Abstract
Bone morphogenetic proteins have been implicated in the development of oligodendrocytes and astrocytes, however, a role for endogenous BMP signaling in glial development has not been demonstrated in a genetic model. Using mice in which signaling via type I BMP receptors Bmpr1a and Bmpr1b have been inactivated in the neural tube, we demonstrate that BMP signaling contributes to the maturation of glial cells in vivo. At P0, mutant mice exhibited a 25-40% decrease in GFAP+ or S100β+ astrocytes in the cervical spinal cord. The number of oligodendrocyte precursors and the timing of their emergence was unchanged in the mutant mice compared to the normals, however myelin protein expression and mature oligodendrocyte numbers were significantly reduced. These data indicate that BMP signaling promotes the generation of astrocytes and mature, myelinating oligodendrocytes in vivo but does not affect oligodendrocyte precursor development, thus suggesting tight regulation of BMP signaling to ensure proper gliogenesis.
Introduction
The development of oligodendrocytes, the myelinating cells of the CNS, is controlled by inductive and repressive extracellular signals secreted in locally restricted regions. These signals regulate specification as well as cellular processes such as proliferation, migration, survival, differentiation, and myelin production. Oligodendrocytes initially appear as oligodendrocyte precursor cells (OPCs) that first originate from the ventral ventricular zone which lies dorsal to the floor plate (Ono et al., 1995; Pringle and Richardson, 1993). The expression of sonic hedgehog specifies the ventral location and induces the expression of transcription factors, such as Olig1 and 2, necessary for oligodendrocyte specification and development (Lu et al., 2002; Lu et al., 2000; Zhou and Anderson, 2002; Zhou et al., 2000). Inhibitors of oligodendrocyte development in the roof plate have been hypothesized to repress OPC formation in the dorsal neural tube (Wada et al., 2000). Mounting evidence exists, however, for an additional dorsal contribution of oligodendrocytes arising later than the ventral one (Cai et al., 2005; Kessaris et al., 2006; Vallstedt et al., 2005).
Bone morphogenetic proteins (BMPs), members of the TGFβ family of signaling molecules, have numerous functions in nervous system development (for reviews see (Chen et al., 2004; Fukuda and Taga, 2005). Several BMP proteins expressed in roof plate regulate the specification of dorsal neuronal cell types (Panchision et al., 2001; Timmer et al., 2002). BMPs have also been hypothesized to regulate the development of both oligodendrocytes and astrocytes. In vitro, addition of BMPs to culture systems promotes astrogliogenesis and inhibits oligodendrogliogenesis (Grinspan et al., 2000; Mabie et al., 1997; See et al., 2004). In vivo, application of BMPs in developing spinal cord decreases the number of differentiated oligodendrocytes (Mekki-Dauriac et al., 2002; Miller et al., 2004). Transgenic mice that overexpress BMP4 exhibit both decreased oligodendrogenesis and increased astrogliogenesis (Gomes et al., 2003; Mekki-Dauriac et al., 2002; Miller et al., 2004). However, these experiments do not address whether endogenous levels of BMP signaling are involved in glial development in vivo.
To investigate the role of endogenous BMP signaling in glial development, we have used mutant mouse pedigrees in which BMP receptor signaling has been genetically eliminated in the neural tube. BMPs signal through serine-threonine kinase receptors composed of Type I and Type II receptor subunits (for review, see von Bubnoff and Cho, 2001). Studies presented here use Bmpr double knockouts that are functionally null for two BMP type I receptor genes, Bmpr1a and Bmpr1b, in the neural tube by E10.5 (Ahn et al, 2001, Wine-Lee et al, 2004).
We assessed the role of BMP signaling in astrocyte and oligodendrocyte development by comparing cervical spinal cord sections from normal and Bmpr double knockout mice. As expected, the numbers of astrocytes expressing either glial fibrillary acidic protein or S100β in the double knockout animals was decreased 25-40% by P0 compared to the normal animals. Surprisingly, the number of oligodendrocyte precursors and their distribution was unaffected in the Bmpr double knockout spinal cords. However, the cords exhibited significantly reduced numbers of cells labeling with myelin protein markers and galactocerebroside at P0. These data indicate that BMP signaling supports astrogliogenesis and oligodendrocyte maturation but does not appear to be required for OPC generation.
Results
BMP signaling in the oligodendrocyte lineage is disrupted in Bmpr double knockout animals
To characterize the role of BMP signaling during gliogenesis, we have used a mouse mutant in which signaling via BMP type I receptors has been abrogated in the neural tube (Wine-Lee et al, 2004). In these mutants, a floxed allele of the Bmpr1a gene has been functionally inactivated using the Bcre-32 transgenic allele. In the Bcre-32 transgenic pedigree, Cre recombinase is expressed in the overwhelming majority of neural tube ventricular cells, thereby efficiently eliminating Bmpr1a gene function in all cell types in the spinal cord and hindbrain (Ahn et al, 2001, Wine-Lee et al, 2004). Bmpr1b gene function is eliminated using a classical knockout (Yi et al., 2000). Previously, it has been demonstrated that these Bmpr double knockout embryos completely abrogate BMP signaling in the neural tube, as evidenced by the complete loss of Smad1, 5 and 8 phosphorylation (Wine-Lee et al., 2004). In addition, these animals exhibit a complete loss of dorsal progenitor cells, dp1, as demonstrated by a loss of Math1 expression and a subsequent loss of DI1 interneurons. Additionally, there is a reduction in the number of DI2 interneurons and a dorsal expansion of the DI3 and DI4 population (Wine-Lee et al, 2004).
To determine that BMP signaling in OPCs was completely disrupted in the oligodendrocyte lineage of double mutant animals, we cultured brains from normal and Bmpr double knockout mice at P0 as described (see methods). Cultures of OPCs were treated with 50ng/ml BMP4 for 24 hours or left untreated. We then examined downstream signaling to Smad proteins. Cultures were immunolabeled with antibodies which recognize the phosphorylated form of Smads 1, 5 and 8 and to A2B5, a surface ganglioside that labels oligodendrocyte precursor cells (LeVine and Goldman, 1988). Oligodendrocytes from normal animals exhibited low levels of phospho-Smad labeling in control conditions and extensive nuclear phospho-Smad labeling when treated with BMP (Figure 1 A-D). The double knockout cultures, however, exhibited no phospho-Smad labeling in control or BMP-treated conditions, indicating that BMP signaling through R-Smads was eliminated by disruption of both Bmpr1a and Bmpr1b. Double immunolabeling with antibody to PDGFRα and phospho-Smad gave the same results (data not shown). Treatment of the cells with BMP4 for 1 hour gave the same results (data not shown). The low level of phospho-Smad labeling seen in untreated cultures from normal animals may reflect autocrine signaling, because oligodendrocyte lineage cells themselves express BMPs (Kondo and Raff, 2004, Zhang and Grinspan, unpublished).
Figure 1.
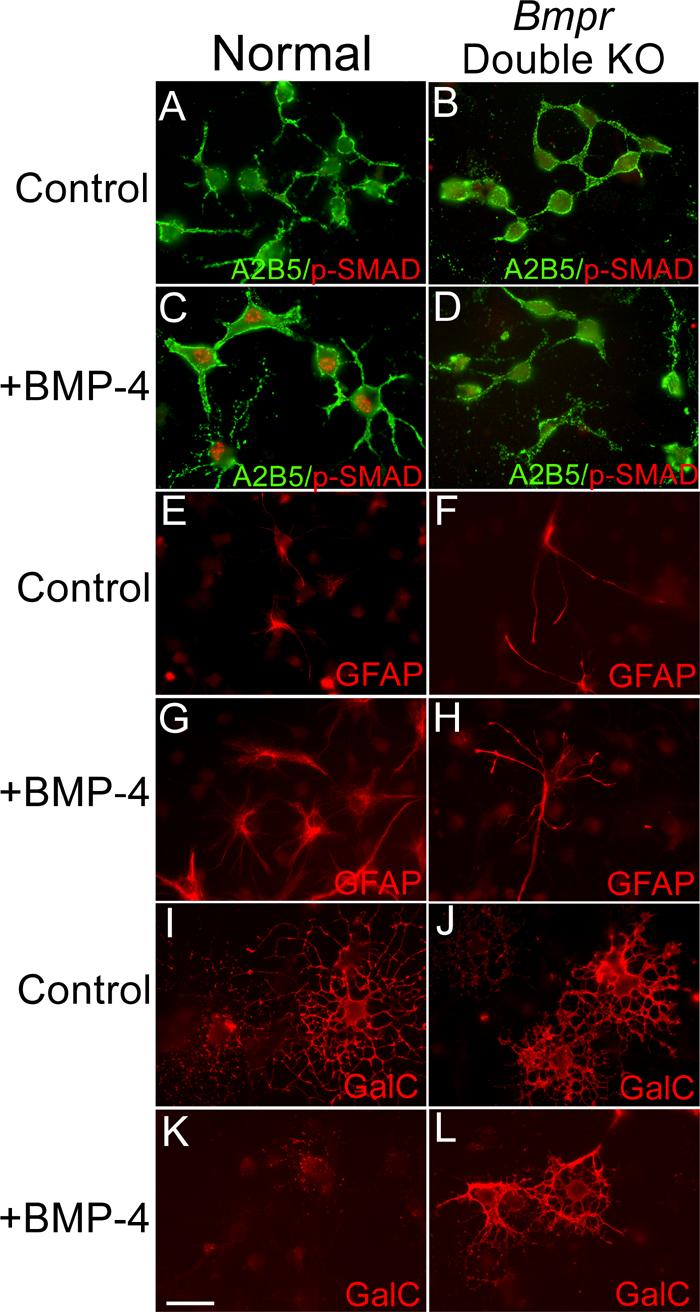
BMP signaling in the oligodendrocyte lineage is disrupted in conditional double knockout Bmpr mice.
