Abstract
We found that β-lapachone (β-lap), a novel bioreductive drug, caused rapid apoptosis and clonogenic cell death in A549 human lung epithelial cancer cells in vitro in a dose-dependent manner. The clonogenic cell death caused by β-lap could be significantly inhibited by dicoumarol, an inhibitor of NAD(P)H:quinone oxido-reductase (NQO1), and also by siRNA for NQO1, demonstrating that NQO1-induced bioreduction of β-lap is an essential step in β-lap-induced cell death. Irradiation of A549 cells with 4 Gy caused a long-lasting upregulation of NQO1, thereby increasing NQO1-mediated β-lap-induced cell deaths. Although the direct cause of β-lap-induced apoptosis is not yet clear, β-lap treatment reduced the expression of p53 and NF-κB, whereas it increased cytochrome C release, caspase-3 activity, and γH2AX foci formation. Importantly, β-lap treatment immediately after irradiation enhanced radiation-induced cell death, indicating that β-lap sensitizes cancer cells to radiation, in addition to directly killing some of the cells. The growth of A549 tumors induced in immunocompromised mice could be markedly suppressed by local radiation therapy when followed by β-lap treatment. This is the first study to demonstrate that combined radiotherapy and β-lap treatment can have a significant effect on human tumor xenografts.
Keywords: NQO1, radiation, β-lapachone, A549 cells, Bioreductive drug
Introduction
Certain chemotherapeutic drugs, such as mitomycin C [1–5], tirapazamine (3-amino-1,2,4-benzotriazine 1,4-di-N-oxide; SR4233) [6], E09 (3-hydroxy-5-aziridinyl-1-methyl-2-(1-H-indole-4,7-dione)-prop-β-en-α-ol) [1,7], RH1 (3-hydroxymethyl-6-methyl-2,5(diaziridinyl)-1,4 benzoquinone) [1,8], and β-lapachone (3,4-dihydro-2,2-dimethyl-2H-naphtho[1,2-b]pyran-5,6-dione; β-lap) [9–14], are prodrugs and become cytotoxic to cancer cells following bioreduction. The major enzymes involved in the bioreduction of quinone-containing drugs are as follows: NAD(P)H:quinone oxidoreductase (NQO1), known as DT-diaphorase; NAD(P)H:cytochrome P450 reductase, known as P450R; and NADH:cytochrome b5 reductase, known as b5R [1–16]. Whereas NQO1 mediates two-electron reduction, P450R and b5R mediate one-electron reduction of quinone compounds. NQO1 is most abundant among the aforementioned reductases in mammalian cells [1,9,14], and the level of NQO1 in many human tumors is much greater than that in adjacent normal tissues [16–20]. Accordingly, there have been considerable efforts to develop anticancer drugs that are activated specifically by NQO1 and, thus, are preferentially toxic to tumors [1–5].
β-lap, a quinone-containing compound originally obtained from lapacho trees in South America, is a novel anticancer drug, which has been demonstrated to possess strong cytotoxicity toward a variety of animal and human cancer cell lines in vitro and in vivo [9–13,20–25]. It also synergistically kills cancer cells when combined with paclitaxel (Taxol) [21–23], ionizing radiation [13,24–27], and heat shock [28]. There have been numerous hypotheses proposed and tested to determine the mechanism of β-lap-induced cell death. The most recently proposed hypothesis is that NQO1 mediates a two-electron reduction of β-lap using NADH and NAD(P)H as electron sources [9–13,27,28]. The two-electron reduced form of β-lap [i.e., β-lap (HQ)] is unstable and rapidly undergoes reoxidation to the original β-lap, creating futile cycling between oxidized and reduced β-lap. As a consequence, when cells are treated with β-lap, cellular NADH, NAD(P)H, and adenosine triphosphate are severely depleted, and cytoplasmic Ca2+ is increased. This chaotic environment activates Ca2+-dependent calpain or similar proteases, resulting in degradation of vital proteins, including p53 and poly(ADP-ribose) polymerase (PARP), leading to caspase-independent apoptosis [10–12]. However, under certain circumstances, the two-electron reduced β-lap may be oxidized first to a one-electron reduced intermediate (i.e., the semiquinone form of β-lap), which then induces redox cycling and generates reactive oxygen species (ROS), thereby causing caspase-dependent apoptosis [10,23]. Bentle et al. [29] have recently reported that β-lap treatment induces NQO1-dependent ROS formation, Ca2+-dependent hyperactivation of PARP-1, and DNA double-strand breaks indicated by an increase in γH2AX foci formation. However, it was also hypothesized that β-lap-induced apoptosis is due to the β-lap-induced activation of cell cycle checkpoints resulting in blockage of cell cycle progression [21–23]. Based on this checkpoint activation hypothesis, phase I/II clinical trials aiming to reveal the feasibility of using β-lap in combination with other checkpoint-inhibiting drugs against human solid tumors have recently been launched by a group of investigators [30].
Boothman et al. [24,25] and Boothman and Pardee [26] reported that β-lap inhibits the repair of potentially lethal DNA damage caused by radiation and enhances the various effects of radiation on cells. Using a split-irradiation method, we observed that β-lap sensitizes cells to radiation by inhibiting the repair of sublethal radiation damage (SLD) [13,27]. In addition, we have previously observed that synergistic cell death caused by β-lap and irradiation occurs even when β-lap treatment was delayed by as long as 10 hours after irradiation, at a time when most SLDs should have already been repaired [13,27]. Importantly, ionizing radiation causes a long-lasting elevation of NQO1 activity in cells [9,13,27]. These observations led us to hypothesize that the synergistic cell death caused by ionizing radiation and β-lap treatment results from inhibition of radiation damage repair and from sensitization to β-lap exposure by radiation-induced upregulation of NQO1 [13,27]. Related to this hypothesis, we have observed that the exposure of cancer cells to mild heating at 41°C to 42°C for 1 hour increased NQO1 activity and cell sensitivity to β-lap for over 24 hours after heating [28]. We also observed that the growth of experimental tumors in syngeneic mice could be significantly suppressed by combining β-lap treatment with irradiation [13] or hyperthermia [28]. These results clearly demonstrated that β-lap is a potentially useful bioreductive drug, particularly in combination with conventional cancer treatment regimens.
In our previous studies [13,27,28], we have used dicoumarol [3-3′ methylene-bis(4 hydroxycoumarin)], an inhibitor of NQO1, to investigate the mechanism of NQO1 in β-lap-induced cell death. Unfortunately, dicoumarol demonstrates other activities besides antagonizing NQO1 function in cells. Therefore, in the present study, we used siRNA-NQO1, in addition to dicoumarol, to more rigorously test our hypothesis on the relationship between the NQO1-mediated cytotoxic effects and the NQO1-mediated radiosensitizing effects of β-lap on A549 human lung epithelial cancer cells. We also studied the kinetics of apoptosis development in A549 cells after β-lap treatment and investigated DNA double-strand breaks caused by β-lap alone or in combination with ionizing radiation by examining γH2AX expression [31–33]. Lastly, we studied the response of A549 xenograft tumors grown in nude mice to β-lap treatment alone or in combination with radiation therapy.
