Abstract
The in vivo hollow fiber assay, in which semipermeable hollow fibers filled with tumor cells, are implanted into animals, was originally developed to screen for anticancer compounds before assessment in more complex tumor models. To enhance screening and evaluation of anticancer drugs, we have applied optical imaging technology to this assay. To demonstrate that tumor cells inside hollow fibers can communicate with the host mice, we have used fluorescence imaging in vivo and CD31 immunostaining ex vivo to show that angiogenesis occurs around cell-filled hollow fibers by 2 weeks after subcutaneous implantation. Bioluminescence imaging has been used to follow the number of luciferase-expressing tumor cells within implanted hollow fibers; proliferation of those cells was found to be significantly inhibited by docetaxel or irinotecan. We also used bioluminescence imaging of hollow fibers to monitor the nuclear factor κB (NFκB) pathway in vivo; NFκB activation by lipopolysaccharide and tumor necrosis factor-α was evaluated in tumor cell lines genetically engineered to express luciferase controlled by an NFκB-responsive element. These results demonstrate that optical imaging of hollow fibers containing reporter tumor cells can be used for the rapid and accurate evaluation of antitumor activities of anticancer drugs and for measurement of molecular pathways.
Keywords: Optical imaging, hollow fiber, angiogenesis, bioluminescence imaging, NFκB
Introduction
A recurrent challenge in the drug discovery of chemotherapeutic agents is to expedite the in vivo evaluation of in vitro leads (i.e., to investigate whether a compound capable of inhibiting tumor growth or acting on a target in vitro will also do so in vivo). The initial in vivo evaluation of compounds that show in vitro activity against tumor cell lines has traditionally involved transplanting these cells into immunocompromised host animals and then measuring the size of developing tumors in the presence and in the absence of the test agent. This valuable method is well-established and allows interactions between tumor cells and host animals to occur. Measurement of subcutaneous tumor size by external calipers is noninvasive; therefore, longitudinal studies are possible, but it takes some time for tumors to grow to the point that they become palpable without sacrificing animals. In addition, some cell lines, although derived from human tumors, are not tumorigenic in animal models, limiting the utility of this traditional xenograft method in studying the effects of chemotherapeutic agents on these cell lines in vivo.
Recently, tumor cell lines have been genetically engineered with constitutive bioluminescent (i.e., firefly luciferase) or fluorescent (i.e., green fluorescent protein or Ds-Red) reporter vectors; with the parallel development of instrumentation for optical imaging of live animals, it is now possible to assess the size of developing tumor xenografts before they reach a size sufficient to become palpable. In a further refinement of this approach, by replacing the constitutive promoter for an optical reporter with a responsive element of certain transcriptional factors (e.g. p53, E2F, or hypoxia-inducible factors) [1–4], it becomes possible to monitor a particular pathway or process regulated by a transcriptional factor. It is also possible to genetically reengineer bioluminescent or fluorescent proteins themselves (e.g., as fusion proteins) to respond to specific molecular process. This approach has been used to make reporters that respond to proteasome inhibition [5], to phosphorylation by particular kinases [6], or to cleavage by specific proteases such as caspases [7]. Such reporters have greatly enhanced our ability to use noninvasive molecular imaging to monitor the contribution of molecular pathways to tumorigenesis and tumor progression.
The in vivo hollow fiber assay was developed by Hollingshead et al. [8] at the National Cancer Institute as a tool for the preliminary screening of novel anticancer drugs before assessment in more complex tumor models. In this assay, semipermeable hollow fibers filled with tumor cells are implanted into the subcutaneous or intraperitoneal compartments of host mice. The mice are then treated with novel compounds to evaluate the in vivo activity of these compounds and their potential to inhibit cell growth in vivo. Hollow fibers are retrievable for subsequent assessment of cell viability (MTT assay) [9], flow cytometry [10], histology [11], and/or Western blot analysis [12]. The hollow fiber assay was not meant to replace classic xenograft systems, in part because it does not model the complex interactions and phenomena that occur when tumor cells grow in and interact with normal host tissues. However, in comparison with a traditional tumor xenograft model, the hollow fiber assay does offer several advantages: 1) it allows retrieval of tumor cells uncontaminated by host cells for subsequent analysis; 2) it permits shortened evaluation time and, therefore, reduced consumption of test compounds; 3) there is no significant change in the volume of the implant or in the weight of the animal; 4) there is no limitation on cell type (nontumorigenic cells can be evaluated); and 5) it allows for multiplexing (several hollow fibers, each filled with a different cell type, can be implanted in one animal, and the in vivo effects of a test compound on these cell types can be evaluated in simultaneously) [1]. Unlike the xenograft model, tumor cells are separated from surrounding tissues by a semipermeable fiber wall, limiting the complex interaction between tumor cells and host tissues, and the growth of tumor cells is limited by the geometric constraint of the fibers. In addition, implantation of hollow fibers requires minimally invasive surgery.
