Abstract
Most models to predict biochemical recurrence (BCR) of prostate cancer use pretreatment serum prostate-specific antigen (PSA), clinical stage and prostate biopsy Gleason grade. We investigated whether human glandular kallikrein 2 (hK2) and free prostate-specific antigen (fPSA) measured in pretreatment serum enhance prediction. We retrospectively measured total PSA (tPSA), fPSA and hK2 in preoperative serum samples from 461 men with localized prostate cancer treated with radical prostatectomy between 1999 and 2001. We developed a regression model to predict BCR using preoperative tPSA, clinical stage and biopsy Gleason grade. We then compared the predictive accuracy of this “base” model with a model with fPSA and hK2 as additional predictors. BCR was observed in 90 patients (20%), including 48 patients with a pretreatment tPSA ≤ 14 ng/ml (13%), and 28 patients (10%) with a pretreatment tPSA ≤ 10 ng/ml. Overall, the predictive accuracy of the base model (bootstrap-corrected concordance index of 0.813) was not improved after the addition of fPSA or hK2 (0.818). However, for men with moderate tPSA-elevation (tPSA ≤ 10 ng/ml), addition of fPSA and hK2 data increased predictive accuracy (from a base model concordance index of 0.756–0.815, p = 0.005). The improvement in accuracy was not sensitive to the threshold for “moderately elevated” PSA. For patients with a moderate tPSA-elevation (tPSA ≤ 10 ng/ml), which closely corresponds to concurrent disease demographics, BCR-prediction was enhanced when fPSA and hK2 were added to the conventional model. Measurements of fPSA and hK2 improve on our ability to counsel patients prior to treatment as to their risk of BCR.
Keywords: prostate cancer, biochemical recurrence, PSA, hK2, free PSA, radical prostatectomy
Radical prostatectomy (RP) provides excellent cancer control of clinically localized prostate cancer (PCa).1 However, approximately 30% of surgically treated men will experience a detectable serum prostate-specific antigen (PSA) as an unequivocal indicator of cancer progression within 10 years of surgery.2,3 These men with biochemical recurrence (BCR) are at significant risk for clinical cancer progression (metastases) and have the need for institution of systemic therapy.
Clinical stage, pretreatment PSA levels and prostate biopsy Gleason grade have been shown to be reliable and independent predictors of treatment failure. The combination of these 3 parameters markedly enhances the ability to predict treatment outcome. Several investigators have included these parameters into clinical risk profiles designed to identify patients who can safely avoid aggressive therapy or to select potential candidates for neoadjuvant clinical trials.4,5
The usefulness of these models, however, depends on their predictive accuracy. Recently, it has been claimed that preoperative PSA levels may reflect primarily benign prostate hyperplasia (BPH) rather than the presence of PCa in populations in which PSA is regularly used for screening.6 Recent studies have suggested that in patients with a pretreatment PSA <10 ng/ml, PSA levels did not predict treatment outcome after RP.7,8 This trend may affect the predictive accuracy of most currently available models, which utilize PSA as the primary predictive parameter. Hence, there is a compelling need to identify novel markers that are specifically linked to the presence of biologically aggressive prostate cancers for improved prediction of outcome in populations with moderately elevated PSA levels.
Because of these current limitations with models based primarily on total PSA levels, we investigated alternative prostate related biomarkers such as free PSA (fPSA) and human glandular kallikrein 2 (hK2) and their association to BCR in patients with clinically localized PCa. This approach is based on previous data, which indicates that a low ratio of free to total PSA (%fPSA)9,10 or increasing levels of hK211,12 are linked to advanced prostate pathology, which is well established as risk factor for progression after surgery. To address this issue, we developed a regression model to assess PSA recurrence using preoperative data on clinical stage, biopsy Gleason grade and tPSA from a cohort of men who underwent RP between 1999 and 2001. We then compared the predictive accuracy of this “base” model to a model that included hK2 and fPSA measurements as additional predictors.
Material and methods
Patients and serum samples
Between the years 1999 and 2001, 1172 patients with clinically localized PCa underwent RP with or without staging lymphadenectomy at University Hospital Hamburg-Eppendorf, Germany. For 505 of these patients, a serum sample was available for evaluation. Blood samples were drawn prior to RP 8 or more weeks after any prostatic manipulation (DRE, TRUS guided biopsy) and immediately processed and frozen at −80°C until analysis. Patients with any neoadjuvant therapy (n = 6), prior surgical treatment for BPH (n = 10) or lack of follow-up information (n = 28) were omitted from this study, leaving 461 patients with corresponding blood samples eligible for analysis. Informed consent was obtained from all of the participating patients, and the protocol was approved by the institutional review board.
Clinical and pathologic evaluation
For each patient, the operating surgeon assigned a clinical stage according to the 1997 edition of the AJCC.13 Biopsy material was histologically graded according to the Gleason grading system.14 Grades of all externally obtained biopsy specimens were reviewed at the Department of Pathology, University Hospital Hamburg-Eppendorf. All prostatectomy specimens were surface-inked and processed using serial transverse sections at 3-mm intervals according to the Stanford protocol.15 Pathologic stage was defined according to the 1997 AJCC staging classification.13
Definition of biochemical recurrence
All patients treated with RP are scheduled for an annual follow-up visit at our institutional outpatient clinic. Information about external posttreatment PSA testing (usually performed every 6 months) is added to the institutional database. BCR was defined as postoperative levels of tPSA ≥ 0.40 ng/ml. The selection of this cut point was based on a previous evaluation, which demonstrated that a significant proportion of patients with a lower PSA (e.g. 0.2 ng/ml or 0.3 ng/ml) did not experience further PSA rises.16 None of the patients received adjuvant therapy before the evidence of cancer recurrence.