Materials and Methods
Cells and β-Lap
A549 human lung cancer cells purchased from the American Type Culture Collection (Manassas, VA) were used. Cells were cultured using Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (Hyclone Laboratories, Inc., Logan, UT), penicillin (50 U/ml), and streptomycin (50 µg/ml) at 37°C under a humidified 95% air-5% CO2 atmosphere. All experiments were conducted using cells in exponential growth phase by seeding cells into culture flasks 15 to 18 hours before use. β-lap purchased from a commercial source (Sigma Chemical Co., St. Louis, MO) was dissolved in dimethyl sulfoxide solution at 10 mM and diluted to desired concentrations in a culture medium immediately before use.
Clonogenic Cell Death By β-Lap and Irradiation Alone or in Combination
Cells were plated in 25-cm2 plastic T-type culture flasks with 5 ml of complete DMEM, incubated overnight at 37°C, and treated with β-lap for varying lengths of time at 37°C. After washing twice with a culture medium, the cells were incubated at 37°C with a complete medium for 8 to 9 days; resultant colonies were fixed with a mixture of methanol and acetic acid (10:1, vol/vol) and stained with 1% crystal violet. The number of colonies containing > 50 cells was counted, and the surviving fraction of clonogenic cells was calculated. For the study of radiation effects on clonogenic survival, cells were irradiated with a Cesium-137 irradiator (Model 68; J. L. Shepherd and Associates, Glenwood, CA) at a dose rate of 0.9 Gy/min and incubated for 8 to 9 days; colonies were counted; and the surviving fraction of clonogenic cells was obtained. When cells were irradiated first and later treated with β-lap, cells were maintained at 37°C during the interval between the two treatments.
Effect of Dicoumarol and siRNA-NQO1 on β-Lap Cytotoxicity
Effect of dicoumarol
Cells were incubated with 50 µM dicoumarol (NQO1 inhibitor; Sigma Chemical Co.) for 30 minutes and then treated with 5 µM β-lap for 4 hours at 37°C [9,10,13,17]. As controls, cells were treated with β-lap alone or with dicoumarol alone. After washing, the cells were cultured for 8 to 9 days, and the surviving fraction of clonogenic cells was calculated.
Effect of siRNA-NQO1
siRNA targeting NQO1 (National Center for Biotechnology Information accession no. NM000903) was synthesized by Ambion, Inc. (Austin, TX; Ambion ID no. 212878). siRNA was 21 nucleotides long and contained symmetric 3′ overhangs of two deoxythymidines. A549 cells were seeded in a 3.5-cm-diameter well of a six-well culture plate (Sarstedt, Newton, NC) at a concentration of 4 x 105 cells with 2 ml/well medium, incubated overnight, and transfected with siRNA-NQO1. siRNA-NQO1 was diluted with a serum-free medium to a final concentration of 100 nM, and siPORT NeoFX (Ambion, Inc., Austin, TX) was diluted with a serum-free medium at a ratio of 1:20 [34–36]. The diluted siRNA was mixed with the same volume of diluted siPORT NeoFX and incubated for 10 minutes at room temperature. To each culture well in which cells were seeded with 2 ml of medium, 200 µl of the siRNA-NQO1 transfection mixture complex was added and cultured for 48 hours at 37°C. Transfected cells were then harvested, and their sensitivity to β-lap was investigated.
Western Blot Analysis
Cells were dissolved in solubilizing buffer [pH 7.4; 1% Triton X-100, 0.1% sodium dodecyl sulfate (SDS), 20 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 1 mM sodium orthovanadate, 1 mM sodium fluoride, 2 mM phenylmethanesulfonylfluoride, 10 mM iodoacetamide, 10 µg/ml aprotinin, and 10 µg/ml leupeptin]. Aliquots containing 50 µg of protein were subjected to electrophoresis on 7.5% SDS polyacrylamide gel electrophoresis, and separated polypeptides were transblotted onto Hybond-P (Amersham Life Sciences, Arlington Heights, IL) in a transfer buffer (192 mM glycine, 25 mM SDS, and 10% methanol). Blots were then blocked with 3% nonfat dry milk in Tris-buffered saline Tween-20 (pH 7.4) incubated with one of the following: anti-NQO1 antibody (1:1000 dilution; KOMA Biotech, Seoul, South Korea); anti-p53 antibody (1:500 dilution; Calbiochem, San Diego, CA); anti-NF-κB antibody (1:1000 dilution; Zymed, San Francisco, CA); or anti-cytochrome C antibody (1:1000 dilution; UBI, Lake Placid, NY). The blots were then treated with horseradish peroxidase-conjugated anti-mouse or antigoat IgG secondary antibody (1:1000 dilution; Santa Cruz Biotech, Santa Cruz, CA), and immunoreactive bands were visualized using chemiluminescence [13,37].
Caspase-3 Activity
Caspase-3 activity was determined using the BD ApoAlert Caspase-3 Fluorescent Assay Kit (BD Biosciences Clontech, Mountain View, CA). Briefly, cells were suspended in 1 ml of lysis buffer on ice for 30 minutes and centrifuged at 15,000g for 20 minutes at 4°C, and supernatants were collected. The reaction buffer, consisting of 50 µM DEVD-AFC Asp-Glu-Val-Asp-AFC fluorophore (caspase-3 substrate) and 2 mM dithiothreitol, was added to the supernatants and then incubated at 37°C for 3 hours. Fluorescence from lysates was measured with a fluorometer (Molecular Devices, Palo Alto, CA) with a 400-nm excitation filter and a 505-nm emission filter [34].
Gel Electrophoresis for Apoptotic DNA Fragmentation
Cells were treated overnight with lysis buffer [10 mM Tris-HCl, pH 7.4; 10 mM NaCl; 10 mM EDTA; 0.1 mg/ml proteinase K; and 1% (wt/vol) sodium dodecyl sulfate] at 48°C. A cold (4°C) 5-M NaCl solution was then added to the lysate, vortexed for several seconds, and centrifuged at 10,000g for 5 minutes. The supernatant was mixed with isopropanol (1:1) and incubated overnight at -20°C. After centrifugation at 12,000g for 20 minutes, pellets were suspended in TE buffer (10 mM Tris-HCl, pH 7.4; and 1 mM EDTA), and RNA was digested by mixing with 0.2 mg/ml DNase-free RNase. An aliquot of 15 to 20 µg of DNA from each sample and a DNA molecular weight marker were subjected to electrophoresis on a 1.5% agarose gel in TBE (89 mM Tris base, 89 mM boric acid, and 2 mM EDTA) and stained with ethidium bromide [37].
NQO1 Expression Study with Confocal Microscopy
Cells grown on tissue culture chamber slides were irradiated with 4 Gy, rinsed with phosphate-buffered saline (PBS) at various times thereafter, and fixed with a mixture of acetone and methanol (1:1) for 20 minutes. After blocking with 1% bovine serum albumin, cells were incubated with anti-NQO1 antibody (1:100 dilution in PBS; KOMA Biotech) for 2 hours, rinsed, and incubated for 1 hour with secondary antibody (anti-mouse IgG) conjugated with fluorescein isothiocyanate (FITC; Jackson ImmunoResearch, West Grove, PA). Labeled cells were rinsed four times with PBS, and NQO1 expression was assessed by visualizing with a laser scanning confocal microscope [13].
