Abstract
Background
Dietary flavone was previously shown to increase the expression of deleted in liver cancer–1 gene (DLC-1) in HT-29 colon carcinoma cell line (Proteomics 2004;4:2455-64). DLC-1 that encodes a Rho GTPase-activating protein, functions as a tumor suppressor gene and is frequently inactivated or down-regulated in several common cancers. Restoration of DLC-1 expression suppresses in vitro tumor cells proliferation and tumorigenicity in vivo.
Methods
Here, the effect of flavone was examined in several DLC-1-deficient cell lines derived from different types human cancer using assays for cell proliferation, gene expression and transfer.
Results
We show that exposure to 150μM flavone increased DLC1 expression in breast but not in liver or prostate carcinoma cells or a nonmalignant breast epithelial cell line. Flavone restored the expression of DLC1 in the breast carcinoma cell lines MDA-MB-468, MDA-MB-361, and BT20 as well as in the colon carcinoma cell line HT-29 all of which are DLC-1-negative due to promoter hypermethylation. We further show that flavone inhibited cell proliferation, induced cell cycle arrest at G2-M, increased p21 Waf1 gene expression, and caused apoptosis. Microarray analysis of these aggressive and metastatic breast carcinoma cells revealed 29 flavone-responsive genes, among which the DNA damage–inducible GADD genes were up-regulated and the proto-oncogene STMN1 and IGFBP3 were down-regulated.
Conclusions
Flavone-mediated alterations of genes that regulate tumor cell proliferation, cell cycle, and apoptosis contribute to chemopreventive and antitumoral effects of flavone. Alone or in combination with demethylating agents, flavone may be an effective adjunct to chemotherapy in preventing breast cancer metastasis.
Author's key words: Flavone, breast cancer, colon cancer, DLC-1 gene, tumor suppressor genes, protooncogenes, DNA methylation, cell cycle, cell growth inhibition, apoptosis
1. Introduction
The DLC1 (deleted in liver cancer–1) gene [1] encodes a Rho GTPase-activating protein and is expressed in most human tissues, but its expression is frequently down-regulated or silenced in various types of human cancer. Indeed, DLC1 is emerging as a bona fide tumor suppressor gene, given that ectopic expression of DLC-1 in several common types human cancer cells that do not express the endogenous gene inhibits cell proliferation and induces caspase-3-mediated apoptosis in vitro as well as abolishes or reduces tumorigenicity in vivo [2,3,4,5,6,7,8]. Limited information is available on factors that regulate transcription of endogenous DLC1, however. Activation of peroxisome proliferator–activated receptor γ (PPARγ), which inhibits the growth of breast and prostate cancer cells as well as metastasis of lung tumor cells, was shown to increase expression of DLC1 in association with inhibition of the invasiveness of lung cancer cells that overexpress PPARγ [9]. All-trans retinoic acid inhibits the proliferation of normal cells as well as that of various types of tumor cells, and culture of Wilms' tumor cells with all-trans-retinoic acid induced expression of DLC1 [10]. Comprehensive gene expression profiling also revealed that doxorubicin, the most widely used drug for treatment of breast cancer, increased the expression of genes important in apoptosis, cell proliferation, cell cycle checkpoints, and suppression of metastasis, including that of DLC1, in breast cancer patients [11].
Progesterone confers protection against ovarian cancer. Gene expression profiling of human immortal nontumorigenic ovarian epithelial cells and ovarian cancer cells revealed that progesterone up-regulated the expression of several genes in the cancer cells [4]. Among these genes, four with known antitumorigenic function—those for ATF3, caveolin-1, NM23-H2, and DLC-1—were selected for post hoc functional analysis. Progesterone induced up-regulation of the expression of all four genes in the cancer cells but not in the nontumorigenic cells. Of these four genes, those for caveolin-1 and DLC-1 exhibited a broad spectrum of antitumor activity similar to that of bona fide tumor suppressor genes. Overexpression of DLC-1 in the ovarian cancer cells thus resulted in inhibition of cell growth, of colony formation in soft agar, and of cell migration as well as in the induction of apoptosis [4].
A protein and mRNA profiling study showed that flavone, a dietary constituent, increased the expression of DLC-1 in HT-29 human colon carcinoma cells [12], which constitutively express DLC1 at only a low level as a result of promoter methylation [13]. We have now confirmed that flavone increases the amount of DLC-1 mRNA in HT-29 cells and have investigated whether this compound exerts a similar effect in other cell lines derived from several types of solid tumors deficient in DLC-1. We found that flavone reactivated DLC1 expression in three breast carcinoma cell lines derived from aggressive or metastatic breast tumors. Given that DLC-1 exhibits marked antitumoral and antimetastatic activity in breast cancer cells, with DLC1 being a candidate breast cancer susceptibility gene [2,6,14,15], we analyzed cellular and molecular alterations resulting from flavone exposure in the three breast carcinoma cell lines and a nontumorigenic breast epithelial cell line. Our results show that flavone targets additional genes important in regulation of cancer cell growth, repair of DNA damage, and apoptosis, effects that likely contribute to its antitumoral activity in breast cancer.
2. Materials and Methods
2.1. Cell culture
The breast carcinomas and immortalized breast epithelial cell lines MDA-MB-468, MDA-MB-361, BT20, MCF10F, colon carcinoma HT-29 cells, and prostate carcinoma 22RVi were obtained from American Type Culture Collection (Manassas, VA). The hepatocellular carcinoma (HCC) cell lines, Focus and 7703, were maintained in our laboratory repository. Cells were cultured under an atmosphere of 5% CO2 at 37°C in Dulbecco's modified Eagle's medium–F12 supplemented with 10% fetal bovine serum and antibiotics (Biosource, Rockville, MD).
2.2. Assay of cell proliferation
Based on previous results with protein and mRNA profiling and other studies, in all experiments the concentration of 150μM flavone (2-phenyl-4H-1-benzopyran-4-one; Sigma, St. Louis, MO) was used [12,16]. For cell proliferation assay, cells were seeded in triplicate in 96-well plates (1 × 104 cells per well) and cultured with flavone for up to 4 days. Cell proliferation was evaluated with the colorimetric MTT reduction assay (Promega, Madison, WI) and measurement of absorbance at 490 nm with a microplate reader (Spektra Plus; PGC Scientific, Gaithersburg, MD).
2.3. Flow cytometry
Control or flavone-treated cells (1 × 106 per sample) were fixed in 70% ethanol, incubated for 5 minutes at 4°C with 0.1% Triton X-100 and RNase A (100 U/ml, Sigma), and then stained with propidium iodide (50 μg/ml, Sigma). The cells were analyzed for cell cycle distribution and apoptosis by flow cytometry with a FACSort instrument (Becton Dickinson, Boston, MA) and FlowJo 6.4.1 software (Tree Star, Inc, Ashland, OR).
2.4. Assay of caspase-3 activity
The activity of caspase-3 was determined by using EnzChek Caspase-3 Assay Kit (Molecular Probes, Eugene, OR) according to the manufacturer's protocol. Fluorescence was measured with a Spektra Max Gemini instrument (PGC Scientific) at excitation and emission wavelengths of 480 and 520 nm, respectively. Serial dilutions of the R110 dye were used as a standard.