In an effort to establish a screening method that will allow a rapid and accurate evaluation of anticancer drugs in animals, we have attempted to leverage the advantages of the in vivo hollow fiber model and the in vivo imaging of cell lines genetically engineered with optical reporters by integrating these two approaches. We here show that tumor cells inside semipermeable hollow fibers do communicate with and affect surrounding host tissues, that the effect of antitumor agents on tumor cells within an implanted hollow fiber can be rapidly assessed after administration to host animals, and that activation of specific signaling pathways can be studied in vivo in tumor cells within an implanted hollow fiber. We expect that this approach will shorten the time for the validation of drug targets in living animals before assessment in more complex tumor models (e.g., metastatic mouse models, transgenic mouse models).
Materials and Methods
Chemicals and Reagents
Human recombinant tumor necrosis factor-α (TNF-α) and lipopolysaccharide (LPS; Escherichia coli serotype 055) were purchased from Sigma (St. Louis, MO). Both TNF-α and LPS were dissolved in distilled water (20 µg/ml and 1.5 mg/ml, respectively) and stored at -20°C. d-Luciferin (Xenogen, Alameda, CA) was dissolved in phosphate-buffered saline (PBS) (15 mg/ml) and stored at -20°C. Docetaxel (Taxotere; Aventis, Bridgewater, NJ) was diluted with an entire diluent supplied by the manufacturer to obtain a 10-mg/ml solution and was stored at room temperature. Irinotecan hydrochloride (Camptosar, 20 mg/ml; Pharmacia and Upjohn Co., New York, NY) was stored at room temperature.
Cell Culture and Transfection
MAT B III rat mammary adenocarcinoma cells were maintained in McCoy's 5A medium supplemented with 10% fetal bovine serum (FBS), and MCF7 human breast cancer cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% FBS. Plasmid transfection was performed in 10-cm tissue culture plates with Lipofectamine 2000 (Invitrogen, Carlsbad, CA). To make stable cell lines, cells were cotransfected with 5 µg of pGL3-control (Promega, Madison, WI) or pTL-NFκB (Panomics, Redwood City, CA) and 0.5 µg of empty pcDNA3 (Invitrogen). Twenty-four hours later, transfected cells were selected and maintained in a medium containing G418 (1 mg/ml). Single clones of transfected cells were established by single cell deposition (manuscript in preparation). The clones MAT B III-Luc-3H9 and MCF7-Luc-10C11 were characterized as exhibiting a high expression of luciferase.
Preparation and Implantation of Hollow Fibers
Polyvinylidene fluoride (PVDF) hollow fibers were purchased from Spectrum Laboratories, Inc. (Rancho Dominguez, CA). Encapsulation of cells in hollow fibers was performed as described before [13]. Briefly, a hollow fiber (o.d. = 1.2 mm; i.d. = 1 mm; molecular mass cutoff = 500 kDa) was filled with cells and sealed in 1.5-cm segments with a heat sealer (Outsource 2000, Albertville, AL). A long fiber was subsequently cut at the sealed portion separating the segments. The 1.5-cm sealed fibers were then cultivated in six-well culture plates for 24 hours before implantation into nude mice: nu/nu nude mice (Charles River, Wilmington, MA) were anesthetized by intraperitoneal administration of ketamine (140 mg/kg) and xylazine (12 mg/kg). The 1.5-cm sealed hollow fiber segments filled with tumor cells were finally implanted subcutaneously using an 11-gauge trocar inserted through a neck incision. All animal studies described in this study were approved by the Merck Research Laboratories Institutional Animal Care and Use Committee.
Subcutaneous Tumor Xenograft
MAT B III rat mammary tumor cells were trypsinized and resuspended in PBS at a concentration of 107 cells/ml. A total of 106 cells in 100 µl of PBS was subcutaneously injected into each flank of anesthetized nude mice. Tumor size was calculated using caliper measurements with the equation: (length x width2)/2.
Fluorescence Imaging of Angiogenesis
AngioSense750 probe (VisEn Medical, Inc., Woburn, MA) is a blood pool probe based on a near-infrared fluorochrome-labeled long circulating synthetic graft copolymer. This fluorescent probe allows localization of the vasculature for extended periods of time and for tracking of angiogenesis. To detect angiogenesis in nude mice bearing hollow fibers, 2 nmol of AngioSense750 in 150 µl of PBS per mouse was administered by tail vein injection. Twenty-four hours later, fluorescence imaging was acquired with an acquisition time of 4 seconds, an excitation wavelength in the range of 710 to 760 nm, and an emission wavelength in the range of 810 to 870 nm, using the IVIS200 imaging system (Xenogen).