NQO1 Enzyme Activity
Cells were washed twice with phenol red-free Hank's balanced salt solution, resuspended in PBS (pH 7.2) containing 10 µg/ml aprotinin, sonicated four times using 10-second pulses on ice, and centrifuged at 14,000g for 20 minutes. The resulting S9 supernatants were collected, transferred into microcentrifuge tubes, and stored at -80°C until NQO1 enzyme activity has been assessed, as previously described [10,13]. Briefly, the reaction medium contained 77 µM cytochrome C (Sigma Chemical Co.) as substrate, 200 µM NADH as immediate electron donor, 10 µM menadione as intermediate electron acceptor, and 0.14% bovine serum albumin in Tris-HCl buffer (50 mM, pH 7.5). Assays were initiated by adding S9 supernatants to the reaction mixture, and enzyme activity was calculated as nanomoles of cytochrome C reduced per minute per milligram of protein, based on the initial rate of change in optical density at 550 nm, read with a Beckman DU 640 spectrophotometer (Beckman Coulter, Fullerton, CA). The extinction coefficient for cytochrome C was 21.1 mM/cm at 37°C. Each assay was performed in the presence and in the absence of dicoumarol, and the activity that was reduced by dicoumarol was taken as NQO1 activity [10,13,14,38].
Flow Cytometric Analysis of Apoptosis
Cells were fixed overnight with 80% (vol/vol) ethanol at 4°C, centrifuged, washed with PBS, and resuspended in 2 ml of PBS containing 30 U of DNase-free RNase. A 5-µg aliquot of propidium iodide in 100 µl was added and incubated in the dark at 37°C for 1 hour, and fluorescence was measured using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA). The fraction of cells in sub-G1 phase (i.e., apoptotic cells and those in other cell cycle phases) was estimated from cellular DNA content [37,39].
Determination of γH2AX Expression
Cells were plated on Lab-Tek chamber slides (Naperville, IL) coated with 0.2% gelatin, incubated overnight at 37°C, and treated with β-lap and irradiation alone or in combination. At various times after treatment, cells were gently rinsed with PBS and fixed with 4% paraformaldehyde for 20 minutes. After washing with PBS twice, cells were incubated with 1% bovine serum albumin (BSA) in PBS for 1 hour and then with monoclonal anti-γH2AX antibody (1:500 dilution in 1% BSA; UBI) for 2 hours at 4°C. After washing twice in PBS, the cells were treated with secondary antibody (anti-mouse IgG) conjugated with FITC (1:500 dilution in 1% BSA; Jackson Immuno-Research) for 1 hour at 37°C, and then washed with PBS containing 0.1% Tween-20 (PBST). The cells were then covered with cover slips using Vectashield mounting medium (Vector Laboratories, Burlingame, CA), and γH2AX foci were examined with a confocal fluorescent microscope. The number of γH2AX foci in each cell was counted in > 60 cells from each experimental group [31–33].
Antitumor Effect of β-Lap
Tumor and β-lap treatment
Eight-week-old female athymic (nu/nu) mice were obtained from Charles River Laboratories (Wilmington, MA) and maintained in microisolator cages in pathogen-free conditions, with laboratory chow and water available ad libitum. A549 cells in exponential growth phase were harvested from culture using trypsin treatment and washed, and 6 x 106 cells in 0.1 ml were injected subcutaneously into the right hind leg of each mouse. Two weeks later, when the tumors had grown to 80 to 120 mm3, they were irradiated with 10-Gy X-rays in a single exposure, and host mice were immediately injected intraperitoneally with 50 mg/kg β-lap dissolved in 0.2 ml of β-hydroxypropyl-β-cyclodextrin (Sigma Chemical Co.). The effects of 10-Gy irradiation or β-lap alone on tumor growth were also studied. Tumor diameters were measured with a caliper, and tumor volumes were obtained using the formula: V = a2b/2, where a is the shortest tumor diameter and b is the longest tumor diameter. Mice were euthanized by CO2 aspiration when tumor volume had reached 1.5 cm3. All tumor experiments were performed following the protocol approved by the University of Minnesota Institutional Animal Care Use Committee (protocol no. 0112A13064).
X-irradiation of tumors
Mice were anesthetized with an intraperitoneal injection of a mixture of 100 mg/kg ketamine and 10 mg/kg xylazine. With the exception of tumor-bearing legs, mice were covered with a 4-mm-thick lead shield, and tumors were irradiated with 250-kVp orthovoltage X-rays with added filtration of 0.35 mm of Cu (Philips Medical System, Brookfield, WI) at a dose rate of 1.4 Gy/min.
Statistical Analysis
Two-tailed Student's t test was used to determine the statistical significance of all data sets.
Results
Effect of β-Lap on Clonogenic Cell Survival
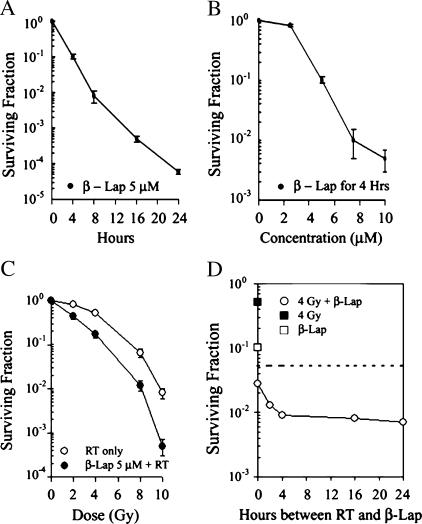
As shown in Figure 1A, treating A549 human lung cancer cells with 5 mM β-lap decreased clonogenic cell survival as a function of incubation time: incubation for 4 and 8 hours reduced clonogenic survival to about 10% and 1.0%, respectively. Figure 1B shows clonogenic cell death caused by a 4-hour treatment with different concentrations of β-lap. Treatment with 2.5 µM β-lap for 4 hours caused little change in cell survival; however, treatment with 5 and 7.5 µM β-lap for 4 hours decreased cell survival to about 10% and 1.0%, respectively.
Figure 1.
Effect of β-lap alone or in combination with irradiation on the survival of A549 cells. (A) Survival curve of A549 cells treated with 5 µM β-lap for various lengths of time. A549 cells were treated with 5 µM β-lap for 4 to 24 hours at 37°C and cultured for 7 to 9 days, and surviving fractions were calculated. The averages of seven experiments with 1 SE are shown. (B) Survival curve of A549 cells treated with different concentrations of β-lap for varying lengths of time. Cells were treated with 2 to 10 µM β-lap for 4 to 24 hours at 37°C and cultured for 7 to 9 days, and surviving fractions were calculated. The averages of seven experiments with 1 SE are shown. (C) Survival curves of cells treated with radiation alone or in combination with β-lap. Cells were treated with different doses of radiation alone (RT only) or irradiated and immediately incubated with 5 µM β-lap for 4 hours (β-lap 5 µM + RT). The cells were then cultured for 7 to 9 days, and surviving fractions were calculated. The survival curve for the combined treatment was normalized for cell deaths caused by β-lap alone. The averages of seven experiments with 1 SE are shown. (D) Effects of β-lap treatment applied at different times after the irradiation of cells. Cells were treated with 4-Gy radiation alone (4 Gy) at 4-hour incubation with 5 µM β-lap alone (β-lap), or irradiated with 4 Gy and treated with 5 µM β-lap for 4 hours at different times after irradiation (4 Gy + β-lap). Cells were then incubated for 7 to 9 days, and surviving fractions were calculated. The averages of six experiments with 1 SE are shown. The dotted line corresponds to a hypothetical cell survival when the combined effect of 4-Gy irradiation and 5 µM β-lap treatment is assumed to be additive.