Cultures of OPCs were established from brains of P0 normal and double knockout mice. The cultures were placed in differentiation medium, treated with 50ng/ml BMP for 24 hours and then labeled with the A2B5 antibody and antibody to phosphorylated Smad1. Two coverslips were examined from each of three separate preparations. Normal OPCs have only a low background level of phosphoSmad labeling (panel A). Upon BMP treatment, OPCs demonstrate nuclear labeling of phospho-Smad (Panel C). Cultures of OPCs from double mutant animals do not show phospho-Smad labeling with or without BMP treatment (Panels B and D). In addition, some cultures were treated with 50ng/ml BMP for 2 days and then processed for immunofluorescence using an antibody to detect GFAP and the 01 antibody to detect GalC. Two coverslips were examined from each of three separate preparations. In the cultures from normal animals without BMP, only a few contaminating cells are GFAP+ (panel E) whereas many cells express GalC and demonstrate the morphology of mature oligodendrocytes (panel I). When cells from the normal cultures are treated with BMP, many cells express GFAP (panel G) and GalC expression is inhibited (panel K). In the cultures from the Bmpr double knockout animals, BMP treatment has no effect; the cultures contain few GFAP+ cells in general (panel F) and BMP treatment does not increase this number (panel H). GalC+ process-bearing oligodendrocytes are present in control as well as BMP treated cultures (panels J and L). The scale bar in K equals 30 μm.
To further confirm that BMP signaling was completely disrupted in the oligodendrocyte lineage in double mutant animals, we investigated whether BMP4 was able to inhibit oligodendrocyte differentiation or induce type II astrogliogenesis of OPCs from double mutant animals, both well-known effects of BMPs on cultured OPCs (Grinspan et al., 2000; Mabie et al., 1997). Cultures from normal and Bmpr double knockout animals were placed in differentiation medium and treated with 50ng/ml BMP or left untreated for two days. Cells were labeled for galactocerebroside (GalC), a marker of oligodendrocyte differentiation and for glial fibrilary acidic protein (GFAP), an astrocyte structural filament (Figure 1 E-L). BMP-treated cultures from normal animals did not express Gal C but showed a large increase in the number of GFAP+ cells (203% ± 57%), as previously shown for BMP treatment of rat OPC cultures (Grinspan et al., 2000a). BMP-treated cultures from Bmpr double knockout animals treated with the same regimen demonstrated no increase in the number of GFAP+ cells and 50% of the cells expressed GalC after 2 days in differentiation medium. Thus OPCs from Bmpr double knockout mice do not respond to BMP.
These data demonstrate that disruption of BMP signaling in the neural tube via Cre-mediated recombination abolishes both the immediate downstream signaling through BMP receptors and the well-studied effects of BMP treatment on the oligodendrocyte lineage in vitro.
Disruption of BMP signaling decreases astrogliogenesis
Addition of BMPs, both in vitro and in vivo, has been shown to increase numbers of astrocytes, while decreasing oligodendrogliogenesis. These results suggest that BMP signaling promotes astrogliogenesis, but do not rigorously demonstrate that BMP signaling is required for astrogliogenesis. To address this issue, we investigated the extent of astrocyte development in normal and Bmpr double knockout mice. Because the double knockout mice die at P0, we have chosen to study the area of the CNS that would exhibit the greatest amount of myelin protein expression at this time point, the cervical spinal cord. Immunolabeling of P0 cervical spinal cord with anti-GFAP revealed a decrease in mature astrocytes in double mutant animals (Fig 2). Cervical spinal cord sections were found to contain 50% fewer GFAP+ cells than those from normal animals (p<0.003). In addition, immunoblotting revealed a similar decrease in GFAP expression in double mutant spinal cord when compared to GFAP expression in normal animals. These data indicate that BMP signaling promotes the development of at least some populations of astrocytes, but it is unclear how or at what stage in astrocyte development BMP signaling operates.
Figure 2.
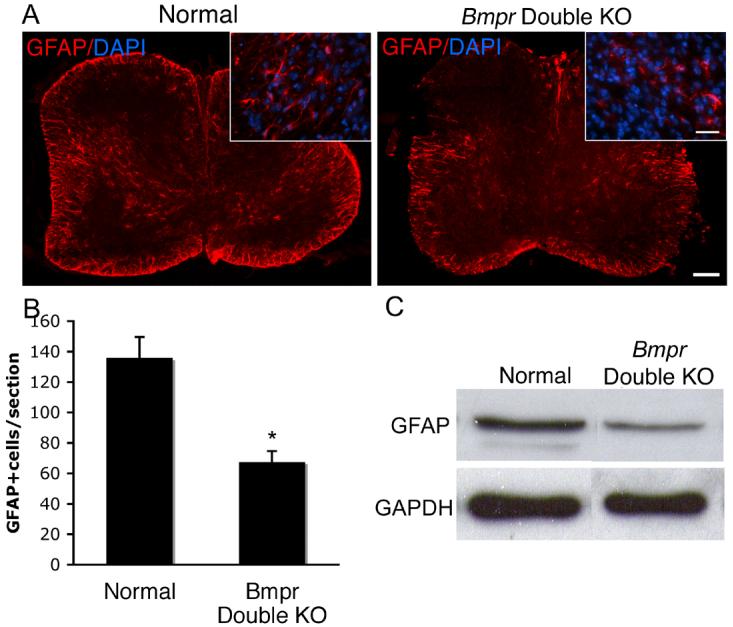
Spinal cord sections from Bmpr double knockout animals have half as many astrocytes as spinal cords from normal animals at P0.
Spinal cords were removed from P0 normal and Bmpr double knockout mice and were prepared for cyrosectioning and labeling. A) GFAP expression is markedly decreased in the double knockouts compared to the normals. Shown are sections of whole spinal cord photographed at 10X and higher power insets at 40X. The scale bar for the 10X sections equal 100μm. The scale bar for the 40X inserts equals 30μm. B) Cell counts were performed at 20 X magnification on 3 cervical spinal cord sections from each of four double knockout animals from four separate litters. Cells were deemed positive if they had a DAPI+ nucleus surrounded by a rim of GFAP+ cytoplasm (see inset in A). The Bmpr double knockout animals had 37% fewer GFAP+ cells than the controls (p< 0.02). C) Western blotting was performed on whole spinal cord lysates from three double knockout and normal animals from at least three litters and demonstrate an equivalent reduction of GFAP protein.
Because Smads have been shown to bind directly to the GFAP promoter (Nakashima et al., 1999), we wanted to determined that the double mutant animals were actually lacking in astrocytes and not just in expression of GFAP. We labeled sections with an antibody to S100β, a slightly earlier marker of astrogliogenesis (Ghandour et al., 1981) and saw a trend towards a decease in the Bmpr double knockout spinal cord sections (Figure 3). Cell counts of S100β+ cells in Bmpr double knockout spinal cords from 5 litters were decreased 25% as compared to wildtype but the variability was extremely high and the statistical significance was not achieved. Recently, S100β expression has been observed in oligodendrocyte lineage cells as they transition from OPCs to mature oligodendrocytes (Deloulme et al., 2004; Hachem et al., 2005) and could potentially account for some of the S100β labeling we see, increasing the variability. However, the S100β staining in our sections is almost exclusively in the grey matter and lateral white matter tracks whereas the maturing oligodendrocytes appear in the ventral and dorsal- most white matter.
Figure 3.
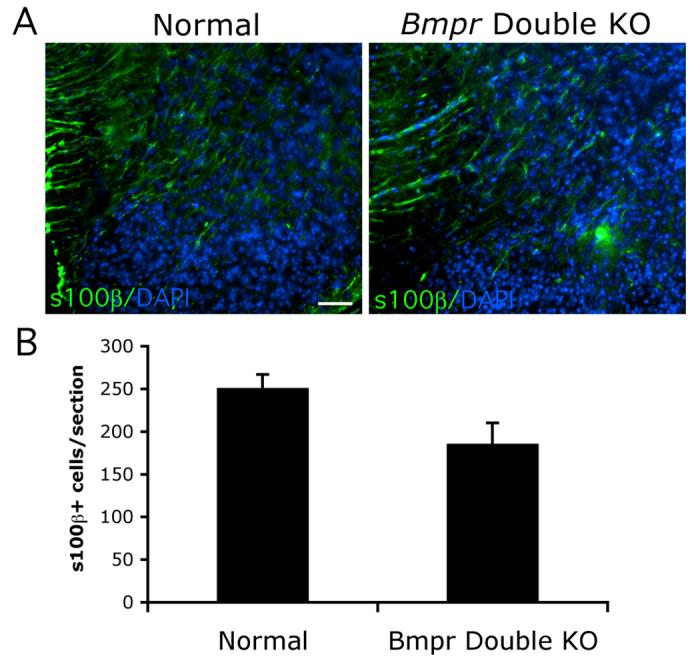
Numbers of astrocytes expressing S100β in the Bmpr double knockout mice is reduced as compared to normal mice but does not achieve statistical significance.
Spinal cords were removed from normal and Bmpr double knockout animals at P0 and processed for cyrosectioning and immunocytochemistry using antibody to S100β. A) Section of dorsolateral spinal cord shows that the number of S100β+ cells is somewhat decreased, especially in the grey matter. The scale bar in A equals 50μm. B) Cell counts were performed at 20 X magnification on 3 cervical spinal cord sections from each of five double knockout animals from five separate litters. Cells were deemed positive if they had a DAPI+ nucleus surrounded by a rim of S100β+ cytoplasm. A decrease of 25% is noted which is not statistically significant (P = 0.052).
