Abstract
Malignant peripheral nerve sheath tumors (MPNST) are sarcomas with poor prognosis and limited treatment options. Factors contributing to tumor progression are largely unknown. We therefore examined MPNST from 22 neurofibromatosis type 1 (NF1) patients, 14 non-NF1 patients, and 14 neurofibroma patients for matrix metalloproteinase 13 (MMP-13) expression. Because wild-type and mutant p53 were shown to differentially regulate MMP-13 expression, TP53 status and protein levels were also determined. MMP-13 expression was detected in 58% of MPNST and was significantly associated with recurrent MPNST (P = .019). p53 was observed in 78% of MPNST and was found to be strongly associated with MMP-13 expression (P = .005). In contrast, 14 neurofibromas lacked MMP-13 and p53 expressions. TP53 mutations were found in only 11% of MPNST and were associated with high tumor grades (P = .029). No significant association between mutant TP53 and MMP-13 was observed, indicating that other factors drive MMP-13 expression in MPNST. The presence of metastasis was linked to p53Pro72 polymorphism (P = .041) and shorter survival. In summary, our data suggest that MMP-13 expression in nerve sheath tumors is coupled with malignant progression. Therefore, MMP-13 may serve as a marker for progression and as a therapeutic target.
Keywords: Malignant peripheral nerve sheath tumor, matrix metalloproteinase 13, neurofibromatosis type 1, TP53, malignant progression
Introduction
Malignant peripheral nerve sheath tumors (MPNST) are aggressive soft-tissue sarcomas with poor prognosis. MPNST grow invasively and often metastasize to the lungs and other organs. With an incidence of 1:100,000, MPNST are rare in the general population [1]. However, 8% to 13% of neurofibromatosis type 1 (NF1) patients develop MPNST. In NF1 patients, MPNST are the major cause of reduced life expectancy, with only 21% of patients surviving longer than 5 years after diagnosis [2].
NF1 is a tumor syndrome caused by mutations in the NF1 tumor-suppressor gene and occurs with an incidence of 1:3500 [3]. A hallmark of NF1 is the development of multiple benign dermal neurofibromas (dNF). Approximately one third of NF1 patients develop plexiform neurofibromas (pNF). MPNST in NF1 patients generally arise by malignant progression of preexisting pNF. Knowledge on molecular alterations causing malignant transformation is limited. However, TP53 mutations likely contribute to the development of some MPNST [4–6]. Our previous screening for progression-associated genes identified matrix metalloproteinase 13 (MMP-13) [7], which was later confirmed by another study [8]. Matrix metalloproteinases (MMP) are endopeptidases involved in the degradation of extracellular matrix (ECM) components. MMP-13, also known as collagenase-3, degrades a wide spectrum of substrates, including collagens of types I, II, III, IV, V, X, and XIV; aggrecan; versican; fibronectin; tenascin; and fibrillin-1 [9–12]. Degradation of the ECM is a prerequisite for tumor cell invasion and development of metastasis. MMP can be expressed either by tumor cells or by surrounding stromal cells, thereby promoting tumor cell invasion. In squamous cell carcinomas, MMP-13 transcripts have been primarily detected in tumor cells at the invading edge [13]. Meanwhile, MMP-13 expression has been detected in different tumor entities and has been shown to correlate with invasive and metastatic behaviors [13–16]. In vitro studies demonstrated that overexpression of MMP-13 leads to increased invasion of fibrosarcoma cells [17]. Inhibition of MMP-13 in squamous cell carcinoma cells resulted in impaired invasion through Matrigel and reduced tumor growth in mice [18].
A regulatory link between MMP-13 and the tumor-suppressor p53 has been reported. Wild-type p53 repressed MMP-13 transcription [19], whereas mutant p53 lacked this inhibitory effect. It is worth noting that “gain-of-function” p53 mutants even stimulated MMP-13 expression [20]. To investigate whether mutant p53 is responsible for MMP-13 expression in vivo, we studied a panel of MPNST and neurofibromas for both features and compared them with clinical and pathological findings.
Materials and Methods
Tumor Samples and DNA Extraction
Tumor samples were collected at the University Hospital Eppendorf (Hamburg, Germany), Robert-Rössle-Hospital (Berlin, Germany), Otto-von-Guericke-University (Magdeburg, Germany), and Charité-Universitätsmedizin Berlin (Berlin, Germany). Following initial diagnosis in local neuropathologies, all tumor samples were reviewed by the same pathologist (A.F.O.). Histologic grading was based on the modified and updated French Federation Nationale des Centres de Lutte Contre Ie Cancer (FNCLCC) system [21,22]. A second surgery after clinical progression was defined as recurrence. The study examined MPNST from 22 NF1 patients, 14 non-NF1 patients (Table 1), and 14 neurofibroma patients (five pNF and nine dNF). MPNST cell lines S462 and ST88-14 (kindly provided by Dr. Andreas Kurtz; Charité-Universitätsmedizin Berlin) were also analyzed. Cell line S462 was established from MPNST 24472. Investigations were carried out with informed consent. Tumor samples were examined histologically before the extraction of DNA and lysates. Tumor areas were scraped from slides for subsequent extraction. In case of frozen tissues, DNA was extracted using Trizol reagent (Invitrogen, Karlsruhe, Germany). DNA extraction from paraffin-embedded material was carried out according to the QIAamp DNA Mini Kit protocol (Qiagen, Hilden, Germany).
Table 1.