Measurements of tPSA, fPSA and hK2
Total and free PSA
To measure tPSA and fPSA, we used the commercialized version of a previously reported dual-label assay (DELFIA Prostatus Dual Assay, PerkinElmer, Turku, Finland) that measures tPSA and fPSA on an equimolar basis.17 Detection limits were 0.04 ng/ml for fPSA (coefficient of variation [CV] 3.7% at 0.44 ng/ml and 17.9% at 0.10 ng/ml), and 0.05 ng/ml for tPSA (CV 5.0% at 2.32 ng/ml and 13.9% at 0.34 ng/ml).
Total hK2
Total hK2 among patients treated in 1999 was measured with a previously reported 3-step assay design.18 It uses a combination of PSA-specific blocking monoclonal antibodies (MAbs) together with capture MAbs and Europium-labeled detection MAbs recognizing both PSA and hK2. Addition of PSA-specific blocking MAbs enables specificity for hK2, as it reduces cross-reaction of PSA to <0.05%. The functional detection limit is 0.04 ng/ml, defined as the concentration at which total (i.e. inter and intraassay) CV is <20%.18 For patients treated in 2000 and 2001, we used a slightly modified and improved assay as was recently described.19 The assay uses 100 μl sample and the functional detection limit is 0.03 ng/ml. Total assay imprecision ranged from 12.9% for low (0.033 ng/ml) to 7.5% for high (0.48 ng/ml) hK2 levels.
Statistical methods
Cox proportional hazard models were used to determine the association between predictors and biochemical failure after RP. All bio-markers were used as log-transformed continuous variables. The base model included only the currently validated predictors (tPSA, clinical stage, biopsy Gleason grade). We then constructed multivariable models with hK2 and/or fPSA as additional predictors. Predictive accuracy for each model was defined in terms of the concordance index. In brief, the concordance index is a generalization of the area under the ROC curve for survival time data, and quantifies discrimination for a single variable or a multivariable model.20 The concordance index ranges from 0.5 (chance or a coin flip) to 1.0 (perfect ability to rank). Bootstrapping was used to calculate standard errors and to correct concordance indices for overfit. Subgroup analysis focused on men with moderately elevated PSA levels, who are more typical of PCa patients in the US and in other countries where tumors are generally detected by PSA screening. Analyses were performed using STATA (Version 8.2, StataCorp., College Station, TX).
Results
Median patient age was 63 years, with an interquartile range of 59–66 years. There were 90 cases of BCR (20%). Median follow-up for patients without BCR was 44 months. The 3- and 5-year recurrence-free probabilities for the study cohort were 84 and 75%, respectively (Fig. 1). Twenty-eight patients with tPSA ≤ 10 ng/ml experienced BCR (10%). Table I and II gives pretreatment levels of tPSA, fPSA, hK2, Gleason score, clinical and pathological characteristics of the study cohort.
Figure 1.
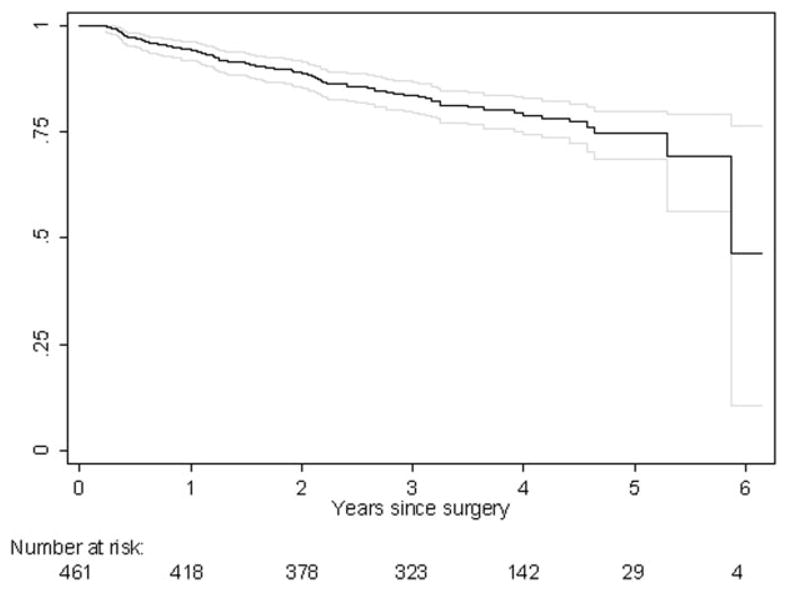
Kaplan-Meier estimates of recurrence-free probability and 95% confidence interval for 461 patients with clinically localized prostate cancer who underwent radical prostatectomy between 1999 and 2001.
TABLE I.