Immunohistochemistry
CD31 expression was immunohistochemically assessed in three mice from each group of mice harboring hollow fibers (with or without tumor cells). Hollow fibers and surrounding tissues were dissected and immersed in zinc-Tris fixative (BD Pharmingen, San Diego, CA) for 24 hours. Six equally spaced sections were processed to cover the entire length of the fibers. Five-micron paraffin-embedded sections were incubated with rat anti-mouse CD31 monoclonal antibody (clone MEC13.3; BD Pharmingen). Subsequently, sections were incubated with biotinylated polyclonal goat anti-rat IgG (mouse adsorbed) and streptavidin-horseradish peroxidase. The substrate 3,3′-diaminobenzidine tetrahydrochloride was used to detect CD31 immunoreactivity. Finally, the sections were counterstained withMayer's hematoxylin.Omission of the primary antibody served as a negative control.
Bioluminescence Imaging of Hollow Fibers
For in vitro studies, d-luciferin was added to a tissue culture medium in plates containing cell-filled hollow fibers up to a final concentration of 50 µg/ml. Five minutes later, photons were counted using the IVIS200 imaging system (Xenogen) according to the manufacturer's instructions. Data were analyzed using LivingImage software (version 2.50.1; Xenogen). Bioluminescence intensities from manually selected region(s) of interest (ROI) were calculated, and data were expressed as total flux (photons/sec). For evaluation of anticancer drugs, nude mice bearing hollow fibers filled with MAT B III-Luc-3H9 or MCF7-Luc-10C11 cells were treated with Taxotere (20 mg/kg) or Camptosar (100 mg/kg) for three doses, with a 4-day interval between doses. Longitudinal imaging was acquired with an acquisition time of 5 minutes at 15 minutes after the administration of d-luciferin (90 mg/kg, i.p.), and photons were analyzed as above. To investigate nuclear factor κB (NFκB) activation in vivo, nude mice bearing hollow fibers filled with MAT B III-NFκB cells were administered LPS (2 mg/kg, i.p.) or TNF-α (2 µg/mouse, i.p.). Before and after the administration of LPS or TNF-α, bioluminescence imaging was obtained after d-luciferin injection; photons were counted and analyzed as above.
Results
Kinetics of Luciferase Imaging in Hollow Fiber Models
To determine the time range in which a luciferase signal is at a maximum, nude mice were implanted with hollow fibers filled with MCF7-Luc-10C11 cells. Twenty-four hours after fiber implantation, sequential bioluminescent images of these mice were acquired with an acquisition time of 5 minutes at 5-minute intervals (with no delay time) after luciferin administration. By 20 minutes, a strong bioluminescence signal was observed, which continued to increase up to about 40 minutes. A maximum in the bioluminescence signal was detected between 40 and 45 minutes and decreased gradually thereafter (Figure 1, A and B). A similar kinetic profile was observed when using MAT B III-Luc-3H9 cells. Therefore, in subsequent studies with implanted hollow fibers, in vivo bioluminescence imaging was acquired about 40 to 45 minutes after luciferin administration.
Figure 1.
Kinetics of luciferase imaging. Hollow fibers filled with MCF7-Luc-10C11 cells stably expressing luciferase at 5 x 106 cells/ml (60,000 cells/fiber) were implanted into nude mice in both flanks. (A) Twenty-four hours later, sequential bioluminescence images were acquired at 5-minute intervals after luciferin administration (90 mg/kg, i.p.). (B) Quantitative analysis of sequential images expressed as photons per second. Each time point represents 10 hollow fibers implanted from five mice (error bars represent the standard error of the mean).
Proliferation of Tumor Cells in Hollow Fibers In Vitro and In Vivo
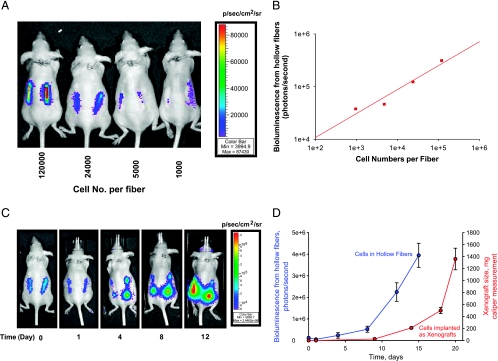
To measure the proliferation of tumor cells in hollow fibers in vitro, MAT B III-Luc-3H9 cells in hollow fibers were cultivated in six-well tissue culture plates. Bioluminescent images were acquired with a 5-minute acquisition time after the addition of luciferin to the medium at a final concentration of 15 µg/ml. A significant linear correlation between bioluminescence and cell number was observed in hollow fibers cultivated in six-well tissue culture plates (data not shown). Furthermore, in nude mice implanted with cell-filled fibers, a linear correlation between bioluminescent signal and cell number was observed (Figure 2, A and B). Longitudinal bioluminescence images demonstrated that bioluminescence decreased by about 30% during the first 24 hours after the implantation of hollow fibers, and then increased at later time points (Figure 2C). By 1 week after implantation into mice, in vivo bioluminescence imaging indicated that tumor cells in hollow fibers had proliferated rapidly (Figure 2D). The proliferation of tumor cells within hollow fibers, determined by bioluminescence, was compared to tumor growth in the xenograft model, determined by caliper measurement. With this cell line, bioluminescent signals may be used to monitor the growth of tumor cells within 10 days of the implantation of hollow fibers, at which time tumors have not yet developed to the point where they are detectable by caliper measurement.