Patient and Tumor Characteristics.
| ID | Sex/Age (Years) | NF1 | Follow-Up Month | Localization | Grade | Metastasis Localization/Month | Relapse Month | MMP-13 IF | p53 IHC | p53 Mut | p53 Pol Codon 72 | TP53 Pol Intron 2 | TP53 Pol Intron 3 |
| 21852 | M/29 | Yes | 24† | Intraspinal | 2 | - | 6 | + | + | - | Arg/Arg | G/G | N/N |
| 24256 | F/21 | Yes | 161† | Arm distal | 3 | Lungs, liver, pancreas, lymph nodes/132 | 108 | ++ | ++ | p53321STOP | Arg/Pro | C/LOH | Dup/LOH |
| 24320 | M/56 | Yes | 46 | Leg | 1 | - | - | + | ++ | - | Arg/Pro | C/G | Dup/N |
| 24626 | M/58 | Yes | 49 | Back | 2 | - | - | - | + | - | Arg/Arg | G/G | N/N |
| 24472 | F/19 | Yes | 11† | Leg proximal | 3 | - | 2 | + | ++ | p53Pro110 | Arg/Arg | G/G | N/N |
| 21914 | F/21 | Yes | 30 | Leg proximal | 2 | - | 4 | + | + | - | Arg/Pro | C/G | Dup/N |
| 24304 | M/27 | Yes | 17† | Plexus cervicobrachialis | 1 | Paravertebral, lumbar, thoracic/0 | 14 | ++ | + | - | Arg/Arg | G/G | N/N |
| 24308 | M/21 | Yes | 14† | Leg proximal | 3 | Lung, thoracic wall/6 | - | + | +++ | - | Arg/Pro | C/G | Dup/N |
| 24310 | M/66 | Yes | 8† | Trunk | 2 | Lung/2 | 5 | - | - | - | Arg/Pro | C/G | N/N |
| 24326 | M/32 | Yes | 8† | Plexus cervicobrachialis | 2 | Lung/2 | - | - | + | - | Arg/Arg | G/G | N/N |
| 24332 | F/30 | Yes | 192 | Arm distal | 2 | - | 10 | ++ | + | - | Arg/Arg | C/G | N/N |
| 24354 | F/33 | Yes | 200 | Leg distal | 1 | - | 96 | + | + | - | Arg/Arg | C/G | Dup/N |
| 24476 | F/13 | Yes | 99† | Arm distal | 2 | - | - | - | - | - | Arg/Arg | G/G | N/N |
| 24480 | F/20 | Yes | 7† | Mediastinal | 2 | - | - | - | - | - | Arg/Arg | G/G | N/N |
| 24484 | F/31 | Yes | 18† | Gluteal | 3 | - | 4 | - | ++ | - | Arg/Arg | G/G | N/N |
| 24534 | F/28 | Yes | 44 | Thoracic wall | 3 | - | - | - | + | - | Arg/Arg | G/G | Dup/N |
| 24668 | F/14 | Yes | 9† | Intraspinal | 3 | Lung/0 | 3 | +++ | +++ | - | Arg/Pro | C/C | Dup/N |
| 24670 | M/31 | Yes | 13† | Inguinal | 3 | Lung/4 | 4 | - | + | - | Arg/Pro | C/G | A11992/N |
| 24694 | F/79 | Yes | 29 | Leg proximal | 2 | - | - | + | ++ | - | Arg/Arg | G/G | N/N |
| 24748 | M/34 | Yes | 12† | Gluteal | 2 | - | 2 | +++ | ++ | - | Arg/Arg | G/G | N/N |
| 24772 | M/15 | Yes | 42† | Retroperitoneal | 2 | - | - | - | + | - | Arg/Arg | C/G | N/N |
| 24776 | M/39 | Yes | 48 | Right axilla | 1 | - | - | ++ | + | - | Arg/Arg | G/G | N/N |
| 26580 | F/78 | No | 4† | Gluteal | 3 | Lung/0 | - | - | - | - | Arg/Arg | G/G | N/N |
| 26582 | M/43 | No | 126 | Os ileum | 3 | - | - | + | ++ | p53Ala258 | Arg/Pro | C/G | A11992/N |
| 26584 | M/41 | No | 47 | Plexus cervicobrachialis | 2 | - | - | - | ++ | - | Arg/Pro | G/G | N/N |
| 26586 | M/28 | No | 27† | Leg distal | 2 | Lung/0 | - | - | + | - | Arg/Arg | G/G | N/N |
| 26588 | F/73 | No | 63 | Leg proximal | 3 | - | - | + | +++ | p53Met173 | Arg/Pro | C/G | Dup/N |
| 26590 | F/50 | No | 11† | Gluteal | 2 | Lung/0 | - | - | - | - | Arg/Arg | G/G | Dup/N |
| 26592 | F/72 | No | 0† | Liver | 2 | - | - | + | + | - | Arg/Pro | C/G | A11992/N |
| 26594 | F/55 | No | 29† | Leg proximal | 3 | Retroperitoneal/25 | - | - | - | - | Arg/Pro | G/G | N/N |
| 28650 | F/16 | No | 12 | Intraspinal, lumbar | 2 | - | 12 | + | + | - | Arg/Arg | G/G | N/N |
| 27722 | M/69 | No | 3 | Leg proximal | 3 | - | 3 | + | +++ | - | Arg/Arg | G/G | N/N |
| 28652 | M/73 | No | 15 | Arm distal | 1 | - | 15 | ++ | - | - | Arg/Arg | G/G | N/N |
| 27724 | M/47 | No | 14† | Leg proximal | 3 | Lung/7 | - | + | + | - | Arg/Pro | G/G | Dup/N |
| 30342 | F/34 | No | 5† | Intraspinal | 2 | Skin/4 | 2 | +++ | + | - | Arg/Pro | C/G | Dup/N |
| 27732 | M/55 | No | 23 | Gluteal | 3 | Lung/0 | - | - | - | - | Arg/Pro | G/G | N/N |
ID = tumor identification number; NF1 = NF1 status of the patient; Grade = tumor grade according to the modified FNCLCC system; IF = immunofluorescence; IHC = immunohistochemistry; p53 mut = p53 mutation status; p53 pol = p53 polymorphism; N = C11992; Dup = 16-bp duplication.
