Abstract
Hes and Hey genes are the mammalian counterparts of the Hairy and Enhancer-of-split type of genes in Drosophila and they represent the primary targets of the Delta–Notch signaling pathway. Hairy-related factors control multiple steps of embryonic development and misregulation is associated with various defects. Hes and Hey genes (also called Hesr, Chf, Hrt, Herp or gridlock) encode transcriptional regulators of the basic helix-loop-helix class that mainly act as repressors. The molecular details of how Hes and Hey proteins control transcription are still poorly understood, however.
Proposed modes of action include direct binding to N- or E-box DNA sequences of target promoters as well as indirect binding through other sequence-specific transcription factors or sequestration of transcriptional activators. Repression may rely on recruitment of corepressors and induction of histone modifications, or even interference with the general transcriptional machinery. All of these models require extensive protein–protein interactions. Here we review data published on protein–protein and protein–DNA interactions of Hairy-related factors and discuss their implications for transcriptional regulation. In addition, we summarize recent progress on the identification of potential target genes and the analysis of mouse models.
INTRODUCTION
Precise temporal and spatial control of gene expression is accomplished by a broad array of sequence-specific transcription factors. Many of these are inefficient transcriptional activators or repressors on their own, but they recruit potent coactivators or corepressors that cannot bind directly to DNA in turn (1). Regulatory mechanisms include chromatin-remodeling factors that mobilize nucleosomes and histone-modifying enzymes. The expression of such regulatory factors is controlled by diverse signaling pathways, other transcription factors and regulatory RNAs, building up a highly complex transcriptional network.
The Notch signaling pathway represents a central regulator of gene expression. This cascade controls cell fate determination and differentiation, making it essential for many aspects of embryonic development as exemplified by a variety of mouse knockout studies. In humans, mutations of Notch ligands or receptors are responsible for a number of diseases like Alagille syndrome, CADASIL, T-cell leukemia, aortic valve calcification and other cardiovascular disorders (2–4).
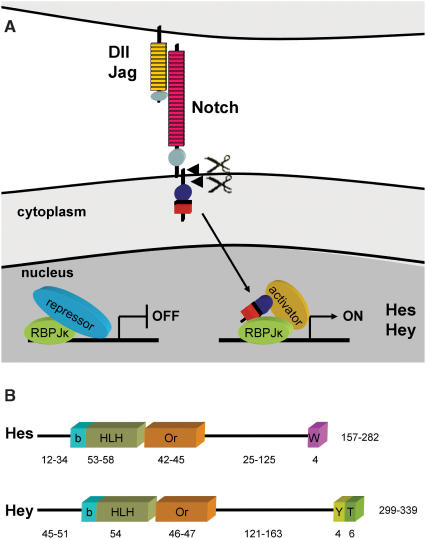
Notch receptors are single-pass transmembrane proteins that become activated upon ligand binding. This leads to two consecutive cleavage events releasing the intracellular domain (NICD), which then translocates to the nucleus. There NICD interacts with the DNA-binding protein RBPJκ (also known as CBF1, Rbpsuh or Su(H) in Drosophila), which is associated with corepressors (e.g. N-CoR, SHARP, CtBP). Interaction with NICD replaces these corepressors and allows recruitment of coactivators like Mastermind/MAML and p300/CBP leading to transcription of target genes (Figure 1) (5). The most extensively studied and best understood targets are Hairy and Enhancer-of-split [E(spl)] genes in Drosophila and the related Hes and Hey genes in mammals (6). Interestingly, there is a crosstalk between Notch and the BMP/TGF-beta, JAK-STAT, Ras and HIF signaling pathways to enhance activation of Hey/Hes expression (7–11), suggesting that these factors transduce and integrate signals from multiple pathways.
Figure 1.
Scheme of Notch signaling. (A) Ligands of the Delta (Dll) or Jagged (Jag) family induce intramembrane cleavage of the Notch receptor. The intracellular domain replaces transcriptional corepressors with activators enabling transcription of Hes and Hey genes by RBPJκ. (B) Domain organization of Hes and Hey proteins. Numbers indicate the amino acid content of the individual protein domains.
Besides the activation of target genes via RBPJκ, referred to as the canonical pathway, additional, non-canonical functions of Notch have been described that are less well characterized. These include e.g. regulation of the actin cytoskeleton, interaction with the wingless pathway, RBPJκ-independent activation of target genes (12), or activation of the RNA-binding protein Musashi (13).
HAIRY AND E(SPL) IN DROSOPHILA
In the fruitfly Drosophila melanogaster, Hairy and seven clustered E(spl) genes (m8, m7, m5, m3, mβ, mγ and mδ) control crucial developmental processes like segmentation, myogenesis or neurogenesis. All of these genes encode basic helix-loop-helix (bHLH) proteins (14). The DNA-binding basic domain (b) is contiguous with one of two amphipathic α-helices separated by a loop (HLH) that serve as a dimerization domain and as a platform for additional protein interactions (15). The HLH region is followed by two additional α-helical stretches (helix3/4), called the Orange domain. This domain is thought to serve as an additional interface for protein interactions and it acts as a transcriptional repressor when fused to a DNA-binding domain (16). A further characteristic of Hairy and E(spl) proteins is the invariant proline residue in the basic domain and a highly conserved carboxyterminal tetrapeptide motif WRPW that recruits the corepressor Groucho (14).
E(spl) genes are activated by Notch signaling and their protein products, as well as Hairy, block neuronal differentiation by inhibiting proneural bHLH activators like Atonal, Daughterless and those of the Achaete–Scute complex (14). The molecular details of how this is achieved are diverse and in part still controversial. Proposed models include sequestering of activator complexes away from DNA (17), direct binding to promoters of target genes and recruitment to promoters without direct DNA binding (18).
MAMMALIAN HES AND HEY PROTEINS
In the mouse and rat genomes, seven Hes (Hes1-7) (19–25) and three Hey genes (Hey1,2,L; also published as Hrt1,2,3; Hesr1,2; Herp2,1 or Chf2,1) have been identified (26–30). Hes proteins are highly similar to Hairy and E(spl), especially within the bHLH, Orange and WRPW domains. Similar to their Drosophila ancestors, Hes proteins are supposed to bind N- and E-box DNA sequences (CACNAG, CANNTG) and they can recruit TLE1-4 corepressors (the orthologs of Groucho) through their WRPW tetrapeptide (31). While Hes1, Hes5 and Hes7 can be induced by the Notch pathway (25,32–35), Hes2, Hes3 (34) and Hes6 (24) appear to be independent of Notch signaling, and further data on Hes4 are lacking.
All members of the Hey gene family can be induced by Notch (26,27,29,30,36–39), they are strongly conserved during evolution (40) and there is also a Drosophila Hey gene with hitherto unknown function (26,27). Especially the bHLH and Orange domains are similar to those of Hes proteins, but the invariant proline residue in their basic domains is replaced by glycine and they do not bind to N-box sequences (41). Hey proteins preferentially bind to an E-box sequence that is also recognized by Hes1, Hes6 and E(spl) proteins (29,41–43). The most striking difference between Hes and Hey proteins is the lack of the WRPW tetrapeptide in the latter. Instead a related YRPW peptide or a further degenerated YXXW (HeyL) sequence can be found, which cannot bind TLE corepressors (41,42). The YXXW motif is followed by a conserved TE(I/V)GAF peptide with presently unknown function (Figure 1B).
There are several additional mammalian proteins that exhibit strong homologies to Hairy and E(spl). Examples are Helt, DEC1 (also known as Stra13, SHARP-2 or BHLHB2) and DEC2. They generally lack WRPW/YRPW motif sequences and there is no evidence for a Notch-dependent expression thus far.
BIOLOGICAL FUNCTIONS OF HES AND HEY GENES
Mammalian Hairy-related proteins are specifically expressed in various tissues and they fulfill important roles during development and adulthood. It is beyond the scope of this manuscript to review all these functions in detail, instead Table 1 provides a short overview of the phenotypes seen in gene targeting experiments in mice.
