Abstract
Background
The aim of the present study was to evaluate the future cumulative risk of prostate cancer in relation to levels of prostate-specific antigen (PSA) in blood and to determine whether this information could be used to individualize the PSA testing interval.
Methods
The study included 5855 of 9972 men (aged 50–66 years) who accepted an invitation to participate in a prospective, randomized study of early detection for prostate cancer. We used a protocol based on biennial PSA measurements starting from 1995 and 1996. Men with serum PSA levels of 3.0 ng/mL or more were offered prostate biopsies.
Results
Among the 5855 men, 539 cases of prostate cancer (9.2%) were detected after a median follow-up of 7.6 years (up to July 1, 2003). Cancer detection rates during the follow-up period in relation to PSA levels were as follows: 0 to 0.49 ng/mL, 0% (0/958); 0.50 to 0.99 ng/mL, 0.9% (17/1992); 1.00 to 1.49 ng/mL, 4.7% (54/1138); 1.50 to 1.99 ng/mL, 12.3% (70/571); 2.00 to 2.49 ng/mL, 21.4% (67/313); 2.50 to 2.99 ng/mL, 25.2% (56/222); 3.00 to 3.99 ng/mL, 33.3% (89/267); 4.00 to 6.99 ng/mL, 38.9% (103/265); 7.00 to 9.99 ng/mL, 50.0% (30/60); and for men with an initial PSA of 10.00 ng/mL or higher, 76.8% (53/69). Not a single case of prostate cancer was detected within 3 years in 2950 men (50.4% of the screened population) with an initial PSA level less than 1 ng/mL.
Conclusions
Retesting intervals should be individualized on the basis of the PSA level, and the large group of men with PSA levels of less than 1 ng/mL can safely be scheduled for a 3-year testing interval.
Prostate cancer is an important and growing health problem particularly facing men in the Western world. There were about 540 000 new cases worldwide in 2000. Nearly 300 000 of these cases were diagnosed in western Europe or North America.1
Most major medical organizations recommend testing for prostate cancer of asymptomatic men only on an individual basis and only after the patient was informed of the pros and cons.2 The most commonly recommended testing interval is annually, but this screening interval seems to have been chosen arbitrarily. Recently, strategies that call for an individualized rescreening interval dependent on baseline levels of prostate-specific antigen (PSA) in serum have been suggested.3 These recommendations are partly based on studies on frozen blood samples, where Gann and coworkers4 noted a 2-fold increase in diagnosis of prostate cancer over time (ie, cumulative risk) if the PSA level was 1.01 to 1.50 ng/mL and 5-fold increased risk if it was 2.01. to 3.00 ng/mL compared with men with levels less than 1 ng/mL. Similar results were reported by Fang and collaborators,5 who used serum from the Baltimore Longitudinal Study of Aging, They found a 3.75-times increased risk for diagnosis of prostate cancer within 25 years when PSA was above the median for their age group (although still within the normal range, ie, ≤4.0 ng/mL). Both of these studies indicate that the lead time (ie, time until symptoms occur) varies even within the normal PSA range. Men with very low PSA level have, on average, longer lead time than men with higher but still normal PSA levels. As a consequence, it should be explored whether men with different PSA levels should have different testing intervals.
The aims of the present study were to evaluate the cumulative prostate cancer risk in men with different PSA levels at baseline and to evaluate the accuracy of using a PSA-dependent screening interval. For this purpose we used data from our ongoing, prospective, randomized, population-based trial evaluating prostate cancer screening with biennial PSA testing in Göteborg, Sweden, as part of the European Randomized Screening for Prostate Cancer study.
METHODS
In the community of Göteborg (population, 440 000), as of December 31, 1994, there were 32298 men alive who had been born between January 1, 1930, and December 31, 1944. After permission was obtained from the ethical committee, with the aid of the Swedish Population Registry, 10 000 men from this group (aged 50–65 years) were randomized by computer to prostate cancer screening and 10 000 men were randomized to serve as the control group. Men with prevalent prostate cancer were excluded, resulting in an intervention group of 9972 men and a control group of 9973 (Figure 1).
