The current vaccine arsenal, developed over two centuries, was implemented in what approximates its present splendor during the 1960s. Since then, worldwide vaccine efforts have done much to defuse the fear of many dreaded infectious diseases, such as smallpox, polio, diphtheria, pertussis, and measles. And yet despite these very real accomplishments, effective vaccines for tough challenges like HIV, malaria, and cancer sometimes seem to be nowhere in sight. Given these current shortcomings, many ask of our modern army of vaccinologists: “What have you done for us lately?” Established principles in vaccine development (like killing or attenuating the infectious agent) have not been direct solutions to these new vaccine challenges. There is currently no consensus about what form these new vaccines will have or how they will be deployed.
One vaccine technology that has gotten many hearts racing is “naked” DNA. Naked DNA vectors are relatively easy to engineer and produce. Unlike vaccines based on recombinant viruses, they need not be covered with proteins or lipids. Irrelevant antigenic components of recombinant immunogens can detract from their function. But what nucleic acid vaccines may gain in elegance, they may lose in power. They simply do not elicit immune responses that are strong enough for the prophylaxis or therapy required in many current experimental models.
That needed power may come from an unexpected source: the induction of apoptotic death in host cells. In this issue, Sasaki et al.1 describe the augmentation of naked DNA vaccine function through the use of specially designed molecules that kill host cells. Their approach involves the expression of model antigens from influenza together with modified versions of caspases—enzymes that mediate programmed cell death. Although others have found that death can accompany enhanced vaccine function, few have induced death so deliberately or so directly.
The authors use a mutated version murine caspase 2 and a chimeric version of the murine caspase 2 prodomain grafted onto human caspase 3 to augment T- and B-cell immune responses to model antigens. Mutated versions of the caspases are used in an attempt to allow requisite antigen production to delay apoptotic death. Once antigen production has occurred, mutant caspases induce the creation of apoptotic bodies that are taken up by macrophages and other antigen-presenting cells including dendritic cells.
A greater enhancement of cellular responses than humoral responses was observed and subsequent work will enable the further testing of this finding in other antigenic systems. The authors claim that increases in T cell responses are one of the strongest effects to date of DNA vaccines, but the approach is not compared directly with (nor is it discussed in the context of) other innovations in the field of nucleic acid immunization.
How are we to understand the events at the nexus of death and immunity? Apoptotic death is distinguishable from necrotic death by a few key factors. Whereas apoptotic death is controlled in large measure from within the dying cell, necrotic death is a process resulting from sudden and catastrophic cellular destruction, such as hypoxia, hyperthermia, or physical injury. Apoptotic cells condense to form apoptotic bodies; necrotic cells swell and the nuclear and cytosolic contents disperse.
Necrosis is consistently described in the literature as “inflammatory” and “immune activating”. Apoptosis is usually described as immunologically invisible or even tolerizing, although others assert that apoptotic death can be associated with inflammation and immunity. But like blind men who describe an elephant as a vastly different beast depending on which part of the elephant’s body they touch, opinions in the debate over apoptosis may depend on an individual’s particular experimental approach. Although apoptosis is often thought of as a single entity, it appears to be a complex process that can have multifarious forms.
As with many debates, part of the problem may stem from workers having different definitions of apoptosis2. The original morphological definition has been replaced by a molecular definition that could be stated thus: apoptosis is a conserved biochemical suicide program that exists in most cells and is turned on by a variety of normal developmental and pathogenic triggers, as well as by experimental manipulations. Genetically described in such organisms as Caenorhabditis elegans, the apoptotic molecular machinery appears to be much more complex in higher organisms3.
Using a molecular definition of apoptosis rather than a morphological one, we can break down apoptotic death into several major types. The first is developmental apoptosis, or the kind of death that occurs during the separation of the digits, the creation of the gyri in the brain, and the formation of a variety of tubular structures, such as those in the digestive and respiratory systems (Fig. 1A). Developmental apoptosis generally does not lead to immunity and thus might be termed “bland” or even tolerizing. Suppression of inflammation by dead cells is complex, but may be mediated in part by the phosphatidylserine (PS) receptor4. The paper by Sasaki et al. suggests, however, that such death can also lead to immunity if key immune-activating signals, such as CpG sequences, are present (Fig. 1B)1. Other candidate signals include lipopolysaccharide and CD40L (ref. 5).
Figure 1.