Deceased patient.
Immunofluorescence and Immunohistochemistry
Monoclonal MMP-13 antibody (AB-4; 1:50 dilution) from Oncogene (Bad Soden, Germany) and anti-mouse Cy3-conjugated antibody (1:100 dilution) from Dianova (Hamburg, Germany) were used for immunofluorescence. p53 (monoclonal antibody DO-7, 1:100 dilution; DakoCytomation, Hamburg, Germany) was detected by immunohistochemistry using a Ventana Benchmark immunostainer (Ventana, Strasbourg, France). Tissues were counterstained with hematoxylin. Antigen presentation was enhanced by heating. Negative controls without primary antibodies were performed and did not produce signals. Scoring was performed according to the percentage of immunopositive cells. Two different scoring systems were used for p53 immunohistochemistry [(+) 1–5% positive cells; (++) 6–25% positive cells; (+++) > 25% positive cells] and MMP-13 immunofluorescence [(+) 5–30% of stained cells; (++) 31–60% of stained cells; (+++) > 60% of stained cells].
Immunocytochemistry
MPNST (2 x 104 cells/well) were seeded on Permanox chamberslides (Nunc, Wiesbaden, Germany). Cells were fixed with methanol on the following day. p53 and MMP-13 antibodies were diluted 1:50 and incubated overnight at 4°C. DAPI I (Vysis, Inc., Downers Grove, IL) and secondary Cy3-labeled antibody (1:100 dilution; Dianova) were incubated simultaneously for 1 hour at room temperature. Negative controls without primary antibodies were carried out and did not produce signals.
Western Blot Analysis
Tumor protein lysates were heat-denatured and run on a 7.5% acrylamide gel. After transfer of proteins, the nitrocellulose membrane was blocked in 5% nonfat milk with 0.5% Tween-Tris-buffered saline for 1 hour and incubated overnight at 4°C with p53 (DO-7, 1:300 dilution; DakoCytomation) or MMP-13 (AB-4, 1:300 dilution). Membranes were then incubated for 1 hour with biotin-conjugated second antibodies, washed, and incubated for 1 hour with Extravidin (1:2000 dilution) from Sigma (Munich, Germany). Visualization was performed with ECL (Amersham Biosciences, Freiburg, Germany). Lysates were adjusted to β-actin expression levels. The anti-β-actin antibody AC-15 (1:6000 dilution) was obtained from Sigma.
Single-Strand Conformation Polymorphism and Sequencing
Electrophoresis of polymerase chain reaction (PCR) products was performed on a polyacrylamide gel at 500 V and 6 mA for 18 hours. All PCR products showing mobility shift were reanalyzed by an independent PCR and were compared with PCR products generated from blood DNA of corresponding patients. Aberrantly migrating bands were excised, and DNA was extracted. After reamplification, PCR products were sequenced bidirectionally on a semiautomated sequencer (model 377; Applied Biosystems, Foster City, CA). TP53 sequence [X54156; National Center for Biotechnology Information (NCBI) database] was used as the reference sequence. Primer sequences, amplifications, and gel conditions are available on request.
Statistical Methods
SPSS version 14.0 (SPSS, Inc., Chicago, IL) was used for statistical analysis. Survival rates were determined using the Kaplan-Meier method and the log rank test. The mean age differences between groups were examined using t test. Association of parameters was assessed with Pearson correlation, Fisher's exact test, or chi-square test. P < .05 was considered significant.
Results
Information on 36 patients and MPNST is provided in Table 1. Twenty-two MPNST patients were diagnosed with NF1, whereas 14 patients developed sporadic MPNST. The female/male ratio in both groups was 1:1. The mean age at diagnosis was 32.6 years for patients with NF1 and 52.4 years for patients without NF1 (t test, P = .003).
MMP-13 Expression and p53 Accumulation
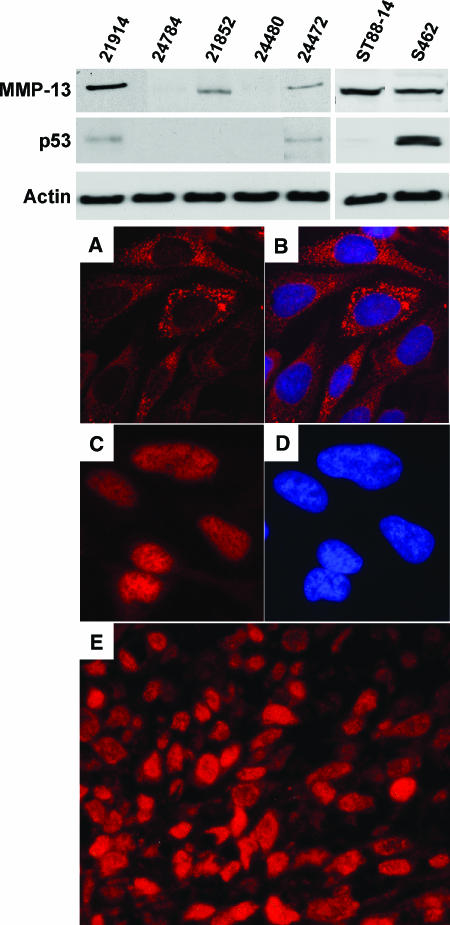
MMP-13 and p53 expressions were analyzed by immunohistochemistry and/or Western blot analysis. MMP-13 was detected in 58% (21 of 36) of MPNST and was generally restricted to distinct areas of the tumor. However, MPNST from three patients (8%) showed homogeneous MMP-13 distribution, including > 60% of the cells (Figure 1C). p53 was detected in 78% (28 of 36) of MPNST. Five pNF cases and nine dNF cases that were also analyzed for the presence of MMP-13 and p53 were negative for both (data not shown). The frequencies of MMP-13 expression in sporadic (8 of 14; 57%) and NF1-associated (13 of 22; 59%) MPNST were similar. Although not significant, p53 was detected more often in NF1-associated tumors (19 of 22; 86%) than in sporadic ones (9 of 14; 64%) (Fisher's exact test, P = .216). Immunocytochemistry of S462 cells demonstrates nuclear accumulation of p53 (Figure 1C) and cytoplasmic localization of MMP-13 (Figure 1, A and B).