Table 1.
Phenotypes of Hes or Hey gene deficient mice
| Notch regulated | Mouse knockout phenotype | References | |
|---|---|---|---|
| Hes1 | Yes | Neurulation defects, premature differentiation of neural progenitor cells | (120–122) |
| Eye and inner ear defects | (123–125) | ||
| Pancreas defects and defective endocrine differentiation | (126) | ||
| Disturbed T-cell differentiation, lack of thymus | (127) | ||
| Hes3 | No | Viable, fertile | (128) |
| Hes5 | Yes | Viable, fertile | (35,121) |
| Eye and inner ear defects | (129,104) | ||
| Elevated myelin levels in central nervous system | (117) | ||
| Hes6 | No | Viable, fertile | (24) |
| Hes7 | Yes | Somitogenesis defects | (108) |
| Hes1/5 | Enhancement of Hes1−/− phenotype | (35,121,125,130) | |
| Defects in cranial and spinal nerves | (131) | ||
| Hes1/3 | Missing midbrain and anterior hindbrain due to premature neuronal differentiation | (128) | |
| Hes1/3/5 | More severe than Hes1/5 loss | (125) | |
| Hey1 | Yes | Viable, fertile | (132,133) |
| Hey2 | Yes | Congenital heart defects | (134–138) |
| Decreased arterial neointima formation | (87) | ||
| HeyL | Yes | Viable, fertile | (47) |
| Hey1/2 | Angiogenesis and arterial differentiation defects | (132,133) | |
| Hey1/L | Congenital heart defects | (47) |
Hes1 plays an essential role in the development of the nervous system, sensory organs (eye, inner ear), pancreas and endocrine cells, as well as lymphocytes. Loss of Hes5 or Hes3 is less severe, but combined with Hes1 deficiency leads to more profound pathologies, as there is partial redundancy among these genes. Hes7 is important for somitogenesis. In contrast, Hey genes play critical roles in the cardiovascular system. Hey2−/− mice and those with a combined Hey1/L loss suffer from severe congenital heart defects. While Hey1−/− mutants are viable, a combined Hey1/2 deficiency phenocopies the vascular defects of Notch1−/− embryos, including impaired angiogenic remodeling and a lack of arterial differentiation. The known overlap in expression sites (31) suggests that there may be additional genetic interactions to be uncovered in compound Hes and Hey deficient mutants.
PROTEIN INTERACTIONS AND MODES OF TRANSCRIPTIONAL REPRESSION
Hairy-related proteins can interact with a large number of HLH proteins, but they also recruit transcriptional corepressors like histone deacetylases. Furthermore, Hes and Hey proteins can form complexes with other transcription factors, which often turns these into transcriptional repressors. Currently known interacting proteins are summarized in Table 2 and a schematic overview of the functional roles of such complexes is presented in Figure 2.
Table 2.
Summary of protein-protein interactions of Hes (A) and Hey (B) proteins
| Interaction partner | Hes/Hey protein | Interacting Hes/Hey domain | Method | Comments | References |
|---|---|---|---|---|---|
| (A) | |||||
| Homo/Heterodimers | |||||
| Hes1 | Hes1 | bHLH-Or | GST, IP, Y2H | (41,44) | |
| Hey1,2 | Hes1 | bHLH (Or stabilizes) | GST, IP, Y2H | Stronger than homodimers | (41,44–46) |
| Hes6 | Hes1 | ND | IP | Repression of Hes1 activity | (22) |
| Helt | Hes5 (not Hes1) | (Orange of Helt) | IP | (48) | |
| HLH factors | |||||
| E47 E2-2 | Hes1,5 | ND | IP, M2H | Repression of transcriptional activity | (21,51) |
| Id1,2,3,4 | Hes1 | ND | IP*, M2H | Sequestration | (51) |
| ITF1,2 | Hes1 | bHLH | GST, Y2H | (44) | |
| Mash1 (Ascl1) | Hes5 | ND | IP | Repression, sequestration | (21,117) |
| Ptf1-p48 | Hes1 | ND | GST, IP, Y2H | Repression of transcriptional activity | (53) |
| Other transcription fators | |||||
| c-myb | Hes1 | ND | IP* | Repression of transcriptional activation of CD4 promoter | (94) |
| GATA1 | Hes1 | ND | GST, IP | Represses GATA1 activity, but not DNA-binding capacity. | (85) |
| RBPJκ | Hes1 | bHLH (H1) | IP | Repression of transcriptional activity | (75) |
| Runx2 (Cbfa1) | Hes1 | C-terminus (not WRPW) | GST, IP, Y2H | Enhances Runx2 activity, interferes with TLE1 and HDAC1 recruitment | (63,92,93) |
| Runx1 (Cbfa2) | Hes1 | ND | GST, IP | (92) | |
| Sox10 | Hes5 | ND | IP | Repression, sequestration | (116) |
| STAT3 JAK2 | Hes1,5 | bHLH-Or | IP* | Promotes STAT3 phosphorylation and nuclear translocation | (11) |
| Transcriptional cofactors | |||||
| TLE1,2,3,4 | Hes1,5,6 | WRPW | GST, IP*, Y2H | Function as a corepressor | (46,59–65,68) |
| SIRT1 | Hes1 | bHLH | GST, IP | Augments repression capacity | (77) |
| HDAC1 | Hes1 | ND | IP | (93) | |
| CBP | Hes1 | ND | IP | Turns Hes1 into transcriptional activator | (68) |
| Others | |||||
| pRB | Hes1 | ND | IP | Enhances Runx2/Hes1 activity | (139) |
| Ubiquilin 1 | Hes1 | ND | M2H | (140) | |
| (B) | |||||
| Homo/Heterodimers | |||||
| Hey1,2,L | Hey1,2,L | bHLH (Or stabilizes) | GST, IP, Y2H | (41,44,47) | |
| Hes1 | Hey1,2 | bHLH (Or stabilizes) | GST, IP, Y2H | Stronger than homodimers | (41,44–46) |
| Helt | Hey2 | (bHLH of Helt) | IP | (48) | |
| HLH factors | |||||
| ARNT | Hey1,2 | ND | Y2H | Repression of ARNT/EPAS induction of VEGF promoter | (28) |
| HAND1,2 | Hey1,2,L | ND | GST | (54) | |
| Id1 | Hey1 | ND | IP | Reduced half-life of Id1 | (119) |
| ITF1,2 | Hey1,2 | bHLH | GST, Y2H | (44) | |
| MyoD | Hey1,2 | ND | IP | Repression of MyoD activity, sequestration | (52) |
| Ptf1-p48 | Hey1,2 | ND | IP | Repression of Ptf1-p48/E47-induced gene expression | (53) |
| Other transcription fators | |||||
| AR SRC | Hey1 | ND | GST, IP* | Repression of AR/SCR-induced gene expression | (74) |
| GATA1,2 | Hey1,2 | bHLH | GST, IP* | Repression of transcriptional activity | (83) |
| GATA4,6 | Hey1,2,L | bHLH | IP | Repression of transcriptional activity | (72,73,76,88) |
| RBPJκ | Hey1,2,L | bHLH (H1) | IP | Repression of transcriptional activity | (75) |
| Runx2 (Cbfa1) | Hey2 | ND | GST | Repression of transcriptional activity | (4) |
| SRF | Hey2 | bHLH | GST, IP | Prevents SRF interaction with CArG box | (89) Contradictory result: (84) |
| STAT3 | Hey1,2 | ND | IP | Enhances transcriptional activity | (11) |
| Transcriptional cofactors | |||||
| Sin3A N-CoR | Hey1,2 | bHLH | GST, IP | Augments repression capacity, recruitment of HDAC1 | (41) |
| SIRT1 | Hey2 | bHLH | GST, IP | Augments repression capacity | (77) |
| Others | |||||
| BOIP | Hey1 (not Hey2) | Or | IP, Y2H | (45) | |
Abbreviations: H1, helix 1; IP, co-immunoprecipitation, IP*; co-immunoprecipitation with endogenous proteins; GST, GST pull-down assay; M2H, mammalian two-hybrid assay; Or, Orange domain; Y2H, yeast two-hybrid assay.