CLINICAL AND PATHOLOGIC CHARACTERISTICS OF THE STUDY COHORT (n = 461)
| Variable | No. patients |
|---|---|
| Pretreatment PSA (ng/ml) | |
| ≤4.0 | 47 (10)a |
| 4.1–10.0 | 248 (54) |
| 10.1–20 | 131 (28) |
| >20 | 35 (8) |
| Clinical stage (1997 TNM) | |
| T1c | 311 (67) |
| T2a/b | 147 (32) |
| T3 | 3 (1) |
| Biopsy Gleason sum | |
| ≤6 | 321 (70) |
| 7 | 128 (27) |
| ≥8 | 12 (3) |
| Pathologic stage (1997 TNM) | |
| pT2a/b | 305 (66) |
| pT3a | 100 (22) |
| pT3b | 56 (12) |
| Pathology Gleason sum | |
| ≤6 | 226 (49) |
| 7 | 225 (49) |
| ≥8 | 10 (2) |
Values in parentheses indicate the percentage of biochemical recurrence of Prostrate cancer.
TABLE II.
MEDIAN TOTAL PSA, FREE PSA, AND HK2 LEVELS OF PROSTATE CANCER PATIENTS, AMONG THE WHOLE COHORT AND AMONG PATIENTS WITH PSA ≤ 10 ng/ml
| Biomarker | Median |
|---|---|
| All patients (n = 461) | |
| tPSA | 8.09 (5.59–12.55)a |
| fPSA | 0.95 (0.64–1.42) |
| hK2 | 0.091 (0.054–0.130) |
| tPSA ≤ 10 ng/ml (n = 294) | |
| tPSA | 6.4 (4.7–7.9) |
| fPSA | 0.75 (0.53–1.08) |
| hK2 | 0.070 (0.045–0.100) |
Values in parentheses indicate interquartile range in ng/ml.
Patients with a pretreatment hK2 level below the median had greater recurrence-free survival than those with higher hK2 (Fig. 2). Likewise, patients with fPSA levels below the median had higher BCR-free survival (Fig. 3).
Figure 2.
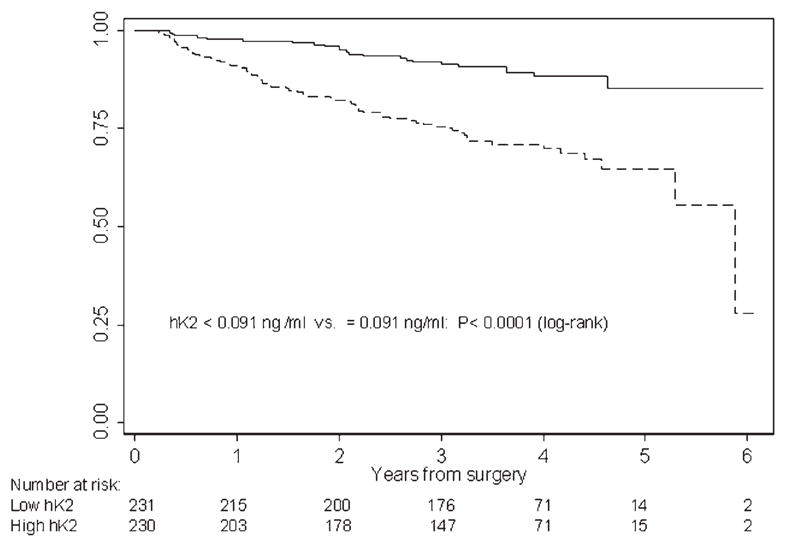
Kaplan-Meier estimates of recurrence-free probability according to pretreatment hK2 levels. Recurrence-free probabilities for patients with pre-treatment hK2 levels below (solid line) and above (dashed line) the median of 0.091 ng/ml within the whole cohort (n = 461).
Figure 3.
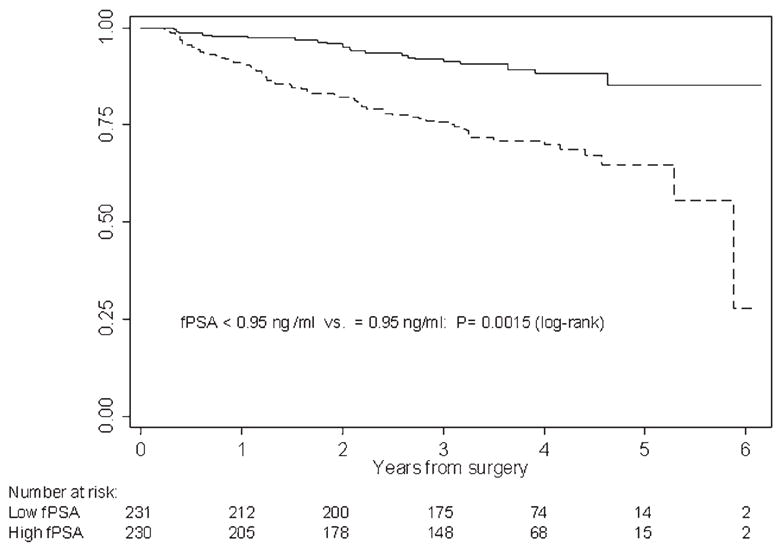
Kaplan-Meier estimates of 3- and 5-year recurrence-free probability. Recurrence-free probabilities for patients with pre-treatment fPSA levels below (solid line) and above (dashed line) the median of 0.95 ng/ml within the whole cohort (n = 461).