Figure 1.
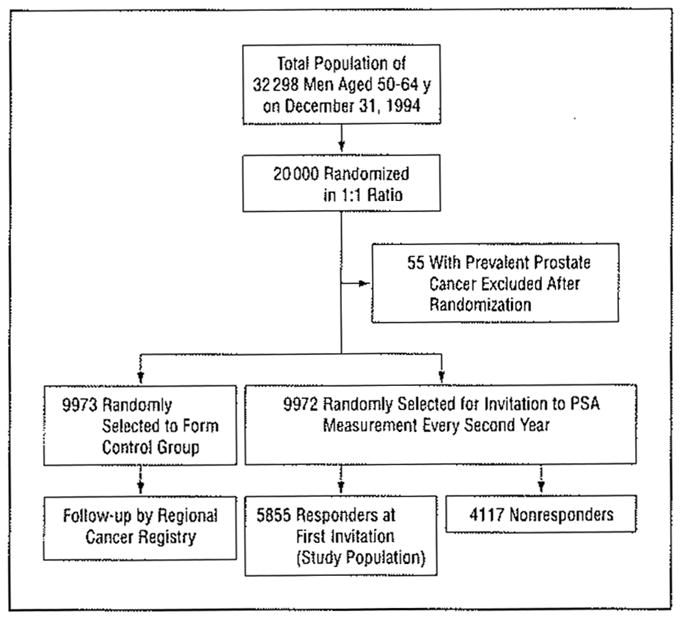
Study design. See the “Methods” section for details. PSA indicates prostate-specific antigen.
The screening group received first-time invitations for PSA testing from January 1995 to December 1996. Serum was separated from blood tells, frozen, and stored at −20°C within 3 hours. The PSA was measured (Delfia Prostatus PSA F/T Dual Assay; Perkin Elmer Life Sciences, Turku, Finland) within 2 weeks from sample collection and less than 3 hours after thawing, Free and complex PSA forms are detected in an equimolar fashion by this assay. The lower limit of detection is 0.05 ng/mL; coefficients of variation are 13.9% at low (0.34 ng/mL), 5.6% at medium (2.30 ng/mL), and 5.5% at high (20.60 ng/mL) PSA levels. Men with PSA levels less than 3.00 ng/mL were not further studied, while men with PSA levels of 3.00 ng/mL or more were invited for further workup by an experienced urologist, consisting of digital rectal examination (DRE), transrectal ultrasound, and laterally directed sextant biopsies.6 Men with benign results of biopsies and PSA levels of 7.00 ng/mL or more were invited for new PSA testing after 6 months, and repeat biopsies were offered when PSA levels remained elevated.
Men with PSA levels less than 3.00 ng/mL and men with benign results of biopsies were invited for repeated PSA-based screening after 2 years (during 1997 and 1998), after 4 years (during 1999 and 2000), and after 6 years (during 2001 and 2002). Invitations for the second and fourth rounds of screening followed the same algorithm as the first. In the third round of screening, men with PSA levels less than 1.00 ng/mL during the second round of screening were not invited, The reason for this was that none of the 2950 men with PSA levels less than 1.00 ng/mL in the first round were found to have PSA levels greater than 3.00 ng/mL during the second round. However, the PSA cutoff that resulted in further investigations was lowered from 3.00 to 2.54 ng/mL for consistency within the European Randomized Screening Study for Prostate Cancer. Therefore, men with negative results of biopsies and persistently elevated PSA levels in the subsequent biennial testing may have been invited for biopsy up to 4 times during 7 to 8 years.
The present study is based on all 5855 men who participated in the first screening round in 1995 and 1996. The cancer detection rates have been related to their baseline PSA level measured in 1995 and 1996. To ensure inclusion of all cancer cases diagnosed outside the screening program (ie, interval cancers or “wild” extra screening), the study data were cross-matched against the regional cancer registry to identify and include any interval cancers diagnosed up to July 1, 2003.