Combined Effect of β-Lap and Irradiation on Clonogenic Cell Survival
Figure 1C shows the survival curve of A549 cells treated with radiation alone or of cells irradiated first and then immediately exposed to 5 µM β-lap for 4 hours. The radiation-survival curve of cells treated with irradiation immediately followed by β-lap treatment, particularly in the initial shoulder region, was steeper than that of cells receiving irradiation alone. This result suggested that repair of SLD is reduced when irradiated cells are immediately treated with β-lap. The combined effect of the two modalities was further elucidated by irradiating the cells with 4 Gy and then treating the cells with 5 µM β-lap for 4 hours at various times after irradiation. As presented in Figure 1D, the clonogenic cell death caused by irradiation immediately followed by a 4-hour incubation with β-lap treatment was significantly greater than the cell death expected to occur had the two modalities acted merely additively (dotted line) (P < .05). The survival of cells that were irradiated first and then treated with β-lap decreased even further as the time interval between irradiation and β-lap treatment was increased to 4, 16, or 24 hours.
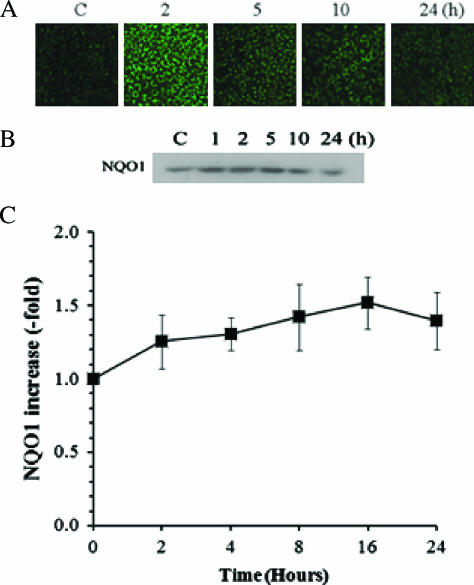
Effect of Irradiation on NQO1 Activity
Both immunostaining of cells (Figure 2A) and Western blot analysis (Figure 2B) showed that a considerable amount of constitutive NQO1 exists in A549 cells and that 4-Gy irradiation caused a significant increase in NQO1 expression for 2 to 24 hours. The enzyme activity of NQO1 before irradiation, as determined by the amount of NQO1-induced reduction in cytochrome C [10,13], was 9.5 ± 1.3 µmol of cytochrome C reduced per minute per milligram of protein. NQO1 activity progressively increased, reaching about 1.5-fold of the control value 16 to 24 hours after 4-Gy irradiation (Figure 2C).
Figure 2.
Effects of radiation on the expression and enzymatic activity of NQO1 in A549 cells. (A) NQO1 expression at various times after 4-Gy irradiation in A549 cells. Control and irradiated cells were labeled with anti-NQO1 antibody, followed by labeling with secondary antibody conjugated with FITC and examined with confocal microscopy. (B) Typical example of Western blot analysis for NQO1 at various times after 4-Gy irradiation in A549 cells. The cells were dissolved in solubilizing buffer, and lysates were subjected to Western blot analysis. Blots from control and irradiated cells were labeled with anti-NQO1 antibody and then with horseradish peroxidase-conjugated secondary antibody, and immunoreactive bands were visualized using chemiluminescence. (C) Enzymatic activity of NQO1 at various times after 4-Gy irradiation in A549 cells. Control and irradiated cells were sonicated, and enzyme activity in S9 supernatants was determined. The enzyme activity in irradiated cells relative to that in control cells (9.5 ± 1.3 µmol of cytochrome C reduced per minute per milligram of protein) is shown. The averages of six experiments with 1 SE are shown.
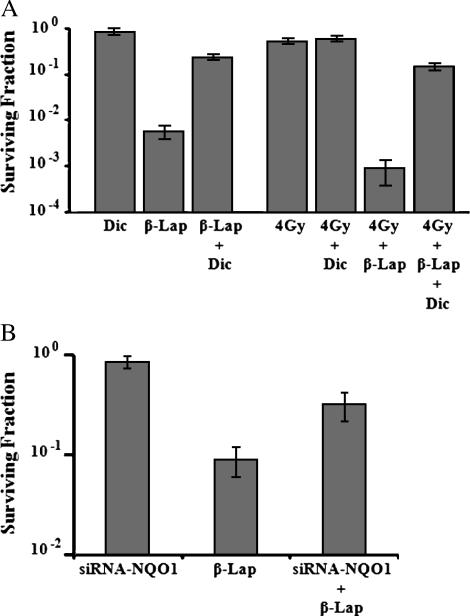
Suppression of β-Lap Cytotoxicity By Dicoumarol and siRNA-NQO1
Figure 3A shows that a 4-hour incubation with 50 µM dicoumarol, an inhibitor of NQO1, exerted little effect on clonogenic cell survival. When cells were incubated with 10 µM β-lap for 4 hours, 0.51 ± 0.18% of cells survived, whereas as much as 25.9 ± 2.9% of cells survived when cells were incubated with 10 µM β-lap and 50 µM dicoumarol. A 4-hour incubation with 50 µM dicoumarol starting immediately after 4-Gy irradiation exerted no influence on radiation-induced cell death. Irradiation with 4 Gy followed by a 4-hour incubation with 10 µM β-lap reduced cell survival to 0.09 ± 0.05%, whereas irradiation with 4 Gy followed by a 4-hour incubation with 10 µM β-lap, together with 50 µM dicoumarol, reduced cell survival to 14.5 ± 3.0%. Pertinent to this observation is that NQO1 activity in A549 cells treated with 50 µM dicoumarol for 4 hours was about 74% that of control cells (data not shown). Figure 3B shows that transfection with siRNA-NQO1 exerted no effect on clonogenic cell survival, whereas siRNA-NQO1 significantly reduced the cytotoxicity of β-lap. The survival of cells transfected with siRNA-NQO1 was about three-fold greater than that of nontransfected cells after treatment with 5 µM β-lap for 4 hours.
Figure 3.
Effects of dicoumarol or siRNA-NQO1 on β-lap-induced clonogenic death in A549 cells. (A) Effects of dicoumarol: Dic—cells were treated with 50 µM dicoumarol for 4 hours; β-lap—cells were treated with 10 µM β-lap for 4 hours; β-lap + Dic—cells were treated with 10 µM β-lap plus 50 µM dicoumarol for 4 hours; 4 Gy—cells were irradiated with 4 Gy; 4 Gy + Dic—cells were irradiated and then treated with 50 µM dicoumarol for 4 hours; 4 Gy + β-lap—cells were irradiated with 4 Gy and immediately incubated with 10 µM β-lap for 4 hours; 4 Gy + β-lap + Dic—cells were irradiated with 4 Gy and immediately treated with 10 µM β-lap plus 50 µM dicoumarol for 4 hours. Cells were washed after the treatments and incubated for 7 to 9 days, and surviving fractions were calculated. The averages of six experiments with 1 SE are shown. (B) Effects of siRNA-NQO1: siRNA-NQO1—cells transfected with siRNA-NQO1 were incubated with regular medium for 4 hours; β-lap—cells were incubated with 5 µM β-lap for 4 hours; siRNA-NQO1 + β-lap—cells transfected with siRNA-NQO1 were incubated with 5 µM β-lap for 4 hours. After the treatments, cells were washed and incubated for 7 to 9 days, and surviving fractions were calculated. The averages of four experiments with duplicate cultures with 1 SE are shown.