In univariate analysis, all variables were significantly associated with biochemical failure at p <0.0001 (Table III). In the multivariable model of the entire cohort, grade and tPSA, but not stage, hK2, or fPSA were statistically significant predictors of BCR. However, for patients with tPSA ≤ 10 ng/ml, only clinical stage, biopsy Gleason score and hK2 were independent predictors of biochemical failure (Table III).
TABLE III.
UNIVARIATE AND MULTIVARIABLE ANALYSIS TO ASSESS THE ASSOCIATION OF PREDICTORS WITH BIOCHEMICAL RECURRENCE AFTER RADICAL PROSTATECTOMY FOR LOCALIZED PROSTATE CANCER AMONG ALL PATIENTS AND AMONG PATIENTS WITH PSA ≤ 10 ng/ml
| Predictor variables | Univariate analysis
|
Multivariable analysis
|
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| All patients (n = 461) | ||||||
| Clinical stage (T1c vs.T2/3) | 2.87 | 1.89, 4.36 | <0.0001 | 1.43 | 0.91, 2.23 | 0.12 |
| Biopsy Gleason sum | ||||||
| ≤6 (reference) | ||||||
| 7 | 3.93 | 2.59, 5.96 | <0.0001 | 3.11 | 1.87, 5.14 | <0.0001 |
| ≥8 | 7.19 | 3.71, 13.93 | <0.0001 | 6.48 | 2.95, 14.21 | <0.0001 |
| log tPSA | 4.57 | 3.3, 6.32 | <0.0001 | 4.33 | 2.35, 7.98 | <0.0001 |
| log fPSA | 2.25 | 1.63, 3.09 | <0.0001 | 0.67 | 0.37, 1.24 | 0.2 |
| log hK2 | 2.70 | 2.02, 3.61 | <0.0001 | 1.16 | 0.79, 1.69 | 0.4 |
| tPSA ≤ 10 ng/ml (n = 294) | ||||||
| Clinical stage (T1c vs.T2/3) | 3.47 | 1.63, 7.39 | 0.001 | 2.23 | 1.01, 4.93 | 0.048 |
| Biopsy Gleason sum | ||||||
| ≤6 (reference) | ||||||
| 7 | 3.70 | 1.74, 7.88 | 0.0007 | 3.16 | 1.36, 7.32 | 0.007 |
| ≥8 | 12.09 | 3.62, 40.42 | <0.0001 | 8.19 | 2.22, 29.92 | 0.002 |
| log tPSA | 6.72 | 1.67, 27.07 | 0.007 | 3.72 | 0.95, 14.45 | 0.06 |
| log fPSA | 1.24 | 0.61, 2.53 | 0.6 | 0.41 | 0.14, 1.20 | 0.1 |
| log hK2 | 4.47 | 2.3, 8.69 | <0.0001 | 4.77 | 2.19, 10.40 | <0.0001 |
HR, hazard ratio; CI, confidence interval; tPSA, total prostate-specific antigen; fPSA, free prostate-specific antigen; hK2, human glandular kallikrein 2.
The established predictors (tPSA, clinical stage and biopsy Gleason score) were combined in a prediction base model. The bootstrap-corrected predictive accuracy of this model was high, with a concordance index of 0.813 for the entire study cohort. Adding the predictor variables fPSA and hK2 levels only marginally affected the predictive accuracy to 0.818 (Table IV). However, for men with PSA ≤ 10 ng/ml, the predictive value of the base model decreased to a bootstrap-corrected concordance index of 0.756. Addition of hK2 and fPSA to the model provided a marked increment in predictive accuracy (from 0.756 to 0.815, p = 0.005). As the number of BCR events in patients with tPSA ≤ 10 ng/ml was relatively low (n = 28), it might be argued that a model with 6 predictors would be at risk of overfitting. Accordingly, we combined tPSA, clinical stage and biopsy Gleason score (raw data) to obtain a predicted probability of 3-year BCR-free survival using a published nomogram5 (nomogram probability), and used the nomogram probability as a single predictor instead of raw covariate values. This reduces the number of variables in the full model from 6 (PSA, clinical stage, 2 Gleason grade categories, hK2, fPSA) to only 3 (nomogram probability, hK2, fPSA). As shown in Table IV, results were similar whether raw covariate or nomogram-predicted probabilities were used. To determine whether our choice of threshold for “moderately elevated” tPSA might affect our conclusions, we varied the tPSA threshold from 8 (BCR-events, n = 18) to 20 ng/ml (BCR-events, n = 65), and computed concordance indices using the nomogram probability of BCR as the covariate (Table V). Results were comparable up to tPSA levels < 14 ng/ml, while the number of BCR events at tPSA ≤ 10 ng/ml up to < 14 ng/ml markedly increased from 28 to 44 subjects.
TABLE IV.
PREDICTIVE ACCURACY (CONCORDANCE INDEX) OF A NOMOGRAM INCLUDING CLINICAL STAGE, BIOPSY GLEASON SUM, AND PRE-TREATMENT PSA (BASE MODEL) AND OF THE BASE MODEL SUPPLEMENTED BY FPSA AND/OR HK2 LEVELS
| Raw data as covariates | Bootstrap corrected | Nomogram probability as covariate | Bootstrap corrected | |
|---|---|---|---|---|
| All 461 patients (n = 461) | ||||
| Base model | 0.819 | 0.813 | 0.806 | 0.806 |
| + fPSA | 0.824 | 0.787 | ||
| + hK2 | 0.820 | 0.807 | ||
| + fPSA & hK2 | 0.827 | 0.818 | 0.802 | 0.797 |
| tPSA ≤ 10 ng/ml (n = 294) | ||||
| Base model | 0.771 | 0.756 | 0.763 | 0.763 |
| + fPSA | 0.782 | 0.739 | ||
| + hK2 | 0.815 | 0.802 | ||
| + fPSA & hK2 | 0.835 | 0.815 | 0.815 | 0.81 |
tPSA, total prostate-specific antigen; fPSA, free prostate-specific antigen; hK2, human glandular kallikrein 2.