2.5. Quantitative RT-PCR analysis
Quantitative RT-PCR analysis of DLC-1 and p21Waf1 mRNA was performed with primers using and a TaqMan probe as described previously [17,18]. Standard curves revealed similar reaction efficiencies for all PCR amplifications. PCR was performed with an ABI PRISM 7900 instrument, and the abundance of DLC-1 and p21Waf1 mRNAs was normalized by that of GAPDH mRNA. Differences in gene expression induced by flavone treatment were evaluated by the 2−ΔΔct method [19]; differences were considered significant when 2−ΔΔct was ≥2 or ≤0.5.
2.6. Cell transfection
Breast cancer cell lines MDA-MB-468, MDA-MB-361, and BT20 at 70% confluence were transfected for 48 h with the adenoviral vector pAd/CMV/V5-GW (Invitrogen, Carlsbad, CA) containing full-length cDNAs for human DLC-1 or β-galactosidase (LacZ).
2.7. Immunoblot analysis
Control or flavone-treated cells (5 × 106) were lysed with the use of the M-PER Mammalian Protein Extraction Reagent (Pierce, Rockford, IL) and Protease Inhibitor Cocktail (Sigma). Proteins were fractionated by SDS-polyacrylamide gel electrophoresis on a 4 to 20% gradient gel (Invitrogen) and then transferred to a nitrocellulose membrane (Invitrogen). After incubation with 5% skim milk (Bio-Rad, Hercules, CA) for 1 h, the membrane was incubated overnight with F-5 and DO-1 mouse monoclonal antibodies (1 μg/ml) to p21Waf1 and p53 (Santa Cruz Biotechnology, Santa Cruz, CA), respectively. The membrane was reprobed with mouse monoclonal antibodies to GAPDH (MAB374; Chemicon, Tamacula, CA) at 0.5 μg/ml for 1 h as a control for sample loading. Immune complexes were detected by incubation of the membrane for 1 h with horseradish peroxidase–conjugated goat secondary antibodies (Santa Cruz Biotechnology) at a dilution of 1:20,000 and with the use of a SuperSignal West Pico Chemiluminescent Substrate Kit (Pierce). Blots were subjected to densitometric analysis with Gene Snap software (Syngene, Cambridge, UK).
2.8. cDNA microarray analysis
Microarrays (Operon Human Version 3.0) containing 34,580 probes were obtained from the NCI array facility (NCI ATC, Gaithesburg, MD). Total RNA was isolated from control or flavone-treated breast carcinoma cell lines with an RNeasy Mini Kit and was purified with a Qiaquick PCR Purification Kit (Qiagen). Samples of the purified total RNA (20 μg) were subjected to RT with a SuperScript Indirect cDNA Labeling System (Invitrogen) and the resulting cDNA was labeled with Cy3 or Cy5 fluorescent dyes (Pharmacia, Piscataway, NJ). The labeled cDNA preparations were combined and allowed to hybridize overnight to the same microarray. Experiments were repeated with the same cDNA preparations labeled with the opposite combination of dyes. The ratio of the Cy3 and Cy5 signals was calculated for each spot on the array with GenePix 5.1 software (Axon Instruments, Sunnyvale, CA) and was further analyzed with the NCI mAdb array analysis tool.
3. Results
To confirm the previous finding by microarray analysis that flavone increased the amount of DLC-1 mRNA in HT-29 colon carcinoma cells [12], we examined the effect of flavone on DLC-1 mRNA by quantitative RT-PCR analysis. Exposure of HT-29 cells to 150 μM flavone for 24 h induced a nearly five fold increase in the amount of DLC-1 mRNA (Fig. 1)
Figure 1.
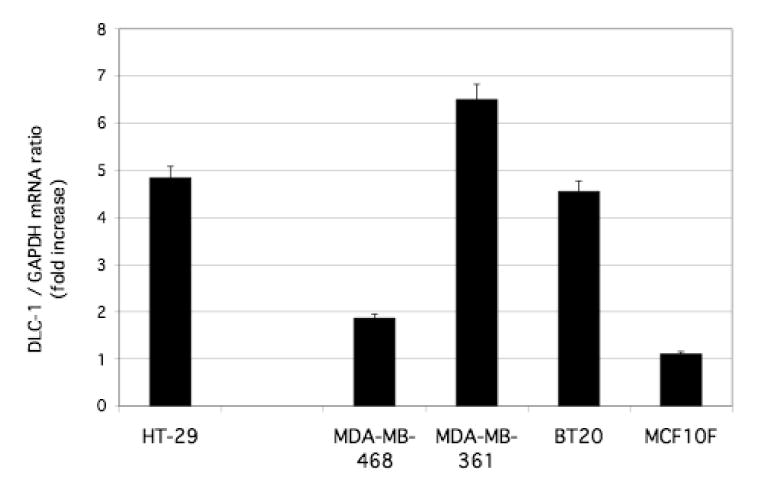
Effects of flavone on DLC1 expression in HT-29 colon carcinoma cells, breast carcinoma cells (MDA-MB-468, MDA-MB-361, BT20), and a normal breast epithelial cell line (MCF10F). Cells were incubated in the absence or presence of 150 μM flavone for 24 h, after which total RNA was isolated and subjected to quantitative RT-PCR analysis of DLC-1 mRNA. The level of normalized DLC1 expression for flavone-treated cells is shown relative to that in nontreated cells (see materials and methods). Data are the means ± SEM from independent experiments.
We next examined the effect of the same treatment in several other human cancer cell lines that, like HT-29 [13], express DLC1 at a low level [6,8,17]. Among these cell lines, flavone induced up-regulation of DLC-1 mRNA in MDA-MB-468, MDA-MB-361, and BT20 breast carcinoma cell lines but not in the immortal nontumorigenic breast epithelial cell line MCF10F (Fig. 1) or in HCC cell lines (Focus, 7703) or prostate (22RVi) cancer cell lines (unpublished observations).
Given that ectopic expression of DLC-1 in various tumor cell types has been shown to perturb the cell cycle, inhibit cell growth, or induce apoptosis [4,6,7,8], we examined whether flavone exerts similar effects in breast carcinoma or nontumorigenic breast epithelial cell lines. Flavone treatment resulted in inhibition of the proliferation of MDA-MB-468, MDA-MB-361, BT20, and MCF10F cells that was apparent as early as 24 h and increased progressively for up to 4 days (Fig. 2).
Figure 2.
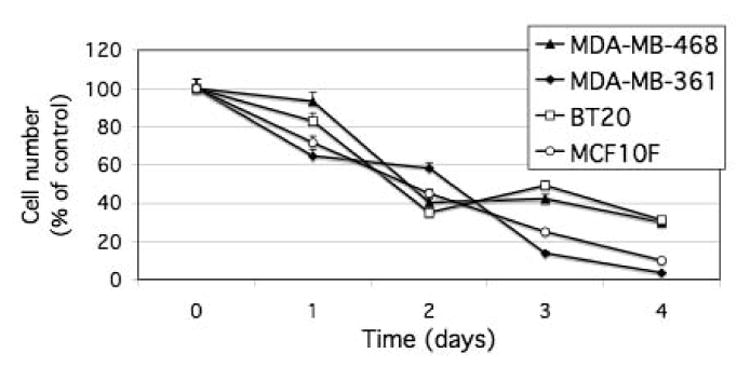
Inhibition of cell proliferation by flavone in breast carcinoma cell lines and a normal breast epithelial cell line. Cells were incubated in the absence or presence of 150 μM flavone for the indicated times, after which cell number was determined by the MTT assay. Cell number in flavone-treated cultures was expressed as a percentage of that in control cultures. Data are means ± SD of triplicates from a representative experiment.