Projected cumulative estimates of the risk of being diagnosed as having prostate cancer were calculated with the Kaplan-Meier method, and the difference between groups was tested with the Mantel-Cox log-rank test.
RESULTS
All 5855 men included in the study had a mean age at the time of PSA testing of 57.9 years (median, 57.6 years; range, 50–66 years). During the follow-up until July 1, 2003, 418 men had died. In consequence, 5437 men (92.9%) were alive and eligible at the end of our study period. Median follow-up was 2769 days (7.6 years) from the time of PSA testing. During this period, the PSA level of 5325 men was tested on at least 2 occasions, while 4523 men underwent blood sampling followed by PSA testing on at least 3 occasions.
As a result of the initial baseline PSA measurements in 1995 and 1996, 661 men with PSA levels of 3.00 ng/mL or more were offered transrectal ultrasound and prostate biopsy. In total, 5194 men were offered repeated PSA measurements according to the study protocol. Until July 1, 2003, 539 men (9.2% of the study cohort) were diagnosed as having prostate cancer; 487 (90.4%) of these men were diagnosed within the screening program. In the Table, the absolute risk of prostate cancer detection during the study period is related to the baseline PSA level measured at the initial invitation in 1995 or 1996. No men with PSA levels less than 0.50 ng/mL were detected as having prostate cancer during the study period. Similarly, the risk was very low (0.9%) for men with PSA levels from 0.50 to 0.99 ng/mL at baseline, but the cancer risk increased to 4.7% for men with baseline levels of 1.00 to 1.49 ng/mL. For men with baseline PSA level greater than or equal to 1.50 μg/L, the risk of detection of prostate cancer varied from 12.3% to 76.8%. A higher PSA level at baseline paralleled increased cancer risk. Because of low competing mortality, the Kaplan-Meier estimates generate similar outcomes, but they depict, in a more readily interpretable fashion, the time elapsed from baseline until the cancers were diagnosed (Figure 2).
Figure 2.
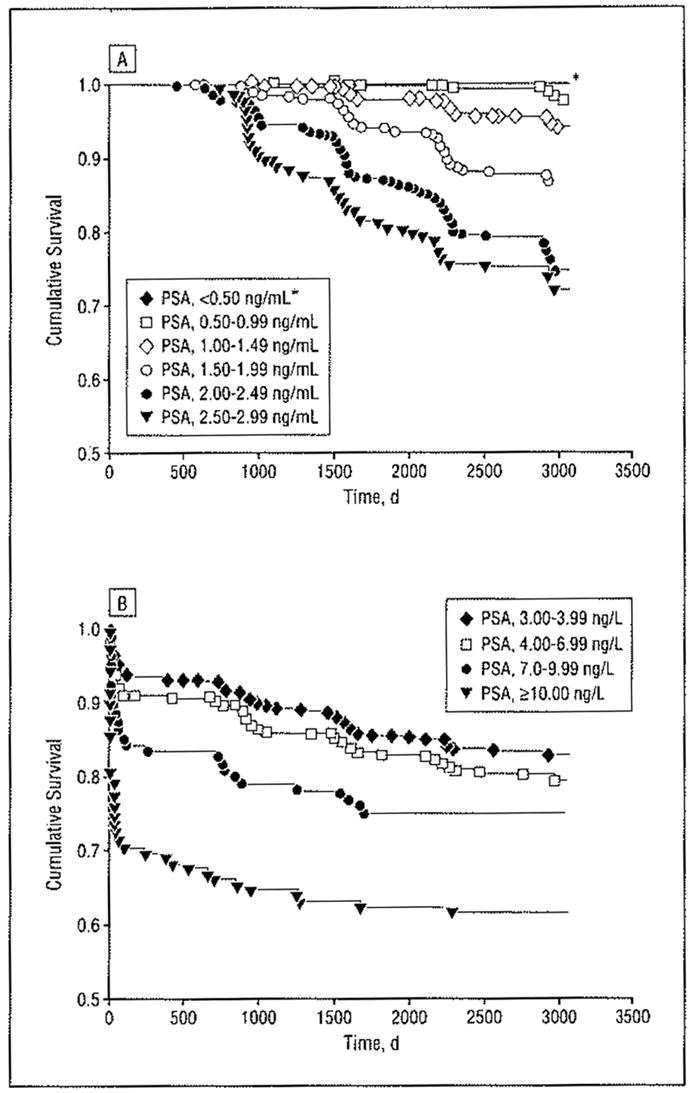
Cumulative risk of being diagnosed as having prostate cancer up to July 1, 2003, in relation to the serum levels of prostate-specific antigen (PSA) in 1995 to 1996. A, PSA levels of 0 to 2.99 ng/mL (cumulative survival is from 0.5–1.0). The asterisk indicates that no patients with a PSA level less than 0.50 ng/mL developed prostate cancer. B, PSA levels of 3 ng/mL or more (men at risk at 0 years, 5855; at 5 years, 5463; and at 8 years, 1599).