Figure 2.
Proliferation of MAT B III-Luc-3H9 cells determined by bioluminescence imaging in hollow fibers. (A) Bioluminescence imaging of nude mice harboring hollow fibers filled with indicated numbers of MAT B III-Luc-3H9 cells per fiber. (B) Linear correlation between bioluminescence and the numbers of cells implanted. (C) Longitudinal bioluminescence imaging of a representative mouse was acquired on the days indicated after hollow fiber implantation. (D) Mean bioluminescent flux (photons/sec) was obtained from 10 hollow fibers in five mice (each bearing two hollow fibers; blue line), and mean tumor volume was determined with caliper measurement in a tumor xenograft (mean from 10 tumors in five mice; red line) (error bars represent the standard error of the mean).
Angiogenesis Associated with Hollow Fibers
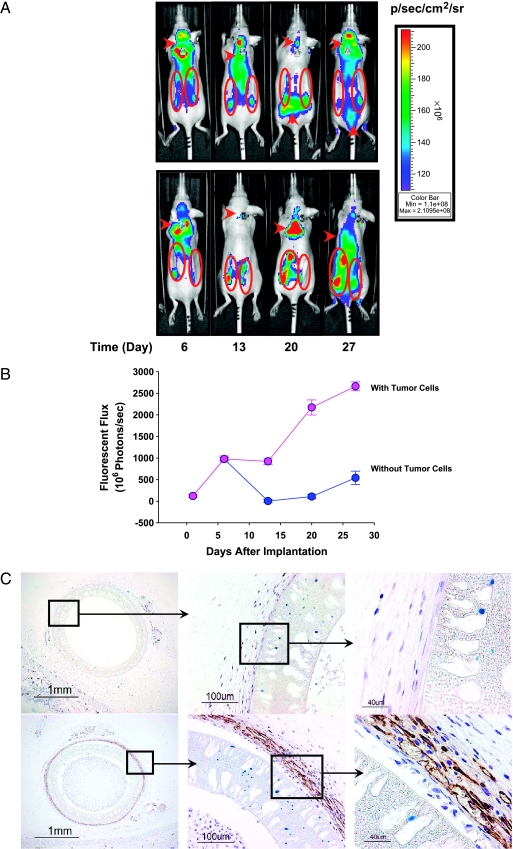
Although the hollow fiber assay does not model the complex interactions and phenomena that occur when tumor cells are grown in normal host tissues, some interactions do take place. To investigate one such interaction, we attempted to detect angiogenesis in tissues adjacent to hollow fibers. Hollow fibers filled with either MAT B III-Luc-3H9 cells or with medium only were implanted in both flanks of two groups of nude mice. AngioSense750, a near-infrared labeled copolymer that localizes in the vasculature, was administered by intravenous injection to nude mice harboring hollow fibers, and fluorescent images were acquired. Starting from day 6 after hollow fiber implantation, fluorescent signals derived from AngioSense750 were detected in both groups of mice (with or without MAT B III-Luc-3H9 cells) (Figure 3A). Thereafter, fluorescent signals from the area around hollow fibers without tumor cells dropped rapidly to baseline, whereas signals from the area around hollow fibers containing MAT B III-Luc-3H9 cells remained stable for another week and increased from that time until the end of the study (Figure 3, A and B). To confirm the angiogenesis detected by in vivo imaging, immunohistochemical staining of tissue sections, including hollow fibers, was also performed with antibodies to CD31 (a marker for endothelial cells, which predominate in neovascular tissues) [14]. Strong CD31 immunoreactivity was detected outside hollow fibers that contained tumor cells, but no immunoreactivity was detected adjacent to hollow fibers without tumor cells (except that some immunoreactivity was observed in sweat glands; Figure 3C).
Figure 3.
Detection of angiogenesis around hollow fibers. (A) Fluorescent images acquired 24 hours after the intravenous administration of AngioSense750 into nude mice harboring hollow fibers without tumor cells (upper panel, ROI encircled) and with MAT B III-Luc-3H9 tumor cells (24,000 cells/fiber; lower panel, ROI encircled). (B) Average fluorescence imaging (photons/sec) was calculated from six hollow fibers in three mice (error bars represent the standard error of the mean). (C) CD31 immunohistochemical staining in hollow fiber sections on day 28 (upper panel, no tumor cells; lower panel, with tumor cells). Magnification is shown on the lower left corner.