This inhibitory effect on cell growth in malignant cell lines (MDA-MB-468, MDA-MB-361, BT20) was accompanied by changes in cell cycle progression and induction of apoptosis. Flavone treatment thus induced accumulation of cells with a sub-G1 DNA content (apoptotic cells) as well as of cells arrested at the G2-M transition of the cell cycle (Fig. 3).
Figure 3.
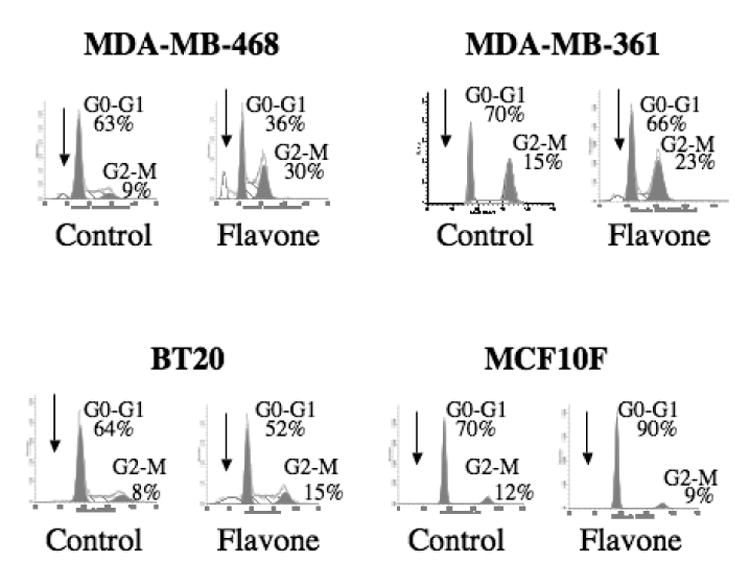
Effect of flavone on cell cycle distribution in breast carcinoma cell lines and a nonmalignant breast epithelial cell line. Cells were incubated in the absence (control) or presence of 150μM flavone for 24 h, after which DNA content was determined by flow cytometry. Arrows indicate the sub-G1 population, and the percentages of cells in G0-G1 or G2-M are shown.
These effects of flavone were not apparent with the nonmalignant cell line MCF10F, which instead manifested G0-G1 arrest in response to this agent. The induction of apoptosis in the malignant cell lines by flavone was confirmed by detection of increased caspase-3 activity in the flavone-treated cells (Fig. 4); again, such an effect was not observed in the nonmalignant cells.
Figure 4.
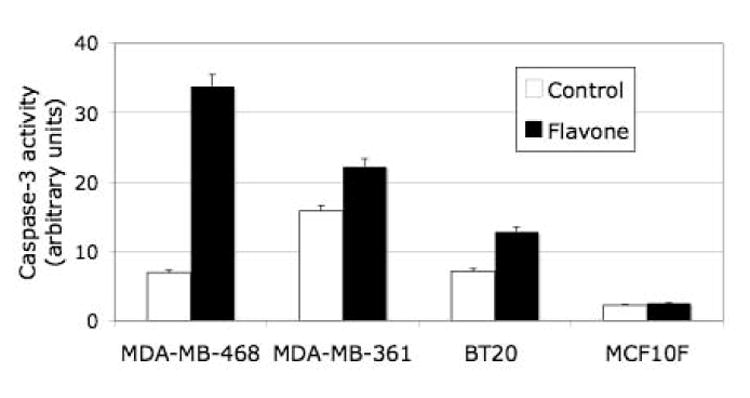
Effect of flavone on caspase-3 activity in breast carcinoma cell and nonmalignant breast epithelial cells. Cells were incubated in the absence or presence of 150μM flavone for 24 h, after which the activity of caspase-3 was determined. Data are expressed in arbitrary units and are means ± SD of triplicates from a representative experiment.
We next examined the effect of flavone on expression of p21Waf1, an inhibitor of cell cycle progression, given that flavone-induced inhibition of the growth of colon tumor cells in vitro and in vivo is associated with both the induction of apoptosis and up-regulation of p21Waf1 gene expression [16]. Furthermore, p21Waf1 is thought to be a downstream target of DLC-2, a member of the DLC family closely related to DLC-1 [20]. Flavone induced marked increases in the amounts of both p21Waf1 mRNA and protein in all three breast cancer cell lines as well as in the nonmalignant breast epithelial cell line (Fig. 5, 6A and 6B).
Figure 5.
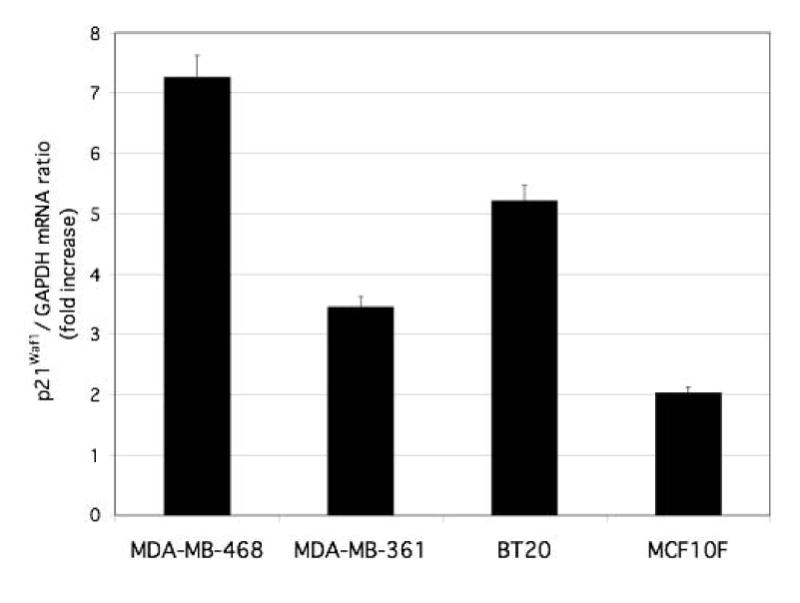
Effect of flavone on p21Waf1 gene expression in breast carcinoma cells and nonmalignant breast epithelial cells. Cells were incubated in the absence or presence of 150 μM flavone for 24 h, after which total RNA was isolated and subjected to quantitative RT-PCR analysis of p21Waf1 mRNA. Data were normalized by the amount of GAPDH mRNA, and the effect of flavone was evaluated by the 2−ΔΔct method and considered significant when 2−ΔΔct was ≥2 or ≤0.5. The abundance of p21Waf1 mRNA in flavone-treated cells is shown relative to that in nontreated cells. Data are the means ± SEM from independent experiments.
Figure 6.