Access to the cumulative number of cancers detected in our study cohort during the observation period (7–8 years) allowed us to study the time elapsed from baseline (1995–1996) until diagnosis of cancer for the men with PSA levels less than 3.00 ng/mL. For men diagnosed as having prostate cancer who had baseline PSA levels of 0.50 to 0.99 ng/mL (n= 17), no cases of cancer were diagnosed within 3 years and only 1 case was diagnosed earlier than 4 years after baseline (1102 days after the baseline PSA level was determined). In men diagnosed as having cancer who had baseline PSA levels of 1.00 to 1.49 ng/mL (n=54), there were 3 cases diagnosed within 3 years (the earliest case was diagnosed 950 days after determination of the baseline PSA level) and 5 cases diagnosed within 4 years. This corresponds to a cumulative detection rate of 0% in 2950 of 5855 men (50.4% of the entire study cohort) in our screening study within 3 years for the men who had baseline PSA levels less than 1.00 ng/mL. For the 4088 of 5855 men with baseline PSA levels less than 1.50 ng/mL (corresponding lo 69.8% of the entire study cohort), 3 men (detection rate, 0.07%) would have had their cancer diagnosis delayed if the screening interval had been 3 years (instead of our biennial screening interval), whereas 6 men (corresponding to a detection rate of 0.15%) would have had their cancer diagnosis delayed if the screening interval had been 4 years. By contrast, the cumulative risk for diagnosis of prostate cancer was significantly higher during the observation period, ie, 12.3% to 25.2% (Table and Figure 2A), for men with baseline PSA levels from 1.50 to 2.99 ng/mL.
COMMENT
The results of the present, study indicate that it seems possible to differentiate the time lo follow-up visits in men aged 50 to 66 years who wish to participate in an early prostate cancer detection program based on their initial (baseline) PSA level. The strength of the study is mainly based on its large, population-based, random sample of men with a long follow-up and no losses to follow-up with regard to the main end point—the diagnosis of prostate cancer.
STUDY POPULATION
The study population was randomly selected from the male population in a defined geographic area. The men were randomized without being informed about the study before randomization, thus preventing preselection bias. We achieved an acceptable attendance rate, with 58.5% of the invited men accepting PSA testing, the majority of whom also continued to participate in the screening program for the whole study period. One might fear that the nonresponders had a higher risk of developing prostate cancer, thus making our results less valid. However, we have recently shown that the nonresponders had a cumulative prostate cancer risk that equated that of our control group, so this seems not to be the case.7 A high acceptance rate for prostate biopsies (83%–90%) of men with an elevated PSA level indicates that most prostate cancer cases will be included.7 Taken together, the results from the study should be valid for men from western Europe and the United States who are in the pertinent age groups for early prostate cancer detection.