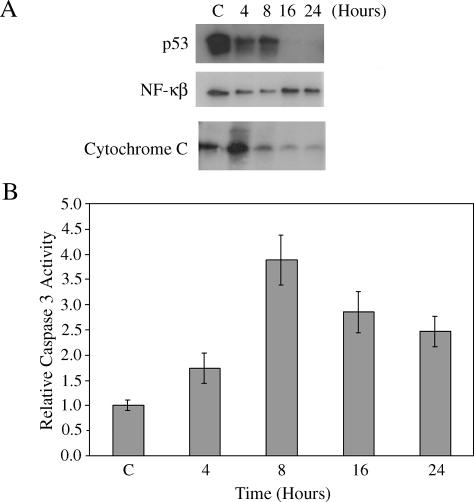
Effects of β-Lap on the Expression of p53 and NF-κB, and on the Release of Cytochrome C
Figure 4 shows the effect of a 4-hour incubation of cells with 5 µM β-lap on the expression of p53 and NF-κB, and on the release of cytochrome C. p53 expression markedly declined immediately after β-lap treatment and had almost completely vanished by 16 hours (i.e., 12 hours after β-lap treatment). NF-κB expression immediately after β-lap treatment was significantly less than that before treatment, but it slightly recovered at 16 hours. The release of cytochrome C increased markedly immediately after β-lap treatment, but progressively decreased from 4 to 20 hours after β-lap treatment.
Figure 4.
Changes in the expression of p53 and NF-κB, release of cytochrome C, caspase-3 activity and DNA laddering by β-lap, or radiation exposure in A549 cells. (A) Cells were treated with 5 µM β-lap for 4 hours, harvested at different times, and washed. The cells were then dissolved in solubilizing buffer, and lysates were subjected to Western blot analysis. The hours shown are those from the start of a 4-hour β-lap incubation. Thus, “4 hours” implies 0 hour after 4 hours of incubation with β-lap. The result shown is representative of five individual experiments. (B) Cells were treated with 5 µM β-lap for 4 hours, harvested, washed, and lysed. Caspase-3 activity was determined using a commercially available assay kit (see text). Relative changes in caspase-3 activity are shown. The hours shown are those from the start of a 4-hour incubation with β-lap. The averages of five measurements with 1 SE are shown.
Effect of β-Lap on Caspase-3 Activity
Relative changes in caspase-3 activity are presented in Figure 4B. Caspase-3 activity was slightly increased immediately after a 4-hour incubation with 5 µM β-lap and increased further to about four times that of control at 8 hours (i.e., 4 hours after β-lap treatment; P > .05). Caspase-3 activity then slowly decreased to about 2.5-fold higher than that of control by 24 hours after β-lap exposure.
Apoptosis Caused By β-Lap Alone or in Combination with Irradiation
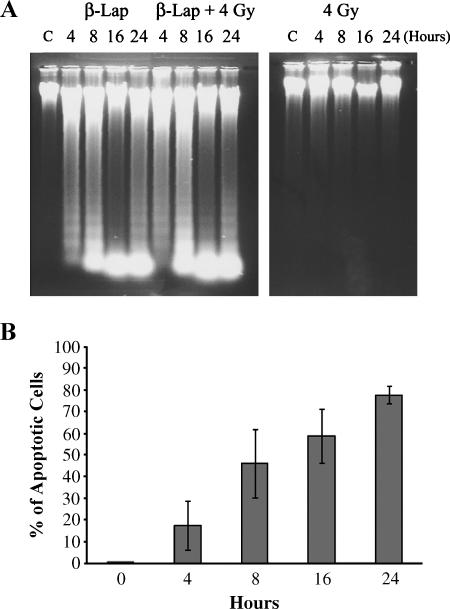
As shown in Figure 5A, a significant degree of apoptotic DNA fragmentation could be seen immediately after a 4-hour incubation with 5 µM β-lap, which progressively increased thereafter. On the contrary, no significant DNA fragmentation was observed by 24 hours after 4-Gy irradiation. The degree of DNA fragmentation in cells that were exposed to β-lap and irradiated with 4 Gy was similar to that in cells exposed to β-lap alone. Figure 5B shows changes in the percentage of apoptotic cells after β-lap treatment, which was obtained based on DNA content as measured with the flow cytometry method. The apoptotic cell population (i.e., cells in sub-G1 phase) progressively increased after a 4-hour incubation with 5 µM β-lap. About 78% of cells were apoptotic at 24 hours (i.e., 20 hours after β-lap treatment).
Figure 5.
Apoptosis caused by β-lap alone or in combination with irradiation in A549 cells. (A) β-Lap—cells were treated with 5 µM β-lap for 4 hours; β-lap + 4 Gy—cells were irradiated with 4 Gy and immediately incubated with 5 µM β-lap for 4 hours; 4 Gy—cells were irradiated with 4 Gy. Cells were washed, lysed, and subjected to agarose electrophoresis for detection of DNA fragmentation. The times shown for β-lap treatment groups are hours from the start of a 4-hour incubation with β-lap. (B) Percentage of apoptotic cells after a 4-hour incubation with 5 µM β-lap. Apoptosis was determined with flow cytometry method. The cells in sub-G1 phase were taken as apoptotic cells. The hours indicate the time from the start of a 4-hour incubation with β-lap. The averages of four experiments with 1 SE are shown.
Effects of β-Lap and Radiation on γH2AX
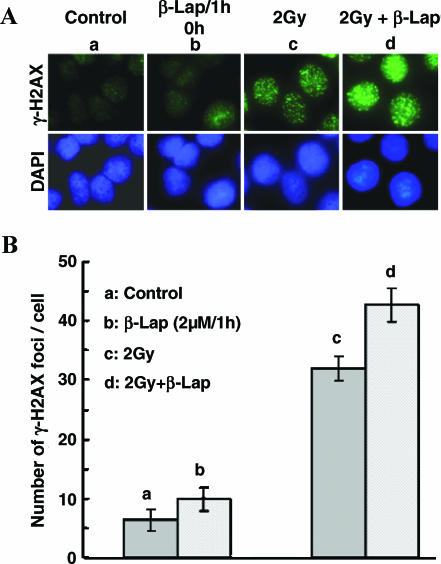
Irradiation of A549 cells with 2 Gy significantly increased the formation of γH2AX foci (a hallmark of DNA double-strand break detection by cells) within 1 hour of exposure (P < .05). The γH2AX foci formation immediately after treatment with 2 µM β-lap for 1 hour was only slightly elevated (Figure 6, A and B), although it significantly increased at 4 hours after treatment (data not shown). The γH2AX foci formation in cells exposed to 2-Gy irradiation and treated with 2 µM β-lap for 1 hour was slightly greater than that in cells treated with irradiation alone.
Figure 6.
Effects of irradiation and β-lap alone or combined on γH2AX foci formation in A549 cells. (A) Upper panel: γH2AX foci expression in representative cells from each experimental group; lower panel: the nucleus of corresponding cells stained with 4′,6-diamidino-2-phenylindole. (a) Control cells; (b) 1-hour treatment with 2 µM β-lap; (c) 1 hour after 2-Gy irradiation; (d) 2-Gy irradiation followed by 1-hour incubation with 2 µM β-lap. (B) Number of γH2AX foci per cell. (a) Control cells; (b) 1-hour treatment with 2 µM β-lap; (c) 1 hour after 2-Gy irradiation; (d) 2-Gy irradiation followed by 1-hour incubation with 2 µM β-lap. The averages of four experiments with 1 SE are shown.