Figure 1.
Western blot analyses of MPNST and MPNST cell lines S462 and ST88-14 with antibodies to MMP-13, p53, and β-actin. (A–D) Immunocytochemistry of S462 cells. (A) Detection of MMP-13. (B) MMP-13 merged with nuclear DAPI staining. (C) Detection of p53. (D) The same section with DAPI filter. (E) MMP-13 immunofluorescence in MPNST 24748. Original magnification, x400.
Detection of MMP-13 and p53 by Western blot analysis was performed with five MPNST and MPNST cell lines S462 and ST88-14 (Figure 1). MMP-13 expression was detected in three MPNST (21914, 24472, and 21852) and in both cell lines. Bands at 48 kDa correspond to the active form of MMP-13. p53 was detected in MPNST 21914 and 24472 and in cell line S462. Western blot analysis results were in accordance with immunohistochemistry and cytochemistry.
TP53 Mutations and Polymorphisms
Ten coding exons (exons 2–11) of TP53, including the exon-intron boundary and promoter sequence (exon 1), were screened for sequence alterations. The results are compiled in Table 1. Somatic mutations were detected in 4 (11%) of 36 MPNST in exons 4, 5, 7, and 9. MPNST 24256 from an NF1 patient carried a nonsense mutation at position 321 (AAA→TAA). MPNST 24472 and the corresponding cell culture S462 carried a mutation in codon 110 (CGT→CCT; Arg→Pro). Two TP53 mutations were detected in sporadic MPNST. MPNST 26582 carried a mutation in codon 258 (GAA→GCA; Glu→Ala), and MPNST 26588 carried a mutation in codon 173 (GTG→ATG; Val→Met).
We detected four different polymorphisms in intron 2, intron 3, and exon 4 of TP53 (Table 2). Thirteen patients were heterozygous, and one was homozygous for the C11827 allele in intron 2. The allele frequency was f(C11827) = 0.21. Eleven patients were heterozygous for the 16-bp duplication in intron 3 [f(dup16 bp) = 0.15], and three patients were heterozygous for the A11992 allele corresponding to f(A11992) = 0.041. Fifteen patients were heterozygous for the p53Pro72 allele corresponding to f(Pro72) = 0.21 and f(Arg72) = 0.79. To exclude the possibility of loss of heterozygosity (LOH) in tumors, corresponding blood DNA was examined in the case of codon 72 polymorphism.
Table 2.
TP53 Polymorphisms in MPNST Patients.
| Localization | DNA Alteration | Cases (n) | Allele Frequency of MPNST Patients | Allele Frequency of Controls | |
| Intron 2 | Nucleic acid 11827 | G→C | 13 (heterozygous), 1 (homozygous) | 0.21 (C11827) | 0.31 (C11827) |
| Intron 3 | Nucleic acid 11951 | Duplication, GGGGACCTGGAGGCT | 11 (heterozygous) | 0.15 (16-bp dup) | 0.16 (16-bp dup) |
| Intron 3 | Nucleic acid 11992 | C→A | 3 (heterozygous) | 0.032 (A11992) | 0.041 (A11992) |
| Exon 4 | Codon 72 | CGC→CCC | 15 (heterozygous) | 0.21 (p53Pro72) | 0.26 (p53Pro72) |
The position of polymorphisms is given according to reference X54156 (NCBI database).
Statistical Analysis
A highly significant association between p53 immunopositivity and MMP-13 immunopositivity was found (Fisher's exact test, P = .005). Taking different staining levels into account, the association was still significant (Pearson correlation, P = .02). TP53 mutations were not significantly associated with MMP-13 expression (Fisher's exact test, P = .141) but with histologic grade (chi-square test, P = .029). All MPNST with mutant TP53 were of histologic grade 3. MMP-13 expression was significantly associated with relapse (Fisher's exact test, P = .019). When MMP-13 staining levels were taken into account, the association was even more significant (Fisher's exact test, P = .013). In detail, MPNST without MMP-13 expression relapsed in only 20% of cases. With increasing MMP-13 expression, the proportion of patients with relapse increased [(+) 46% with relapse; (++) 80% with relapse; and (+++) 100% with relapse].