RESULTS IN COMPARISON WITH OTHER SERIES
Our results are consistent with those of earlier series based on frozen blood samples. In those series, men with the lowest PSA values at baseline had the lowest risk of developing a clinical prostate cancer.4,5 In this study we showed that this group also had a lower risk of having cancer detected by screening. Men with high-normal PSA values had a higher risk than men with low-normal values of developing an elevated PSA level and being diagnosed as having prostate cancer.
Ito and coworkers8 studied men aged 50 to 78 years (mean age, 64.3 years) in Japan with methods similar to ours. They had different PSA cutoff values to trigger a prostate biopsy, and, overall, about 50% of men with an indication for a biopsy actually underwent biopsy. In patients with a baseline PSA level of 0 to 1.00 ng/mL, they found a cumulative detection rate of 0.2% within 4 years after the PSA measurement. For patients with a baseline value of 1.10 to 2.00 ng/mL, the corresponding detection rate was 1.6%. In men with PSA levels of 2.10 to 3.00 and 3.10 to 4.00 ng/mL, their 5- to 8-year cancer detection rate was lower than ours (9.2% and 18.8% detection rates, respectively, compared with 23.3% and 33.3% in our series). This difference is probably due to their lower rate of prostate biopsies (50% vs 90%) and higher cutoff values to trigger a biopsy.8 However, other explanations may also exist, such as ethnic differences between Japanese and Swedish or Western men.
Crawford and coworkers9 presented unpublished data from the Prostate, Lung, Colorectal, and Ovarian Cancer trial and noted that the cumulative risk of having an elevated PSA level above 4.00 ng/mL (with annual screening) was 1.4% at 4 years if the PSA level was less than 1.00 ng/mL at baseline. There were no data on actual cancer detection rate, but the authors suggested that PSA testing could be delayed for 4 years in patients with a PSA level of less than 1.00 ng/mL, in line with our results.
Of equal importance is the fate of men who had a high-normal PSA level (between 1.50 and 2.99 ng/mL). These patients carried a substantial risk of being diagnosed as having prostate cancer over time, and a close follow-up (shorter screening intervals or early biopsy) seemed indicated. Also consistent with this finding, Harris el al10 stated that patients with a PSA level of less than 2.00 ng/mL could safely forgo repeat testing for 3 years, while patients with a PSA level between 2.00 and 4.00 ng/mL needed annual monitoring.
POSSIBLE LIMITATIONS OF THE STUDY
It may be argued that eliminating DRE from our screening procedure and limiting our biopsy strategy to 6 laterally directed sextant biopsies may underestimate the prostate cancer risk. However, in the situation with a low PSA value, a positive DRE result is associated with prostate cancer in only 3% to 5% of cases.11,12 A positive DRE result is also comparatively uncommon in these younger age groups, and Vis and colleagues13 calculated that one would have to perform 289 DREs to find 1 case of clinically significant prostate cancer in patients with a PSA level less than 3.00 ng/mL, Regarding the use of only 6 cores for biopsy, it has been shown that by directing these cores laterally, as in our study, 94% of cases detectable with a more extensive biopsy protocol could be found.14 Because we are evaluating a repeated screening program and have registered all cancer cases outside the screening program, cases that would have been missed in one round would probably be caught in the next, thus further reducing the possible limitations already mentioned.
One important aspect of this study is that biopsy indication is PSA driven, and it could be argued that the findings are due to the algorithm of the study. If we had lowered the cutoff level to less than 2.54 ng/mL, we would probably have detected prostate cancers even in men with extremely low PSA levels, as was shown in the control group of the Prostate Cancer Prevention Trial.15 The question is whether these tumors represent earlier stages and safely could be left undiagnosed until the PSA level rises above a certain level and then still be diagnosed at a curable stage. Because almost half of men in this age group have microscopic foci of prostate cancer but only 3% to 5% of men in the Western world die of the disease, it is obvious that the diagnosis should not be made as early as possible but instead as late as possible but while cancers are still curable. Whether a PSA-driven biopsy indication alone (no DRE) will fail to diagnose some cancers early enough cannot entirely be ruled out from this study. However, the stage and grade distribution of cancers detected at repealed screening in this study is extremely favorable.8 Similar results were presented by Carter and coworkers,16 who noted that 89% of tumors still were curable when the PSA level was less than 5.00 ng/mL, resulting in an acceptable window of curability in men with a PSA level between 3.00 and 5.00 ng/mL.