The decrease in number of astrocytes could be due to decreased proliferation or increased cell death. To determine the extent of astrocyte proliferation in the absence of BMP signaling, we double labeled with antibodies to phospho-histone 3. a marker of mitosis, beginning in the G2 phase (Hendzel et al., 1997), or Ki67, a marker of the S, G2 and M phases of the cell cycle (Gerdes et al, 1984) and GFAP. There was no difference in the number of double labeled cells in both the normal and double knockouts, suggesting that altered proliferation at P0 is not responsible for the lack of astrocytes in double knockout animals (data not shown).
To determine whether cell death is responsible for the decrease in astrocytes in the absence of BMP signaling, we performed the TUNEL assay in addition to GFAP labeling on normal and Bmpr spinal cord sections. Although the number of TUNEL+ cells per section was small, TUNEL labeling was increased at least three fold in the Bmpr double knockouts, however only rare GFAP+ cells in either the double knockouts or the normals were also TUNEL+ (Figure 4). We then labeled sections from the same animals with antibody to caspase 3 and GFAP, because dying GFAP+ cells may be more apparent using this slightly earlier cell death marker. There was no difference between the number of caspase 3+/GFAP+ cells in normal when compared to double mutant animals (Figure 4). These data suggest that astrocyte survival is unlikely to require BMP signaling, but that the decrease in astrocytes may be the result of diversion from the astrocyte lineage.
Figure 4.
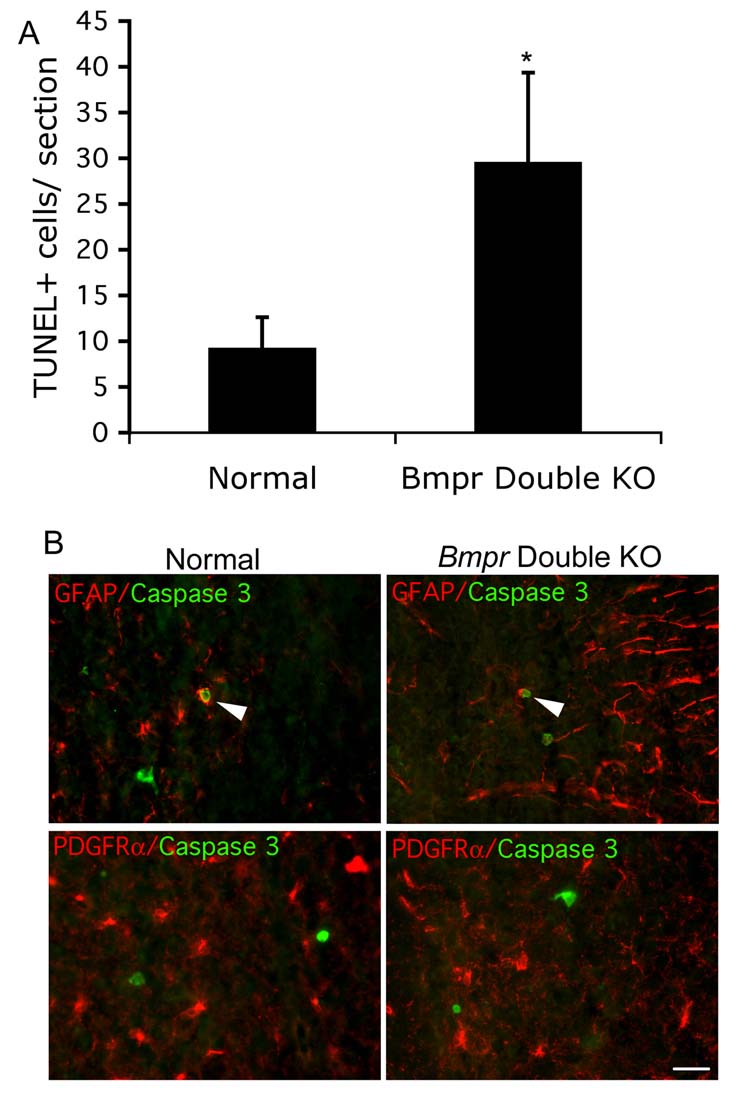
Although overall cell death is increased in the Bmpr mutants, few astrocytes and oligodenodrocytes are labeled with cell death markers.
A) The TUNEL assay was performed on P0 spinal cord sections from pairs of normal and Bmpr double knockout mice from three separate litters and the number of TUNEL+ cells per sections was determined. There was a three fold increase. B) Rare GFAP+ cell co-label with caspase 3 (arrow). Although caspase 3+ cells are present, PDGFRα + cell do not co-label with them. The scale bar equals 30μm.
Disruption of BMP signaling in neural tube decreases oligodendrogenesis
To determine the extent of oligodendrocyte development in normal and double mutant animals, we immunolabeled the myelin proteins, MBP and PLP, in cervical spinal cord sections of normal and double mutant animals at P0. In normal animals, the expression of MBP and PLP at this early time point is primarily concentrated in peripheral ventral spinal cord and in a small area of medial dorsal spinal cord at P0 (Figure 5). In Bmpr double mutant animals, however, MBP and PLP expression was significantly reduced. To count PLP+ cells, we visualized the sections by fluorescence microscopy and counted only those cells in which a DAPI+ nucleus was visible surrounded by labeled cytoplasm and processes. The number of PLP+ cells was decreased an average of 56% (p< 0.008) in the Bmpr double knockout animals compared to the normal animals (Figure 5). Confirmation of this result via Western blot showed a 74±11.7% decrease in MBP expression using spinal cords from Bmpr double knockouts from three separate litters. These surprising results demonstrate a significant decrease in the number of fully mature oligodendrocytes in the absence of BMP signaling, suggesting that BMP signaling contributes to oligodendrocyte development. Since a reduced number of mature oligodendrocytes are present at P0 in the double knockouts, BMP appears not be absolutely required for their formation.
Figure 5.
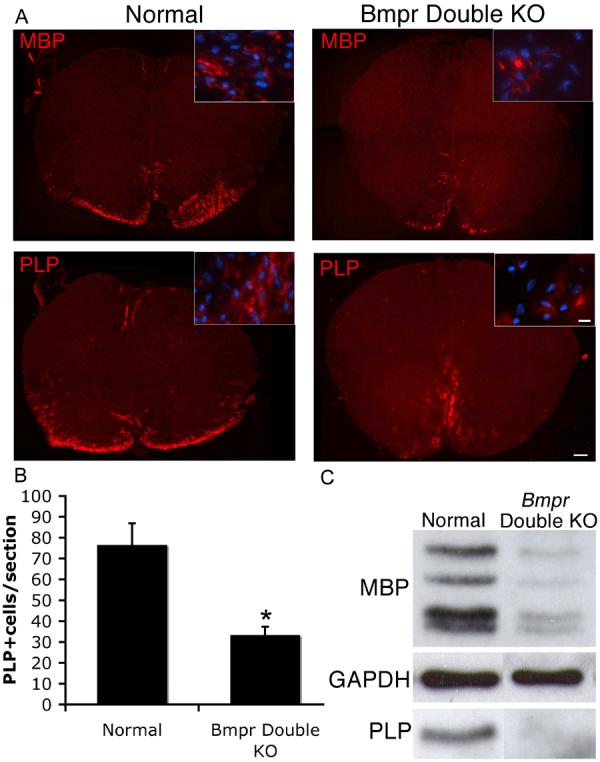
Spinal cords from Bmpr double knockout animals have reduced numbers of cells expressing myelin proteins PLP and MBP.
A) Spinal cords were removed from normal and Bmpr double knockout mice at P0 and processed for cryosectioning and immunolabeling with antibodies to MBP and PLP. Shown are sections of whole spinal cord photographed at 10X and high magnification insets photographed at 40X. The Bmpr double knockout demonstrates no dorsal staining and severely reduced staining in the ventral areas of the cord. The scale bar for the 10X sections equals 100μm. The scale bar for the 40X insets equals 10μm. B) Cell counts were performed at 20X magnification on three sections per animal from four animals taken from four different litters. Cells were deemed positive if a rim of PLP+ cytoplasm could be seen around a DAPI+ nucleus. The number of cells per section is reduced more than 50% in the Bmpr double knockouts. C) Western blots performed on whole spinal cord sections from three double knockout and normal animals from at least three litters showed a similar reduction in PLP and MBP protein in the Bmpr double knockout animals.
The first step in oligodendrocyte differentiation is classically defined by the expression of the surface antigen GalC, whereas myelin protein expression occurs as much as several days after GalC expression (Grinspan et al., 1993). To determine at which stage BMP signaling was acting, we immunolabeled vibratome sections of spinal cords from pairs of Bmpr double knockout and normal mice for GalC. We found a significant decrease of 37% (p<0.05) in GalC labeling in the double knockouts as compared to the normals (Figure 6). This is somewhat less than the decrease in myelin proteins, suggesting that some proportion of cells in the Bmpr double knockout spinal cords are able to express Gal C but not progress to myelin protein expression.
Figure 6.