Tumor Growth
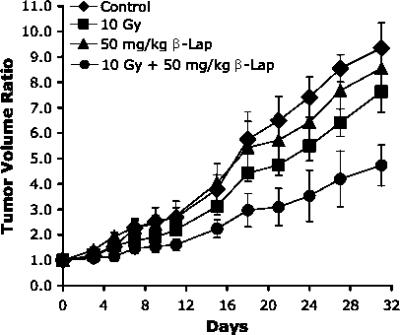
The relative volume of A549 tumors as a function of days after treatment is shown in Figure 7. Tumor growth was slightly suppressed by a single intraperitoneal injection of 50 mg/kg β-lap and was noticeably suppressed by a single 10-Gy irradiation. However, tumor growth was markedly suppressed when the tumors were first irradiated with 10 Gy and the host mice were subsequently injected intraperitoneally with 50 mg/kg β-lap. The volume of control and that of β-lap-treated tumors increased four times in 15.2 ± 1.7 and 15.4 ± 2.4 days, respectively, and the volume of irradiated tumors increased four times in 16.8 ± 1.4 days. The volume of tumors receiving the combined treatment increased four times in 25.7 ± 3.5 days, which was nearly 10 days longer than that caused by irradiation or β-lap treatment alone (P < .05).
Figure 7.
Effects of various treatments on the growth of A549 human lung tumor xenografts in the hind legs of nude mice. 10 Gy—tumors were locally irradiated with 10 Gy in a single exposure; 50 mg/kg β-lap—the host mice were injected intraperitoneally with β-lap at 50 mg/kg; 10 Gy + 50 mg/kg β-lap—tumors were locally irradiated with 10 Gy in a single exposure and then the host mice were injected intraperitoneally with β-lap at 50 mg/kg. The averages of five to seven tumors per group with 1 SE are shown.
Discussion
We have demonstrated that the combination of irradiation with the bioreductive drug β-lap effectively kills A549 human lung cancer cells in vitro and also suppresses the growth of A549 xenografts—a result that has not been reported yet in human tumor models. Treatment of A549 cells with β-lap alone in vitro caused clonogenic cell death (Figure 1, A and B) and rapid apoptosis (Figure 5). Furthermore, β-lap synergistically interacted with ionizing radiation in causing clonogenic cell death (Figure 1, C and D). Killing of A549 cells by β-lap alone or in combination with radiation could be significantly suppressed with dicoumarol, an inhibitor of NQO1 (Figure 3A), indicating that NQO1 is an important mediator of cell death caused by β-lap alone or in combination with ionizing radiation. Dicoumarol is not specific against NQO1 [40] and, thus, it was possible that dicoumarol reduced the cytotoxicity of β-lap through mechanisms other than inhibition of NQO1 activity. Therefore, we further investigated the role of NQO1 in β-lap-induced cell death using siRNA-NQO1 to transiently suppress NQO1 expression in A549 cells. As shown in Figure 3B, siRNA-NQO1 could significantly reduce the effect of β-lap to cause clonogenic cell death. These results clearly demonstrated that NQO1 is a critical determinant for cell deaths caused by β-lap alone and in combination with irradiation in A549 human lung cancer cells.
It has been suggested that futile cycling between an oxidized and a two-electron reduced form of β-lap, mediated by NQO1 and requiring NADH and NAD(P)H as electron sources, causes depletion or oxidation of NADH and NAD(P)H, resulting in activation of Ca2+-dependent calpain or similar proteases [11,12,29,41]. In cells treated with β-lap, vital proteins such as p53 and PARP were found to be degraded, probably due to the β-lap-induced activation of calpain or similar proteases [11,12,29]. As shown in Figure 4A, we also observed that β-lap treatment markedly reduced p53 expression in A549 human lung cancer cells. It has been previously reported that p53 is stabilized by NQO1 and, thus, inhibition of NQO1 led to degradation of p53 [42]. It may then be hypothesized that β-lap, a substrate of NQO1, deprives NQO1 of its ability to protect p53 from calpain or similar proteases. We are currently testing this hypothesis by studying the effect of calpain inhibitors on β-lap-induced downregulation of p53. As shown in Figure 5, as much as 60% to 80% of cells were already apoptotic at 16 to 24 hours after β-lap treatment. It is, therefore, probable that the lack of p53 expression 16 to 24 hours after β-lap treatment (Figure 3A) was due to apoptotic disintegration of cells. Massive release of cytochrome C and inactivation of NF-κB (Figure 4A) may account for the activation of caspase-3 and the rapid induction of apoptosis after β-lap exposure, as determined with the DNA laddering method (Figure 5A) and the flow cytometry method (Figure 5B). However, activation of caspase-3 in A549 cells by β-lap treatment is at odds with other reports [9–12,29] indicating that the effect of β-lap on p53 expression may be dependent on cell line.
The slope of the initial part of the radiation-survival curve of cells irradiated and immediately treated with β-lap was steeper than that of cells receiving irradiation only (Figure 1C), which indicates that β-lap increased the radiosensitivity of cells. With the use of split-dose irradiation, we have previously demonstrated that β-lap inhibits SLD repair in rodent cancer cell lines [13,27]. Inhibition of SLD repair may account for the greater-than-additive clonogenic cell death that occurs when cells are irradiated and immediately treated with β-lap (0-hour interval between 4-Gy irradiation and β-lap treatment, as shown in Figure 1D). However, inhibition of SLD repair by β-lap could not account for the synergistic cell death caused by irradiation and β-lap treatment applied 4 to 24 hours after irradiation because repair of SLD is usually completed in 2 to 4 hours. This observation supports our hypothesis [13,27] that the synergistic cell death caused by exposure to radiation and β-lap treatment applied 4 to 24 hours later is due to a long-lasting upregulation of NQO1 caused by radiation exposure.
It has been demonstrated that DNA double-strand breaks lead to phosphorylation of H2AX at serine 139, thereby forming γH2AX [31–33]. As shown in Figure 6, significant γH2AX foci formation could be observed immediately after 2-Gy irradiation in A549 cells. However, γH2AX foci formation was negligible when examined immediately after treatment with 2 µM β-lap for 1 hour, although significant γH2AX foci formation was observed 4 hours after a 1-hour treatment with 2 µM β-lap (data not shown), which indicates that β-lap is a potent inducer of DNA DSB, as recently reported by other investigators [29]. The γH2AX foci formation in cells irradiated with 2 Gy and treated with 2 µM β-lap for 1 hour was slightly greater than that in cells receiving irradiation alone. However, the combined effect of 2 Gy irradiation and 1-hour treatment with 2 µMβ-lap on increasing γH2AX foci formation appeared to be no more than additive. The specific relationship between γH2AX formation and induction of apoptosis caused by β-lap treatment alone or in combination with irradiation remains to be elucidated further.
