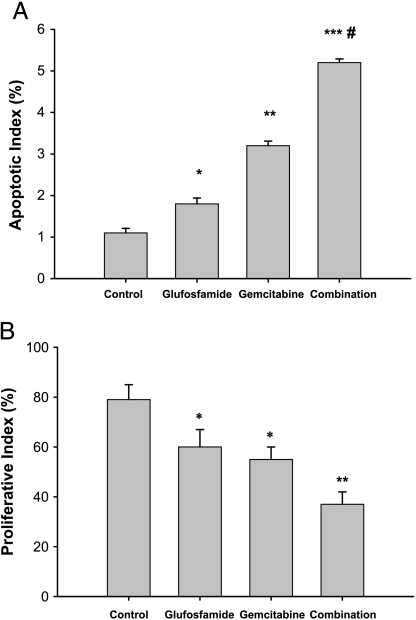
Figure 6 shows the apoptotic and proliferative indexes derived from TUNEL-stained and PCNA-stained sections of tumors from mice treated with saline, glufosfamide (30 mg/kg, iv, daily for 2 weeks), with gemcitabine (300 mg/kg, once a week for 2 weeks), or with both agents in combination. Tumors were removed 24 hours after the last treatment (at 2 weeks) and then weighed. Similar to the RFP model described above, treatment with gemcitabine alone yielded a reduction in tumor weight from 2.2 ± 0.5 to 1.4 ± 0.5 g. Glufosfamide at 10 mg/kg decreased only to 1.9 ± 0.6 g, whereas 30 mg/kg glufosfamide reduced weight to 1.6 ± 0.4 g. Again, combination therapy was most effective. Although the addition of 10 mg/kg glufosfamide to gemcitabine added little effect, combination with gemcitabine and 30 mg/kg reduced weight to 0.9 ± 0.3 g, a significant effect. In these tumors, apoptosis occurred at a low level in control mice, but was significantly increased by glufosfamide and, to a greater degree, by gemcitabine (Figure 6). Combination treatment resulted in a level of apoptosis that was significantly greater than that of either agent alone. PCNA staining was significantly greater with both gemcitabine alone and glufosfamide alone compared to control, indicating significant antiproliferative effects. Combination treatment resulted in significantly greater PCNA staining compared to either treatment alone. CD31 staining failed to reveal any effects of either treatment alone or combination treatment (data not shown).
Figure 6.
Apoptotic and proliferative indices measured by TUNEL and PCNA staining. Tumors were removed at the end of treatment and processed for immunohistochemistry. *P < .05 vs control; P < .01 vs control. ***P < .001 vs control. #P < .05 vs gemcitabine.
Discussion
This study was conducted to provide information on the potential value of the use of glufosfamide in combination with gemcitabine in the treatment of pancreatic cancer. Although gemcitabine is considered the standard of care as first-line treatment for pancreatic cancer [18], the benefit of such treatment is limited and, thus, alternative or additional treatments are needed. Among alternative agents of interest, glufosfamide, an alkylating agent in which the alkylating metabolite of ifosfamide is linked to β-d-glucose, has been studied in phase I and phase II trials. Part of the rationale for the potential advantage of glufosfamide over ifosfamide was that the active compound of ifosfamide, iphosphoramide mustard, must be released from the parent compound by liver microsomal enzymes [10]. In addition, other toxic metabolites (such as acrolein), which are both nephrotoxic and urotoxic, are released [19,20]. By coupling iphosphoramide mustard to glucose, in theory, metabolic activation should be avoided, toxic metabolites should be limited, and the active compound should be taken up preferentially by cells with upregulated sugar transporters and then released by hydrolysis by intracellular glucosidases. Thus, glufosfamide may take advantage of the observation of the overexpression of glucose transporters by pancreatic carcinomas [11].