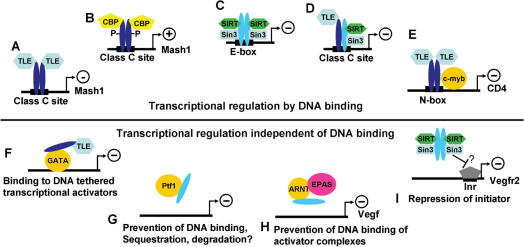
Figure 2.
Proposed models of how Hairy-related factors affect gene expression. (A and B) Binding of Hes1 (dark blue) to a class C E-box can repress or activate the Mash1 promoter depending on the recruited cofactors. (C) Hey proteins (light blue) recruit cofactors different than Hes1 and bind to E-box sequences in vitro. (D) DNA binding of a Hes/Hey heterodimer. (E) Combined DNA and protein binding turning a transcriptional activator into a repressor. (F–I) Transcriptional regulation independent of DNA binding includes turning activators into repressors (F), prevention of DNA binding, sequestration, degradation (G and H) or interference with the basal transcriptional machinery (I).
Hes and Hey factors form homo- and heterodimers
Drosophila E(spl) proteins form homodimers and heterodimers with each other via their HLH domains (17) and this has also been found for Hes and Hey proteins (41,44–47). Heterodimers between Hes and Hey family members appear to be even more stable than the corresponding homodimers. The Orange domain significantly improves interaction strength (44) and such heterodimers bind to DNA target sequences with even higher affinity than the corresponding homodimers (41). As there is overlapping expression between Hes and Hey genes in several tissues (31), it is conceivable that such heterodimers are formed in vivo. Indeed, affinity purification of Hes1 from preadipocytes led to copurification of Hey1 as well as Helt, a new Hey-related protein that associates with Hey2 and Hes5 (46). Surprisingly, Helt appears to use its bHLH domain to bind Hey2, but its Orange domain to interact with Hes5 (48). As Hes and Hey factors differ in the recruitment of corepressors as discussed below, heterodimers broaden the respective repression capacity ( Figure 2D). In rare cases heterodimerization may even be antagonistic, e.g. during neural differentiation, where Hes6 counteracts Hes1 repression activity by forming Hes1/Hes6 heterodimers (22).
Interaction with other bHLH proteins
The bHLH family consists of about 125 members (49). Hes and Hey proteins have been shown to interact specifically with some of the ubiquituosly expressed E-proteins (21,44,50,51). The latter tend to form homodimers or heterodimers with lineage-specific bHLH factors and they activate transcription by binding to E-box DNA sequences (CANNTG). Interaction with Hes1, 5 or 7, however, strongly reduces transcriptional activity of E-proteins, presumably in a squelching type of action (19,21,25,50).
Hairy-related proteins also interact with lineage-specific bHLH factors. Hey1 forms dimers with the muscle-specific factor MyoD and prevents its activity during myogenic differentiation of 10T1/2 cells. Here, Hey1 is supposed to counteract formation of a critical MyoD/E47 heterodimer (52). Hey2 was shown to antagonize activation of the VEGF promoter by the ARNT/EPAS (HIF2) complex (28) and the activity of Ptf1-p48, a bHLH protein important for pancreas development, is likewise strongly decreased by Hes1, Hey1 or Hey2 (53) ( Figure 2G and H). Finally, interaction of Hey proteins with Hand1 and Hand2, two important bHLH regulators of heart development, has also been described (54), but functional data are still lacking.
How can Hairy-related factors alter the functional properties of many different bHLH proteins? The promiscuity of HLH heterodimerization already generates a spectrum of pairings with varying affinities that may indirectly reduce the amount of specific dimers. Work in Drosophila further suggests that inhibition of bHLH proteins like Scute does not rely on formation of compromised or novel HLH dimers but may be due to binding of E(spl) repressors to the Scute transactivation domain. Thus, even within the bHLH family of proteins multiple modes of interaction are possible (17,18,55).
Recruitment of Groucho/TLE
The WRPW motif of Hairy and E(spl) proteins has been assumed to mediate transcriptional repression in Drosophila as it was only found in repressor proteins (56). Its functional importance was underscored by the fact that two mutant Hairy alleles carry mutations of the WPRW sequence (57). In Drosophila, the WRPW motif of Hairy and E(spl) proteins binds the corepressor Groucho (58) and in mammals Hes proteins recruit the Groucho homologs TLE1-4 to generate a transcriptional repressor complex (46,59–65). Groucho/TLE is supposed to attract further corepressors like histone deacetylases and members of the Sin3 complex, suggesting that such interactions lead to strong transcriptional repression (66,67). The interaction with TLE proteins appears to be quite stable, as affinity purification of Hes1 containing nuclear complexes from mouse preadipocytes yielded only a small number of interacting proteins that include TLE1, 3 and 4 (46).
A recent report on the control of Mash1 expression in differentiating neural stem cells suggested that the Hes1/TLE interaction can be dissociated by cellular signaling pathways that may even convert Hes1 into a transcriptional activator (68). In this case the corepressor complex disassembles, but Hes1 remains bound to the Mash1 promoter and after CaMKIIdelta-dependent phosphorylation recruits coactivators including p300/CBP ( Figure 2A and B). It remains to be seen, however, to what extend this scenario can be generalized.
Another surprise came from a genome-wide chromatin profiling analysis in Drosophila, where 59 putative target genes were detected for Hairy and 155 for Groucho. Quite unexpectedly, only a single gene was targeted by both proteins, while there was a strong overlap of binding sites for Hairy and other corepressors like CtBP and Sir2 (69). As the screen for Groucho targets was only performed in Kc cells, this may not necessarily be representative for other cell types and differentiation states, but decoration of larval polytene chromosomes rather supported the conclusions of little overlap in targeted genes. This clearly suggests that Groucho is not the primary cofactor for Hairy and perhaps for E(spl) proteins alike, but this may well depend on the cellular context.
In contrast to Hes proteins, the Hey proteins cannot bind to TLE proteins (41,42). Hey1 and Hey2 lack a WRPW motif, but harbor a related YRPW (YQPW) motif. The crystal structure of the WRPW–TLE1 interaction revealed that the N-terminal tryptophan residue binds in a hydrophobic pocket (70), where the tyrosine residue of Hey proteins cannot efficiently interact. Indeed, a corresponding tyrosine to tryptophan (Y→W) exchange in Hey1 allows TLE1 binding (42). Consistent with these data deletion of the YXXW-TE(I/V)GAF motif of Hey proteins was found to have no effect on repression capacity and the reason for its strong conservation remains to be established (4,52,71–76).
Interaction with other corepressors—localization of repression domains
Despite the lack of TLE recruitment, Hey proteins are strong transcriptional repressors, and this prompted searches for interacting corepressors other than TLE. Strong repression activity could be mapped within the bHLH domain of Hey1 and Hey2, which directly interacts with N-CoR and mSin3A. These corepressors then indirectly recruit histone deacetylase-1 (HDAC1) (41).
The bHLH domains of Hey2 and Hes1 can recruit yet another histone deacetylase, SIRT1 (77). This direct interaction is evolutionary conserved as Drosophila Hairy interacts with the SIRT1 homolog Sir2, a NAD+-dependent histone deacetylase associated with gene silencing, control of metabolism and aging (78). Loss of Sir2 function leads to reduced activity of Hairy repressor activity (79).