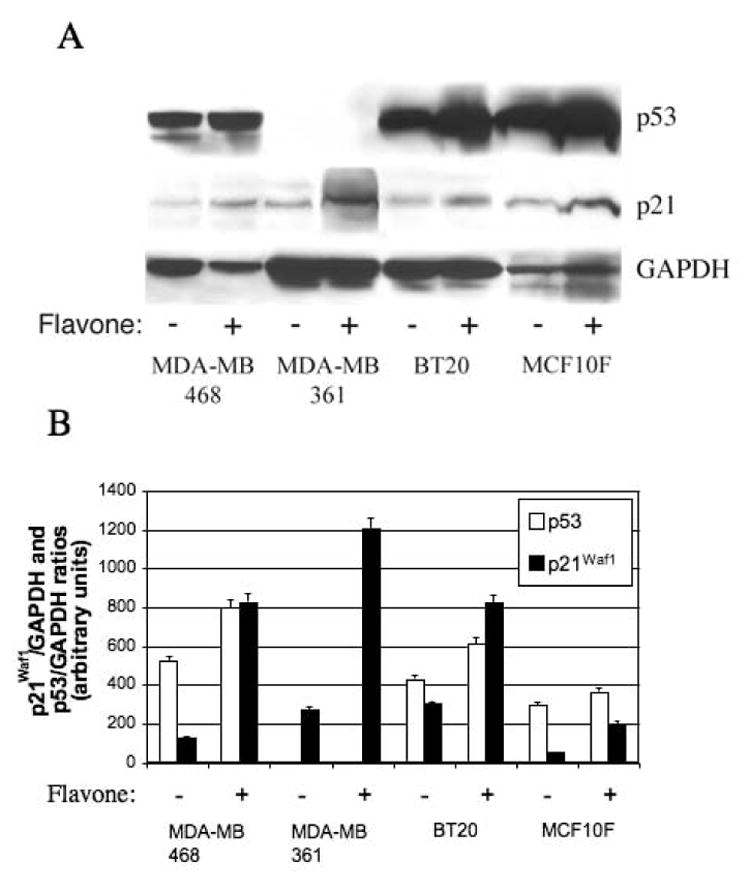
Effects of flavone on p21Waf1 and p53 abundance in breast cancer cell lines) and a normal breast epithelial cell line. (A) Cells were incubated in the absence or presence of 150μM flavone for 24 h, after which cell lysates were subjected to immunoblot analysis with antibodies to p53, to p21Waf1, or to GAPDH. (B) Blots similar to that in (A) were subjected to densitometric analysis, and the amounts of p21Waf1 and p53 were normalized by that of GAPDH.
Given that p53 binds directly to and increases the expression of p21Waf1 [21], we also examined the effect of flavone on the abundance of p53. Flavone increased the amount of p53 in MDA-MB-468 and BT20 cells, which harbor a mutant form of p53, but not in MDA-MB-361 cells, which are p53 negative, or in MCF10F cells, which express wild-type p53 (Fig. 6A and 6B).
To determine whether overexpression of p21 Waf1 was mediated by DLC-1 in the breast cancer cell lines, we transfected the cells with an adenoviral vector encoding DLC-1. Restoration of DLC-1 expression in these cells did not increase the expression of p21Waf1 (Fig. 7).
Figure 7.
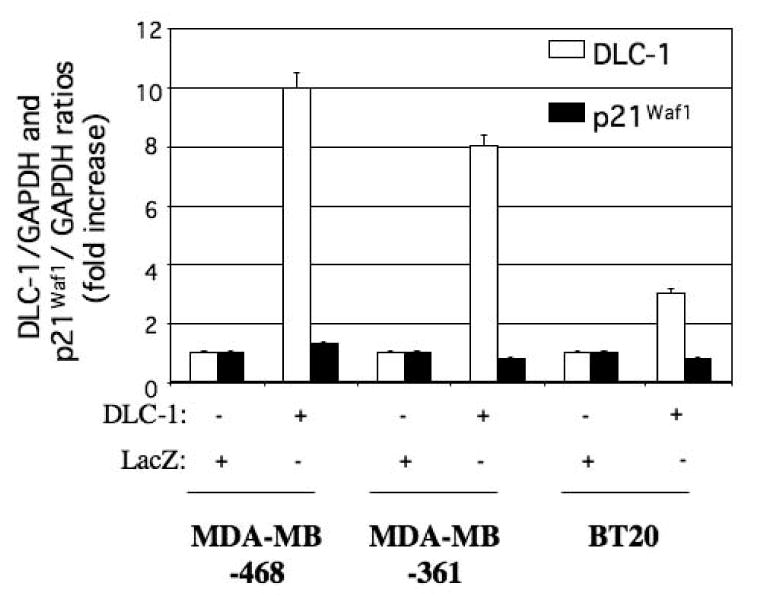
Effect of ectopic DLC-1 expression on p21Waf1 abundance in the breast cancer cell lines MDA-MB-468, MDA-MB-361, and BT20. Cells were transfected with expression vectors for DLC-1 or LacZ (control), and the abundance of DLC-1 and p21Waf1 mRNAs was subsequently determined by quantitative RT-PCR analysis. Data were normalized by the amount of GAPDH mRNA, and the normalized data were expressed relative to the corresponding value for cells transfected with the control vector. Differences between cells transfected with the DLC-1 vector and those transfected with the control vector were evaluated by the 2−ΔΔct method and considered significant when 2−ΔΔct was ≥2 or ≤0.5. Data are the means ± SEM from independent experiments.
Finally, we used high-density oligonucleotide microarrays to identify genes differentially expressed in each of the three breast cancer cell lines before and after flavone treatment. Only genes that were expressed in all three cell lines and which showed at least a two fold change in expression level in response to flavone treatment were selected for further analysis. The output data set contained 29 genes whose expression was significantly up-or down-regulated by flavone in all three breast cancer cell lines (Table I). The Correlation Summary Report revealed that the flavone signatures in the estrogen receptor–negative cell lines MDA-MB-468 and BT20 were more similar to each other (R = 0.92) than to that in the estrogen receptor–positive cell line MDA-MB-361 (R = 0.82 and 0.83, respectively).
Table I.
List of flavone-responsive genes in breast cancer cell lines MDA-MB-468, MDA-MB-361 and BT20 revealed by micro-array analysis. Gene involvement in the cellular biology was determined by KEEG/ BioCarta pathway and Gene Ontology reports. Their relationship to human disease was obtained from NCI Gene Report and online Gene Cards database.