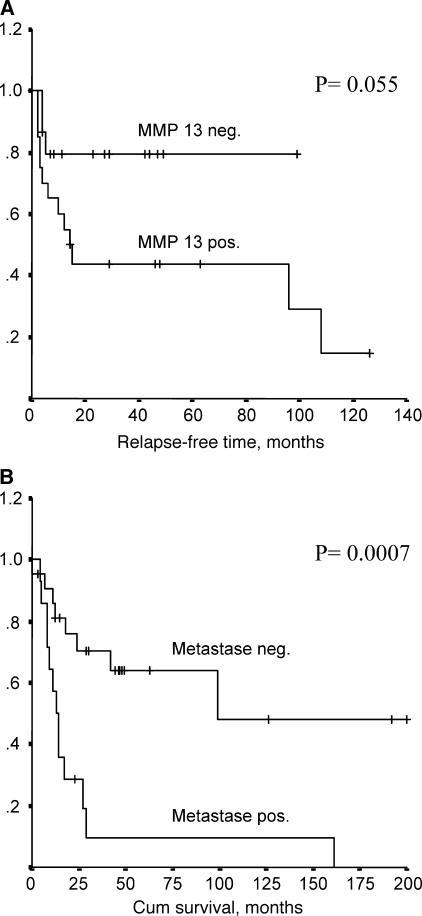
Cumulative survival analysis was of borderline significance, as shown in Figure 2A (log rank test, P = .055). The presence of metastasis was associated with p53Pro72 polymorphism (Fisher's exact test, P = .041) and correlated with a shorter survival of patients (log rank test, P = .0007) (Figure 2B). No significant association was detected for MMP-13 expression with p53Pro72 polymorphism. Furthermore, MMP-13 was not linked to metastasis.
Figure 2.
Kaplan-Meier curves showing (A) relapse-free survival in patients with and without MMP-13 expression, and (B) cumulative survival of patients with and without metastasis.
Discussion
MMP-13 Expression in MPNST
More than half of the 36 MPNST analyzed expressed MMP-13. MMP-13 expressions were similar in NF1-associated and sporadic MPNST, and were associated with a higher risk for recurrence. We detected the expression of MMP-13 in MPNST but not in 14 neurofibromas, further supporting an association with malignancy. This assumption is in accordance with a study reporting that MMP-13 is expressed in carcinomas but is generally absent in premalignant or benign lesions [23].
TP53 Mutation and MMP-13 Expression
Previous studies have reported that MPNST harbor TP53 mutations. However, the proportion of MPNST carrying mutant TP53 differs strongly among those studies [4–6,24]. This may be explained by the analysis of small tumor panels. In addition, most studies restricted their analysis to selected TP53 exons. We analyzed TP53 mutation frequency in a larger panel of MPNST screening all coding exons. Furthermore, we determined whether an association between TP53 status and MMP-13 expression is present. It has been previously shown that wild-type p53 represses the MMP-13 promoter and that this effect could be reversed by the overexpression of several p53 mutants [20]. p53Gly281 mutant even stimulated MMP-13 promoter up to two-fold to three-fold. Based on these observations, we asked whether MMP-13 expression in MPNST is linked to mutant TP53. Our analysis revealed that mutant TP53 is rare in MPNST. Although all four MPNST with TP53 mutation (11%) expressed MMP-13, the association was not significant. Because most MMP-13-positive MPNST carried wild-type TP53, mutant p53 is not likely to be a major driver of MMP-13 expression in MPNST. Factors such as interleukin-1, tumor necrosis factor-α, tumor growth factor-β (TGF-β), keratinocyte growth factor, basic fibroblast growth factor, acidic fibroblast growth factor, platelet-derived growth factor, and epidermal growth factor have been reported to drive MMP-13 expression in different tumors [25,26]. Most of these growth factors have been shown to be expressed in nerve sheath tumors [27] and may, therefore, induce MMP-13 expression in MPNST. TP53 mutation frequency in sarcomas has been evaluated in many studies and occurs in 10% to 30% [28,29]. Therefore, our data showing that 11% of MPNST carry TP53 mutations fit within this range. TP53 mutations correlate with histologic grade (all MPNST with mutant TP53 were of histologic grade 3). An NF1 mouse model is based on the haploinsufficiency of Nf1 and Trp53. These mice develop high-grade sarcomas, including MPNST [30,31]. However, MPNST are uncommon in mice and humans with hereditary defects in TP53 (Li-Fraumeni syndrome). Taken together, these observations suggest that mutant p53 plays a minor role in human MPNST and that other gene alterations must also contribute to their development.
p53 Immunoreactivity Is Linked to MMP-13 Expression
Although no significant association between mutant TP53 DNA sequence and MMP-13 expression existed, we detected a strong association between p53 and MMP-13 expression. The majority of MPNST were p53-immunopositive (78%). This is in accordance with a previous study that found p53 positivity in 83% of MPNST [5]. We provide evidence that p53 expression in peripheral nerve sheath tumors is, similar to MMP-13, restricted to MPNST and absent in neurofibroma. Immunodetection of p53 may hint toward mutant TP53. Mutant p53 often accumulates in the nucleus because its degradation is impaired. However, we and others did not find a significant association between nuclear p53 and mutant TP53 [5,29,32]. In fact, we show that the number of p53-positive MPNST exceeds, by far, those carrying mutant TP53. Nevertheless, all MPNST with mutant p53 were p53-immunopositive. p53 positivity without an underlying mutation suggests a stronger expression or a longer half-life of p53. The strong overlap of p53 immunoreactivity and MMP-13 expression may be explained by cellular stresses (such as hypoxia) known to induce the expression or stabilization of these proteins [33,34]. Our results fit to an immunohistochemical study that detected increased levels of p53 and TGF-β in areas of MPNST compared to adjacent neurofibroma areas [35]. Notably, TGF-β is an inducer of MMP-13 [36]. At first sight, it may appear contradictory that p53 and MMP-13 are often coexpressed because wild-type p53 has been described as a repressor of MMP-13 transcription. However, cytokines and growth factors, often expressed in cancers, are MMP-13 inducers and may override the inhibitory effect of p53.