The region between the Orange domain and the WRPW/YXXW motif also possesses some repression capacity, but its mode of action is unclear (,4,41,52,72–74,76). In Drosophila, Hairy and E(spl)mδ interact with the C-terminal binding protein (CtBP) via the pentapeptides PLSLV or PVNLA that are located close to the WRPW motif, but the physiological relevance of this interaction has been questioned (80,81). CtBP is assumed to again recruit chromatin-modifying enzymes like histone deacetylases to mediate repression (82). The fact that there was a striking overlap of genomic targets for Hairy, Sir2 and CtBP in the above-mentioned screen of Drosophila Kc cell chromatin strongly argues for a common molecular pathway and perhaps physical interaction of the proteins (69).
Interaction with distinct HDAC classes
Studies using the histone deacetylase inhibitor trichostatin A (TSA) that inhibits HDACs, but not sirtuins like Sir2/SIRT1, revealed that recruitment of both types of histone deacetylases is necessary for full Hes and Hey repression activity. A plausible scenario suggests that Hey and Hes factors can recruit HDAC1 using the bHLH domain and the C-terminus, while SIRT1 is only bound by the bHLH domain. If this model is correct, TSA treatment would only partially block repression by inhibiting HDAC1 without affecting SIRT1. Indeed, many reports describe only moderate effects of TSA on modulation of Hes or Hey repression activity (29,73,74,77,83,84) with one exception (85). In contrast, repression capacity of the carboxyterminal half of the Hes1 and Hey2 proteins, which does not bind SIRT1, is almost abolished by TSA treatment (74,77).
Taken together, these observations suggest that Hey and Hes factors use combinations of both, TSA sensitive and insensitive histone deacetylases to mediate repression by histone modification.
Repression of GATA factors
The zinc finger transcription factors GATA1, 2 and 3 play crucial roles e.g. in the hematopoietic system, whereas GATA4, 5 and 6 are important regulators in the cardiovascular system. GATA factor activity is tightly regulated by interaction with cofactors (86). There is convincing evidence that Hes and Hey proteins interact with GATA factors ( Figure 2F) to strongly repress GATA transcriptional activity (72,73,76,83,85,87,88). In hematopoietic progenitor cells there seems to be competition between Hes1 and the coactivator p300 for GATA1 interaction (85), but the molecular mode of repression is not clear, as studies on the influence on DNA binding by GATA are controversial (72,83,85). In the developing heart, Hey2 appears to limit GATA4/6 activity and this is consistent with an elevated expression of GATA target genes in Hey2 knockout mice (73).
Interference with the Myocardin/SRF complex
Hey2 does not only interfere with GATA4/6, it also seems to interfere with another master regulator in vascular smooth muscle cells (VSMCs), Myocardin. Hey2 is coexpressed with Myocardin in VSMCs of arteriosclerotic lesions as well as after vascular wall injury, where it represses Myocardin-induced upregulation of the smooth muscle myosin heavy chain promoter (84,89). Myocardin forms a ternary complex with serum response factor (SRF) on CArG-boxes (90), but the question of how Hey2 interferes with the function of this Myocardin/SRF complex is open. While one study found a direct Hey2/SRF interaction that inhibited DNA binding of the ternary complex, another found no evidence for this and invoked independent parallel pathways acting on smooth muscle genes (84,89).
Other protein interactions
Besides the more classical interaction partners that were seen in multiple organisms, there are several additional interactions that may be of functional relevance. Runx2 (Cbfa1), a central regulator of bone development, physically interacts with Hairy-related factors, but Hes and Hey proteins seem to have opposing effects in this case. While Hey1 and Hey2 strongly inhibit Runx2 activity (4,91), Hes1 cooperates with Runx2 to stimulate the Osteopontin or Osteocalcin promoters (92,93). In the latter case, interaction of Runx2 or Runx1 (AML1, Cbfa2) with Hes1 inhibits formation of the Hes1/TLE complex and thus blocks Hes1 repression capacity (63,92).
Hey1 was also shown to interact with the androgen receptor (AR) that binds the coactivator SRC1 to activate transcription of androgen-responsive promoters. Recruitment of Hey1 to the AR/SRC1 complex, however, prevents activation of AR (74). Similarly, there is evidence that c-myb is turned from a transcriptional activator into a repressor of the CD4 enhancer in the presence of Hes1 and both proteins form a stable nuclear complex in T-cells (94) ( Figure 2E).
Finally, there is compelling evidence that the Notch pathway interacts with other important signaling cascades like BMP/TGF-beta (7) or hypoxia-induced signaling (8,9). It appears that Notch enhances signaling through the JAK-STAT pathway since Hes1, Hes5, Hey1 and Hey2 bind to STAT3, which enhances phosphorylation and nuclear translocation of STAT3 (11).
Hey1 was even postulated to directly act on the basal transcriptional machinery to repress promoters that contain an initiator element, but lack a TATA box, where introduction of a TATA box would relieve repression by Hey1 (95) ( Figure 2I). This hypothesis is based on repression by Hey1 and Hey2 of the minimal Vegfr2 promoter (96–98) that lacks E-box sequences and it has precedence in the binding of the Hairy-related factor Stra13 to the basal transcription factor TFIIB (99). Nevertheless, it remains to be seen whether these findings hold up to further scrutiny and can be generalized.
In summary, there is a plethora of interactions based on classical HLH dimerization as well as interactions with corepressors or histone deacetylases. The exact target sites of such bHLH heterodimers are currently unknown. In several cases, Hes and Hey proteins appear to bind to other DNA-binding transcription factors to modulate their activity. Quite surprisingly they often seem to rely on the DNA-binding capacity of their respective partners and do not need E- or N-box type target sequences. With probably rare exceptions, Hes and Hey proteins act as genuine transcriptional repressors.
POTENTIAL TARGET GENES OF HAIRY-RELATED PROTEINS
The expression levels of multiple genes are affected by Hairy-related transcription factors. Here we present a short summary of potential target genes that have at least been verified using promoter-reporter assays ( Table 3). These data suggest that Hes factors use both, DNA-binding dependent and independent functions to regulate transcription, whereas Hey factors seem to primarily repress the function of activating transcription factors by forming protein–protein complexes with them. However, this is not simply a tritrating-out mechanism or prevention of DNA binding by activators. It appears more likely that DNA-bound activators are turned into repressors by recruitment of Hey factors associated with corepressors.
Table 3.