In contrast to our previous results in rodent tumors [13], a single β-lap treatment at 50 mg/kg alone exerted little effect on the growth of A549 human tumor xenografts in the present study. However, combined treatment with irradiation and β-lap suppressed the growth of A549 human lung tumor xenografts to a greater-than-additive extent (Figure 7). It should be noted that radiation-induced upregulation of NQO1 and the resulting increase in β-lap toxicity, as seen in our in vitro studies (Figure 1D), probably did not play a significant role in the synergistic antitumor effect in the present in vivo study because β-lap treatment was administered immediately after tumor irradiation and, thus, β-lap was likely cleared before radiation-induced upregulation of NQO1 became significant. Therefore, we suspect that synergistic suppression of tumor growth was due mostly to β-lap-induced radiosensitization rather than to an increase in direct β-lap toxicity mediated by NQO1. The effect of β-lap injections several hours after tumor irradiation, as well as the effect of multiple β-lap injections, is currently being studied in our laboratory.
We conclude that NQO1 is an important player in cell death caused by the naturally occurring product β-lap and that ionizing radiation synergistically increases β-lap-induced clonogenic cell death by upregulating NQO1. Lastly, β-lap increases cellular radiosensitivity by inhibiting SLD repair. Importantly, our results clearly demonstrate for the first time that combined radiation and β-lap therapy has a significant antitumor effect on human tumor xenografts. Therefore, further study of this therapeutic mechanism is warranted considering that NQO1 levels in many human tumors have been documented to be much higher than those in adjacent normal tissues [1,2,16–20]. It may also be valuable to investigate the feasibility of specifically exploiting radiation-induced NQO1 activity to enhance the cytotoxicity of bioreductive drugs other than β-lap, such as mitomycin C.
Footnotes
This work was supported by grants from the Molecular and Cellular BioDiscovery Research Program (2006) of the Ministry of Science and Technology, Korean Government, and Korea Research Foundation (KRF-2005-042-E00107); the National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (0620340-1); and the National Institutes of Health, United States (RO1 CA116721).
References
- 1.Begleiter A, Fourie J. Induction of NQO1 in cancer cells. Methods Enzymol. 2004;382:321–351. doi: 10.1016/S0076-6879(04)82018-4. [DOI] [PubMed] [Google Scholar]
- 2.Rauth AM, Goldberg Z, Mirsa V. DT-diaphorase: possible roles in cancer chemotherapy and carcinogenesis. Oncol Res. 1997;9:339–349. [PubMed] [Google Scholar]
- 3.Ross D, Siegel D. NAD(P)H:quinone oxidoreductase 1 (NQO1, DT-diaphorase), functions and pharmacogenetics. Methods Enzymol. 2004;382:115–144. doi: 10.1016/S0076-6879(04)82008-1. [DOI] [PubMed] [Google Scholar]
- 4.Siegel D, Beall H, Senekowitach C, Kasai M, Arai H, Gibson NW, Ross D. Bioreductive activation of mitomycin C by DT-diaphorase. Biol Chem. 1992;31:7879–7885. doi: 10.1021/bi00149a019. [DOI] [PubMed] [Google Scholar]
- 5.Robertwon N, Stratford IJ, Houlbrook S, Carmichael J, Adams GE. The sensitivity of human tumour cells to quinone bioreductive drugs: what role for DT-diaphorase? Biochem Pharmacol. 1992;44:409–412. doi: 10.1016/0006-2952(92)90429-m. [DOI] [PubMed] [Google Scholar]
- 6.Brown JM. The hypoxic cell: a target for selective cancer therapy-eighteenth Bruce F. Cain Memorial Award Lecture. Cancer Res. 1994;59:5863–5870. [PubMed] [Google Scholar]
- 7.Loadman PM, Bibby MC, Phillips RM. Pharmacological approach towards the development of indolequinone bioreductive drugs used on the clinically inactive agent EO9. Br J Pharmacol. 2002;137:701–709. doi: 10.1038/sj.bjp.0704916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winski SL, Swann E, Hargreaves RH, Dehn DL, Butler J, Moody CJ, Ross D. Relationship between NAD(P)H:quinone oxidoreductase 1 (NQO1) levels in a series of stably transfected cell lines and susceptibility to antitumor quinones. Biochem Pharmacol. 2001;61:1509–1516. doi: 10.1016/s0006-2952(01)00631-1. [DOI] [PubMed] [Google Scholar]
- 9.Planchon SM, Pink JJ, Tagliarino C, Bornmann WG, Varnes ME, Boothman DA. β-lapachone-induced apoptosis in human prostate cancer cells: involvement of Nqo/xip3. Exp Cell Res. 2001;267:95–106. doi: 10.1006/excr.2001.5234. [DOI] [PubMed] [Google Scholar]
- 10.Pink JJ, Planchon SM, Tagliarino C, Varnes ME, Siegel D, Boothman DA. NAD(P)H:quinone oxidoreductase activity is the principal determinant of beta-lapachone cytotoxicity. J Biol Chem. 2000;275:5416–5424. doi: 10.1074/jbc.275.8.5416. [DOI] [PubMed] [Google Scholar]
- 11.Pink JJ, Wuerzberger-Davis S, Tagliarino C, Planchon SM, Yang X, Froelich CJ, Boothman DA. Activation of cysteine protease in MCF-7 and T47D breast cancer cells during β-lapachone mediated apoptosis. Exp Cell Res. 2000;255:144–155. doi: 10.1006/excr.1999.4790. [DOI] [PubMed] [Google Scholar]
- 12.Tagliarino C, Pink JJ, Dubyak GR, Nieminen AL, Boothman DA. Calcium is a key signaling molecule in beta-lapachone medicated cell death. J Biol Chem. 2001;276:19150–19159. doi: 10.1074/jbc.M100730200. [DOI] [PubMed] [Google Scholar]
- 13.Park HJ, Ahn KJ, Ahn SD, Choi E, Lee SW, Williams B, Kim EJ, Griffin R, Bey EA, Bornmann WG, et al. Susceptibility of cancer cells to β-lapachone is enhanced by ionizing radiation. Int J Radiat Oncol Biol Phys. 2005;61:212–219. doi: 10.1016/j.ijrobp.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Fitzsimmons SA, Workman P, Grever M, Paull K, Camalier R, Lewis AD. Reductase enzyme expression across the National Cancer Institute tumor cell line panel: correlation with sensitivity to mitomycin C and EO9. J Natl Cancer Inst. 1996;88:259–269. doi: 10.1093/jnci/88.5.259. [DOI] [PubMed] [Google Scholar]
- 15.Hodnick WF, Satorelli AC. Reductive activation of mitomycin C by NADH:cytochrome b5 reductase. Cancer Res. 1993;53:4907–4912. [PubMed] [Google Scholar]
- 16.Marin A, Lopez de Cerain A, Hamilton E, Lewis AD, Martinez-Penuela JM, Idoate MA, Bello J. DT-diaphorase and cytochrome b5 reductase in human lung and breast tumors. Br J Cancer. 1997;76:923–929. doi: 10.1038/bjc.1997.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siegel D, Franklin WA, Ross D. Immunohistochemical detection of NAD(P)H:quinone oxidoreductase in human lung and lung tumors. Clin Cancer Res. 1998;4:2065–2070. [PubMed] [Google Scholar]