Western blot analysis results show that MMP-13 and p53 signals were stronger in the S462 cell line than in the original MPNST 24472 (Figure 1). Mutant TP53 in these samples presumably results in accumulation of p53 most likely due to impaired degradation. Stronger signals of MMP-13 and p53 in S462 cells compared to the primary tumor hint toward a selection of subclones with these features during culturing conditions.
TP53 Polymorphisms and Correlation with Clinical Characteristics
The frequencies of TP53 variants in MPNST were compared with data from controls published in earlier studies (Table 2). The 16-bp duplication variant in intron 3 and the polymorphism in exon 4 were compared to a German control group (n = 549) [37]. Intron 2 polymorphism was compared to that of a study containing 154 individuals [38], and the frequency of A11992 (intron 3) was compared to that of Caucasian controls from the NCBI database. The allele distribution in MPNST patients was similar to that observed in control groups. This observation indicates that these polymorphisms are unlikely to contribute to the development of MPNST, although the 16-bp duplication in intron 3 has previously been associated with an increased risk of cancers (Wang-Gohrke et al. [37], no. 2654). However, p53Pro72 was more frequently detected in MPNST patients with metastasis (Fisher's exact test, P = .041), and metastasis correlated with shorter survival (log rank test, P = .0007) (Figure 2B). This observation fits the functional differences reported for this variant. p53Pro72 suppresses cellular transformation less efficiently [39] and is less susceptible than p53Arg72 to degradation by the human papillomavirus 18-encoded protein E6 [40]. In addition, p53Arg72 induces apoptosis markedly better than p53Pro72 [41]. Therefore, p53Pro72 may promote the development of metastasis more than p53Arg72. Although not significant, an association between increasing histologic grade and p53Pro72 polymorphism (chi-square test, P = .084) was present, further supporting the idea of p53Pro72 contribution to malignant progression. In summary, our data are in accordance with previous inconsistent reports that did not find a clear correlation between general cancer risk and codon 72 polymorphism, but an association between p53Pro72 and cancer progression, survival, age of onset, and response to therapy [42].
Taken together, our data suggest that MMP-13 and p53 immunopositivities, but also p53Pro72 allele, are associated with tumor progression. Therefore, MPNST patients with these determinants may need a closer follow-up and more aggressive therapy. Especially the absence of MMP-13 expression in healthy tissues of adults makes MMP-13 an attractive therapeutic target. Furthermore, MMP-13 may serve as a marker for malignant progression in MPNST.
Acknowledgements
We thank Kathrein Stichling and Petra Matylewski for their technical assistance, and Lope Estevez-Schwarz for providing tumor tissues.
Abbreviations
- dNF
dermal neurofibromas
- MMP-13
matrix metalloproteinase 13
- MPNST
malignant peripheral nerve sheath tumors
- NF1
neurofibromatosis type 1
- pNF
plexiform neurofibromas
Footnotes
This work was supported by Berliner Krebsgesellschaft and Deutsche Krebshilfe.
References
- 1.Ducatman BS, Scheithauer BW, Piepgras DG, Reiman HM, Ilstrup DM. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer. 1986;57:2006–2021. doi: 10.1002/1097-0142(19860515)57:10<2006::aid-cncr2820571022>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 2.Evans DG, Baser ME, McGaughran J, Sharif S, Howard E, Moran A. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet. 2002;39:311–314. doi: 10.1136/jmg.39.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huson SM. Neurofibromatosis 1: a clinical and genetic overview. In: Huson SM, Hughes RAC, editors. The Neurofibromatoses. London: Chapman and Hall Medical; 1994. pp. 160–203. [Google Scholar]
- 4.Birindelli S, Perrone F, Oggionni M, Lavarino C, Pasini B, Vergani B, Ranzani GN, Pierotti MA, Pilotti S. Rb and TP53 pathway alterations in sporadic and NF1-related malignant peripheral nerve sheath tumors. Lab Invest. 2001;81:833–844. doi: 10.1038/labinvest.3780293. [DOI] [PubMed] [Google Scholar]
- 5.Mawrin C, Kirches E, Boltze C, Dietzmann K, Roessner A, Schneider-Stock R. Immunohistochemical and molecular analysis of p53, RB, PTEN in malignant peripheral nerve sheath tumors. Virchows Arch. 2002;440:610–615. doi: 10.1007/s00428-001-0550-4. [DOI] [PubMed] [Google Scholar]