| Gene | UniGene ID | Description | Biological Pathway and Function | Disease relationship |
|---|---|---|---|---|
| Table 1: Up-regulated genes | ||||
| DDIT3 (GADD153) | Hs.505777 | DNA-damage-inducible transcript 3 | B, CC/AP, p38 MAPK SP, TR | Cell injury, colon carcinoma, liposarcoma myxoid, malignant neoplasm musculoskeletal, melanoma, myeloid leukemia, osteosarcoma |
| SRXN1 | Hs.516830 | Sulfiredoxin 1 homolog (S. c.) | B, CA, response to oxidative stress | |
| SQSTM1 | Hs.437277 | Sequestosome 1 | Response to stress, RANK SP | Paget disease of bone |
| HMOX1 | Hs.517581 | Heme oxygenase (decycling) 1 | B, CA, IL-10 anti-inflammatory SP, porphyrin and chlorophyll metabolism, regulation of I-kappa B /NF-kappa B SP, response to oxidative stress | Atherosclerosis, cell injury, cerebral vasospasm, inflammation, ischemia, jaundice neonatal, malaria cerebral, shock |
| PPP1R15A (GADD34) | Hs.76556 | Protein phosphatase 1, regulatory (inhibitor) subunit 15A | CC/AP, response to DNA damage stimulus | Herpes simplex, malignant neoplasm |
| SLC20A1 | Hs.187946 | Solute carrier family 20 (phosphate transporter), member 1 | Regulation of I-kappa B /NF-kappa B SP | |
| AKR1C3 | Hs.78183 | Aldo-keto reductase family 1, member C3 | CA, prostaglandin and leukotriene metabolism | |
| STC2 | Hs.233160 | Stanniocalcin 2 | B, cell-cell signaling, response to nutrient | Malignant neoplasm of breast |
| OSIL | Ubiquitin-binding protein p62 | |||
| HERPUD1 | Hs.146393 | Homocysteine-inducible, endoplasmic reticulum stress-inducible, ubiquitin-like domain member 1 | Destruction of misfolded proteins by the ER-associated protein degradation system, unfolded protein response | |
| PRNP | Hs.472010 | Prion protein (p27-30) | B, response to oxidative stress | Creutzfeldt-Jakob disease, fatal familial insomnia, Gerstmann-Straussler disease, Huntington disease-like 1, kuru |
| RABGAP1 | Hs.271341 | RAB GTPase activating protein 1 | ||
| GADD45A | Hs.80409 | Growth arrest and DNA-damage-inducible | CC/AP, hypoxia, p53 SP, response to stress | Ataxia telangiectasia, lung carcinoma, leukemia, malignant neoplasm of breast, melanoma, ovarian epithelial carcinoma |
| PBEF1 | Hs.489615 | Pre-B-cell colony enhancing factor 1 | B, CA, nicotinate and nicotinamide metabolism, positive regulation of cell proliferation, ST | Systemic infection |
| TMEM38B | Hs.411925 | Transmembrane protein 38B | ||
| NEU1 | Hs.520037 | Sialidase 1 | CA, glycosphingolipid metabolism, N-Glycan degradation | Influenza, malignant neoplasm of breast and metastasis, sialidosis |
| SEC63 | Hs.529957 | SEC63-like (S. c.) | B, ST | Autosomal dominant polycystic liver disease (pcld) |
| DKFZP434 F0318 | Hs.23388 | Hypothetical protein | ||
| DNAJB9 | Hs.6790 | DNAJ (Hsp40) homolog, subfamily B, member 9 | B, chaperon regulator | |
| Table 1: Down-regulated genes | ||||
| RAD51L3 | Hs.125244 | RAD51-like 3 (S. c.) | B, CA, recombination repair of DNA | Malignant neoplasm of breast |
| RARRES3 | Hs.17466 | Retinoic acid receptor responder | Tumor suppressor, growth regulator | Malignant neoplasm, psoriasis |
| STMN1 | Hs.209983 | Stathmin 1/oncoprotein 18 | B, drug resistance, MAPK SP | Acute erythroblastic leukemia, lymphoma, lung carcinoma, osteosarcoma, plasmacytoma |
| FBXO4 | Hs.165575 | F-box protein 4 | CA, ubiquitination | |
| SUB1 | Hs.229641 | SUB1 homolog (S. c.) | B, TR | |
| S100A2 | Hs.516484 | S100 calcium binding protein A2 | B, hypothetical tumor suppressor | Basal cell carcinoma, malignant neoplasm of mouth, melanoma, non-small cell lung carcinoma, papillary carcinoma, psoriasis, squamous carcinoma |
| IGFBP3 | Hs.450230 | Insulin-like growth factor binding protein 3 | B, CC/AP, enzyme regulator, food intake and Energy homeostasis, hypoxia, p53 SP | Acromegaly, hypoglycemia, malignant neoplasm of breast, pituitary dwarfism ii, prostate carcinoma |
| E2F8 | Hs.523526 | E2F transcription factor 8 | B, TR | |
| AMOT | Hs.528051 | Angiomotin | B, regulation of vascular permeability and angiogenesis | |
| TUBB | Hs.533059 | Beta tubulin | B, CA, gap junction, ST | Cerebral amyloid angiopathy familial, ciliary motility disorders, non-small cell lung carcinoma, ovarian epithelial carcinoma, polyneuropathy, prostate carcinoma, |
B= binding activity, CA= catalytic activity, CC/AP= cell cycle/apoptosis, SP = signaling pathway, ST= signal transducer, TR= transcription regulator activity
The flavone-responsive genes were functionally categorized according to the Gene Ontology classification. Genes were classified on the basis on their cellular localization and molecular function and were mapped to biological pathways with the use of the KEGG and BioCarta Pathways databases. The listed genes encoded intracellular products with the exception of those for the extracellular proteins stanniocalcin 2 (STC2) and insulin-like growth factor–binding protein 3 (IGFBP3). The Gene Ontology classification revealed the output dataset to contain genes whose products possess binding activity (15 proteins), act as signal transducers (12 proteins), exhibit catalytic activity (9 proteins), participate in the response to stress (7 proteins), contribute to metabolism (6 proteins), regulate cell growth or apoptosis (5 proteins), control transcription (3 proteins), function as chaperones (3 proteins), or are important in DNA repair (1 protein) or drug resistance (1 protein). Most of the flavone-responsive genes have already been implicated in the pathogenesis of malignant neoplasms of the breast (6 proteins) or lung (4 proteins), leukemia (3 proteins), melanoma (3 proteins), or prostate cancer, ovarian cancer, osteosarcoma, or psoriasis (2 proteins each).
The flavone-responsive genes known to be involved in breast cancer development include GADD genes, STC2, STMN1 (stathmin 1), and IGFBP3. Among these genes, the DNA damage–inducible GADD34, GADD153, GADD45A, and STC2 were significantly up-regulated in response to flavone treatment. Furthermore, flavone up-regulated the expression of STC2 in both the estrogen receptor positive (MDA-MB-361) and estrogen receptor negative (MDA-MB-468, BT20) cell lines. The proto-oncogene STMN1 and IGFBP3 were significantly down-regulated by flavone treatment in all three cell lines.
4. Discussion
Both genetic and external factors are important in the development and prevention of breast cancer. Among such external factors, diet is thought to play an important role. A low incidence of breast cancer in Asia has been attributed in part to a high intake of flavonoids [22,23]. The incidence of cancer increases when Asian women move to the United States, where the average diet is rich in fat and confers a strong significant protective effect during adolescence. Flavonoids and flavone exert a broad spectrum of biological effects by targeting genes involved in regulation of tumor cell proliferation, the cell cycle, and apoptosis, thus reducing the risk of cancer or inhibiting the growth of tumor cells [16,24,25,26,27]. Such effects were apparent in the present study. Flavone thus affected the expression of a variety of genes that regulate cell proliferation, the response to DNA damage, and apoptosis. Restoration of DLC-1 expression in breast tumor cells in which expression of endogenous DLC1 is down-regulated or silenced has been shown to inhibit cell growth in vitro and to suppress the formation of tumors and metastases in athymic nude mice [2,6]. The effects of flavonoids on the expression of proto-oncogenes and tumor suppressor genes in vitro or in vivo have been examined in only a relatively small number of studies with human or rat mammary cells. The induction of apoptosis in mammary gland cells from young adult female rats by the soy isoflavone genistein was accompanied by an increase in the expression of the tumor suppressor gene PTEN [28]. A diet rich in two other soy isoflavones increased the expression of BRCA1 and BRCA2 in the mammary glands of ovariectomized rats [29]. In immortalized human breast epithelial cells, genistein inhibited cell proliferation by down-regulating expression of the MET proto-oncogene and up-regulating that of the tumor suppressor gene EGR1 as well as that of the immediate-early response genes FOS and JUN [30]. The inhibition by genistein of the growth of human breast tumor cells derived from an invasive carcinoma was associated with a marked increase in BRCA2 expression [31]. Collectively, these observations indicate that up-regulation of the expression of tumor suppressor genes by flavone or flavonoids is a contributing factor to their antitumoral activity.