Summary of known promoters and DNA-binding sites of Hes (A) and Hey (B) transcription factors
| Target gene | Hes/Hey protein | Method | Mode of action/binding site | Comment | References |
|---|---|---|---|---|---|
| (A) | |||||
| Direct | |||||
| Acid-α-Glucosidase | Hes1 | CAT EMSA | Class C E-box binding in first intron | Repression or activation depending on cell type | (141,142) |
| Calcipressin | Hes1 (not Hey1,2) | ChIP | DNA binding | Repression of endogenous gene expression | (143) |
| CD4 | Hes1 | EMSA Luc | N-box binding in CD4 silencer, interference with c-myb | Repression of endogenous gene in T-cells | (94,144) |
| E2F1 | Hes1 | EMSA Luc | N-box binding (homodimer and Hes1/Hey1) | Repression of endogenous gene | (145) |
| Hes1 | Hes1 | CAT FP ChIP EMSA | Direct: N-box binding Indirect: Binding to RBPJκ | Mutation of N-boxes prevents inhibition | (75,109,146,147) |
| Mash1 (Ascl1) | Hes1 | ChIP EMSA Luc | Class C E-box binding | Repression of endogenous gene. Activator by CBP binding instead of TLE1 | (68,114,115) |
| MBP | Hes5 | ChIP Luc | Direct: DNA binding and HDAC1 recruitment; Indirect: repression of Mash1 and Sox10 | Elevated myelin levels in Hes5−/− mice | (117) |
| Neurogenin3 | Hes1 | EMSA Luc | N-box binding | (148) | |
| p27(KIP1) | Hes1,6 | ChIP EMSA Luc | Class C E-box binding | Higher p27(KIP1) levels in Hes1−/− mice | (43,116) |
| PGDS | Hes1 | ChIP EMSA Luc | E-box binding | (149) | |
| Indirect | |||||
| APRE (acute phase resp. element) | Hes1,5 | EMSA Luc | Promotion of STAT3 phosphorylation by interaction with JAK2/STAT3 | Suppression of endogenous Hes1 reduces STAT3 phosphorylation | (11) |
| ATH5 | Hes1 | CAT | Competition with Ngn2 and ATH5 | Dominant negative regulator of ATH5 in retina | (150) |
| Fatty acid synthase | Hes1 | Luc | Repression of SREBP transcriptional activity | Blocks adipogenesis in preadipocytes | (46) |
| E-box promoter | Hes1 | CAT | Binding to MyoD inhibits MyoD-driven transcription | Diminished myogenic conversion of C3H10T1/2 cells induced by MyoD | (19) |
| E-box promoter | Hes1,5,7 | CAT FP Luc | Binding to E47 inhibits transcriptional activity and DNA binding | Interference with B-cell differentiation | (19,21,25,50) |
| E-box promoter | Hes1 | CAT Luc | Binding to Mash1 inhibits transcriptional activity; Rapid degradation of Mash1 | Neuronal differentiation is dependent of Hes1 downregulation | (19,115,151) |
| GATA-binding elements | Hes1 | ChIP, EMSA, Luc | Binding to GATA1 does not interfere with DNA binding, but recruitment of p300 is inhibited | Inhibition of erythroid and megakaryocytic differentiation | (85) |
| Osteopontin Osteocalcin | Hes1 | Luc | Binding to Runx2 interferes with recruitment of TLE1/ HDAC1; enhanced by Vit.-D3 and pRB | Potentiation of Runx2-induced Osteopontin expression (Activation!) | (63,92,93,139) |
| p21 | Hes1 | Luc | Repression of MASH/ E47-driven p21 expression | Suppression of endogenous gene; inhibition of proliferation | (147) |
| Ptf1-binding elements | Hes1 | Luc | Binding to Ptf1-p48 interferes with Ptf1-p48 DNA binding | Ectopic Hes1 represses acinar cell differentiation | (53,152) |
| Unknown | |||||
| DLK-Pref1 | Hes1 | Luc | ND | Repression of endogenous gene | (153) |
| Mdm2 | Hes1 | Luc | ND | Hes1 causes p53 upregulation | (154) |
| p57 | Hes1 | CAT | ND | Higher p57 levels in Hes1−/− mice | (155) |
| (B) | |||||
| Direct | |||||
| DAT1 | Hey1 | Luc, Y1H | Binding 3′ UTR | Repression of endogenous gene; upregulation in Hey1−/− mice | (156,157) |
| Vegfr2 | Hey1 | ChIP, Luc | Interference with initiator element | Repression of endogenous gene in endothelial cells | (95,96) |
| Indirect | |||||
| αMyHC | Hey1,2,L | Luc | Binding to GATA4 represses transcriptional activity | (72) | |
| ANF | Hey1,2,L | EMSA, Luc | Binding to GATA4/6 represses transcriptional activity. DNA binding of GATA can occur (72), or is prevented (88) | Repression of endogenous gene in cardiomyocytes. Ectopic expression in Hey2−/- hearts | (72,73,88) |
| APRE (acute phase resp. element) | Hey1,2 | Luc | Promotion of STAT3 phosphorylation by interaction with JAK2/STAT3 | Activation | (11) |
| E-box promoter | Hey1,2 | Luc | Prevent dimer formation of Mash1/E47 and Math3/E47 | Interference with neural differentiation | (158) |
| GATA-binding elements | Hey1 | EMSA, Luc | Binding to GATA1. No interference with DNA binding of GATA1 | Inhibition of erythroid differentiation of K562 cells | (83) |
| Myogenin | Hey1,2 | EMSA, Luc | Binding to MyoD prevents MyoD/E47 complex formation | Hey1 inhibits myogenic conversion of 10T1/2 cells induced by MyoD | (52) |
| Nkx2.5 | Hey1,2,L | Luc | Binding to GATA4 represses transcriptional activity | (72) | |
| Osteocalcin | Hey1 | Luc | Binding to Runx2 represses transcriptional activity | Hey1 downregulation enhances mineralization of MC3T3 cells | (4,91) |
| Probasin | Hey1,2 | Luc | Binding to AR and SRC1 interferes with AR/SRC1- transcriptional activity | Downregulation of Hey1 inhibits repression of AR-driven gene expression | (74) |
| Ptf1-binding elements | Hey1 | EMSA, Luc | Binding to Ptf1-p48 interferes with Ptf1-p48 DNA binding | Repression of acinar cell differentiation | (53,152) |
| SM22α SM-MHC | Hey1,2 | Luc | Binding to Myocardin or GATA6 represses transcript. activity or prevents SRF binding to CArG box | Repression of endogenous gene in smooth muscle cells and 10T1/2 cells | (76,84,89) |
| VEGF | Hey2 | EMSA, Luc | Binding to ARNT prevents ARNT/EPAS DNA binding | (28) | |
| Unknown | |||||
| Coup-TFII | Hey1,2 | Luc | Downregulation under hypoxia (Hey induction) | (9) | |
| GATA4,6 | Hey1,2,L | Luc | (73) | ||
| Hey1,2,L | Hey1,2,L | Luc | Indirect: Binding to RBPJκ | (29,38,75) | |
| Hypoxia response element | Hey1,2 | Luc | (9,159) | ||
| Mdm2 | Hey1 | Luc | Hey1 causes p53 upregulation | (154) | |
| RTA (virus) | Hey1 | ChIP, Luc | Independent of DNA binding | (160) | |
| Tbx2 | Hey1 | Luc | ND | Tbx2 repression in Hey1 or Hey2 overexpressing mice | (161) |
Abbreviations: CAT, chloramphenicol acetyl transferase assay; ChIP, chromatin immunoprecipitation; EMSA, electrophoretic mobility shift assay; FP, DNA footprint; Luc, luciferase assay.
Potential DNA-binding sites
Studies in Drosophila have shown that Hairy and E(spl) are capable of DNA binding. While initial studies suggest that these factors bind to N-box (CACNAG) sequences and a variant thereof, the class C E-box site (CACGCG), later studies revealed that the class B E-box sequence CACGTG is the preferred binding site for E(spl) proteins (56,100–102). The flanking nucleotides of such a core sequence may also influence binding as shown by site selection experiments, which revealed tggCACGTGcca as the optimal binding site (103).
In mammals Hes1, 2, 3, 5 and 7 can bind to N-box sequences (19–21,25,41). Hes1 also interacts strongly with class C sites, but the binding affinities of Hes1, 2 and 7 towards class B E-box sequences appears to be somewhat lower (20,25,41). Interestingly, it was proposed that Hes6, an antagonist of Hes1, does not bind to standard N- or E-boxes (22), but to the E(spl) specific class B site (43). In the case of Hes1, DNA binding can be inhibited by PKC-mediated phosphorylation of serine residues in the basic domain (118), but it is unclear if this is physiologically relevant and the site is not conserved in other Hes and Hey proteins.
The preferred binding site for Hey1 and Hey2 is the same class B site as described for the E(spl) proteins (29,41–43,52,71), and even the preference for the flanking nucleotides is conserved (42). N-box binding of Hey1 and Hey2 is very weak, but binding to class C sites can be enhanced by formation of a Hey2/Hes1 heterodimer (41) ( Figure 2A–D). Thus it is still difficult to reconcile the diverse biological functions with the apparent overlaps in potential genomic targets as identified by binding-site selection schemes. Selectivity may thus depend on additional cofactors, unappreciated differences in in vivo affinities and spatio-temporal expression patterns.