In Vivo Inhibition of Tumor Cell Growth in Hollow Fibers By Anticancer Drugs
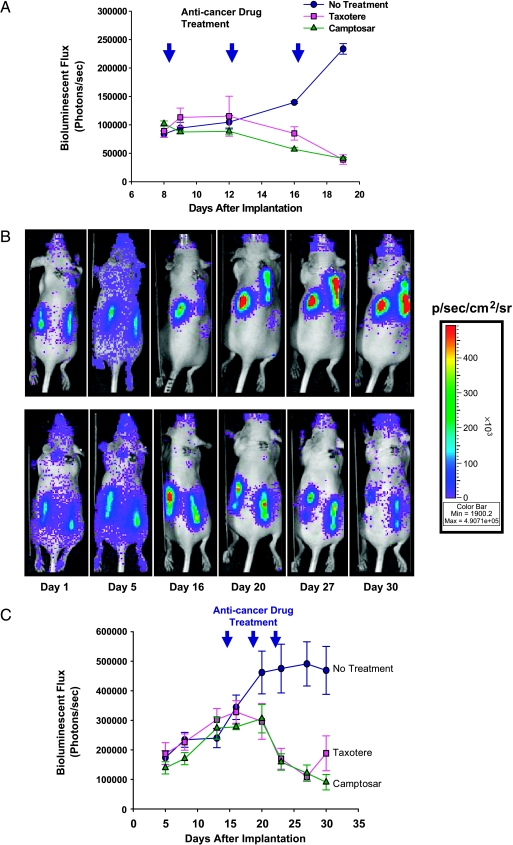
To assess the ability of the in vivo hollow fiber/optical reporter cell line model to report antitumor activities of known anticancer drugs, two commercial drugs chosen for their different mechanisms of action (Taxotere and Camptosar) were selected. For the first study, hollow fibers filled with MAT B III-Luc-3H9 cells were implanted into each flank of nude mice. Mice were divided into three groups with three mice in each group, and each group was treated either with a vehicle (control) or with Taxotere (20 mg/kg) or Camptosar (100 mg/kg) at 4-day intervals starting on day 8 after the implantation of hollow fibers. The results show that tumor cell proliferation was inhibited by both Taxotere and Camptosar (Figure 4A). Moreover, by day 12 after the initial dose, tumor cell numbers had decreased by about 50%, as detected by bioluminescence imaging. In a second study, nude mice harboring hollow fibers filled with MCF7-Luc-10C11, an estrogen receptor-positive human breast cancer cell line stably expressing luciferase, were also treated with Taxotere or Camptosar as above. As in the case of MAT B III cells, both Taxotere and Camptosar significantly inhibited the proliferation of MCF7 cells (Figure 4, B and C).
Figure 4.
Inhibition of the proliferation of mammary adenocarcinoma cells in hollow fibers in vivo by Taxotere and Camptosar. Nude mice bearing hollow fibers were either vehicle-treated or treated with Taxotere (20 mg/kg) or Camptosar (100 mg/kg) at indicated times (at 4-day intervals; arrowheads) after the implantation of hollow fibers filled with MAT B III-Luc-3H9 (A) or MCF7-Luc-10C11 (C) cells. Bioluminescence imaging was acquired longitudinally in these mice over the period shown. Data points show the means of six hollow fibers from three mice (each bearing two hollow fibers; error bars represent the standard error of the mean). (B) Longitudinal bioluminescence imaging of a representative mouse with either vehicle treatment (upper panel) or Taxotere treatment (lower panel) on days 15, 19 and 23, which harbors hollow fibers filled with MCF7-Luc-10C11 cells, was acquired on the days indicated after hollow fiber implantation.
NFκB Induction in Hollow Fibers In Vivo
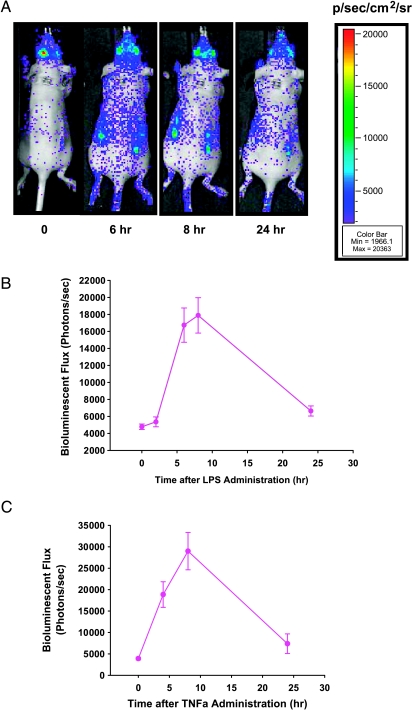
To assess the ability of the in vivo hollow fiber/optical reporter cell line model to report pathway-specific effects of administered compounds, cells reporting the activity of the transcription factor NFκB were constructed. NFκB plays an important role in tumor development and progression. NFκB and the signaling pathways involved in its activation are, therefore, attractive targets for cancer prevention and therapeutics. Thus, a cell line expressing an optical reporter to monitor NFκB activity is expected to be a powerful tool for the target validation of anticancer drugs that affect the NFκB pathway. To investigate whether NFκB induction can be imaged in the hollow fiber model, LPS was administrated intraperitoneally into nude mice harboring hollow fibers filled with MAT B III-NFκB-Luc cells—a cell line stably transfected with luciferase driven by an NFκB-responsive element. When compared to imaging before the administration of LPS, bioluminescence from NFκB reporter cells was significantly increased after LPS administration. NFκB activity was increased four-fold between 6 and 8 hours after treatment with LPS, whereas NFκB activity dropped significantly after 24 hours (Figure 5, A and B). To provide additional verification that this optical reporter is an appropriate indicator of NFκB activation, TNF-α was administered intraperitoneally (2 µg/mouse). As observed with LPS, TNF-α increased NFκB reporter activity in hollow fibers in vivo, and this increase reached a maximum at 8 hours after TNF-α injection (Figure 5C).