In addition to the increase in the expression of DLC1 in all three breast carcinoma cell lines flavone inhibited tumor cell proliferation, arrested the cells in G2-M of the cell cycle, induced up-regulation of p21Waf1 and caspase-3–mediated apoptosis was detected. A recent study suggested that DLC-2, which is closely related to DLC-1, functions in a Rho-independent manner and that p21Waf1 is a downstream effector of Rho-DLC-2 signaling. Introduction of a DLC-2 vector into breast cancer cells already transfected with p21Waf1 cDNA did not further inhibit cell proliferation [20]. In a study with HCC cells, it has been concluded that DLC-2 inhibition of cell growth occurs via a pathway that does not involve p21Waf1 [32]. Our present results with breast cancer cells suggest that p21Waf1 is not a downstream effector of DLC-1 and that flavone stimulation of p21Waf1 expression is independent of that of DLC-1 expression.
Although ectopic expression of DLC-1 in HCC cells was previously shown to induce accumulation of hypodiploid (apoptotic) cells, arrest of the cell cycle at G2-M was not observed [8]. The flavone-induced G2-M arrest apparent in breast cancer cells in the present study was likely mediated by p21Waf1, which induces such arrest in a variety of cell types [33,34,35,36]. In contrast to its effects in malignant cells, flavone induced G0-G1 arrest without apoptosis in nonmalignant breast epithelial cells, suggesting that nonmalignant cells might be able to regain their proliferative capacity.
Microarray analysis revealed that flavone induced deregulation of several genes already implicated in breast cancer. The GADD family of genes is implicated in the induction of apoptosis [37]. The observed up-regulation of GADD34, GADD153, and GADD45A as well as that of DLC1, which also induces apoptosis, might be responsible for the induction of programmed cell death in flavone-treated breast tumor cells. Although a contribution of p21Waf1 cannot be ruled out, the induction of apoptosis does not appear to be a consequence of increased p21Waf1 expression in most cell types examined [38]. STC2 possesses growth-inhibitory activity and its expression correlates with that of the estrogen receptor in breast tumors [39,40]. Flavone-induced up-regulation of STC2 was apparent in both estrogen receptor–positive and –negative breast cancer cell lines in the present study.
Among the genes whose expression was down-regulated by flavone treatment were IGFBP3, which encodes a positive regulator of cell proliferation and whose overexpression predicts an unfavorable prognosis in breast cancer [41], and STMN1, an important proto-oncogene in breast cancer progression [42,43]. STMN1 is overexpressed in breast tumors harboring mutant p53 and is responsible for resistance to microtubule-based anticancer treatment [42], suggesting that down-regulation of STMN1 expression by flavone may reduce breast tumor cell resistance to such treatment. Silencing of STMN1 expression by RNA interference in breast cancer cell lines harboring p53 mutations was found to inhibit cell proliferation, restore cell cycle regulation, and induce apoptosis [43]. Targeting of STMN1 in breast cancers with p53 mutations, which are frequently more aggressive and refractory to therapy, may thus represent a potential new therapeutic approach. In this context and given that more than half of all human cancers lack p53 function [44,45], our observations on the effects of flavone in breast cancer cells with p53 mutations may also be relevant and have therapeutic implications. Our data indicate that flavone-induced up-regulation of the expression of p21Waf1 is independent of the expression of its binding partner p53, given that increased expression of p21Waf1 was detected in tumor cells in which the p53 gene is mutated. Flavone also induced apoptosis in these cells. The flavonoid apigenin was also shown to induce apoptosis in HT-29 colon carcinoma cells [in which the p53 gene is also mutated [46].
Flavone and flavonoids have been shown to reactivate the expression of genes silenced by methylation, including tumor suppressor genes. (–)Epigallocatechin-3-gallate, the major polyphenol in green tea, was shown to inhibit DNA methyltransferase activity and to reverse the methylation of several genes including that of a tumor suppressor gene in cancer cell lines [47]. Genistein and related soy isoflavones were also shown to reactivate the gene for the tumor suppressor p16INK4a as well as those for retinoic acid receptor β, O6-methylguanine methyltransferase, and mut L homolog 1 in colon, esophageal, and prostate cancer cell lines [48]. Our results now suggest that flavone manifests a similar activity in breast cancer cells. Flavone thus restored the expression of DLC1 in breast and colon carcinoma cell lines all of which manifest silencing of the endogenous gene as a result of promoter hypermethylation. Promoter hypermethylation is a major mechanism of DLC1 down-regulation and inactivation in many other types of solid tumors and hematological malignancies [13,17,49], and reactivation of DLC1 expression by dietary constituents thus warrants further investigation.
Various dietary compounds have been shown to have potential therapeutic benefits [48,50,51,52]. Our present results obtained with cell lines derived from aggressive or metastatic breast tumors suggest the possibility that administration of flavone, alone or in combination with DNA methyltransferase and histone deacetylase inhibitors, may be an effective adjunct to chemotherapy.