Autoregulation and oscillating gene expression
During formation of the somites in the embryo several components of the Notch signaling system are expressed in a cyclical fashion. Waves of expression move through the presomitic mesoderm and finally arrest in a newly formed somite, which then buds off. This repeated process, referred to as the somite segmentation clock (105), was first discovered in chicken, where an oscillatory expression of the Hes1 ortholog c-Hairy1 (106) and of c-Hey2 (44) was observed. In mice, Hes1 and Hes7 are critical cycling genes (107,108) and loss of Hes7 causes a loss of somite segmentation (108).
Mathematical models suggest that Hes proteins are translated and subsequently repress their own transcription. Due to the short half-life of Hes proteins, autorepression is relieved allowing a new wave of Hes transcription and translation every 90–120 min. Indeed there are reports showing that Hes1, 7 and Hey1, 2 and L proteins can repress their own promoters (29,38,108,109). Elegant work from Ryoichiro Kageyama's group showed that the short half-life of Hes7 (∼22 min) is indeed crucial for oscillating gene expression during somitogenesis, since mutant mice expressing a more stable Hes7 protein (half-life ∼30 min) lose synchronization of the clock (110).
There is some evidence that cycling Hes1 expression is an even more general process, since it was observed after serum stimulation in cultured cell lines (111), however, it seems that exact synchronization and stabilization of the oscillation is dependent upon sufficient cell–cell contacts to keep neighbors in phase (112). Proposed mechanisms how Hes and Hey proteins repress their own promoters include direct DNA binding (109) and interaction with RBPJk (75), but none of those has been fully verified.
Regulation of endogenous Hes and Hey target genes
In recent years, a few dozen potential target genes of Hes and Hey proteins have been described in the literature (Table 3). Most of these were identified by candidate gene approaches. In future studies whole transcriptome analyses should reveal a more global view of the complex regulatory network. First attempts have been undertaken to elucidate targets of Hey2 in endothelial cells or Hes1 in preadipocytes by microarray analyses (46,113). However, such experiments will have to be extended to differentiate direct targets from indirectly regulated genes. This may have to rely on techniques like chromatin-IP followed by large-scale tag-sequencing or whole genome microarray analyses.
At least some studies have already employed more rigorous tests to show that e.g. Hes1 binds to the Mash1 and p27(KIP1) promoters to regulate their activity (see Table 3). Importantly, these functions are well in line with the phenotypes observed in Hes1 or Hes5 knockout mice. Loss of Hes1 leads to premature neuroendocrine differentiation due to absence of Hes1-mediated inhibition of the promoter of the neurogenic gene Mash1 (68,114,115). In the context of this promoter Hes1 may even function as an integrator of additional signals and become the core of an activator complex in later developmental steps (68). Another link to explain gene knockout phenotypes comes from the reduced inhibition of the cyclin-dependent kinase (CDK) inhibitor p27(KIP1), which seems to be a key factor leading to strongly reduced thymocyte proliferation and reduced thymus size in Hes1−/− embryos (43,116).
For Hes5, the direct and indirect repressive effects on the myelin basic protein (MBP) promoter correlate well with the upregulation of MBP in Hes5−/− brains. This on the other hand is consistent with the limited remyelination in patients with multiple sclerosis, where Hes5 is highly expressed in immature oligodendrocytes of lesions (117). These examples clearly suggest that a set of several direct Hes or Hey target genes will be needed to fully explain the phenotypes of gain or loss of function alleles in various tissues.
CONCLUDING REMARKS
Almost thirty years of research on Hairy and related factors, lead to the publication of a few hundred papers, providing a wealth of information. This survey gathers data on the progress on mouse models, protein–protein and protein–DNA interactions to faciliate future studies and to highlight questions that are still unresolved.
Although quite a number of cofactors are now known that are recruited by Hairy-related proteins, little is known about the dynamics of these interactions. While the Hes1/TLE1 interaction is necessary for neural stem cell maintenance, it dissociates during neuronal differentiation and Hes1 is turned from a repressor into an activator, which is at least partly mediated by post-translational modifications like phosphorylation (68). In addition, the repressive functions of Hes6 can be blocked by phosphorylation of a specific serine residue by CK2 (65). It appears obvious that such strategies might also be employed for tightly regulated complex formation and dissociation of Hes and Hey proteins with other factors dependent on the cellular context.
Furthermore, DNA-binding affinities of Hes and Hey factors may be regulated by kinases. Phosphorylation of a residue in the basic domain of Hes1 decreases DNA-binding affinity (118), implying that Hairy-related factors can be influenced by growth factor signaling cascades. During the last years it already became clear that there is a crosstalk between Notch and the BMP/TGF-beta, JAK/STAT, Ras and hypoxia signaling cascades in the regulation of Hes and Hey genes (7–11). Elucidation of the biological effects as well as the associated post-translational modifications of those interactions will be a challenge in the future.
The question of how Hes and Hey factors act as repressors when they bind to other transcription factors is also not fully answered. We have schematically summarized the current models in Figure 2. Another possible mode of repression, which was not explicitly mentioned so far, is Hes/Hey-mediated protein degradation of transcriptional activators. It has been proposed that the WRWP peptide of Hes6 mediates proteins degradation and Hes1 seems to induce rapid degradation of the Mash1 transcription factor (68,114,115). Furthermore, the half-life of BMP-induced Id1 is reduced upon Hey1 binding (119). Again, knowledge of protein modifications like ubiquitinylation or SUMOylation is elusive.
Finally, it will be challenging to analyze the cellular localization of these factors in more detail. It already became clear that Hey1 is located in the cytoplasm and the nucleus of benign prostate hyperplasia, but is excluded from the nucleus in many malignant prostate cancers (74). The cytoplasmic functions of bHLH transcription factors are unknown. It will be interesting to see, if these proteins possess additional functions besides transcriptional regulation.
ACKNOWLEDGEMENTS
We wish to apologize to researchers in the field whose work could not be cited due to space limitations. Work on Notch signaling in our laboratory is supported by the Deutsche Forschungsgemeinschaft (DFG). Funding to pay the Open Access publication charges for this article was provided by University of Wuerzburg and Deutsche Forschungsgemeinschaft (DFG) to M.G.
Conflict of interest statement. None declared.