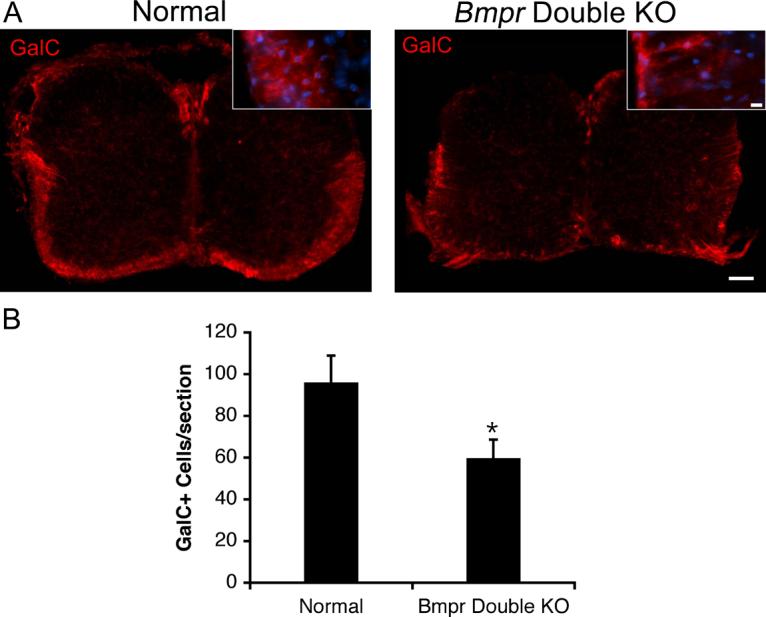
The number of cells expressing GalC is decreased in the Bmpr double knock out animals.
Spinal cords were removed from normal and Bmpr double knockout mice and processed for vibratome sectioning and immunolabeling with antibody to GalC. A) Shown are sections of whole spinal cord photographed at 10X and high magnification insets photographed at 40X. The scale bar for the 10X sections equals 100μm. The scale bar for the 40X insets equals 10μm. B) Cell counts performed at 20X magnification on three sections per animal from three animals taken from three separate litters. There is a 37% decrease in expression of GalC in the double knockout animals.
These results demonstrate that the presence of functional BMP receptors and BMP signaling contributes to the development of mature oligodendrocytes.
Disruption of BMP signaling does not affect oligodendrocyte precursors
The decrease in mature oligodendrocytes in double mutant animals may reflect a role for BMP signaling in formation of oligodendrocyte precursors. To investigate this possibility, we labeled P0 spinal cord sections from normal and double mutant animals with the O4 antibody which recognizes the POA antigen, and is expressed in the late precursor period through maturation (Bansal et al, 1992). Cells were counted as positive when they contained a DAPI+ nucleus surrounded by a rim of O4+ cytoplasm. The numbers of O4+ cells in the cervical spinal cords of the normal and double knockout animals did not differ significantly (Figure 7). To mark the entire precursor stage, we used antibody against PDGFRα, (Grinspan and Franceschini, 1995; Hall et al., 1996; Pringle and Richardson, 1993). Counts of PDGFRα+ cells in cervical spinal cord revealed no difference in the number of PDGFRα+ cells between normal and double mutant animals (Figure 8). Similar studies using antibody to NG2, another marker of oligodendrocyte precursors in neonatal animals, also showed no difference in the number of precursors between double knockouts and normals (data not shown).
Figure 7.
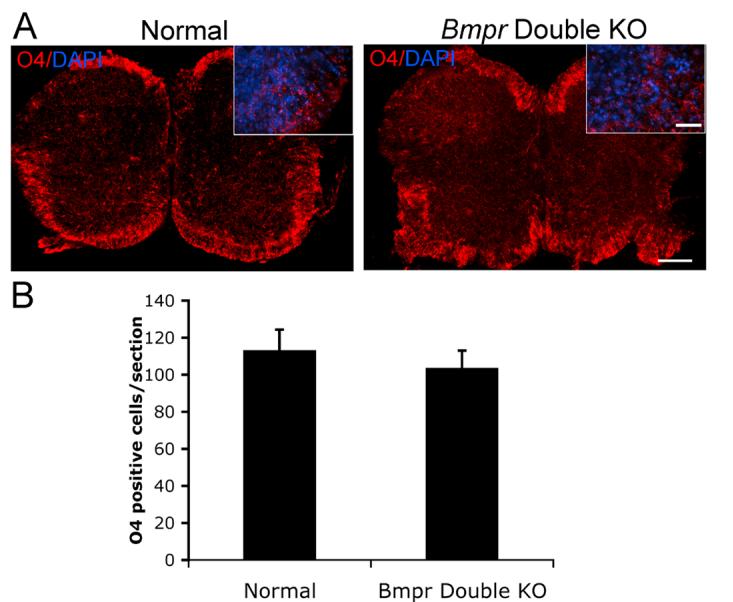
The number of cells expressing the late progenitor marker O4 is the same in control and Bmpr double knockout animals.
Spinal cords were removed from normal and Bmpr double knockout mice and processed for vibratome sectioning and immunolabeling with O4 antibody. A) Shown are sections of whole spinal cord photographed at 10X and high magnification insets photographed at 40X. The scale bar for the 10X sections equals 100μm. The scale bar for the 40X insets equals 30μm. B) Cell counts performed at 20X magnification on three sections per animal from three animals taken from three separate litters. There is no statistically significant difference between the number of cells in the mutant and normals.
Figure 8.
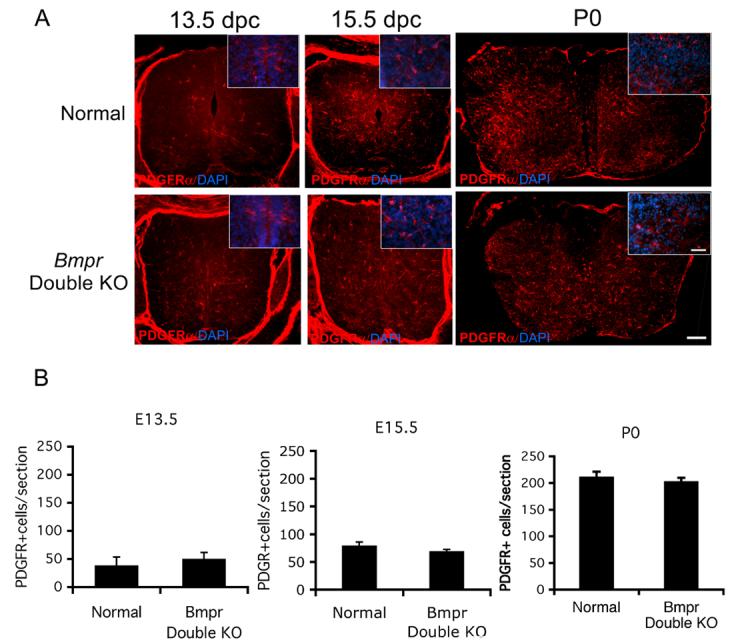
The number and distribution of oligodendrocyte precursors is the same in the normal mice as in the Bmpr double knockouts.
Spinal cords were removed from normal and Bmpr double knockout mice at E13.5, E15.5 and P0 and were prepared for cyrosectioning and labeling with antibody to PDGFRa. A) Shown are sections of whole spinal cord at 5X (scale bar = 100μm) and higher magnification inserts at 40X (scale bar= 50μm). Cells exhibiting cytoplasmic labeling with PDGFRα are visible throughout the cord in both normal and mutant animals. No differences in the number or distribution of precursors is noted at any of the time points (for E13.5, P<0.374, for E15.5, P<0.406, for P0, P<0.185. B) Cell counts were performed at 20 X magnification on three cervical spinal cord sections from three Bmpr knockout and normal mice from three litters at E13.5, E15.5 and P0. Cells were counted as positive if they had a DAPI+ nucleus and a rim of PDGFαR+ cytoplasm. There was no significant difference between the number of cells in the normals and mutants.
The paucity of mature oligodendrocytes in the double knockout animals may represent a delay in oligodendrogenesis rather than a block of oligodendrocyte development. To investigate this possibility, we immunolabeled cryosections of 12.5 dpc, 13.5dpc and 15.5dpc Bmpr double knockout and normal embryos for PDGFRα, which labels OPCS. At 12.5, PDGFRα cells could not be identified in either normal or Bmpr double knockout animals (data not shown). At 13.5dpc, we observed PDGFRα labeling near the ventral midline of the subventricular zone in both normal and double knockout embryos, suggesting that OPCs are generated at the appropriate time point even in the absence of BMP signaling (Figure 8). At 15.5dpc, there was no difference between normal and Bmpr animals in the number or distribution of PDGFRα+ cells (79±3 cells per section and 75.5±3 cells per section, respectively, P<0.4, Figure 8). These results demonstrate that BMP signaling does not alter the timing of OPC emergence or the numbers of OPCs generated. BMP signaling during oligodendrocyte development may only play a role in differentiation and myelin formation.
BMP signaling and oligodendrocyte proliferation and cell death
A decrease in myelin protein expression without a change in the number of OPCs suggests several possibilities. There could be a change in the rate of OPC proliferation. To determine the extent of oligodendrocyte precursor proliferation in normal and double mutant animals at P0, we double-labeled cryosections with phospho-histone 3 antibody and with an antibody to PDGFRα. The number of phospho-histone 3+/ PDGFRα+ cells was the same in normal and double mutant animals (5.8 ± 1.2 cells/section versus 4.6 ± 1.6 cells per section, p=0.573 for sections from 4 normal and 4 double knockout animals from 4 litters). Labeling for the Ki-67 protein gave us the same results (data not shown). Labeling of embryonic sections at E13.5 and E15.5 with both phosphohistone 3 and Ki67 also did not show significant differences between normal and double mutant animals (supplemental figure 1). This indicates that decreased myelination in double mutant animals is not the result of altered precursor proliferation.