Acknowledgments
This work was supported by Intramural Research Program of the NIH, National Cancer Institute. Bethesda, Maryland, USA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yuan BZ, Miller MJ, Keck CL, Zimonjic DB, Thorgeirsson SS, Popescu NC. Cloning, characterization, and chromosomal localization of a gene frequently deleted in human liver cancer (DLC-1) homologous to rat RhoGAP. Cancer Res. 1998;58:2196–99. [PubMed] [Google Scholar]
- 2.Goodison S, Yuan J, Sloan D, Kim R, Li C, Popescu NC, et al. The RhoGAP protein DLC-1 functions as a metastasis suppressor in breast cancer cells. Cancer Res. 2005;65:6042–53. doi: 10.1158/0008-5472.CAN-04-3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seng TJ, Low JS, Li H, Cui Y, Goh HK, Wong ML, et al. The major 8p22 tumor suppressor DLC1 is frequently silenced by methylation in both endemic and sporadic nasopharyngeal, esophageal, and cervical carcinomas, and inhibits tumor cell colony formation. Oncogene advance online publication. 2006 July 24; doi: 10.1038/sj.onc.1209839. [DOI] [PubMed] [Google Scholar]
- 4.Syed V, Mukherjee K, Lyons-Weiler J, Lau KM, Mashima T, Tsuruo T, et al. Identification of ATF-3, caveolin-1, DLC-1, and NM23-H2 as putative antitumorigenic, progesterone-regulated genes for ovarian cancer cells by gene profiling. Oncogene. 2005;24:1774–87. doi: 10.1038/sj.onc.1207991. [DOI] [PubMed] [Google Scholar]
- 5.Wong CM, Yam JW, Ching YP, Yau TO, Leung TH, Jin DY, et al. Rho GTPase-activating protein deleted in liver cancer suppresses cell proliferation and invasion in hepatocellular carcinoma. Cancer Res. 2005;65:8861–68. doi: 10.1158/0008-5472.CAN-05-1318. [DOI] [PubMed] [Google Scholar]
- 6.Yuan BZ, Zhou X, Durkin ME, Zimonjic DB, Gumundsdottir K, Eyfjord JE, et al. DLC-1 gene inhibits human breast cancer cell growth and in vivo tumorigenicity. Oncogene. 2003;22:445–450. doi: 10.1038/sj.onc.1206064. [DOI] [PubMed] [Google Scholar]
- 7.Yuan BZ, Jefferson AM, Baldwin KT, Thorgeirsson SS, Popescu NC, Reynolds SH. DLC-1 operates as a tumor suppressor gene in human non-small cell lung carcinomas. Oncogene. 2004;23:1405–11. doi: 10.1038/sj.onc.1207291. [DOI] [PubMed] [Google Scholar]
- 8.Zhou X, Thorgeirsson SS, Popescu NC. Restoration of DLC-1 gene expression induces apoptosis and inhibits both cell growth and tumorigenicity in human hepatocellular carcinoma cells. Oncogene. 2004;23:1308–13. doi: 10.1038/sj.onc.1207246. [DOI] [PubMed] [Google Scholar]
- 9.Bren-Mattison Y, Van P V, Chan D, Winn R, Geraci MW, Nemenoff RA. Peroxisome proliferator-activated receptor-gamma (PPAR(gamma)) inhibits tumorigenesis by reversing the undifferentiated phenotype of metastatic non-small-cell lung cancer cells (NSCLC) Oncogene. 2005;24:1412–22. doi: 10.1038/sj.onc.1208333. [DOI] [PubMed] [Google Scholar]
- 10.Zirn B, Samans B, Spangenberg C, Graf N, Eilers M, Gessler M. All-trans retinoic acid treatment of Wilms tumor cells reverses expression of genes associated with high risk and relapse in vivo. Oncogene. 2005;24:5246–51. doi: 10.1038/sj.onc.1208725. [DOI] [PubMed] [Google Scholar]
- 11.Folgueira MA, Carraro DM, Brentani H, Patrao DF, Barbosa EM, Netto MM, et al. Gene expression profile associated with response to doxorubicin-based therapy in breast cancer. Clin Cancer Res. 2005;11:7434–43. doi: 10.1158/1078-0432.CCR-04-0548. [DOI] [PubMed] [Google Scholar]
- 12.Herzog A, Kindermann B, Doring F, Daniel H, Wenzel U. Pleiotropic molecular effects of the pro-apoptotic dietary constituent flavone in human colon cancer cells identified by protein and mRNA expression profiling. Proteomics. 2004;4:2455–64. doi: 10.1002/pmic.200300754. [DOI] [PubMed] [Google Scholar]
- 13.Yuan BZ, Durkin ME, Popescu NC. Promoter hypermethylation of DLC-1, a candidate tumor suppressor gene, in several common human cancers. Cancer Genet Cytogenet. 2003;140:113–17. doi: 10.1016/s0165-4608(02)00674-x. [DOI] [PubMed] [Google Scholar]
- 14.Tang K, Oeth P, Kammerer S, Denissenko MF, Ekblom J, Jurinke C, et al. Mining disease susceptibility genes through SNP analyses and expression profiling using MALDI-TOF mass spectrometry. J Proteome Res. 2004;3:218–27. doi: 10.1021/pr034080s. [DOI] [PubMed] [Google Scholar]
- 15.van den Boom D, Beaulieu M, Oeth P, Roth R, Honisch C, Nelson MR, et al. MALDI-TOF MS: a platform technology for genetic discovery. Int J Mass Spectrom. 2004;238:173–188. [Google Scholar]
- 16.Chen YC, Shen SC, Chow JM, Ko CH, Tseng SW. Flavone inhibition of tumor growth via apoptosis in vitro and in vivo. Int J Oncol. 2004;25:661–70. [PubMed] [Google Scholar]
- 17.Guan M, Zhou X, Soulitzis N, Spandidos DA, Popescu NC. Aberrant methylation and deacetylation of deleted in liver cancer-1 gene in prostate cancer: potential clinical applications. Clin Cancer Res. 2006;12:1412–19. doi: 10.1158/1078-0432.CCR-05-1906. [DOI] [PubMed] [Google Scholar]
- 18.Ullmannova V, Stockbauer P, Hradcova M, Soucek J, Haskovec C. Relationship between cyclin D1 and p21(Waf1/Cip1) during differentiation of human myeloid leukemia cell lines. Leuk Res. 2003;27:1115–23. doi: 10.1016/s0145-2126(03)00103-6. [DOI] [PubMed] [Google Scholar]
- 19.Mattarucchi E, Marsoni M, Passi A, Lo CF, Pasquali F, Porta G. Establishment and study of different real-time polymerase chain reaction assays for the quantification of cells with deletions of chromosome 7. J Mol Diagn. 2006;8:218–24. doi: 10.2353/jmoldx.2006.050111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagaraja GM, Kandpal RP. Chromosome 13q12 encoded Rho GTPase activating protein suppresses growth of breast carcinoma cells, and yeast two-hybrid screen shows its interaction with several proteins. Biochem Biophys Res Commun. 2004;313:654–65. doi: 10.1016/j.bbrc.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Takimoto R, El-Deiry WS. DNA replication blockade impairs p53-transactivation. Proc Natl Acad Sci USA. 2001;98:781–83. doi: 10.1073/pnas.98.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shu XO, Jin F, Dai Q, Wen W, Potter JD, Kushi LH, et al. Soyfood intake during adolescence and subsequent risk of breast cancer among Chinese women. Cancer Epidemiol Biomarkers Prev. 2001;10:483–88. [PubMed] [Google Scholar]
- 23.Wu AH, Wan P, Hankin J, Tseng CC, Yu MC, Pike MC. Adolescent and adult soy intake and risk of breast cancer in Asian-Americans. Carcinogenesis. 2002;23:1491–96. doi: 10.1093/carcin/23.9.1491. [DOI] [PubMed] [Google Scholar]
- 24.Le Bail JC, Aubourg L, Habrioux G. Effects of pinostrobin on estrogen metabolism and estrogen receptor transactivation. Cancer Lett. 2000;156:37–44. doi: 10.1016/s0304-3835(00)00435-3. [DOI] [PubMed] [Google Scholar]