REFERENCES
- 1.Kadonaga JT. Regulation of RNA polymerase II transcription by sequence-specific DNA binding factors. Cell. 2004;116:247–257. doi: 10.1016/s0092-8674(03)01078-x. [DOI] [PubMed] [Google Scholar]
- 2.Shawber CJ, Kitajewski J. Notch function in the vasculature: insights from zebrafish, mouse and man. Bioessays. 2004;26:225–234. doi: 10.1002/bies.20004. [DOI] [PubMed] [Google Scholar]
- 3.Weng AP, Ferrando AA, Lee W, Morris JPT, Silverman LB, Sanchez-Irizarry C, Blacklow SC, Look AT, Aster JC. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306:269–271. doi: 10.1126/science.1102160. [DOI] [PubMed] [Google Scholar]
- 4.Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, Grossfeld PD, Srivastava D. Mutations in NOTCH1 cause aortic valve disease. Nature. 2005;437:270–274. doi: 10.1038/nature03940. [DOI] [PubMed] [Google Scholar]
- 5.Bray SJ. Notch signalling: a simple pathway becomes complex. Nat. Rev. Mol. Cell. Biol. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 6.Fischer A, Gessler M. Hey genes in cardiovascular development. Trends Cardiovasc. Med. 2003;13:221–226. doi: 10.1016/s1050-1738(03)00082-3. [DOI] [PubMed] [Google Scholar]
- 7.Kluppel M, Wrana JL. Turning it up a Notch: cross-talk between TGF beta and Notch signaling. Bioessays. 2005;27:115–118. doi: 10.1002/bies.20187. [DOI] [PubMed] [Google Scholar]
- 8.Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U, et al. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev. Cell. 2005;9:617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Diez H, Fischer A, Winkler A, Hu CJ, Hatzopoulos AK, Breier G, Gessler M. Hypoxia-mediated activation of Dll4-Notch-Hey2 signaling in endothelial progenitor cells and adoption of arterial cell fate. Exp. Cell Res. 2007;313:1–9. doi: 10.1016/j.yexcr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Stockhausen MT, Sjolund J, Axelson H. Regulation of the Notch target gene Hes-1 by TGFalpha induced Ras/MAPK signaling in human neuroblastoma cells. Exp. Cell Res. 2005;310:218–228. doi: 10.1016/j.yexcr.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Kamakura S, Oishi K, Yoshimatsu T, Nakafuku M, Masuyama N, Gotoh Y. Hes binding to STAT3 mediates crosstalk between Notch and JAK-STAT signalling. Nat. Cell Biol. 2004;6:547–554. doi: 10.1038/ncb1138. [DOI] [PubMed] [Google Scholar]
- 12.Lai EC. Notch signaling: control of cell communication and cell fate. Development. 2004;131:965–973. doi: 10.1242/dev.01074. [DOI] [PubMed] [Google Scholar]
- 13.Okabe M, Imai T, Kurusu M, Hiromi Y, Okano H. Translational repression determines a neuronal potential in Drosophila asymmetric cell division. Nature. 2001;411:94–98. doi: 10.1038/35075094. [DOI] [PubMed] [Google Scholar]
- 14.Fisher A, Caudy M. The function of hairy-related bHLH repressor proteins in cell fate decisions. Bioessays. 1998;20:298–306. doi: 10.1002/(SICI)1521-1878(199804)20:4<298::AID-BIES6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 15.Massari ME, Murre C. Helix-loop-helix proteins: regulators of transcription in eucaryotic organisms. Mol. Cell. Biol. 2000;20:429–440. doi: 10.1128/mcb.20.2.429-440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dawson SR, Turner DL, Weintraub H, Parkhurst SM. Specificity for the hairy/enhancer of split basic helix-loop-helix (bHLH) proteins maps outside the bHLH domain and suggests two separable modes of transcriptional repression. Mol. Cell. Biol. 1995;15:6923–6931. doi: 10.1128/mcb.15.12.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alifragis P, Poortinga G, Parkhurst SM, Delidakis C. A network of interacting transcriptional regulators involved in Drosophila neural fate specification revealed by the yeast two-hybrid system. Proc. Natl Acad. Sci. USA. 1997;94:13099–13104. doi: 10.1073/pnas.94.24.13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giagtzoglou N, Alifragis P, Koumbanakis KA, Delidakis C. Two modes of recruitment of E(spl) repressors onto target genes. Development. 2003;130:259–270. doi: 10.1242/dev.00206. [DOI] [PubMed] [Google Scholar]
- 19.Sasai Y, Kageyama R, Tagawa Y, Shigemoto R, Nakanishi S. Two mammalian helix-loop-helix factors structurally related to Drosophila hairy and Enhancer of split. Genes Dev. 1992;6:2620–2634. doi: 10.1101/gad.6.12b.2620. [DOI] [PubMed] [Google Scholar]
- 20.Ishibashi M, Sasai Y, Nakanishi S, Kageyama R. Molecular characterization of HES-2, a mammalian helix-loop-helix factor structurally related to Drosophila hairy and Enhancer of split. Eur. J. Biochem. 1993;215:645–652. doi: 10.1111/j.1432-1033.1993.tb18075.x. [DOI] [PubMed] [Google Scholar]
- 21.Akazawa C, Sasai Y, Nakanishi S, Kageyama R. Molecular characterization of a rat negative regulator with a basic helix-loop-helix structure predominantly expressed in the developing nervous system. J. Biol. Chem. 1992;267:21879–21885. [PubMed] [Google Scholar]
- 22.Bae S, Bessho Y, Hojo M, Kageyama R. The bHLH gene Hes6, an inhibitor of Hes1, promotes neuronal differentiation. Development. 2000;127:2933–2943. doi: 10.1242/dev.127.13.2933. [DOI] [PubMed] [Google Scholar]
- 23.Pissarra L, Henrique D, Duarte A. Expression of hes6, a new member of the Hairy/Enhancer-of-split family, in mouse development. Mech. Dev. 2000;95:275–278. doi: 10.1016/s0925-4773(00)00348-8. [DOI] [PubMed] [Google Scholar]
- 24.Koyano-Nakagawa N, Kim J, Anderson D, Kintner C. Hes6 acts in a positive feedback loop with the neurogenins to promote neuronal differentiation. Development. 2000;127:4203–4216. doi: 10.1242/dev.127.19.4203. [DOI] [PubMed] [Google Scholar]
- 25.Bessho Y, Miyoshi G, Sakata R, Kageyama R. Hes7: a bHLH-type repressor gene regulated by Notch and expressed in the presomitic mesoderm. Genes Cells. 2001;6:175–185. doi: 10.1046/j.1365-2443.2001.00409.x. [DOI] [PubMed] [Google Scholar]
- 26.Kokubo H, Lun Y, Johnson RL. Identification and expression of a novel family of bHLH cDNAs related to Drosophila hairy and enhancer of split. Biochem. Biophys. Res. Commun. 1999;260:459–465. doi: 10.1006/bbrc.1999.0880. [DOI] [PubMed] [Google Scholar]
- 27.Leimeister C, Externbrink A, Klamt B, Gessler M. Hey genes: a novel subfamily of hairy- and Enhancer of split related genes specifically expressed during mouse embryogenesis. Mech. Dev. 1999;85:173–177. doi: 10.1016/s0925-4773(99)00080-5. [DOI] [PubMed] [Google Scholar]
- 28.Chin MT, Maemura K, Fukumoto S, Jain MK, Layne MD, Watanabe M, Hsieh CM, Lee ME. Cardiovascular basic helix loop helix factor 1, a novel transcriptional repressor expressed preferentially in the developing and adult cardiovascular system. J. Biol. Chem. 2000;275:6381–6387. doi: 10.1074/jbc.275.9.6381. [DOI] [PubMed] [Google Scholar]
- 29.Nakagawa O, McFadden DG, Nakagawa M, Yanagisawa H, Hu T, Srivastava D, Olson EN. Members of the HRT family of basic helix-loop-helix proteins act as transcriptional repressors downstream of Notch signaling. Proc. Natl Acad. Sci. USA. 2000;97:13655–13660. doi: 10.1073/pnas.250485597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iso T, Sartorelli V, Chung G, Shichinohe T, Kedes L, Hamamori Y. HERP, a new primary target of Notch regulated by ligand binding. Mol. Cell. Biol. 2001;21:6071–6079. doi: 10.1128/MCB.21.17.6071-6079.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iso T, Kedes L, Hamamori Y. HES and HERP families: multiple effectors of the Notch signaling pathway. J. Cell. Physiol. 2003;194:237–255. doi: 10.1002/jcp.10208. [DOI] [PubMed] [Google Scholar]