An increase in cell death before or during differentiation could also account for the lack of mature oligodendrocytes despite a full complement of OPCs in the double knockout animals. Maturing oligodendrocytes are highly susceptible to programmed cell death in the absence of survival cues. To determine whether cell death occurs in the absence of BMP signaling in the oligodendrocyte lineage, we measured apoptosis by the TUNEL assay and labeling for PDGFRα, PLP, and Olig2 in spinal cords from P0 Bmpr and normal mice. We also labeled with antibody against activated caspase 3 and PDGFRα or O4. Virtually none of the oligodendrocyte lineage cells identified with any of the markers showed TUNEL or caspase labeling in normal or double knockout animals (Figure 4b and supplemental figure 2). This suggests that the lack of oligodendrocytes expressing myelin proteins cannot be accounted for by apoptosis using these methods. Because we had identified an overall increase in TUNEL labeling, we also double labeled spinal cord sections from the double knockouts and normals with caspase 3 and NeuN to rule out neuronal cell death (data not shown), only occasional NeuN+ cells also labeled with caspase and there was no difference between the normals and mutants.
Oligodendrocytes from double knockout animals are able to express myelin proteins in culture
The significant decrease in the number of cells expressing myelin proteins in the double knockout animals could reflect either a block to differentiation or a developmental delay. However, the double knockout animals die shortly after birth so we can only assess the early stages of myelination in vivo in these animals. To compensate for this, we cultured oligodendrocytes from normal and Bmpr double knockout animals and determined whether myelin proteins were produced. After 4 days of incubation in differentiation medium, cells cultured from either the normal or knockout animals expressed both PLP and MBP with extensive labeling of cell processes (Figure 9). Cell counts showed that the percentage of GalC+ and PLP+ cells did not differ statistically in cultures from the normal and Bmpr double knockout animals (Figure 9). Thus expression of myelin proteins can be rescued by in vitro conditions.
Figure 9.
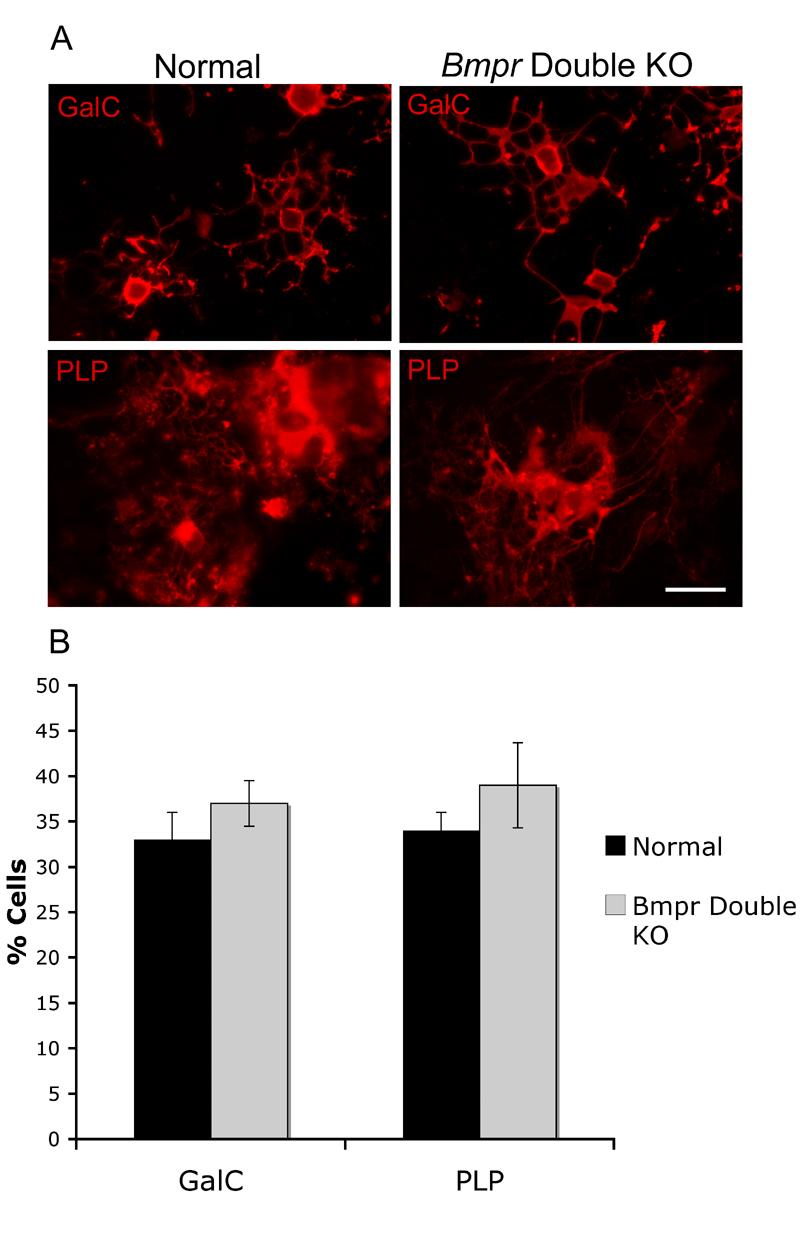
Oligodendrocyte precursors from Bmpr double knockout mice can differentiate in vitro.
Forebrain cells from normal and Bmpr double knockout animals were cultured in vitro and allowed to differentiate. A) After 4 days, both types of cultures contained mature cells with extensive branched processes labeling with antibody to GalC and PLP. B) Cell counts were performed by counting the number of GalC or PLP+ cells as a function of the number of DAPI+ nuclei on two coverslips each on cultures taken from three or more separate litters. There was no significant difference in the numbers of mature cells between the normal and the mutants. The scale bar in A equals 30μm.
Discussion
We investigated the role of BMPs during gliogenesis in the developing spinal cord. Using mutant mice in which both Bmpr 1a and Bmpr 1b have been inactivated in the neural tube by 10dpc, we show a significant decrease in the number of mature astrocytes. Though the number of OPCs is normal, a reduction in oligodendrocyte differentiation and myelin protein expression was observed at P0. Thus, disruption of BMP signaling in the spinal cord does not appear to affect OPC generation but does result in decreased or delayed oligodendrocyte and astrocyte development, suggesting that BMP signaling contributes to proper gliogenesis.
The decreased expression of the astrocyte marker, GFAP, demonstrates that disruption of BMP signaling results in decreased astrogliogenesis in mouse spinal cord. A slightly earlier astrocytes marker, S100β, is also decreased 25% but this was not statistically significant. The decrease in astrocytes is consistent with previous data showing the converse result that overexpression of BMPs both in vitro and in vivo increases astrogliogenesis (Gomes et al., 2003; Gross et al., 1996; Mekki-Dauriac et al., 2002). This study provides the first direct evidence that BMP signaling is involved in the generation of appropriate numbers of astrocytes during the development of the spinal cord.
What could account for the decrease in astrocytes upon disruption of BMP signaling? One possibility is the prevention of astrogliogenesis by apoptosis of precursors. Although we did see an overall increase in apoptosis by TUNEL and caspase 3 labeling, virtually none of the dying cells co-labeled with GFAP, indicating that they were either not astrocytes or were astrocyte precursors that had not yet achieved GFAP expression.
It is also possible that an astrocyte precursor population is present in these animals, but unable to differentiate to the point of expressing more mature markers. S100β expression occurs just prior to GFAP expression during astrocyte maturation (Ghandour et al., 1981). The Bmpr double knockout animals showed a small but non-statistically significant decrease in S100β compared to the normal animals, suggesting that BMP signaling may be required at a stage in astrocyte development during or just following S100β expression but prior to the expression of GFAP. Therefore, we suggest that BMPs may contribute to the maturation of astrocytes, but not their specification. Loss of BMP signaling may, in addition, specifically decrease GFAP expression because SMADs have been shown to regulate the GFAP promoter (Nakashima et a, 1999).
Because BMP expression in vitro can divert OPCs to the astrocyte lineage, there may be a population of oligodendrocyte precursors in the double knockouts that would normally become astrocytes by BMP signaling. Disruption of BMP signaling within the glial-restricted precursor population could allow the oligodendrocyte pathway to become predominant and generate an increase in the number of OPCs and/or mature oligodendrocytes. However, our data indicates that the numbers of OPCs in the double knockout animals do not change and that mature oligodendrocytes fail to form, suggesting that diversion of OPCs is not likely.
Our observation that BMP signaling contributes to myelination contrasts other studies in which overexpression of BMPs inhibited the progression of the oligodendrocyte lineage and inhibition of BMPs, via specific inhibitors, resulted in increased oliogdendrogliogenesis. Overexpression studies of BMPs include the addition of BMPs to in vitro culture systems which inhibits differentiation of OPCs and myelin protein expression but promotes astrocyte development (Grinspan et al., 2000; Gross et al., 1996; Mabie et al., 1997; See et al., 2004). In vivo experiments in which BMPs are overexpressed from neuron-specific promoters, or are over-expressed locally via bead implantation in Xenopus or implantation of BMP-overexpressing cells in chick indicate that BMPs promote astrogliogenesis while inhibiting oligodendrocyte development (Gomes et al., 2003; Mekki-Dauriac et al., 2002; Miller et al., 2004). However, each of these observations reflects the effects of BMP overexpression and does not investigate the role of endogenous BMP signaling in glial development. Gain of function experiments in the nervous system do not necessarily predict the actual role of growth factors shown in loss of function experiments. Examples of this include the roles of both TGF-beta and neuregulin in myelination in the peripheral nerve (D'Antonio et al., 2006; Taveggia et al., 2005)
Other studies have attempted to suppress BMP signaling in vivo using BMP inhibitors and have demonstrated an increase in oligodendrocytes or ectopic formation of oligodendrocytes. Miller et al (2004) placed beads containing function-blocking antibodies to BMP4 adjacent to dorsal spinal cord in Xenopus embryos and observed a small number of ectopic oligodendrocytes. Mekki-Dauriac et al (2002) found ectopic oligodendrocytes in embryonic chick spinal cord after transplanting cells secreting the BMP-specific inhibitor noggin or ablating the BMP-rich dorsal areas. A significant difference in our system is that we genetically eliminated BMP signaling altogether rather than reduced signaling via inhibitors. Any remaining low level of BMP signaling in the models using inhibitors may promote oligodendrocyte development instead of repressing it. In addition, oligodendrocytes express BMPs themselves (Kondo and Raff, 2004) and Grinspan and Zhang, unpublished). It may be that this autocrine or juxtaparacrine expression promotes proper myelination. Since oligodendrocytes from double knockout animals lack BMP receptors, they cannot respond to their own BMPs.