TABLE V.
SENSITIVITY ANALYSIS TO DETERMINE WHETHER THE CHOICE OF CUT-OFF VALUE FOR LOW TPSA MIGHT INFLUENCE OUR FINDINGS
| PSA cut-off ng/ml | No. Patients | No. BCR | Risk (%) | Base model | Base model 1 fPSA and hK2 | Increment |
|---|---|---|---|---|---|---|
| 8 | 226 | 18 | 8 | 0.771 | 0.840 | 0.069 |
| 9 | 261 | 21 | 8 | 0.760 | 0.836 | 0.076 |
| 10 | 294 | 28 | 10 | 0.764 | 0.815 | 0.051 |
| 11 | 325 | 36 | 11 | 0.777 | 0.828 | 0.051 |
| 12 | 339 | 39 | 12 | 0.782 | 0.829 | 0.047 |
| 13 | 356 | 44 | 12 | 0.785 | 0.817 | 0.032 |
| 14 | 371 | 48 | 13 | 0.790 | 0.804 | 0.014 |
| 15 | 388 | 51 | 13 | 0.778 | 0.771 | −0.007 |
| 20 | 426 | 65 | 15 | 0.776 | 0.780 | 0.004 |
| All pt. | 461 | 90 | 20 | 0.806 | 0.802 | −0.004 |
The tPSA cut-off was varied (8–20 ng/ml) and corresponding concordance indices computed. The nomogram probability of BCR was used as the covariate.
BCR, biochemical recurrence; fPSA, free prostate-specific antigen; hK2, human glandular kallikrein 2.
To test whether the diagnostic value of fPSA and hK2 was dependent on the level of tPSA, we first created a linear prediction from a model including hK2 and fPSA only. We added the interaction between this multivariable prediction and tPSA to our full model. The interaction term was less than one and statistically significant (p = 0.015), demonstrating that the diagnostic value of hK2 and fPSA increases as tPSA decreases.
Discussion
In recent years, statistical models have evolved as diagnostic tools that incorporate independent biochemical and clinical findings to predict the risk of BCR following RP.4,5 BCR is the most common sign of cancer recurrence after surgical treatment for localized PCa. The preoperative Kattan nomogram is a predictive model for BCR widely used in clinical practice.5,21 This nomogram combines the variables clinical stage, biopsy Gleason score and PSA. Subsequently, various additional clinical factors have been incorporated into statistical models aiming to improve prediction accuracy.22,23 These parameters include prostate biopsy features (percentage of positive biopsies, percentage of biopsy tissue with cancer), MRI T-stage, PSA adjusted for prostate volume (PSA density) and plasma levels of transforming growth factor β1 and soluble interleukin-6 receptor. However, to date only 2 studies have attempted to measure the incremental benefit gained by additional predictors. A diagnostic model combining the standard clinical predictors with features from systematic biopsy only marginally improved diagnostic accuracy for BCR prediction (concordance index of 0.79 for the standard model vs. 0.81 for the model including biopsy features).24 In contrast, platelet-free plasma levels of transforming growth factor β1 (TGF-β1) and interleukin-6 soluble receptor, which have been reported to be markedly elevated in patients with distant metastases from PCa, significantly enhanced prediction of BCR over the standard Kattan pretreatment nomogram (concordance index of 0.75 vs. 0.83 for a model supplemented by TGF-β1 and interleukin-6 receptor).25
Measurements of prostate-specific tissue kallikreins such as multiple PSA-forms and hK2 in blood have been shown to improve early detection and staging of PCa.11,12,26 However, there are no previous data reported whether these measurements may enhance the predictive accuracy of the standard pretreatment model to assess risk for BCR. Using a carefully designed protocol to avoid fPSA-decay ex vivo after the blood draw, we conducted this study to evaluate whether measurements of hK2 and fPSA could enhance the predictive accuracy of a standard model to assess the risk of BCR. In our current analysis, we confirmed the high predictive accuracy of a regression model that included clinical stage, biopsy Gleason score and PSA (base model). Addition of hK2 and fPSA to the base model did not contribute enhanced predictive value. Yet the accuracy of a model, and the relative benefit of additional predictors, is crucially dependent on the heterogeneity of the population. PSA is a powerful predictor when the serum levels vary widely: in this cohort, tPSA ranged from 0.3 to 86 ng/ml. In populations regularly exposed to screening, levels of tPSA decrease significantly and eventually lose association with important disease characteristics. This is exemplified by recent reports from Stamey et al. who found that tPSA did not predict treatment outcome after RP in men with tPSA from 2 to 9 ng/ml,8 and a series of 1095 men with tPSA ≤ 10 ng/ml studied by Freedland et al. who concluded that PSA was not an independent predictor of biochemical failure following RP in a multivariable model (p = 0.09).7 Hence, it is highly controversial whether tPSA remain as a significant predictor of treatment failure among men with a more narrow range of serum tPSA. Our study supports these findings, as tPSA was unable to predict BCR (p = 0.06) in a multivariable analysis of men with tPSA ≤ 10 ng/ml. Moreover, the predictive accuracy of a model using only stage, Gleason grade and tPSA decreased markedly in this tPSA range. However, in this subset of men, hK2 was an independent predictor of BCR and substantially increased predictive accuracy of our model, whether alone or in combination with fPSA. We further explored the benefits of using hK2 and fPSA and found it to be applicable to pretreatment tPSA-levels of up to14 ng/ml, at which BCR occurred in 44 patients. Thus, hK2 and fPSA appear to compensate for the diluted prognostic capacity of tPSA based nomograms in men with moderately elevated PSA levels.