- 25.Pagliacci MC, Smacchia M, Migliorati G, Grignani F, Riccardi C, Nicoletti I. Growth-inhibitory effects of the natural phyto-oestrogen genistein in MCF-7 human breast cancer cells. Eur J Cancer. 1994;30A:1675–82. doi: 10.1016/0959-8049(94)00262-4. [DOI] [PubMed] [Google Scholar]
- 26.Wang C, Kurzer MS. Phytoestrogen concentration determines effects on DNA synthesis in human breast cancer cells. Nutr Cancer. 1997;28:236–47. doi: 10.1080/01635589709514582. [DOI] [PubMed] [Google Scholar]
- 27.Zava DT, Duwe G. Estrogenic and antiproliferative properties of genistein and other flavonoids in human breast cancer cells in vitro. Nutr Cancer. 1997;27:31–40. doi: 10.1080/01635589709514498. [DOI] [PubMed] [Google Scholar]
- 28.Dave B, Eason RR, Till SR, Geng Y, Velarde MC, Badger TM, et al. The soy isoflavone genistein promotes apoptosis in mammary epithelial cells by inducing the tumor suppressor PTEN. Carcinogenesis. 2005;26:1793–1803. doi: 10.1093/carcin/bgi131. [DOI] [PubMed] [Google Scholar]
- 29.Vissac-Sabatier C, Coxam V, Dechelotte P, Picherit C, Horcajada MN, Davicco MJ, et al. Phytoestrogen-rich diets modulate expression of Brca1 and Brca2 tumor suppressor genes in mammary glands of female Wistar rats. Cancer Res. 2003;63:6607–12. [PubMed] [Google Scholar]
- 30.Singletary K, Ellington A. Genistein suppresses proliferation and MET oncogene expression and induces EGR-1 tumor suppressor expression in immortalized human breast epithelial cells. Anticancer Res. 2006;26:1039–48. [PubMed] [Google Scholar]
- 31.Vissac-Sabatier C, Bignon YJ, Bernard-Gallon DJ. Effects of the phytoestrogens genistein and daidzein on BRCA2 tumor suppressor gene expression in breast cell lines. Nutr Cancer. 2003;45:247–255. doi: 10.1207/S15327914NC4502_15. [DOI] [PubMed] [Google Scholar]
- 32.Leung TH, Ching YP, Yam JW, Wong CM, Yau TO, Jin DY, et al. Deleted in liver cancer 2 (DLC2) suppresses cell transformation by means of inhibition of RhoA activity. Proc Natl Acad Sci USA. 2005;102:15207–12. doi: 10.1073/pnas.0504501102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lepley DM, Li B, Birt DF, Pelling JC. The chemopreventive flavonoid apigenin induces G2/M arrest in keratinocytes. Carcinogenesis. 1996;17:2367–75. doi: 10.1093/carcin/17.11.2367. [DOI] [PubMed] [Google Scholar]
- 34.Matsukawa Y, Marui N, Sakai T, Satomi Y, Yoshida M, Matsumoto K, et al. Genistein arrests cell cycle progression at G2-M. Cancer Res. 1993;53:1328–31. [PubMed] [Google Scholar]
- 35.Sato F, Matsukawa Y, Matsumoto K, Nishino H, Sakai T. Apigenin induces morphological differentiation and G2-M arrest in rat neuronal cells. Biochem Biophys Res Commun. 1994;204:578–84. doi: 10.1006/bbrc.1994.2498. [DOI] [PubMed] [Google Scholar]
- 36.Yang JH, Hsia TC, Kuo HM, Chao PD, Chou CC, Wei YH, et al. Inhibition of lung cancer cell growth by quercetin glucuronides via G2/M arrest and induction of apoptosis. Drug Metab Dispos. 2006;34:296–304. doi: 10.1124/dmd.105.005280. [DOI] [PubMed] [Google Scholar]
- 37.Scott DW, Mutamba S, Hopkins RG, Loo G. Increased GADD gene expression in human colon epithelial cells exposed to deoxycholate. J Cell Physiol. 2005;202:295–303. doi: 10.1002/jcp.20135. [DOI] [PubMed] [Google Scholar]
- 38.Ludwig RL, Bates S, Vousden KH. Differential activation of target cellular promoters by p53 mutants with impaired apoptotic function. Mol Cell Biol. 1996;16:4952–60. doi: 10.1128/mcb.16.9.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bouras T, Southey MC, Chang AC, Reddel RR, Willhite D, Glynne R, et al. Stanniocalcin 2 is an estrogen-responsive gene coexpressed with the estrogen receptor in human breast cancer. Cancer Res. 2002;62:1289–95. [PubMed] [Google Scholar]
- 40.Gagliardi AD, Kuo EY, Raulic S, Wagner GF, DiMattia GE. Human stanniocalcin-2 exhibits potent growth-suppressive properties in transgenic mice independently of growth hormone and IGFs. Am J Physiol Endocrinol Metab. 2005;288:E92–105. doi: 10.1152/ajpendo.00268.2004. [DOI] [PubMed] [Google Scholar]
- 41.Rocha RL, Hilsenbeck SG, Jackson JG, Lee AV, Figueroa JA, Yee D. Correlation of insulin-like growth factor-binding protein-3 messenger RNA with protein expression in primary breast cancer tissues: detection of higher levels in tumors with poor prognostic features. J Natl Cancer Inst. 1996;88:601–6. doi: 10.1093/jnci/88.9.601. [DOI] [PubMed] [Google Scholar]
- 42.Alli E, Bash-Babula J, Yang JM, Hait WN. Effect of stathmin on the sensitivity to antimicrotubule drugs in human breast cancer. Cancer Res. 2002;62:6864–69. [PubMed] [Google Scholar]
- 43.Alli E, Yang JM, Hait WN. Silencing of stathmin induces tumor-suppressor function in breast cancer cell lines harboring mutant p53. Oncogene advance online publication. 2006 August 14; doi: 10.1038/sj.onc.1209864. [DOI] [PubMed] [Google Scholar]
- 44.Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 45.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–10. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 46.Takagaki N, Sowa Y, Oki T, Nakanishi R, Yogosawa S, Sakai T. Apigenin induces cell cycle arrest and p21/WAF1 expression in a p53-independent pathway. Int J Oncol. 2005;26:185–89. [PubMed] [Google Scholar]
- 47.Fang MZ, Wang Y, Ai N, Hou Z, Sun Y, Lu H, et al. Tea polyphenol (-)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003;63:7563–70. [PubMed] [Google Scholar]
- 48.Fang MZ, Chen D, Sun Y, Jin Z, Christman JK, Yang CS. Reversal of hypermethylation and reactivation of p16INK4a, RARbeta, and MGMT genes by genistein and other isoflavones from soy. Clin Cancer Res. 2005;11:7033–41. doi: 10.1158/1078-0432.CCR-05-0406. [DOI] [PubMed] [Google Scholar]
- 49.Shi H, Guo J, Duff DJ, Rahmatpanah F, Chitima-Matsiga R, Al-Kuhlani M, Taylor KH, Sjahputera O, Andreski M, Wooldridge JE, Caldwell CW. Discovery of novel epigenetic markers in non-Hodgkin's lymphoma. Carcinogenesis. 2007;28:60–70. doi: 10.1093/carcin/bgl092. [DOI] [PubMed] [Google Scholar]
- 50.Hillman GG, Forman JD, Kucuk O, Yudelev M, Maughan RL, Rubio J, et al. Genistein potentiates the radiation effect on prostate carcinoma cells. Clin Cancer Res. 2001;7:382–90. [PubMed] [Google Scholar]
- 51.Li Y, Ellis KL, Ali S, El-Rayes BF, Nedeljkovic-Kurepa A, Kucuk O, et al. Apoptosis-inducing effect of chemotherapeutic agents is potentiated by soy isoflavone genistein, a natural inhibitor of NF-kappaB in BxPC-3 pancreatic cancer cell line. Pancreas. 2004;28:e90–e95. doi: 10.1097/00006676-200405000-00020. [DOI] [PubMed] [Google Scholar]
- 52.Sarkar FH, Li Y. Using chemopreventive agents to enhance the efficacy of cancer therapy. Cancer Res. 2006;66:3347–50. doi: 10.1158/0008-5472.CAN-05-4526. [DOI] [PubMed] [Google Scholar]