Figure 5.
Induction of NFκB reporter in hollow fibers in vivo by LPS and TNF-α. (A) Bioluminescence imaging of a representative mouse on pretreatment and posttreatment with LPS. (B and C) Nude mice harboring hollow fibers with MAT B III-NFκB-Luc cells were administered LPS (B; 2 mg/kg, i.p.) or TNF-α (C; 2 µg/mouse, i.p.). Bioluminescent images were acquired immediately before the administration of LPS or TNF-α, as well as at the indicated time after the administration of an antitumor agent. Data points show a mean bioluminescent flux (photons/sec) of six hollow fibers from three mice (each bearing two hollow fibers; error bars represent the standard error of the mean).
Discussion
The original hollow fiber assay involves the short-term in vitro cultivation of cells, followed by in vivo implantation into nude mice for short-term evaluation of drug efficacy. However, for a longitudinal study, this method requires the retrieval of hollow fibers at each time point from euthanized mice. Our observations have expanded the application of this assay by combining it with optical imaging, providing a longitudinal noninvasive method to evaluate tumor cell growth in hollow fibers in vivo.
Optical imaging of hollow fiber allows medium-term (several weeks) evaluation of anticancer drugs. In comparison with the kinetics of bioluminescence in the previous study [15], the delayed kinetics observed in this study might be explained by the lower dose of luciferin injected (90 vs 150 mg/kg), smaller cell numbers implanted inside hollow fibers (24,000 vs 1 x 106 cells), and/or different tumor types (mammary versus brain tumors). The proliferation of tumor cells (MAT B III and MCF7 were tested) was followed for at least 4 weeks by noninvasive imaging in this study. Bioluminescence imaging has demonstrated that tumor cells are able to proliferate actively for at least 4 weeks within hollow fibers. Thereafter, tumor cells grow slowly or cease proliferating, and bioluminescence shows a decrease in cell number. Unlike the tumor xenograft model, in which tumor cells are able to invade surrounding tissues and to proliferate without limit [16], the growth inhibition seen in hollow fibers is presumably caused by contact inhibition due to constraints in the internal volume of the hollow fiber model. As tumor cell proliferation within hollow fibers can be tracked for at least 4 weeks, a broad time window to evaluate anticancer drugs is ensured in this model.
Over this time period, the communication of tumor cells within hollow fibers with host tissues is strongly supported by the finding that angiogenesis is detected in tissues surrounding hollow fibers. Angiogenesis around hollow fibers is significantly associated with the presence of tumor cells, suggesting that there is an interaction between tumor cells, even though they are constrained within hollow fibers, and surrounding host cells. The angiogenesis detected in both groups of mice shortly after fiber implantation is most likely due to healing from a minimally invasive surgery when hollow fibers were implanted. The significant difference in AngioSense750 fluorescent signals between hollow fibers with or without tumor cells strongly suggests that angiogenesis is stimulated by tumor cells within hollow fibers.
Our results support previous histologic studies that found that angiogenesis occurs around hollow fibers [17]. Compared to studies using postmortem histologic study as the primary end point, our approach using AngioSense750 is noninvasive; therefore, results can be obtained longitudinally with a small number of animals. Taken together, we have confirmed that communication and interaction occur between tumor cells inside hollow fibers and the host mice. For example, tumor cells may secrete positive angiogenic factors, such as vascular endothelial growth factor, platelet-derived growth factor, and insulin-like growth factor, that freely diffuse out of the fibers into surrounding tissues and promote the development of new vessels [18]. This angiogenesis around hollow fibers increases the supply of oxygen and nutrients to maintain the growth and proliferation of tumor cells inside the fibers.
The hollow fiber in vivo imaging model allows the rapid and accurate evaluation of the antitumor activities of novel anticancer drugs. In comparison with the tumor xenograft model, tumor cell proliferation on the days soon after fiber implantation was detected with noninvasive bioluminescence imaging, suggesting its rationale for early administration of anticancer drugs. In the present study, two known anticancer drugs in clinical use (Taxotere and Camptosar) were evaluated in the hollow fiber model with different tumor cell lines. Taxotere is a microtubule-stabilizing taxane, which binds to free tubulin and promotes the assembly of tubulin into stable microtubules while simultaneously inhibiting their disassembly. This leads to the stabilization of microtubules, which results in apoptosis through mitotic catastrophe in breast cancer cells [19]. The active metabolite of Camptosar, SN-38, inhibits topoisomerase I activity, resulting in DNA double-strand breaks and, ultimately, apoptosis [20]. Administration of Camptosar has been shown to induce antitumor activity in human tumor xenografts in mice [21]. In both cell lines tested, Taxotere and Camptosar significantly inhibited the proliferation of tumor cells within 4 days of their administration. Thus, the proof of concept is well-established, and this model is suitable for evaluating novel anticancer drugs. In addition, as these PVDF hollow fibers have a 500-kDa cutoff, this technology can potentially be used to evaluate antibody (Peter Lassota, Caliper, Inc., personal communication) and siRNA therapies, in addition to small molecules.