The clinical relevance of our findings become more important in consideration of the high proportion of men with serum PSA ≤ 10 ng/ml in contemporary RP series. Particularly in the US, there is a marked shift towards lower PSA levels at diagnosis because of widespread use of tPSA-testing for the detection of PCa. In our study cohort, which represents a RP referral series from a nonscreened population during 1999–2001, the proportion of men with tPSA ≤ 10 ng/ml prior to treatment was 64%. This is similar to the proportion (63–74%) reported from large prostatectomy series from several institutions accrued between year 1983 and 2002.1,3,7 Among patients who more recently underwent RP (from 2002 to 2003) at University Hospital Hamburg-Eppendorf, the percentage with tPSA ≤ 10 ng/ml increased to 82%. This suggests that the widespread use of PSA screening may decrease the proportion of men with tPSA ≥ 10 ng/ml at diagnosis to 10% or less.
The validity of our data might be influenced by the risk of preanalytical bias caused by the well-documented but commonly neglected lack of ex vivo stability of fPSA after the blood draw.27 We meticulously processed our samples (rapid separation of serum from the blood cells, immediate freezing at −80°C and analysis of samples immediately after thawing), to minimize the influence from preanalytical decrease of fPSA-levels.
The role of fPSA as a predictor of biochemical failure remains unclear. The use of fPSA to predict treatment outcome has produced conflicting results. While some authors have reported significant benefit of using %fPSA for staging,9,10 others have not confirmed these findings.28 It is, however, noteworthy that the reference populations which showed a benefit of using fPSA for staging PCa were consistently those with a tPSA <10 ng/ml. Accordingly, models that include fPSA in our study show a modest increase in predictive accuracy. Highest predictive accuracy can be obtained with inclusion of both hK2 and fPSA. This is true for all patients and especially for the subgroup of men with only moderately elevated tPSA levels. In an exploratory analysis (data not shown), we added %fPSA instead fPSA in the full prediction model and found no increment in predictive accuracy. Since %fPSA did not offer any advantage over the application of fPSA alone, %fPSA was not used in the model.
To what extent are previously reported data consistent with our findings that serum levels of hK2 and are linked to biologically aggressive cancers? The tissue studies from Darson and associates reported on more intense immunostaining specific for hK2 in lymphatic metastases and in poorly differentiated (i.e. high grade) cancer compared to well-differentiated tumors and benign tissue, whereas contrary findings were reported for PSA.29 Findings recently reported by Lintula et al., using RT-PCR to quantify relative levels of hK2 and PSA mRNA in benign and malignant prostatic tissue30 are consistent with the previous report from Darson. Higher hK2/PSA mRNA ratios were observed in cancerous tissue samples compared with BPH tissue samples. This suggests that changes in relative expression-levels of hK2 vs. PSA are possibly associated with carcinogenesis and progression. However, it is unclear whether these findings may significantly change the blood levels, as tissue levels are up to 106-fold higher than PSA and hK2-levels in serum. Several other factors such as kidney function, nature of complex ligands and catalytic action can significantly affect the levels in blood. Nevertheless, previous evaluations of hK2 in PCa patients scheduled for RP have indicated that there may be a significant association of the hK2-levels in serum with total cancer volume, volume of high-grade cancer (Gleason grade ≥ 4) and advanced prostate pathology.11,12, 31
In conclusion, this initial data set suggests that the incorporation of fPSA and hK2 levels in blood in pretreatment prediction models for BCR enhances risk prediction. The value of these analytes is particularly marked in patients with only moderately elevated PSA, which is typical of contemporary patient populations. Further research is planned to replicate our findings on a larger study population.
Acknowledgments
We thank Gun-Britt Eriksson and Kerstin Håkansson for expert technical assistance with the immunoassays.
Grant sponsor: NCI; Grant number: P50-CA92629; Grant sponsor: Swedish Cancer Society; Grant number: 3555; Grant sponsor: European Union; Grant number: LSHC-CT-2004-503011; Grant sponsor: Fundacion Federico, the Deutsche Forschungs Gemeinschaft; Grant number: Ste 1429/1-1; Grant sponsor: Finnish Academy; Grant number: 878541.
Footnotes
In full disclosure of potential conflicts of interest, we would like to notify you that Dr. Hans Lilja is a patent holder of the free PSA and hK2 Immunoassay. The authors further confirm that no further relationship exists that could be construed as resulting in an actual, potential or apparent conflict of interest with the manuscript submitted for review.