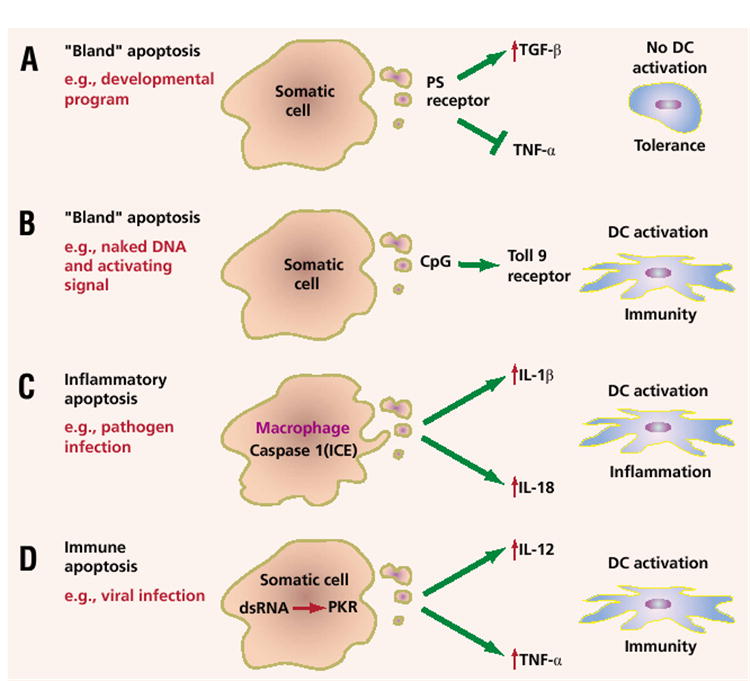
The causes and consequences of apoptotic cell death as they relate to immunity. (A) An immunologically “bland” or even tolerizing apoptosis is induced during development. Engagement of the phosphatidylserine (PS) receptor leads to upregulation of transforming growth factor-β (TGFβ) and the blockade of lipopolysaccharide-induced tumor necrosis factor-α (TNFα). (B) Bland apoptosis, such as that induced by caspase 2 in the paper by Sasaki et al.1, may lead to immunity if the appropriate signals are in place. (C) Inflammatory apoptosis may occur as a result of pathogen infection (e.g., Shigella). Engagement of the death machinery in some cell types, such as macrophages, leads to the activation of caspase 1, which can then cleave IL-1β and IL-18 into their active forms. (D) Immune apoptosis results from engagement of innate immune pathways in cells induced to die, for example, because of viral infection. An example of an innate immune pathway is the type I interferon-inducible, double-stranded RNA–dependent protein kinase (PKR), which can induce both death as well as the upregulation of key cytokines like IL-12 and TNF-α.
Although the original definition of apoptosis excluded inflammation, it has now become apparent that in some cell types under certain circumstances, the generation of inflammatory signals can be a component of the apoptotic machinery (Fig. 1C). For example, activation of caspase 1, also known as interleukin 1–converting enzyme (ICE), can result in cytokine maturation of both IL-1 and IL-18 when the pro- forms of these cytokines are present upon infection with Shigella flexneri6. Other ICE-like caspases that may be important for inflammation are numbered 4, 5, 11, 12, and 13, but their full activities remain incompletely elucidated.
Heightened immunity may also result when apoptotic death is induced by double-stranded RNA and other products of viral replication (Fig. 1D). Self-replicating, “naked” nucleic acid immunogens are 100–1,000 fold more efficient at eliciting immune responses than conventional DNA vaccines7. They accomplish this efficiency through the activity of an RNA replicase from an alphavirus (called a replicon) that is expressed by an RNA- or DNA-based immunogen8. The reasons why replicon-based vaccines may be more efficient is that they mediate the copying of positive-stranded RNA to negative-stranded RNA and back again. Requisite double-stranded RNA species are produced, activating a double-stranded RNA–dependent protein kinase called PKR. This can lead to enhanced activation of the innate immune response and to apoptotic death9. A caspase-dependent death is induced and the increase in the uptake of antigen by dendritic cells is accompanied by a dramatic increase in vaccine function7,8.
Needless to say, the induction of death of the host cell adds a measure of safety to the naked DNA vaccine. But will immune activation deriving from death be enough to finally propel DNA vaccines into forms that are clinically useful? Only time—and many more experiments—will tell. It seems clear that the design of new vaccine approaches will benefit greatly from a better understanding of the relationships between apoptotic death pathways and the innate and adaptive immune response.
References
- 1.Sasaki S, et al. Nat Biotechnol. 2001;19:543–547. doi: 10.1038/89289. [DOI] [PubMed] [Google Scholar]
- 2.Restifo NP. Curr Opin Immunol. 2000;12:597–603. doi: 10.1016/s0952-7915(00)00148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aravind L, Dixit VM, Koonin EV. Science. 2001;291:1279–1284. doi: 10.1126/science.291.5507.1279. [DOI] [PubMed] [Google Scholar]
- 4.Fadok VA, et al. Nature. 2000;405:85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- 5.Larsson M, Fonteneau JF, Bhardwaj N. Trends Immunol. 2001;22:141–148. doi: 10.1016/s1471-4906(01)01860-9. [DOI] [PubMed] [Google Scholar]
- 6.Sansonetti PJ, et al. Immunity. 2000;12:581–590. doi: 10.1016/s1074-7613(00)80209-5. [DOI] [PubMed] [Google Scholar]
- 7.Leitner WW, Ying H, Driver DA, Dubensky T, Restifo NP. Cancer Res. 2001;60:51–55. [PMC free article] [PubMed] [Google Scholar]
- 8.Ying H, et al. Nat Med. 1999;5:823–827. doi: 10.1038/10548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Der SD, Yang YL, Weissmann C, Williams BR. Proc Natl Acad Sci USA. 1997;94:3279–3283. doi: 10.1073/pnas.94.7.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]