Although several small clinical studies [21,22] have indicated that glufosfamide had an activity similar to that indicated by published results for gemcitabine, preclinical studies such as those described here showing that glufosfamide could be used in combination with gemcitabine to provide even greater benefits have not been previously reported. Our results clearly demonstrate that, at a minimum, an additive benefit is associated with a combination treatment with these two agents. In vitro proliferation and apoptosis assays indicated that addition of glufosfamide to gemcitabine enhanced activity. Glufosfamide demonstrated dose-dependent inhibition in the pancreatic orthotopic xenograft model and enhanced the activity of gemcitabine. This model takes advantage of a fluorescent biomarker (RFP) to follow the disease in real time to more accurately follow the effect of therapeutics on the particularly malignant human cancer than can be conducted with traditional flank xenograft models [15]. In this invasive lethal model, only the combination of 30 mg/kg glufosfamide and gemcitabine resulted in a significant increase in survival. This finding is somewhat surprising because treatment with glufosfamide alone or gemcitabine alone in the preliminary study resulted in an increase in 50% survival. However, examination of the control groups in the two studies revealed that control tumors grew much faster and that greater lethality was observed in the second study. Thus, we conclude that single-agent therapy in the second study was inadequate to affect these more rapidly growing tumors significantly and that combination therapy was required to increase survival significantly. Immunohistochemistry assay of apoptosis and proliferation in treated tumors confirmed the additive effects of combination therapy on tumor size and indicated that both activities are involved in the antitumor effects of both agents.
It is generally believed that chemotherapy and radiation therapy exert their effects by apoptosis [23]. We investigated apoptosis in our experiments and found that gemcitabine and glufosfamide both exhibited proapoptotic effects. Bold et al. [24] concluded that Bcl-2 plays a major role in the apoptosis evoked by gemcitabine because increased levels of Bcl-2 correlated with resistance to gemcitabine in various pancreatic cell lines. Interestingly, MiaPaCa-2 cells were most sensitive to gemcitabine and were associated with relatively low levels of Bcl-2 expression. This finding may, in part, explain the high sensitivity of our MiaPaCa-2 tumors to gemcitabine. Similarly, glufosfamide was found to induce apoptosis in association with a decline in Bcl-2 levels [25], and overexpression of Bcl-2 reduced the cytotoxic effect of glufosfamide. Thus, gemcitabine and glufosfamide may have some proapoptotic mechanisms in common. However, other mediators of gemcitabine-mediated apoptosis have been described [26,27], and the precise series of events leading to glufosfamide cell death has not been well studied.
Although glufosfamide and gemcitabine may share some activities, these agents should initiate cell death by different mechanisms. Glufosfamide is hydrolyzed to iphosphoramide mustard in tumor cells and acts as an alkylating agent leading to DNA double-stranded breaks [25,28]. In contrast, gemcitabine is a deoxycytidine analogue that, after intracellular phosphorylation to diphosphate and triphosphate molecular forms, inhibits ribonucleotide reductase, resulting in a reduction in deoxynucleotide concentrations, including dCTP. In addition, metabolites of gemcitabine compete with dCTP for incorporation into DNA. These two mechanisms of action lead to inhibition of DNA synthesis. Recently, an additional activity of gemcitabine, which in our opinion could explain the additional benefit derived from glufosfamide treatment above that achieved with gemcitabine only in our experiments, has been described. Gemcitabine was shown to inhibit homologous recombination mechanisms that are required to repair double-stranded DNA breaks induced by ionizing radiation or chemotherapy [29]. Becker et al. [25] demonstrated that glufosfamide does induce DNA doublestranded breaks. Therefore, it is reasonable to hypothesize that, in the present experiments, gemcitabine may have inhibited the repair of these lesions. Further experiments are required to prove that this mechanism is the primary explanation for our results.
In summary, these in vitro and in vivo studies have demonstrated that glufosfamide enhances the effects of gemcitabine on multiple in vitro and in vivo models of pancreatic cancer. The additional benefits appear to be, at least in part, related to enhanced apoptosis and antiproliferation. These results suggest that glufosfamide could be useful as adjunctive therapy in the treatment of patients with pancreatic cancer.
Acknowledgements
The authors thank Kevin Kaster and Lennert Olssen for helpful advice in the preparation of this manuscript.