References
- 1.Hull GW, Rabbani F, Abbas F, Wheeler TM, Kattan MW, Scardino PT. Cancer control with radical prostatectomy alone in 1,000 consecutive patients. J Urol. 2002;167:528–34. doi: 10.1016/S0022-5347(01)69079-7. [DOI] [PubMed] [Google Scholar]
- 2.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–7. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 3.Gerber GS, Thisted RA, Scardino PT, Frohmuller HG, Schroeder FH, Paulson DF, Middleton AW, Jr, Rukstalis DB, Smith JA, Jr, Schell-hammer PF, Ohori M, Chodak GW. Results of radical prostatectomy in men with clinically localized prostate cancer. JAMA. 1996;276:615–9. [PubMed] [Google Scholar]
- 4.D’Amico AV, Whittington R, Malkowicz SB, Fondurulia J, Chen MH, Kaplan I, Beard CJ, Tomaszewski JE, Renshaw AA, Wein A, Coleman CN. Pretreatment nomogram for prostate-specific antigen recurrence after radical prostatectomy or external-beam radiation therapy for clinically localized prostate cancer. J Clin Oncol. 1999;17:168–72. doi: 10.1200/JCO.1999.17.1.168. [DOI] [PubMed] [Google Scholar]
- 5.Kattan MW, Eastham JA, Stapleton AM, Wheeler TM, Scardino PT. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst. 1998;90:766–71. doi: 10.1093/jnci/90.10.766. [DOI] [PubMed] [Google Scholar]
- 6.Stamey TA. The era of serum prostate specific antigen as a marker for biopsy of the prostate and detecting prostate cancer is now over in the USA. BJU Int. 2004;94:963–4. doi: 10.1111/j.1464-410X.2004.05212.x. [DOI] [PubMed] [Google Scholar]
- 7.Freedland SJ, Aronson WJ, Kane CJ, Terris MK, Presti JC, Jr, Trock B, Amling CL. Biochemical outcome after radical prostatectomy among men with normal preoperative serum prostate-specific antigen levels. Cancer. 2004;101:748–53. doi: 10.1002/cncr.20390. [DOI] [PubMed] [Google Scholar]
- 8.Stamey TA, Johnstone IM, McNeal JE, Lu AY, Yemoto CM. Preoperative serum prostate specific antigen levels between 2 and 22 ng/ml correlate poorly with post-radical prostatectomy cancer morphology: prostate specific antigen cure rates appear constant between 2 and 9 ng/ml. J Urol. 2002;167:103–111. [PubMed] [Google Scholar]
- 9.Southwick PC, Catalona WJ, Partin AW, Slawin KM, Brawer MK, Flanigan RC, Patel A, Richie JP, Walsh PC, Scardino PT, Lange PH, Gasior GH, et al. Prediction of post-radical prostatectomy pathological outcome for stage T1c prostate cancer with percent free prostate specific antigen: a prospective multicenter clinical trial. J Urol. 1999;162:1346–51. [PubMed] [Google Scholar]
- 10.Morote J, Encabo G, de Torres IM. Use of percent free prostate-specific antigen as a predictor of the pathological features of clinically localized prostate cancer. Eur Urol. 2000;38:225–9. doi: 10.1159/000020283. [DOI] [PubMed] [Google Scholar]
- 11.Haese A, Graefen M, Becker C, Noldus J, Katz J, Cagiannos I, Kattan M, Scardino PT, Huland E, Huland H, Lilja H. The role of human glandular kallikrein 2 for prediction of pathologically organ confined prostate cancer. Prostate. 2003;54:181–6. doi: 10.1002/pros.10180. [DOI] [PubMed] [Google Scholar]
- 12.Haese A, Vaisanen V, Lilja H, Kattan MW, Rittenhouse HG, Pettersson K, Chan DW, Huland H, Sokoll LJ, Partin AW. Comparison of predictive accuracy for pathologically organ confined clinical stage T1c prostate cancer using human glandular kallikrein 2 and prostate specific antigen combined with clinical stage and Gleason grade. J Urol. 2005;173:752–6. doi: 10.1097/01.ju.0000152618.38747.dd. [DOI] [PubMed] [Google Scholar]
- 13.American Joint Committee on Cancer (AJCC) AJCC cancer staging manual. 5. Philadelphia: Lippincott-Raven; 1997. [Google Scholar]
- 14.Gleason DF. Histologic grading and clinical staging of prostate carcinoma. Philadelphia: Lea and Febiger; 1977. [Google Scholar]
- 15.McNeal JE, Redwine EA, Freiha FS, Stamey TA. Zonal distribution of prostatic adenocarcinoma. Correlation with histologic pattern and direction of spread. Am J Surg Pathol. 1988;12:897–906. doi: 10.1097/00000478-198812000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Amling CL, Bergstralh EJ, Blute ML, Slezak JM, Zincke H. Defining prostate specific antigen progression after radical prostatectomy: what is the most appropriate cut point? J Urol. 2001;165:1146–51. [PubMed] [Google Scholar]
- 17.Mitrunen K, Pettersson K, Piironen T, Bjork T, Lilja H, Lovgren T. Dual-label one-step immunoassay for simultaneous measurement of free and total prostate-specific antigen concentrations and ratios in serum. Clin Chem. 1995;41:1115–20. [PubMed] [Google Scholar]