Our results, combined with previous data, suggest that overexpression of BMPs is inhibitory to oligodendrocyte development yet BMP signaling is required for timely maturation of oligodendrocytes. There are several possible ways to explain this paradox in the Bmpr double knockout. The effects may be dose dependent. Large amounts of BMPs may be inhibitory for oligodendrocyte maturation, as seen in vitro, whereas smaller amounts promote appropriate myelination. This is potentially controlled by the amount of BMPs secreted in the local area, which may decrease as oligodendrocytes begin to mature. BMP4 expression in Xenopus begins to decrease around the time of birth when the first myelin-protein-expressing oligodendrocytes would appear (Miller et al, 2004). Thus a lowering, but not total absence, of BMP would facilitate oligodendrocyte differentiation. BMP signaling in oligodendrocytes could also demonstrate stage specificity such that BMP signaling is not necessary for precursor development but supports oligodendrocyte differentiation.
A third possible explanation for the lack of oligodendrocyte differentiation and myelination upon inhibition of BMP signaling is that it is an indirect effect due to the loss of astrocytes. Astrocytes may provide signals that activate the differentiation and myelination program in oligodendrocytes (Ishibashi et al., 2006). This activation may require BMP signaling to the astrocytes or just a critical number of astrocytes, but the myelination effect on the oligodendrocytes would be indirect. However, it is not known if the decrease in astrocytes is severe enough to produce this effect. These mutant mice also lack DI1 and some DI2 interneurons and have a dorsal expansion of the DI3 and DI4 population (Wine-Lee et al, 2004). Formally, the altered fate of a specific population of neurons could change the timing of myelination and delay or decrease oligodendrocyte maturation. However, the number of neurons in the DI1 and DI2 population are very small relative to the total and the dorsal spinal cord neuronal subtypes develop within the same time period (Muller et al., 2002), suggesting that the effect would not account for the significant decrease we find. The generation of oligodendrocyte-specific BMP receptor knockouts will begin to address these questions.
Although myelin protein expression is reduced in the double knockout animals, the numbers of oligodendrocyte precursors were unchanged and had the same anatomical distribution. This was also an unexpected observation since administration of BMPs in vitro diverts neuronal stem cells and OPCs to the astrocyte lineage (Gross et al., 1996). However, our data suggests that OPCs are unaffected by BMP signaling even though the BMP receptors are deleted from subventricular zone cells before OPCs first appear. Since even the numbers of O4+ cells, appearing only in the late precursors period, are the same in the normal and mutant spinal cords, the main effect of BMPs in P0 spinal cord oligodenodrogliogenesis appears to be on maturation.
In addition to finding no alterations in numbers or distribution of OPCs after genetically eliminating BMP signaling, we found no changes in OPC proliferation or cell death even with a significant decrease in mature oligodendrocytes. There was also no increase in cell death in immature or mature oligodendrocytes. Three lines of evidence suggest that cell death does not play a role in the lack of mature oligodendrocytes in this mutant either during the precursors stage or at the point of differentiation. One, we could not detect double labeling using the TUNEL assay and antibodies to PDGFRα, PLP or Olig2, which identifies cells throughout the entire oligodendrocyte lineage. Nor could we detect double labeling with antibody to activated caspase 3 and PDGFRα or O4. Two, we do not see a loss of O4+ cells in the Bmpr double knockouts compared to controls. O4 antibody identifies the POA antigen which appears in the late progenitor stage and remains positive throughout maturation (Bansal et al, 1992). If cell death were a mechanism for the lack of mature oligodendrocytes, we would see a decrease in the number of O4+ cells in the mutants as compared to the controls. If the oligodendrocyte precursors were unable to mature or were delayed in maturation, the O4 number would stay the same. Finally, these data mirror those found in mice with genetically eliminated expression of Olig1, Nkx2.2, Sox 10, transcription factors required for oligodendrocyte development (Lu et al., 2002; Qi et al., 2001; Stolt et al., 2002). These knockout models showed normal numbers of OPCs and delayed or decreased oligodendrocyte differentiation without adoption of another cell fate. Qi et al (2001), speculate that the precursors simply do not differentiate and are lost in the neonatal period. In our model, this would account for normal numbers of PDGFRα+ and O4+ cells at birth.
Using our mutant pedigree that only survives until P0, we are unable to determine whether myelin protein expression is blocked or merely delayed. To address this, we cultured P0 oligodendrocyte precursors and demonstrated that they express myelin proteins upon differentiation. We detected PLP and MBP expression by both immunohistochemistry and by Western blotting in cultured cells. Although this suggests that myelin protein expression is only delayed, it is possible that factors present in our culture system can override the block on myelin protein formation or that the in vitro milieu lacks inhibitory in vivo signals. In a recent study of Olig1-null mice, expression of myelin-forming genes was abolished in vivo, whereas expression of myelin proteins and elaboration of membrane sheets was present in vitro (Xin et al., 2005) indicating that expression of myelin proteins in vitro may not be the same as in vivo.
In summary, our results show that BMP signaling contributes to the timely development of glial cells in the spinal cord. BMP signaling is promotes a full complement of astrocytes, as previously suggested by in vitro data. That BMP signaling supports only the maturation but not the generation of normal numbers of OPCs is novel and suggests that levels of BMP are tightly regulated during development to ensure proper levels of myelination.
Experimental Methods
Generation of Bmpr double knockout mutants
The Bmpr1a conditional knockout was generated suing the AB-1 ES cell line and was described previously (Ahn et al., 2001; Mishina et al., 2002) . Tissue specific recombination of Bmpr1a in the neural tube is driven by the Brn4/Pou3f4 promoter region of the Bcre 32 pedigree on a CD-1 strain backgound (Heydemann et al., 2001). The Bmpr1b classical knockout pedigree was a generous gift from Dr. Karen Lyons which was generated using the CCE cell line (Yi et al., 2000). The mating scheme used to generate double knockout mutants was described in detail previously (see Figure 1 of Wine-Lee et al, 2004). Briefly, female animals contained the Bmpr1afn floxed allele and the Bmpr1bKO classical knockout allele, but no Cre transgene (Bmpr1afn/Bmpr1afn;Bmpr1bKO /+). These were crossed with male mice containing the Bcre-32 transgene, the Bmpr1aKO classical knockout allele, and Bmpr1bKO (Bmpr1aKO/+;Bmpr1bKO/+; Bcre-32). The Bcre-32 transgenic allele was homozygosed when available, but hemizygous animals were also used in the study. Double knockout mice die within hours of birth and demonstrate severe limb defects. Normal animals have at least one functional allele of Bmpr1a and Bmpr1b and do not show any phenotype. Because the pedigrees have been maintained by intercross breeding, the background strain is a mixture of strains, including C57BL/6J, CD-1 and the 129/Sv strains used to generate the AB-1 and CCE ES cell lines.
Immunohistochemistry
For frozen sections, P0 animals were perfused with 0.9% saline and 4% paraformaldehyde, then spinal cords were removed, further fixed in 4% paraformadehyde for 1 hour, then placed in 30% sucrose overnight and embedded in OCT (Sakura Finetek). The entire spinal cord was then cryosectioned at 12 μm.
To label oligodendrocytes, sections were washed in phospho-buffered saline (PBS) incubated in block containing 20% fetal calf serum, 2% bovine serum albumin, 0.1% Triton in PBS for 30 minutes, washed in PBS, incubated overnight at 4°C in primary antibody, washed in PBS, incubated for 30 minutes with the appropriate secondary (all secondary antibodies were purchased from Jackson Immunoresearch Laboratories), washed in PBS followed by 5 minutes with DAPI and mounted. The antibody pairs used to label mature oligodendrocytes are anti-PLP (1:2, AA3 hybridoma supernatant, gift of Alex Gow, Wayne State University) with goat anti-rat IgG and anti-MBP (hybridoma supernatant, 1:2, gift of Virginia Lee, University of Pennsylvania) with goat anti-rat IgG (1:100).
To label oligodendrocyte precursors, sections processed as described above were incubated in NG2 antibody (1:100, Chemicon) with goat anti-rabit IgG or anti PDGFRα antibody (1:250, BD Biosciences Pharmigen) with goat anti-rat IgG, treated with DAPI 5 minutes and mounted. Antibody to Olig 2 (1:200, Chemicon) with goat anti-rabbit IgG was used to label both OPCs and mature oligodendrocytes.
To label astrocytes, sections were processed as described above and incubated with anti-glial fibrillary acidic protein (GFAP) antibody (1:2, hybridoma supernatant, gift of Dr. Virginia Lee, University of Pennsylvania) with anti-rat secondary (1:100) or with anti- S100β (1:100, Sigma) with anti-rabbit secondary.