Hollow fiber technology is applicable not only to the evaluation of drug efficacy but also to the study of intracellular molecular pathways. For example, in a previous study [6], a p27-luciferase-expressing tumor cell was used to monitor Cdk2 activity by in vivo bioluminescence imaging in hollow fibers. To further illustrate the utility of this technology in studying specific molecular pathways, we have evaluated NFκB signaling in the present study. NFκB is the most important component of the signaling pathway activated by inflammation and infection [22]. Activation of NFκB in inflammatory cells in response to infectious agents, inflammatory cytokines, and proteins, and danger signals released by necrotic cells lead to the production of secreted factors that enhance the growth, survival, and vascularization of carcinoma cells [22]. In the present study, a luciferase plasmid driven by an NFκB-responsive element was stably transfected into tumor cells, and the resultant reporter cell line was then evaluated in the hollow fiber model after treatment of the host mice with either LPS or TNF-α. Consistent with the data obtained in vitro, TNF-α and LPS activated the NFκB pathway and induced the NFκB reporter. In comparison with a transgenic mouse model, in which NFκB activity was monitored in normal mouse tissues [23], the hollow fiber model described here is capable of providing a rapid evaluation of NFκB activity in both human and mouse/rat tumor cells. Thus, the model presented here can be used to test NFκB inhibitors (small molecule compounds and peptides) under development by pharmaceutical companies. Based on the above results, if a cell line is genetically engineered with a pathwaydependent optical reporter, it could be used in the hollow fiber model to monitor molecular pathways or events.
In summary, the technology of the in vivo imaging of hollow fibers containing tumor cells expressing optical reporters has been applied to the evaluation of antitumor activities of known anticancer drugs in nude mice and can be used in the near future for primary anticancer drug screening in vivo. This technology allows an early evaluation of anticancer drugs because it is not necessary to wait for palpable tumor formation, and it also permits the retrieval of uncontaminated tumor cells for additional analysis. There is no limitation on cell type; even nontumorigenic tumor cells can be used in this model. The use of genetically engineered constitutively expressed optical reporters affords the ability to noninvasively and repetitively image implanted hollow fibers containing tumor cells, enabling longitudinal studies to follow the in vivo antitumor activity of a compound in the same group of mice. Furthermore, with the use of more sophisticated genetically engineered optical reporters, this technology can be applied to monitor specific molecular events and pathways (e.g., apoptosis, cell proliferation, and cell cycle change). In particular, implantation of multiple fibers (each bearing one pathway-specific reporter cell line) will allow investigators to image multiple molecular pathways in one mouse, permitting the determination of whether compounds of interest target one pathway/molecule but not the others.
References
- 1.Wang W, El-Deiry WS. Bioluminescent molecular imaging of endogenous and exogenous p53-mediated transcription in vitro and in vivo using an HCT116 human colon carcinoma xenograft model. Cancer Biol Ther. 2003;2:196–202. doi: 10.4161/cbt.2.2.347. [DOI] [PubMed] [Google Scholar]
- 2.Uhrbom Nerio E, Holland EC. Dissecting tumor maintenance requirements using bioluminescence imaging of cell proliferation in a mouse glioma model. Nat Med. 2004;10:1257–1260. doi: 10.1038/nm1120. [DOI] [PubMed] [Google Scholar]
- 3.Harada H, Kizaka-Kondoh S, Hiraoka M. Optical imaging of tumor hypoxia and evaluation of efficacy of a hypoxia-targeting drug in living animals. Mol Imaging. 2005;4:182–193. doi: 10.1162/15353500200505112. [DOI] [PubMed] [Google Scholar]
- 4.Lungu GF, Li ML, Xie X, Wang LV, Stoica G. In vivo imaging and characterization of hypoxia-induced neovascularization and tumor invasion. Int J Oncol. 2007;30:45–54. [PubMed] [Google Scholar]