- 6.Menon AG, Anderson KM, Riccardi VM, Chung RY, Whaley JM, Yandell DW, Farmer GE, Freiman RN, Lee JK, Li FP, et al. Chromosome 17p deletions and p53 gene mutations associated with the formation of malignant neurofibrosarcomas in Recklinghausen neurofibromatosis. Proc Natl Acad Sci USA. 1990;87:5435–5439. doi: 10.1073/pnas.87.14.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holtkamp N, Mautner V, Friedrich R, Harder A, Hartmann C, Theallier-Janko A, Hoffmann K, von Deimling A. Differentially expressed genes in neurofibromatosis 1-associated neurofibromas and malignant peripheral nerve sheath tumors. Acta Neuropathol Berl. 2004;107:159–168. doi: 10.1007/s00401-003-0797-8. [DOI] [PubMed] [Google Scholar]
- 8.Levy P, Bieche I, Leroy K, Parfait B, Wechsler J, Laurendeau I, Wolkenstein P, Vidaud M, Vidaud D. Molecular profiles of neurofibromatosis type 1-associated plexiform neurofibromas: identification of a gene expression signature of poor prognosis. Clin Cancer Res. 2004;10:3763–3771. doi: 10.1158/1078-0432.CCR-03-0712. [DOI] [PubMed] [Google Scholar]
- 9.Ashworth JL, Murphy G, Rock MJ, Sherratt MJ, Shapiro SD, Shuttleworth CA, Kielty CM. Fibrillin degradation by matrix metalloproteinases: implications for connective tissue remodelling. Biochem J. 1999;340:171–181. [PMC free article] [PubMed] [Google Scholar]
- 10.Knauper V, Cowell S, Smith B, Lopez-Otin C, O'Shea M, Morris H, Zardi L, Murphy G. The role of the C-terminal domain of human collagenase-3 (MMP-13) in the activation of procollagenase-3, substrate specificity, and tissue inhibitor of metalloproteinase interaction. J Biol Chem. 1997;272:7608–7616. doi: 10.1074/jbc.272.12.7608. [DOI] [PubMed] [Google Scholar]
- 11.Knauper V, Lopez-Otin C, Smith B, Knight G, Murphy G. Biochemical characterization of human collagenase-3. J Biol Chem. 1996;271:1544–1550. doi: 10.1074/jbc.271.3.1544. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell PG, Magna HA, Reeves LM, Lopresti-Morrow LL, Yocum SA, Rosner PJ, Geoghegan KF, Hambor JE. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996;97:761–768. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johansson N, Vaalamo M, Grenman S, Hietanen S, Klemi P, Saarialho-Kere U, Kahari VM. Collagenase-3 (MMP-13) is expressed by tumor cells in invasive vulvar squamous cell carcinomas. Am J Pathol. 1999;154:469–480. doi: 10.1016/S0002-9440(10)65293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Airola K, Karonen T, Vaalamo M, Lehti K, Lohi J, Kariniemi AL, Keski-Oja J, Saarialho-Kere UK. Expression of collagenases-1 and -3 and their inhibitors TIMP-1 and -3 correlates with the level ofvasion in malignant melanomas. Br J Cancer. 1999;80:733–743. doi: 10.1038/sj.bjc.6690417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Etoh T, Inoue H, Yoshikawa Y, Barnard GF, Kitano S, Mori M. Increased expression of collagenase-3 (MMP-13) and MT1-MMP in oesophageal cancer is related to cancer aggressiveness. Gut. 2000;47:50–56. doi: 10.1136/gut.47.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pendas AM, Uria JA, Jimenez MG, Balbin M, Freije JP, Lopez-Otin C. An overview of collagenase-3 expression in malignant tumors and analysis of its potential value as a target in antitumor therapies. Clin Chim Acta. 2000;291:137–155. doi: 10.1016/s0009-8981(99)00225-9. [DOI] [PubMed] [Google Scholar]
- 17.Ala-Aho R, Johansson N, Baker AH, Kahari VM. Expression of collagenase-3 (MMP-13) enhances invasion of human fibrosarcoma HT-1080 cells. Int J Cancer. 2002;97:283–289. doi: 10.1002/ijc.1619. [DOI] [PubMed] [Google Scholar]
- 18.Ala-aho R, Ahonen M, George SJ, Heikkila J, Grenman R, Kallajoki M, Kahari VM. Targeted inhibition of human collagenase-3 (MMP-13) expression inhibits squamous cell carcinoma growth in vivo. Oncogene. 2004;23:5111–5123. doi: 10.1038/sj.onc.1207678. [DOI] [PubMed] [Google Scholar]
- 19.Ala-aho R, Grenman R, Seth P, Kahari VM. Adenoviral delivery of p53 gene suppresses expression of collagenase-3 (MMP-13) in squamous carcinoma cells. Oncogene. 2002;21:1187–1195. doi: 10.1038/sj.onc.1205198. [DOI] [PubMed] [Google Scholar]
- 20.Sun Y, Cheung JM, Martel-Pelletier J, Pelletier JP, Wenger L, Altman RD, Howell DS, Cheung HS. Wild type and mutant p53 differentially regulate the gene expression of human collagenase-3 (hMMP-13) J Biol Chem. 2000;275:11327–11332. doi: 10.1074/jbc.275.15.11327. [DOI] [PubMed] [Google Scholar]
- 21.Coindre JM, Trojani M, Contesso G, David M, Rouesse J, Bui NB, Bodaert A, De Mascarel I, De Mascarel A, Goussot JF. Reproducibility of a histopathologic grading system for adult soft tissue sarcoma. Cancer. 1986;58:306–309. doi: 10.1002/1097-0142(19860715)58:2<306::aid-cncr2820580216>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 22.Guillou L, Coindre JM, Bonichon F, Nguyen BB, Terrier P, Collin F, Vilain MO, Mandard AM, Le Doussal V, Leroux A, et al. Comparative study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group grading systems in a population of 410 adult patients with soft tissue sarcoma. J Clin Oncol. 1997;15:350–362. doi: 10.1200/JCO.1997.15.1.350. [DOI] [PubMed] [Google Scholar]