References
- 1.Warshaw AL, Fernandez-del Castillo C. Pancreatic carcinoma. N Engl J Med. 1992;326:455–465. doi: 10.1056/NEJM199202133260706. [DOI] [PubMed] [Google Scholar]
- 2.Evans DB, Abbruzzese JL, Willet CG. Cancer of the pancreas. In: deVita V, Hellman S, Rosenberg SA, editors. Principles and Practice of Oncology. Philadelphia, PA: JB Lippincott; 2000. pp. 1126–1160. [Google Scholar]
- 3.Jemal A, Thomas A, Murray T, Thun M. Cancer statistics, 2002. CA Cancer J Clin. 2002;52:23–47. doi: 10.3322/canjclin.52.1.23. [DOI] [PubMed] [Google Scholar]
- 4.Mangray S, King TC. Molecular pathobiology of pancreatic adenocarcinoma. Front Biosci. 1998;3:D1148–D1160. doi: 10.2741/a351. [DOI] [PubMed] [Google Scholar]
- 5.Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 6.Moore M. Activity of gemcitabine in patients with advanced pancreatic carcinoma: a review. Cancer (Philadelphia) 1996;78:633–638. doi: 10.1002/(SICI)1097-0142(19960801)78:3<633::AID-CNCR44>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 7.Noble S, Goa K. Gemcitabine, a review of its pharmacology and clinical potential in non-small cell lung cancer and pancreatic cancer. Drugs. 1997;54:447–472. doi: 10.2165/00003495-199754030-00009. [DOI] [PubMed] [Google Scholar]
- 8.Huang P, Chubb S, Hertel LW, Grindey GB, Plunkett W. Action of 2′,2′-difluoro-deoxycytidine on DNA synthesis. Cancer Res. 1991;51:6110–6117. [PubMed] [Google Scholar]
- 9.Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkhart C, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. J Am Med Assoc. 2007;297:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 10.Connors TA, Cox PJ, Farmer PB, Foster AB, Jarman M. Some studies of the active intermediates formed in the microsomal metabolism of cyclophosphamide and iphosphamide. Biochem Pharmacol. 1974;23:115–129. doi: 10.1016/0006-2952(74)90318-9. [DOI] [PubMed] [Google Scholar]
- 11.Reske SN, Grillenberger KG, Glatting G, Port M, Hildebrandt M, Gansauge F, Beger HG. Overexpression of glucose transporter 1 and increased FDG uptake in pancreatic carcinoma. J Nucl Med. 1997;38:1344–1348. [PubMed] [Google Scholar]
- 12.Veyhl M, Wagner K, Volk C, Gorboulev M, Baumgarten K, Weber W-M, Schaper M, Bertram B, Wiessler M, Koepsell H. Transport of the new chemotherapeutic agent β-d-glucosylisophosphoramide mustard (D-19575) into tumor cells is mediated by the Na+-d-glucose cotransporter SAAT1. Proc Natl Acad Sci. 1998;95:2914–2919. doi: 10.1073/pnas.95.6.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seker H, Bertram B, Wieber M. Possible role of the cytosolic β-glucosidase in the metabolism of saccharide-coupled platinum and ifosfamide mustard in tumor cells. In: Berkarda B, editor. 10th Mediterranean Congress of Chemotherapy. Monduzzi, Milan: Monduzzi; 1996. pp. 381–385. [Google Scholar]
- 14.Pohl J, Bertram B, Hilgard P, Nowrousian MR, Stuben J, Wiebler M. D-19575—a sugar-linked isophosphoramide mustard derivative exploiting transmembrane glucose transport. Cancer Chemother Pharmacol. 1995;35:364–370. doi: 10.1007/s002800050248. [DOI] [PubMed] [Google Scholar]
- 15.Katz MH, Takimoto S, Spivak D, Moossa AR, Hoffman RM, Bouvet M. A novel red fluorescent protein orthotopic pancreatic cancer model for the preclinical evaluation of chemotherapeutics. J Surg Res. 2003;113:151–160. doi: 10.1016/s0022-4804(03)00234-8. [DOI] [PubMed] [Google Scholar]
- 16.Bouvet M, Wang J, Nardin SR, Nassirpour R, Yang M, Baranov E, Jiang P, Moossa AR, Hoffman RM. Real-time optical imaging of primary tumor growth and multiple metastatic events in a pancreatic cancer orthotopic model. Cancer Res. 2002;62:1534–1540. [PubMed] [Google Scholar]