- 5.Luker GD, Pica CM, Song J, Luker KE, Piwnica-Worms D. Imaging 26S proteasome activity and inhibition in living mice. Nat Med. 2003;9:969–973. doi: 10.1038/nm894. [DOI] [PubMed] [Google Scholar]
- 6.Zhang GJ, Safran M, Wei W, Sorensen E, Lassota P, Zhelev N, Neuberg DS, Shapiro G, Kaelin WG., Jr Bioluminescent imaging of Cdk2 inhibition in vivo. Nat Med. 2004;10:643– 648. doi: 10.1038/nm1047. [DOI] [PubMed] [Google Scholar]
- 7.Laxman B, Hall DE, Bhojani MS, Hamstra DA, Chenevert TL, Ross BD, Rehemtulla A. Noninvasive real-time imaging of apoptosis. Proc Natl Acad Sci USA. 2002;99:16551–16555. doi: 10.1073/pnas.252644499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollingshead MG, Alley MC, Camalier RF, Abbott BJ, Mayo JG, Malspeis L, Grever MR. In vivo cultivation of tumor cells in hollow fibers. Life Sci. 1995;57:131–141. doi: 10.1016/0024-3205(95)00254-4. [DOI] [PubMed] [Google Scholar]
- 9.Hall LA, Krauthauser CM, Wexler RS, Hollingshead MG, Slee AM, Kerr JS. The hollow fiber assay: continued characterization with novel approaches. Anticancer Res. 2000;20:903–911. [PubMed] [Google Scholar]
- 10.Suggitt M, Swaine DJ, Pettit GR, Bibby MC. Characterization of the hollow fiber assay for the determination of microtubule disruption in vivo. Clin Cancer Res. 2004;10:6677–6685. doi: 10.1158/1078-0432.CCR-04-0855. [DOI] [PubMed] [Google Scholar]
- 11.Sadar MD, Akopian VA, Beraldi E. Characterization of a new in vivo hollow fiber model for the study of progression of prostate cancer to androgen independence. Mol Cancer Ther. 2002;1:629–637. [PubMed] [Google Scholar]
- 12.Krauthauser CM, Hall LA, Wexler RS, Slee AM, Mitra J, Enders GH, Kerr JS. Regulation of gene expression and cell growth in vivo by tetracycline using the hollow fiber assay. Anticancer Res. 2001;21:869–872. [PubMed] [Google Scholar]
- 13.Zhang GJ, Kaelin WG., Jr Bioluminescent imaging of ubiquitin ligase activity: measuring Cdk2 activity in vivo through changes in p27 turnover. Methods Enzymol. 2005;399:530–549. doi: 10.1016/S0076-6879(05)99036-8. [DOI] [PubMed] [Google Scholar]
- 14.DeLisser HM, Christofidou-Solomidou M, Strieter RM, Burdick MD, Robinson CS, Wexler RS, Kerr JS, Garlanda C, Merwin JR, Madri JA, et al. Involvement of endothelial PECAM-1/CD31 in angiogenesis. Am J Pathol. 1997;151:671–677. [PMC free article] [PubMed] [Google Scholar]
- 15.Burgos JS, Rosol M, Moats RA, Khankaldyyan V, Kohn DB, Nelson MD, Jr, Laug WE. Time course of bioluminescent signal in orthotopic and heterotopic brain tumors in nude mice. Biotechniques. 2003;34:1184–1188. doi: 10.2144/03346st01. [DOI] [PubMed] [Google Scholar]
- 16.Suggitt M, Bibby MC. 50 years of preclinical anticancer drug screening: empirical to target-driven approaches. Clin Cancer Res. 2005;11:971–981. [PubMed] [Google Scholar]
- 17.Phillips RM, Pearce J, Loadman PM, Bibby MC, Cooper PA, Swaine DJ, Double JA. Angiogenesis in the hollow fiber tumor model influences drug delivery to tumor cells: implications for anticancer drug screening programs. Cancer Res. 1998;58:5263–5266. [PubMed] [Google Scholar]
- 18.Folkman J. Angiogenesis and apoptosis. Semin Cancer Biol. 2003;13:159–167. doi: 10.1016/s1044-579x(02)00133-5. [DOI] [PubMed] [Google Scholar]
- 19.Morse DL, Gray H, Payne CM, Gillies RJ. Docetaxel induces cell death through mitotic catastrophe in human breast cancer cells. Mol Cancer Ther. 2005;4:1495–1504. doi: 10.1158/1535-7163.MCT-05-0130. [DOI] [PubMed] [Google Scholar]
- 20.Bahadori HR, Rocha Lima CM, Green MR, Safa AR. Synergistic effect of gemcitabine and irinotecan (CPT-11) on breast and small cell lung cancer cell lines. Anticancer Res. 1999;19:5423–5428. [PubMed] [Google Scholar]
- 21.Hardman WE, Moyer MP, Cameron IL. Fish oil supplementation enhanced CPT-11 (irinotecan) efficacy against MCF7 breast carcinoma xenografts and ameliorated intestinal side-effects. Br J Cancer. 1999;81:440–448. doi: 10.1038/sj.bjc.6690713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 23.Carlsen H, Alexander G, Austenaa LM, Ebihara K, Blomhoff R. Molecular imaging of the transcription factor NF-kappaB, a primary regulator of stress response. Mutat Res. 2004;551:199–211. doi: 10.1016/j.mrfmmm.2004.02.024. [DOI] [PubMed] [Google Scholar]