CLINICAL IMPLICATIONS
The results of the present study support the use of PSA for risk stratification regarding men’s future risk to be diagnosed as having prostate cancer. The main message is that men with low PSA values do not need close surveillance and could be spared unnecessary tests and visits.
If it is requested that not a single tumor be missed, 50% of the men (those with a baseline PSA level of < 1.00 ng/mL) in ibis age group could be tested every third year. If it is found acceptable lo delay the detection of 3 to 6 cases of prostate cancer (of 539), 70% of the men in actual age groups may be omitted from retesting for 3 or 4 years, maintaining a high diagnostic accuracy of 99.6% to 98.9%.
Men with baseline PSA levels between 1.50 and 2.99 ng/mL are instead al higher risk (12.3%–25.2%) of being diagnosed as having prostate cancer within the next 8 years and need lo be followed up with regular, shorter intervals if these tumors are to be detected early.
CONCLUSIONS
On the basis of the results of the present large, population-based study, individualized retesting intervals depending on the baseline PSA measurement may be recommended lo men who have chosen to undergo PSA testing for possible early detection of prostate cancer. Patients with a baseline PSA level of less than 1.00 ng/mL may safely wait 3 years until their next PSA test, while patients with a value exceeding 1.50 ng/mL will need annual testing.
Table.
Cumulative Risk of Diagnosis of Prostate Cancer in Relation to Baseline PSA Level Obtained in 1995 and 1996
| Baseline PSA Level, ng/ml | No. (%) of Men (N = 5855) | No of Cancer Cases (n = 539) | Cancer Detection Rate, % |
|---|---|---|---|
| 0.00–0.49 | 958 (16.4) | 0 | 0 |
| 0.50–0.99 | 1992 (34.0) | 17 | 0.9 |
| 1.00–1.49 | 1138 (19.4) | 54 | 4.7 |
| 1.50–1.99 | 571 (9.8) | 70 | 12.3 |
| 2.00–2.49 | 313 (5.3) | 67 | 21.4 |
| 2.50–2.99 | 222 (3.8) | 56 | 25.2 |
| 3.00–3.99 | 267 (4.6) | 89 | 33.3 |
| 4.00–6.99 | 265 (4.5) | 103 | 38.9 |
| 7.00–9.99 | 60 (1.0) | 30 | 50.0 |
| ≥10.00 | 69 (1.2) | 53 | 76.8 |
Abbreviation: PSA, prostate-specific antigen.
Acknowledgments
Funding/Support: This study is a part of the European Randomized Screening for Prostate Cancer study and was supported in part by grants 3792 and 3555 from the Swedish Cancer Society, Stockholm; contract LSHC-CT-2004-50301. from the European Union, Brussels, Belgium; and grant P50-CA92629 from the National Cancer Institute, Bethesda, Md (SPORE [Specialized Programs of Research Excellence] Pilot Project 7). The study was also supported by Wallac Oy, Turku, Finland; Schering Plough, Stockholm, Sweden; and Abbott Pharmaceuticals, Solna, Sweden.
Footnotes
Financial Disclosure: Dr Lilja has a patent for free PSA measurements.
CME course available at www.archinternmed.com
Role of the Sponsor: The funding sources were not involved in study design or conduct of the study, nor in data collection, management, analysis, or interpretation of the study. They were not involved in preparation, writing, review, or approval of this manuscript.
References
- 1.Schröder FH. Screening for prostate cancer. Urol Clin North Am. 2003;30:239–251. doi: 10.1016/s0094-0143(02)00180-5. [DOI] [PubMed] [Google Scholar]
- 2.US Preventive Services Task Force. [Accessed January 20, 2005];Recommendations and rationale: screening for prostate cancer. Available at: http://www.ahrq.gov/clinic/uspstf/uspsprca.htm.