- 17.Turk J, Bemis K, Colbert W, Heim R, Helton D, Rush G, Shannon H, Shetler T, Todd G, Wilson B. General pharmacology of gemcitabine hydrochloride in animals. Arzneimittelforschung. 1994;44:1089–1092. [PubMed] [Google Scholar]
- 18.Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy UK, Storniolo AM, Tarassoff P, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 19.Briasoulis E, Pavlidis N, Terret C, Bauer J, Fiedler W, Schoffski P, Raoul J-L, Hess D, Selvais R, Lacombe D, et al. Glufosfamide administered using a 1-hour infusion given as first-line treatment for advanced pancreatic cancer. A phase II trial of the EORTC-new drug development group. Eur J Cancer. 2003;39:2334–2340. doi: 10.1016/s0959-8049(03)00629-4. [DOI] [PubMed] [Google Scholar]
- 20.Bruggerman SK, Kisro J, Wagner T. Ifosfamide cytotoxicity on human tumor and renal cells: role of chloroacetaldehyde in comparison to 4-hydroxyifosfamide. Cancer Res. 1997;57:2676–2780. [PubMed] [Google Scholar]
- 21.Skinner R, Sharkey IM, Pearson AD, Craft AW. Ifosfamide, mesna, and nephrotoxicity in children. J Clin Oncol. 1993;11:173–190. doi: 10.1200/JCO.1993.11.1.173. [DOI] [PubMed] [Google Scholar]
- 22.Briasoulis E, Judson I, Pavlidis N, Beale P, Wander J, Groot Y, Veerman G, Schuessler M, Niebch G, Siamopoulos K, et al. Phase I trial of 6-hour infusion of glufosfamide, an new alkylating agent with potentially enhanced selectivity for tumors that overexpress transmembrane glucose transporters: a study of the European organization for research and treatment of cancer early clinical studies group. J Clin Oncol. 2000;18:3535–3544. doi: 10.1200/JCO.2000.18.20.3535. [DOI] [PubMed] [Google Scholar]
- 23.Kerr JFR, Winteford CM, Harmon BV. Apoptosis: its significance in cancer and cancer therapy. Cancer. 1994;73:2013–2026. doi: 10.1002/1097-0142(19940415)73:8<2013::aid-cncr2820730802>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 24.Bold RJ, Chandra J, McConkey DJ. Gemcitabine-induced programmed cell death (apoptosis) of human pancreatic carcinoma is determined by Bcl-2 content. Ann Surg Oncol. 1999;6:279–285. doi: 10.1007/s10434-999-0279-x. [DOI] [PubMed] [Google Scholar]
- 25.Becker R, Ritter A, Eichhorn U, Lips J, Bertram B, Wiessler M, Zdzlenicka MZ, Kaina B. Induction of DNA breaks and apoptosis in crosslink-hypertensive V79 cells by the cytostatic drug β-d-glucosyl-ifosfamide mustard. Br J Cancer. 2002;86:130–135. doi: 10.1038/sj.bjc.6600027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cascallo M, Calbo J, Capella G, Fillat C, Pastor-Anglada M, Mazo A. Enhancement of gemcitabine-induced apoptosis by restoration of p53 function in human pancreatic tumors. Oncology. 2005;68:179–189. doi: 10.1159/000086772. [DOI] [PubMed] [Google Scholar]
- 27.Koizumi K, Tanno S, Nakano Y, Habiro A, Izawa T, Mizukami Y, Okumura T, Kogho Y. Activation of p38 mitogen-activated protein kinase in necessary for gemcitabine-induced cytotoxicity in human pancreatic cancer cells. Anticancer Res. 2005;25:3347–3353. [PubMed] [Google Scholar]
- 28.Seker H, Bertram B, Burkle A, Kaina B, Pohl J, Koepsell H, Wiesser M. Mechanistic aspects of the cytotoxic activity of glufosfamide, a new tumour therapeutic agent. Br J Cancer. 2000;82:629–634. doi: 10.1054/bjoc.1999.0974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wachters FM, van Putten JWG, Maring JG, Zdzienicka MZ, Groen HJM, Kampinga HH. Selective targeting of homologous DNA recombination repair by gemcitabine. Int J Radiat Oncol Biol Phys. 2003;57:553–562. doi: 10.1016/s0360-3016(03)00503-0. [DOI] [PubMed] [Google Scholar]