- 23.Airola K, Johansson N, Kariniemi AL, Kahari VM, Saarialho-Kere UK. Human collagenase-3 is expressed in malignant squamous epithelium of the skin. J Invest Dermatol. 1997;109:225–231. doi: 10.1111/1523-1747.ep12319441. [DOI] [PubMed] [Google Scholar]
- 24.Lothe RA, Smith-Sorensen B, Hektoen M, Stenwig AE, Mandahl N, Saeter G, Mertens F. Biallelic inactivation of TP53 rarely contributes to the development of malignant peripheral nerve sheath tumors. Genes Chromosomes Cancer. 2001;30:202–206. [PubMed] [Google Scholar]
- 25.Balbin M, Pendas AM, Uria JA, Jimenez MG, Freije JP, Lopez-Otin C. Expression and regulation of collagenase-3 (MMP-13) in human malignant tumors. APMIS. 1999;107:45–53. doi: 10.1111/j.1699-0463.1999.tb01525.x. [DOI] [PubMed] [Google Scholar]
- 26.Uria JA, Stahle-Backdahl M, Seiki M, Fueyo A, Lopez-Otin C. Regulation of collagenase-3 expression in human breast carcinomas is mediated by stromal-epithelial cell interactions. Cancer Res. 1997;57:4882–4888. [PubMed] [Google Scholar]
- 27.Kurtz A, Martuza RL. Antiangiogenesis in neurofibromatosis 1. J Child Neurol. 2002;17:578–584. doi: 10.1177/088307380201700807. (discussion, 602–574, 646–551). [DOI] [PubMed] [Google Scholar]
- 28.Mousses S, McAuley L, Bell RS, Kandel R, Andrulis IL. Molecular and immunohistochemical identification of p53 alterations in bone and soft tissue sarcomas. Mod Pathol. 1996;9:1–6. [PubMed] [Google Scholar]
- 29.Yoo J, Lee HK, Kang CS, Park WS, Lee JY, Shim SI. p53 gene mutations and p53 protein expression in human soft tissue sarcomas. Arch Pathol Lab Med. 1997;121:395–399. [PubMed] [Google Scholar]
- 30.Cichowski K, Shih TS, Schmitt E, Santiago S, Reilly K, McLaughlin ME, Bronson RT, Jacks T. Mouse models of tumor development in neurofibromatosis type 1. Science. 1999;286:2172–2176. doi: 10.1126/science.286.5447.2172. [DOI] [PubMed] [Google Scholar]
- 31.Vogel KS, Klesse LJ, Velasco-Miguel S, Meyers K, Rushing EJ, Parada LF. Mouse tumor model for neurofibromatosis type 1. Science. 1999;286:2176–2179. doi: 10.1126/science.286.5447.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim EL, Yoshizato K, Kluwe L, Meissner H, Warneck G, Zapf S, Westphal M, Deppert W, Giese A. Comparative assessment of the functional p53 status in glioma cells. Anticancer Res. 2005;25:213–224. [PubMed] [Google Scholar]
- 33.An WG, Kanekal M, Simon MC, Maltepe E, Blagosklonny MV, Neckers LM. Stabilization of wild-type p53 by hypoxia-inducible factor 1alpha. Nature. 1998;392:405–408. doi: 10.1038/32925. [DOI] [PubMed] [Google Scholar]
- 34.Koong AC, Denko NC, Hudson KM, Schindler C, Swiersz L, Koch C, Evans S, Ibrahim H, Le QT, Terris DJ, et al. Candidate genes for the hypoxic tumor phenotype. Cancer Res. 2000;60:883–887. [PubMed] [Google Scholar]
- 35.Watanabe T, Oda Y, Tamiya S, Masuda K, Tsuneyoshi M. Malignant peripheral nerve sheath tumour arising within neurofibroma. An immunohistochemical analysis in the comparison between benign and malignant components. J Clin Pathol. 2001;54:631–636. doi: 10.1136/jcp.54.8.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johansson N, Ala-aho R, Uitto V, Grenman R, Fusenig NE, Lopez-Otin C, Kahari VM. Expression of collagenase-3 (MMP-13) and collagenase-1 (MMP-1) by transformed keratinocytes is dependent on the activity of p38 mitogen-activated protein kinase. J Cell Sci. 2000;113(Part 2):227–235. doi: 10.1242/jcs.113.2.227. [DOI] [PubMed] [Google Scholar]
- 37.Wang-Gohrke S, Becher H, Kreienberg R, Runnebaum IB, Chang-Claude J. Intron 3 16 bp duplication polymorphism of p53 is associated with an increased risk for breast cancer by the age of 50 years. Pharmacogenetics. 2002;12:269–272. doi: 10.1097/00008571-200204000-00012. [DOI] [PubMed] [Google Scholar]
- 38.Verselis SJ, Li FP. Common polymorphism in p53 intron 2, IVS2 + 38G→C. Hum Mutat. 2000;16:181. doi: 10.1002/1098-1004(200008)16:2<181::AID-HUMU23>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 39.Thomas M, Kalita A, Labrecque S, Pim D, Banks L, Matlashewski G. Two polymorphic variants of wild-type p53 differ biochemically and biologically. Mol Cell Biol. 1999;19:1092–1100. doi: 10.1128/mcb.19.2.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Storey A, Thomas M, Kalita A, Harwood C, Gardiol D, Mantovani F, Breuer J, Leigh IM, Matlashewski G, Banks L. Role of a p53 polymorphism in the development of human papillomavirus-associated cancer. Nature. 1998;393:229–234. doi: 10.1038/30400. [DOI] [PubMed] [Google Scholar]
- 41.Dumont P, Leu JI, Della Pietra AC, III, George DL, Murphy M. The codon 72 polymorphic variants of p53 have markedly different apoptotic potential. Nat Genet. 2003;33:357–365. doi: 10.1038/ng1093. [DOI] [PubMed] [Google Scholar]
- 42.Pietsch EC, Humbey O, Murphy ME. Polymorphisms in the p53 pathway. Oncogene. 2006;25:1602–1611. doi: 10.1038/sj.onc.1209367. [DOI] [PubMed] [Google Scholar]