- 3.Carter HB. Rationale for earlier and less frequent prostate cancer screening. Urology. 2001;58:639–641. doi: 10.1016/s0090-4295(01)01390-5. [DOI] [PubMed] [Google Scholar]
- 4.Gann PH, Hennekens CH, Stampfer MJ. A prospective evaluation of plasma prostate-specific antigen for detection of prostatic cancer. JAMA. 1995;273:289–294. [PubMed] [Google Scholar]
- 5.Fang J, Metter EJ, Landis P, Chan DW, Morrell CH, Carter HB. Low levels of prostate specific antigen predict long-term risk of prostate cancer: results from the Baltimore Longitudinal Study of Aging. Urology. 2001;58:411–416. doi: 10.1016/s0090-4295(01)01304-8. [DOI] [PubMed] [Google Scholar]
- 6.Aus G, Bergdahl S, Hugosson J, Lodding P, Pihl CG, Pileblad E. Outcome of laterally directed sextant biopsies of the prostate in screened males aged 50–66 years; implications for sampling order. Eur Urol. 2001;39:655–660. doi: 10.1159/000052523. [DOI] [PubMed] [Google Scholar]
- 7.Hugosson J, Aus G, Lilja H, Lodding P, Pihl CG. Results of a randomized, population-based study of biennial screening using serum prostate-specific antigen measurement to detect prostate carcinoma. Cancer. 2004;100:1397–1405. doi: 10.1002/cncr.20126. [DOI] [PubMed] [Google Scholar]
- 8.Ito K, Yamamoto T, Ohi M, et al. Possibility of re-screening intervals of more than one year in men with PSA levels of 4.0 ng/ml or less. Prostate. 2003;57:8–13. doi: 10.1002/pros.10268. [DOI] [PubMed] [Google Scholar]
- 9.Crawford D, Chia D, Andriole GL, et al. PSA testing interval, reduction in screening intervals: data from the prostate, lung, colorectal and ovarian cancer (PLCO) screening trial [abstract] J Urol. 2002;167(suppl 4):99–100. [Google Scholar]
- 10.Harris CH, Dalkin BL, Martin E, Marx PC, Ahmann FR. Prospective longitudinal evaluation of men with initial prostate specific antigen levels of 4.0 ng/ml or less. J Urol. 1997;157:1740–1743. [PubMed] [Google Scholar]
- 11.Yamamoto T, Ito K, Ohi M, et al. Diagnostic significance of digital rectal examination and transrectal ultrasonography in men with prostate-specific antigen levels of 4 ng/ml or less. Urology. 2001;58:994–998. doi: 10.1016/s0090-4295(01)01409-1. [DOI] [PubMed] [Google Scholar]
- 12.Carvalhal GF, Smith DS, Mager DE, Ramos C, Catalona WJ. Digital rectal examination for detecting prostate cancer at prostate specific antigen levels of 4 ng/ml or less. J Urol. 1999;161:835–839. [PubMed] [Google Scholar]
- 13.Vis AN, Hoedemaeker RF, Robool M, van der Kwast TH, Schroder FH. Tumor characteristics in screening for prostate cancer with and without rectal examination as an initial screening test at low PSA values (0.0–3.9 ng/ml) Prostate. 2001;47:252–261. doi: 10.1002/pros.1069. [DOI] [PubMed] [Google Scholar]
- 14.Norberg M, Egevad L, Holmberg L, Sparen P, Norlén BJ, Busch C. The sextant protocol for ultrasound-guided core biopsies of the prostate underestimates the presence of cancer. Urology. 1997;50:562–566. doi: 10.1016/S0090-4295(97)00306-3. [DOI] [PubMed] [Google Scholar]
- 15.Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215–224. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 16.Carter HB, Epstein IJ, Chan DW, Fozard JL, Pearson JD. Recommended prostate-specific antigen testing intervals for the detection of curable cancer. JAMA. 1997;277:1456–1460. [PubMed] [Google Scholar]