- 18.Belinsky M, Jaiswal AK. NAD(P)H:quinone oxidoreductase 1 (DT-diaphorase) expression in normal and tumor tissues. Cancer Metastasis Rev. 1993;12:103–117. doi: 10.1007/BF00689804. [DOI] [PubMed] [Google Scholar]
- 19.Cresteil T, Jaiswal AK. High levels of expression of the NAD(P)H:quinone oxidoreductase (NQO1) gene in tumor cells compared to normal cells of the same origin. Biochem Pharmacol. 1991;42:1021–1027. doi: 10.1016/0006-2952(91)90284-c. [DOI] [PubMed] [Google Scholar]
- 20.Beall HD, Murphy AM, Siegel D, Hargreaves RH, Butler J, Ross D. NAD(P)H:quinone oxidoreductase (DT-diaphorase) as a target for bioreductive antitumor quinones: quinone cytotoxicity and selectivity in human lung and breast cancer cell lines. Mol Pharmacol. 1995;48:499–504. [PubMed] [Google Scholar]
- 21.Li Y, Sun X, LaMont JT, Pardee AB, Li CJ. Selective killing of cancer cells by β-lapachone: direct checkpoint activation as a strategy against cancer. Proc Natl Acad Sci USA. 2003;100:2674–2678. doi: 10.1073/pnas.0538044100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li CJ, Li YZ, Pinto AV, Pardee AB. Potent inhibition of tumor survival in vivo by beta-lapachone plus Taxol: combining drugs imposes different artificial checkpoints. Proc Natl Acad Sci USA. 1999;96:13369–13374. doi: 10.1073/pnas.96.23.13369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pardee AB, Li YZ, Li C. Cancer therapy with β-lapachone. Curr Cancer Drug Targets. 2002;2:227–242. doi: 10.2174/1568009023333854. [DOI] [PubMed] [Google Scholar]
- 24.Boothman DA, Trask DK, Pardee AB. Inhibition of potentially lethal DNA damage repair in human tumor cells by beta-lapachone, an activator of topoisomerase. Cancer Res. 1989;49:605–612. [PubMed] [Google Scholar]
- 25.Boothman DA, Greer A, Pardee AB. Potentiation of halogenated pyrimidine radiosensitizers in human carcinoma cells by beta-lapachone (3,4-dihyrdro-2,2-dimethyl-2H-naptho[1,2-b]pyran-5,6-dione), a novel DNA repair inhibitor. Cancer Res. 1987;47:5361–5366. [PubMed] [Google Scholar]
- 26.Boothman DA, Pardee AB. Inhibition of radiation-induced neoplastic transformation by beta-lapachone. Proc Natl Acad Sci USA. 1989;86:4963–4967. doi: 10.1073/pnas.86.13.4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki M, Amano M, Choi J, Park HJ, Williams B, Ono K, Song CW. Synergistic effects of radiation and β-lapachone in DU-145 human prostate cancer cells in vitro. Radiat Res. 2006;165:525–531. doi: 10.1667/RR3554.1. [DOI] [PubMed] [Google Scholar]
- 28.Park HJ, Choi EK, Choi J, Ahn K, Kim EJ, Ji IM, Kook YH, Ahn S, Williams B, Griffin RJ, et al. Heat-induced up-regulation of NAD(P)H:quinone oxidoreductase potentiates anticancer effects of β-lapachone. Clin Cancer Res. 2005;11:8866–8871. doi: 10.1158/1078-0432.CCR-05-0818. [DOI] [PubMed] [Google Scholar]
- 29.Bentle MS, Reinicke KE, Bery EA, Spitz DR, Boothman DA. Calcium-dependent modulation of poly(ADP-ribose)polymerase-1 alters cellular metabolisms and DNA repair. J Biol Chem. 2006;281:33684–33696. doi: 10.1074/jbc.M603678200. [DOI] [PubMed] [Google Scholar]
- 30.Shapiro GI, Supko JG, Ryan DP, Appelman L, Berkenblit A, Craig AR, Jones D, Yagovane D, Li C, Eder J. Phase I trial of ARQ 501, an activated checkpoint therapy (ACT) agent, in patients with advanced solid tumors. J Clin Oncol. 2005;23 NO165 (June Suppl) Abstract No: 3042. [Google Scholar]
- 31.Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-strand breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 32.Camphausen K, Burgan W, Cerra M, Oswald KA, Trepel JB, Lee MJ, Tofilon PJ. Enhanced radiation-induced cell killing and prolongation of H2AX foci expression by the histone deacetylase inhibitor MS-275. Cancer Res. 2004;64:316–321. doi: 10.1158/0008-5472.can-03-2630. [DOI] [PubMed] [Google Scholar]
- 33.Lowndes NF, Toh GW. DNA repair: the importance of phosphorylating histone H2AX. Curr Biol. 2005;15:R99–R102. doi: 10.1016/j.cub.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 34.Park C, Hong S, Jin S, Kim N, Cho K, Choen J, Ahn J, Yang J, Park R. TRAIL-mediated apoptosis in human liver cells. Cancer Res Treat. 2004;35:341–348. doi: 10.4143/crt.2003.35.4.341. [DOI] [PubMed] [Google Scholar]
- 35.Spankuch-Schmitt B, Bereiter-Hahn J, Kaufmann M, Strebhardt K. RNA effect of RNA silencing of polo-like kinase-1 (PLK1) on apoptosis and spindle formation in human cancer cells. J Natl Cancer Inst. 2002;94:1863–1877. doi: 10.1093/jnci/94.24.1863. [DOI] [PubMed] [Google Scholar]
- 36.Zhang M, Zhang X, Bai C, Chen J, Wei MQ. Inhibition of epidermal growth factor receptor expression by RNA interference in A549 cells. Acta Pharmacol Sin. 2004;25:61–67. [PubMed] [Google Scholar]
- 37.Park HJ, Lyon JC, Ohtsubo T, Song CW. Cell cycle progression and apoptosis after irradiation in an acidic environment. Cell Death Differ. 2000;7:729–738. doi: 10.1038/sj.cdd.4400702. [DOI] [PubMed] [Google Scholar]
- 38.Prochaska HJ, Santamaria AB. Direct measurement of NAD(P)H:quinone reductase from cells cultured in microtiter wells: a screening assay for anticarcinogenic enzyme inducers. Anal Biochem. 1988;169:328–336. doi: 10.1016/0003-2697(88)90292-8. [DOI] [PubMed] [Google Scholar]
- 39.Park HJ, Lee SW, Chung HS, Lee SH, Chung HS, Rhee YH, Lim BW, Ha SW, Song CW, Lee HS, et al. Influence of environmental pH on G2-phase arrest caused by ionizing radiation. Radiat Res. 2003;159:86–93. doi: 10.1667/0033-7587(2003)159[0086:ioepog]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 40.Dehn DL, Siegel D, Swann E, Moody CJ, Ross D. Biochemical, cytotoxic, and genotoxic effects of ES936, a mechanismsbased inhibitor of NAD(P)H:quinone oxidoreductase 1, in cellular systems. Mol Pharmacol. 2003;64:714–720. doi: 10.1124/mol.64.3.714. [DOI] [PubMed] [Google Scholar]
- 41.Tagliarino C, Pink JJ, Boothman DA. Calpains and apoptosis. Korean Biol Sci. 2001;5:267–274. [Google Scholar]
- 42.Asher G, Lotem J, Sachs L, Kahana C, Shaul Y. Mdm-2 and ubiquitin-independent p53 proteasomal degradation regulated by NQO1. PNAS. 2002;99:13125–13130. doi: 10.1073/pnas.202480499. [DOI] [PMC free article] [PubMed] [Google Scholar]