To determine extent of cell proliferation, sections were immunolabeled separately with antibody to the mitosis marker phosphorylated histone H3 and with antibody to the proliferation protein Ki-67 (Hendzel et al., 1997; Nowak and Corces, 2000; Scholzen and Gerdes, 2000). Sections were processed as described above and incubated in anti-phospho-histone 3 antibody (1:250 in block, Upstate Biotechnology) or Ki-67 antibody (1:100. Vector Laboratories) and anti-rabbit secondary antibody (1:100). To determine extent of OPC and astrocyte proliferation specifically, sections were double-labeled for phospho-histone 3 and PDGFRα or GFAP, respectively.
Cell death was measured using the TUNEL analysis as previously described (Grinspan et al., 1998). To determine extent of cell death among oligodendrocyte and astrocyte populations specifically, sections were double-labeled with anti-cleaved caspase 3 (1:200, Cell Signaling) and anti-rabbit secondary antibody (1:100) and anti-PDGFRα or anti-GFAP, respectively.
To enumerate the astrocytes and OPCs, we counted digital images at 20X taken from at least four fields of grey matter per section from three cervical spinal cord sections per animal on one or two animals per litter using at least three litters, comparing normal and Bmpr double mutant animals. To enumerate cells expressing PLP or GalC, we counted digital images at 40X taken from at least 6 fields of white matter per section from three cervical spinal cords sections per animal on one or two animals per litter using at least three litters.
To determine the number of differentiated oligodendrocytes expressing galactocerebroside (GalC) or O4, sections of spinal cord from normal and double mutant animals were fixed in 4% paraformaldehyde for one hour and soaked in 30% sucrose, then cut on a vibratome at 50 μM. These sections were blocked for 30 minutes in block containing 0.1% triton then immunolabeled using O1 (hybridoma supernatant 1:2, (Sommer and Schachner, 1981)) for 1 hour, washed 3x with PBS, incubated with goat anti-mouse IgM secondary antibody (1:100) for 20 minutes, washed 3x with PBS, then treated with DAPI for 10 minutes. O4 labeling was performed in a similar manner using O4 antibody at 1:20 (hybridoma supernatant, Sommer and Schachner, 1981) and a “MOM” immunodetection kit (Vector). Counts were performed as indicated above.
Western Blotting
Spinal cords from P0 animals were dissected and sonicated for 30 seconds in ice-cold lysis buffer (containing 25 mM Tris, pH 7.6, 1 mM MgCl2, 1mM EDTA, 1% TritonX-100, 1% SDS, 1 mM PMSF, 50μg/ml antipain, 2 μg/ml aprotinin, 1μg/ml leupeptin, 1μg/ml pepstatin A), then centrifuged and supernatant preserved. Protein concentration was determined using BCA method. (Pierce Chemical, Rockford, IL). Protein samples (50 μg) were denatured and run on 15% gel as previously described (See et al., 2004). Membranes were incubated in blocking buffer with anti-MBP (1:500) and anti-GFAP (1:100). Samples incubated with anti-PLP (1:100) were not denatured. Membranes were then incubated with appropriate horse radish peroxidase-conjugated secondary antibody. Immunoreactive protein was detected with ECL reagents (Amersham, Piscataway, NJ) and membranes were exposed to hyperfilm (Amersham). Blots were stripped and reprobed with antibody to GAPDH (1;2,500) as a loading control. The ratio of GFAP or MBP to GAPDH was assessed by image ready software.
Cell culture
A mixed population of cells were isolated from brains of normal and double knockout P1 mouse pups. Tails were collected for genotyping via PCR. Individual brains were cultured separately in serum-containing medium as previously described (Grinspan and Franceschini, 1995) and cultures were not combined with like phenotypes until those phenotypes were confirmed by PCR. After one day, the cells were switched to a serum-free growth medium containing 100 μg /ml transferrin, 100 μg /ml bovine serum albumin, 60 ng/ml progesterone, 16 μg/ml putrescine, 40 ng/ml sodium selenite, 5 mg/ml N-acetyl cysteine, 1mM sodium pyruvate, 10 ng/ml d-biotin, 0.5 mg/ml insulin (Sigma-Aldrich, St Louis, MO), 10 ng/ml basic fibroblast growth factor, 2 ng/ml platelet-derived growth factor (R & D, Minneapolis, MN), 1ng/ml neurotrophin-3 (Peprotech, Rocky Hill, NJ). Cultures were maintained in this medium until confluent (approximately one week).
After a week, cells were immunoselected by incubation on 100mm tissue culture plates coated with Thy1.2 antibody (Serotec) to remove contaminating neurons, astrocytes and microglia and then the remaining cells were incubated on plates coated with A2B5 antibody to collect OPCs (Grinspan et al., 2000). These purified OPCs were grown in GM in lysine-coated T75 flasks and plated on lysine-coated coverslips and 100 mm plates with growth factors (10ng/ml fibroblast growth factor (FGF) and 2ng/ml platelet-derived growth factor (PDGF)).
To differentiate the oligodendrocyte precursors, cultures from normal, and Bmpr double knockout animals were treated with differentiation medium containing 50% DMEM, 50% Ham's F12 with 50 μg/ml transferrin, 5μg/ml putresine, 3 ng/ml progesterone, 2.6 ng/ml selenium, 12.5 g/ml insulin, 0.4μg/ml T4, 0.3% glucose, 2mM glutamine, and 10 ng/ml biotin with or without 50ng/ml BMP4 (R&D systems). Antibodies used for immunolabeling cultures cells were anti-phospho-Smad (Cell Signaling), A2B5 (hybridoma supernatant, ATCC, (Eisenbarth et al., 1979), 01 and PLP. Procedures for immunolabeling oligodendrocytes in culture have been described previously (See et al., 2004).
Supplementary Material
Acknowledgments
This work was supported by RO1 NS43422 (JBG), RO1 NS39159 (EBC III), and Nat'l Multiple Sclerosis Society RG 3662 (JBG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn K, Mishina Y, Hanks MC, Behringer RR, Crenshaw EB., 3rd BMPR-IA signaling is required for the formation of the apical ectodermal ridge and dorsal-ventral patterning of the limb. Development. 2001;128:4449–61. doi: 10.1242/dev.128.22.4449. [DOI] [PubMed] [Google Scholar]
- Bansal R, Stefansson K, Pfeiffer SE. Proligodendroblast antigen (POA), a developmental antigen expressed by A007/O4-positive oligodendrocyte progenitors prior to the appearance of sulfatide and galactocerebroside. Journal of Neurochemistry. 1992;58:2221–2229. doi: 10.1111/j.1471-4159.1992.tb10967.x. [DOI] [PubMed] [Google Scholar]
- Cai J, Qi Y, Hu X, Tan M, Liu Z, Zhang J, Li Q, Sander M, Qiu M. Generation of oligodendrocyte precursor cells from mouse dorsal spinal cord independent of Nkx6 regulation and Shh signaling. Neuron. 2005;45:41–53. doi: 10.1016/j.neuron.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth factors. 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- D'Antonio M, Droggiti A, Feltri ML, Roes J, Wrabetz L, Mirsky R, Jessen KR. TGFbeta type II receptor signaling controls Schwann cell death and proliferation in developing nerves. J Neurosci. 2006;26:8417–27. doi: 10.1523/JNEUROSCI.1578-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deloulme JC, Raponi E, Gentil BJ, Bertacchi N, Marks A, Labourdette G, Baudier J. Nuclear expression of S100B in oligodendrocyte progenitor cells correlates with differentiation toward the oligodendroglial lineage and modulates oligodendrocytes maturation. Mol Cell Neurosci. 2004;27:453–65. doi: 10.1016/j.mcn.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Eisenbarth G, Walsh F, Nirenberg M. Monoclonal antibody to a plasma membrane antigen of neurons. Proc. Nat'l Acad. Sci. USA. 1979;76:4913–4917. doi: 10.1073/pnas.76.10.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda S, Taga T. Cell fate determination regulated by a transcriptional signal network in the developing mouse brain. Anat Sci Int. 2005;80:12–18. doi: 10.1111/j.1447-073x.2005.00097.x. [DOI] [PubMed] [Google Scholar]
- Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J. Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- Ghandour MS, Labourdette G, Vincendon G, Gombos G. A biochemical and immunohistological study of S100 protein in developing rat cerebellum. Dev Neurosci. 1981;4:98–109. doi: 10.1159/000112745. [DOI] [PubMed] [Google Scholar]
- Gomes WA, Mehler MF, Kessler JA. Transgenic overexpression of BMP4 increases astroglial and decreases oligodendroglial lineage commitment. Develop Biol. 2003;255:164–177. doi: 10.1016/s0012-1606(02)00037-4. [DOI] [PubMed] [Google Scholar]
- Grinspan J, Wrabetz L, Kamholz J. Oligodendrocyte maturation and myelin gene expression in PDGF-treated cultures from rat cerebral white matter. J. Neurocytol. 1993;22:322–333. doi: 10.1007/BF01195556. [DOI] [PubMed] [Google Scholar]
- Grinspan JB, Coulalglou M, Beesley JS, Carpio DF, Scherer SS. Maturation-dependent apoptotic cell death of oligodendrocytes in myelin-deficient rats. J. Neurosci. Res. 1998;54:623–634. doi: 10.1002/(SICI)1097-4547(19981201)54:5<623::AID-JNR7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Grinspan JB, Edell E, Carpio DF, Beesley JS, Lavy L, Pleasure D, Golden JA. Stage-specific effects of bone morphogenetic proteins on the oligodendrocyte lineage. J Neurobiol. 2000;43:1–17. [PubMed] [Google Scholar]