- 18.Becker C, Piironen T, Kiviniemi J, Lilja H, Pettersson K. Sensitive and specific immunodetection of human glandular kallikrein 2 in serum. Clin Chem. 2000;46:198–206. [PubMed] [Google Scholar]
- 19.Vaisanen V, Eriksson S, Ivaska KK, Lilja H, Nurmi M, Pettersson K. Development of sensitive immunoassays for free and total human glandular kallikrein 2. Clin Chem. 2004;50:1607–17. doi: 10.1373/clinchem.2004.035253. [DOI] [PubMed] [Google Scholar]
- 20.Harrell FE., Jr . Regression modelling strategies with applications to linear models, logistic regression, and survival analysis. New York: Springer; 2001. [Google Scholar]
- 21.Graefen M, Karakiewicz PI, Cagiannos I, Quinn DI, Henshall SM, Grygiel JJ, Sutherland RL, Stricker PD, Klein E, Kupelian P, Skinner DG, Lieskovsky G, et al. International validation of a preoperative nomogram for prostate cancer recurrence following radical prostatectomy. J Clin Oncol. 2002;20:3206–12. doi: 10.1200/JCO.2002.12.019. [DOI] [PubMed] [Google Scholar]
- 22.D’Amico AV, Whittington R, Malkowicz SB, Wu YH, Chen M, Art M, Tomaszewski JE, Wein A. Combination of the preoperative PSA level, biopsy gleason score, percentage of positive biopsies, and MRI T-stage to predict early PSA failure in men with clinically localized prostate cancer. Urology. 2000;55:572–7. doi: 10.1016/s0090-4295(99)00479-3. [DOI] [PubMed] [Google Scholar]
- 23.Freedland SJ, Wieder JA, Jack GS, Dorey F, deKernion JB, Aronson WJ. Improved risk stratification for biochemical recurrence after radical prostatectomy using a novel risk group system based on prostate specific antigen density and biopsy Gleason score. J Urol. 2002;168:110–15. [PubMed] [Google Scholar]
- 24.Graefen M, Ohori M, Karakiewicz PI, Cagiannos I, Hammerer PG, Haese A, Erbersdobler A, Henke RP, Huland H, Wheeler TM, Slawin K, Scardino PT, et al. Assessment of the enhancement in predictive accuracy provided by systematic biopsy in predicting outcome for clinically localized prostate cancer. J Urol. 2004;171:200–3. doi: 10.1097/01.ju.0000099161.70713.c8. [DOI] [PubMed] [Google Scholar]
- 25.Kattan MW, Shariat SF, Andrews B, Zhu K, Canto E, Matsumoto K, Muramoto M, Scardino PT, Ohori M, Wheeler TM, Slawin KM. The addition of interleukin-6 soluble receptor and transforming growth factor β1 improves a preoperative nomogram for predicting biochemical progression in patients with clinically localized prostate cancer. J Clin Oncol. 2003;21:3573–9. doi: 10.1200/JCO.2003.12.037. [DOI] [PubMed] [Google Scholar]
- 26.Becker C, Piironen T, Pettersson K, Hugosson J, Lilja H. Testing in serum for human glandular kallikrein 2, and free and total prostate specific antigen in biannual screening for prostate cancer. J Urol. 2003;170:1169–74. doi: 10.1097/01.ju.0000086640.19892.0b. [DOI] [PubMed] [Google Scholar]
- 27.Piironen T, Pettersson K, Suonpaa M, Stenman UH, Oesterling JE, Lovgren T, Lilja H. In vitro stability of free prostate-specific antigen (PSA) and prostate-specific antigen (PSA) complexed to α1-antichymotrypsin in blood samples. Urology. 1996;48(Suppl 6A):81–7. doi: 10.1016/s0090-4295(96)00616-4. [DOI] [PubMed] [Google Scholar]
- 28.Graefen M, Karakiewicz P, Cagiannos I, Hammerer PG, Haese A, Palisaar J, Huland E, Huland H, Scardino P, Kattan MW. Percentage of free PSA is not an independent predictor or organ confinement or PSA recurrence in unscreened patients with localized prostate cancer treated with radical prostatectomy. J Urol. 2002;167:1306–9. [PubMed] [Google Scholar]
- 29.Darson MF, Pacelli A, Roche P, Rittenhouse HG, Wolfert RL, Saeid MS, Young CYF, Klee GG, Tindall DJ, Bostwick DG. Human glandular kallikrein 2 expression in prostate adenocarcinoma and lymph node metastases. Urology. 1999;53:939–44. doi: 10.1016/s0090-4295(98)00637-2. [DOI] [PubMed] [Google Scholar]
- 30.Lintula S, Stenman J, Bjartell A, Nordling S, Stenman UH. Relative concentrations of hK2/PSA mRNA in benign and malignant prostatic tissue. Prostate. 2005;63:324–9. doi: 10.1002/pros.20194. [DOI] [PubMed] [Google Scholar]
- 31.Haese A, Graefen M, Steuber T, Becker C, Noldus J, Erbersdobler A, Huland E, Huland H, Lilja H. Total and Gleason grade 4/5 cancer volumes are major contributors of human kallikrein 2, whereas free prostate specific antigen is largely contributed by benign gland volume in serum from patients with prostate cancer or benign prostatic biopsies. J Urol. 2003;170:2269–73. doi: 10.1097/01.ju.0000095794.04551.0c. [DOI] [PubMed] [Google Scholar]