- Grinspan JB, Franceschini B. PDGF is a survival factor for PSA-NCAM+ oligodendroglial pre-progenitor cells. J. Neurosci. Res. 1995;41:540–551. doi: 10.1002/jnr.490410414. [DOI] [PubMed] [Google Scholar]
- Gross RE, Mehler MF, Mabie PC, Zang Z, Santschi L, Kessler JA. Bone Morphogenic proteins promote astroglial lineage commitment by mammalian subventricular zone progenitor cells. Neuron. 1996;17:595–606. doi: 10.1016/s0896-6273(00)80193-2. [DOI] [PubMed] [Google Scholar]
- Hachem S, Aguirre A, Vives V, Marks A, Gallo V, Legraverend C. Spatial and temporal expression of S100B in cells of oligodendrocyte lineage. Glia. 2005;51:81–97. doi: 10.1002/glia.20184. [DOI] [PubMed] [Google Scholar]
- Hall A, Giese NA, Richardson WD. Spinal cord oligodendrocytes develop from ventrally derived progenitor cells that express PDGF alpha-receptors. Development. 1996;122:4085–4094. doi: 10.1242/dev.122.12.4085. [DOI] [PubMed] [Google Scholar]
- Hendzel MJ, Wei Y, Mancini MA, Van Hooser A, Ranalli T, Brinkley BR, Bazett-Jones DP, Allis CD. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma. 1997;106:348–60. doi: 10.1007/s004120050256. [DOI] [PubMed] [Google Scholar]
- Heydemann A, Nguyen LC, Crenshaw EB., 3rd Regulatory regions from the Brn4 promoter direct LACZ expression to the developing forebrain and neural tube. Brain Res Dev Brain Res. 2001;128:83–90. doi: 10.1016/s0165-3806(01)00137-7. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Dakin KA, Stevens B, Lee PR, Kozlov SV, Stewart CL, Fields RD. Astrocytes promote myelination in response to electrical impulses. Neuron. 2006;49:823–32. doi: 10.1016/j.neuron.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat. Neurosci. 2006;9:173–179. doi: 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Raff MC. A role for Noggin in the development of oligodendrocyte precursor cells. Developmental Biology. 2004;267:242–51. doi: 10.1016/j.ydbio.2003.11.013. [DOI] [PubMed] [Google Scholar]
- LeVine SM, Goldman J. Embryonic divergence of oligodendrocyte and astrocyte lineages in the developing rat cerebrum. Journal of Neuroscience. 1988;8:3992–4006. doi: 10.1523/JNEUROSCI.08-11-03992.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu QR, Sun T, Zhu ZM, Ma N, Garcia M, Stiles CD, Rowitch DH. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109:75–86. doi: 10.1016/s0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- Lu QR, Yuk D-I, Alberta JA, Zhu Z, Pawlitzky I, Chan J, McMahon AP, Stiles CD, Rowitch DH. Sonic hedgehog-regulated oligodendrocyte lineage genes encoding bHLH proteins in the mamalian central nervous system. Neuron. 2000;25:317–329. doi: 10.1016/s0896-6273(00)80897-1. [DOI] [PubMed] [Google Scholar]
- Mabie PC, Mehler MF, Marmur R, Papavasiliou A, Song Q, Kessler JA. Bone morphogenic proteins induce astroglial differentiation of oligodendroglial-astroglial progenitor cells. Journal of Neuroscience. 1997;17:4112–4120. doi: 10.1523/JNEUROSCI.17-11-04112.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekki-Dauriac S, Agius E, Kan P, Cochard P. Bone morphogenetic proteins negatively control oligodendrocyte precursor specification in the chick spinal cord. Development. 2002;129:5117–30. doi: 10.1242/dev.129.22.5117. [DOI] [PubMed] [Google Scholar]
- Miller RH, Dinsio K, Wang R, Geertman R, Maier CE, Hall AK. Patterning of spinal cord oligodendrocyte development by dorsally derived BMP4. J Neurosci Res. 2004;76:9–19. doi: 10.1002/jnr.20047. [DOI] [PubMed] [Google Scholar]
- Mishina Y, Hanks MC, Miura S, Tallquist MD, Behringer RR. Generation of Bmpr/Alk3 conditional knockout mice. Genesis. 2002;32:69–72. doi: 10.1002/gene.10038. [DOI] [PubMed] [Google Scholar]
- Muller T, Brohmann H, Pierani A, Heppenstall PA, Lewin GR, Jessell TM, Birchmeier C. The homeodomain factor lbx1 distinguishes two major programs of neuronal differentiation in the dorsal spinal cord. Neuron. 2002;34:551–62. doi: 10.1016/s0896-6273(02)00689-x. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Yanagisawa M, Arakawa H, Kimura N, Hisatsune T, Kawabata M, Miyazono K, Taga T. Synergistic signaling in fetal brain by STAT3-Smad1 complex bridged by p300. Science. 1999;284:479–82. doi: 10.1126/science.284.5413.479. [DOI] [PubMed] [Google Scholar]
- Nowak SJ, Corces VG. Phosphorylation of histone H3 correlates with transcriptionally active loci. Genes Dev. 2000;14:3003–13. doi: 10.1101/gad.848800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K, Bansal R, Payne J, Rutishauser U, Miller RH. Early development and dispersal of oligodendrocyte precursors in the embryonic chick spinal cord. Development. 1995;121:1743–1754. doi: 10.1242/dev.121.6.1743. [DOI] [PubMed] [Google Scholar]
- Panchision DM, Pickel JM, Studer L, Lee SH, Turner PA, Hazel TG, McKay RD. Sequential actions of BMP receptors control neural precursor cell production and fate. Genes Dev. 2001;15:2094–110. doi: 10.1101/gad.894701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle NP, Richardson WD. a singularity of PDGFalpha receptor expression in the dorsoventral axis of the neural tube may define the origin of the oligodendrocyte lineage. Development. 1993;117:525–533. doi: 10.1242/dev.117.2.525. [DOI] [PubMed] [Google Scholar]
- Qi Y, Cai J, Wu Y, Wu R, Lee J, Fu H, Rao M, Sussel L, Rubenstein J, Qiu M. Control of oligodendrocyte differentiation by the Nkx2.2 homeodomain transcription factor. Development. 2001;128:2723–33. doi: 10.1242/dev.128.14.2723. [DOI] [PubMed] [Google Scholar]
- Scholzen T, Gerdes J. The Ki-67 protein: from the known and unknown. J. Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- See J, Zhang X, Eraydin N, Mun S-B, Mamontov P, Golden J, Grinspan JB. Oligodendrocyte maturation is inhibited by bone morphogenetic protein. Mol. Cell Neurosci. 2004;26:481–492. doi: 10.1016/j.mcn.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Sommer I, Schachner M. Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces. An immunocytochemical study in the central nervous system. Dev. Biol. 1981;83:311–327. doi: 10.1016/0012-1606(81)90477-2. [DOI] [PubMed] [Google Scholar]
- Stolt CC, Rehberg S, Ader M, Lommes P, Riethmacher D, Schachner M, Bartsch U, Wegner M. Terminal differentiation of myelin-forming oligodendrocytes depends on the transcription factor Sox10. Genes Dev. 2002;16:165–70. doi: 10.1101/gad.215802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taveggia C, Zanazzi G, Petrylak A, Yano H, Rosenbluth J, Einheber S, Xu X, Esper RM, Loeb JA, Shrager P, Chao MV, Falls DL, Role L, Salzer JL. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47:681–94. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmer JR, Wang C, Niswander L. BMP signaling patterns the dorsal and intermediate neural tube via regulation of homeobox and helix-loop-helix transcription factors. Development. 2002;129:2459–72. doi: 10.1242/dev.129.10.2459. [DOI] [PubMed] [Google Scholar]
- Vallstedt A, Klos JM, Ericson J. Multiple dorsoventral origins of oligodendrocyte generation in the spinal cord and hindbrain. Neuron. 2005;45:55–67. doi: 10.1016/j.neuron.2004.12.026. [DOI] [PubMed] [Google Scholar]
- von Bubnoff A, Cho KW. Intracellular BMP signaling regulation in vertebrates: pathway or network? Dev Biol. 2001;239:1–14. doi: 10.1006/dbio.2001.0388. [DOI] [PubMed] [Google Scholar]
- Wada T, Kagawa T, Ivanova A, Zalc B, Shirasaki R, Murakami F, Iemura S, Ueno N, Ikenaka K. Dorsal spinal cord inhibits oligodendrocyte development. Develop Biol. 2000;227:42–55. doi: 10.1006/dbio.2000.9869. [DOI] [PubMed] [Google Scholar]
- Wine-Lee L, Ahn KJ, Richardson RD, Mishina Y, Lyons KM, Crenshaw EB., 3rd Signaling through BMP type 1 receptors is required for development of interneuron cell types in the dorsal spinal cord. Development. 2004;131:5393–403. doi: 10.1242/dev.01379. [DOI] [PubMed] [Google Scholar]
- Xin M, Yue T, Ma Z, Wu FF, Gow A, Lu QR. Myelinogenesis and axonal recognition by oligodendrocytes in brain are uncoupled in Olig1-null mice. J Neurosci. 2005;25:1354–65. doi: 10.1523/JNEUROSCI.3034-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi SE, Daluiski A, Pederson R, Lyons KM. The type I BMP receptor BMPR1B is required for chondrogenesis in the mouse limb. Development. 2000;127:621–630. doi: 10.1242/dev.127.3.621. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Anderson DJ. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell. 2002;109:61–73. doi: 10.1016/s0092-8674(02)00677-3. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Wang S, Anderson DJ. Indentification of a novel family of oligodendrocyte lineage-specific basic helix-loop-helix transcription factors. Neuron. 2000;25:331–343. doi: 10.1016/s0896-6273(00)80898-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