- 32.Jarriault S, Brou C, Logeat F, Schroeter EH, Kopan R, Israel A. Signalling downstream of activated mammalian Notch. Nature. 1995;377:355–358. doi: 10.1038/377355a0. [DOI] [PubMed] [Google Scholar]
- 33.Hsieh JJ, Nofziger DE, Weinmaster G, Hayward SD. Epstein-Barr virus immortalization: Notch2 interacts with CBF1 and blocks differentiation. J. Virol. 1997;71:1938–1945. doi: 10.1128/jvi.71.3.1938-1945.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishimura M, Isaka F, Ishibashi M, Tomita K, Tsuda H, Nakanishi S, Kageyama R. Structure, chromosomal locus, and promoter of mouse Hes2 gene, a homologue of Drosophila hairy and Enhancer of split. Genomics. 1998;49:69–75. doi: 10.1006/geno.1998.5213. [DOI] [PubMed] [Google Scholar]
- 35.Ohtsuka T, Ishibashi M, Gradwohl G, Nakanishi S, Guillemot F, Kageyama R. Hes1 and Hes5 as notch effectors in mammalian neuronal differentiation. EMBO J. 1999;18:2196–2207. doi: 10.1093/emboj/18.8.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leimeister C, Schumacher N, Steidl C, Gessler M. Analysis of HeyL expression in wild-type and Notch pathway mutant mouse embryos. Mech. Dev. 2000;98:175–178. doi: 10.1016/s0925-4773(00)00459-7. [DOI] [PubMed] [Google Scholar]
- 37.Lin MH, Leimeister C, Gessler M, Kopan R. Activation of the Notch pathway in the hair cortex leads to aberrant differentiation of the adjacent hair-shaft layers. Development. 2000;127:2421–2432. doi: 10.1242/dev.127.11.2421. [DOI] [PubMed] [Google Scholar]
- 38.Maier MM, Gessler M. Comparative analysis of the human and mouse Hey1 promoter: Hey genes are new Notch target genes. Biochem. Biophys. Res. Commun. 2000;275:652–660. doi: 10.1006/bbrc.2000.3354. [DOI] [PubMed] [Google Scholar]
- 39.Iso T, Chung G, Hamamori Y, Kedes L. HERP1 is a cell type-specific primary target of Notch. J. Biol. Chem. 2002;277:6598–6607. doi: 10.1074/jbc.M110495200. [DOI] [PubMed] [Google Scholar]
- 40.Winkler C, Elmasri H, Klamt B, Volff JN, Gessler M. Characterization of hey bHLH genes in teleost fish. Dev. Genes Evol. 2003;213:541–553. doi: 10.1007/s00427-003-0360-6. [DOI] [PubMed] [Google Scholar]
- 41.Iso T, Sartorelli V, Poizat C, Iezzi S, Wu HY, Chung G, Kedes L, Hamamori Y. HERP, a novel heterodimer partner of HES/E(spl) in Notch signaling. Mol. Cell. Biol. 2001;21:6080–6089. doi: 10.1128/MCB.21.17.6080-6089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fischer A, Leimeister C, Winkler C, Schumacher N, Klamt B, Elmasri H, Steidl C, Maier M, Knobeloch KP, et al. Hey bHLH factors in cardiovascular development. Cold Spring Harb. Symp. Quant. Biol. 2002;67:63–70. doi: 10.1101/sqb.2002.67.63. [DOI] [PubMed] [Google Scholar]
- 43.Cossins J, Vernon AE, Zhang Y, Philpott A, Jones PH. Hes6 regulates myogenic differentiation. Development. 2002;129:2195–2207. doi: 10.1242/dev.129.9.2195. [DOI] [PubMed] [Google Scholar]
- 44.Leimeister C, Dale K, Fischer A, Klamt B, Hrabe de Angelis M, Radtke F, McGrew MJ, Pourquie O, Gessler M. Oscillating expression of c-Hey2 in the presomitic mesoderm suggests that the segmentation clock may use combinatorial signaling through multiple interacting bHLH factors. Dev. Biol. 2000;227:91–103. doi: 10.1006/dbio.2000.9884. [DOI] [PubMed] [Google Scholar]
- 45.Van Wayenbergh R, Taelman V, Pichon B, Fischer A, Kricha S, Gessler M, Christophe D, Bellefroid EJ. Identification of BOIP, a novel cDNA highly expressed during spermatogenesis that encodes a protein interacting with the orange domain of the hairy-related transcription factor HRT1/Hey1 in Xenopus and mouse. Dev. Dyn. 2003;228:716–725. doi: 10.1002/dvdy.10406. [DOI] [PubMed] [Google Scholar]
- 46.Ross DA, Hannenhalli S, Tobias JW, Cooch N, Shiekhattar R, Kadesch T. Functional analysis of Hes-1 in preadipocytes. Mol. Endocrinol. 2005;20:698–705. doi: 10.1210/me.2005-0325. [DOI] [PubMed] [Google Scholar]
- 47.Fischer A, Steidl C, Wagner TU, Lang E, Jakob PM, Friedl P, Knobeloch KP, Gessler M. Combined loss of Hey1 and HeyL causes congenital heart defects because of impaired epithelial to mesenchymal transition. Circ. Res. 2007;100:856–863. doi: 10.1161/01.RES.0000260913.95642.3b. [DOI] [PubMed] [Google Scholar]
- 48.Nakatani T, Mizuhara E, Minaki Y, Sakamoto Y, Ono Y. Helt, a novel basic-helix-loop-helix transcriptional repressor expressed in the developing central nervous system. J. Biol. Chem. 2004;279:16356–16367. doi: 10.1074/jbc.M311740200. [DOI] [PubMed] [Google Scholar]
- 49.Ledent V, Vervoort M. The basic helix-loop-helix protein family: comparative genomics and phylogenetic analysis. Genome Res. 2001;11:754–770. doi: 10.1101/gr.177001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kawamata S, Du C, Li K, Lavau C. Overexpression of the Notch target genes Hes in vivo induces lymphoid and myeloid alterations. Oncogene. 2002;21:3855–3863. doi: 10.1038/sj.onc.1205487. [DOI] [PubMed] [Google Scholar]
- 51.Jogi A, Persson P, Grynfeld A, Pahlman S, Axelson H. Modulation of basic helix-loop-helix transcription complex formation by Id proteins during neuronal differentiation. J. Biol. Chem. 2002;277:9118–9126. doi: 10.1074/jbc.M107713200. [DOI] [PubMed] [Google Scholar]
- 52.Sun J, Kamei CN, Layne MD, Jain MK, Liao JK, Lee ME, Chin MT. Regulation of myogenic terminal differentiation by the hairy-related transcription factor CHF2. J. Biol. Chem. 2001;276:18591–18596. doi: 10.1074/jbc.M101163200. [DOI] [PubMed] [Google Scholar]
- 53.Ghosh B, Leach SD. Interactions between Hairy/Enhancer of Split-related proteins and the pancreatic transcription factor Ptf1-p48 modulate function of the PTF1 transcriptional complex. Biochem. J. 2006;393:679–685. doi: 10.1042/BJ20051063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Firulli BA, Hadzic DB, McDaid JR, Firulli AB. The basic helix-loop-helix transcription factors dHAND and eHAND exhibit dimerization characteristics that suggest complex regulation of function. J. Biol. Chem. 2000;275:33567–33573. doi: 10.1074/jbc.M005888200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Giagtzoglou N, Koumbanakis KA, Fullard J, Zarifi I, Delidakis C. Role of the Sc C terminus in transcriptional activation and E(spl) repressor recruitment. J. Biol. Chem. 2005;280:1299–1305. doi: 10.1074/jbc.M408949200. [DOI] [PubMed] [Google Scholar]
- 56.Ohsako S, Hyer J, Panganiban G, Oliver I, Caudy M. Hairy function as a DNA-binding helix-loop-helix repressor of Drosophila sensory organ formation. Genes Dev. 1994;8:2743–2755. doi: 10.1101/gad.8.22.2743. [DOI] [PubMed] [Google Scholar]
- 57.Wainwright SM, Ish-Horowicz D. Point mutations in the Drosophila hairy gene demonstrate in vivo requirements for basic, helix-loop-helix, and WRPW domains. Mol. Cell. Biol. 1992;12:2475–2483. doi: 10.1128/mcb.12.6.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paroush Z, Finley RL, Jr, Kidd T, Wainwright SM, Ingham PW, Brent R, Ish-Horowicz D. Groucho is required for Drosophila neurogenesis, segmentation, and sex determination and interacts directly with hairy-related bHLH proteins. Cell. 1994;79:805–815. doi: 10.1016/0092-8674(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 59.Fisher AL, Ohsako S, Caudy M. The WRPW motif of the hairy-related basic helix-loop-helix repressor proteins acts as a 4-amino-acid transcription repression and protein-protein interaction domain. Mol. Cell. Biol. 1996;16:2670–2677. doi: 10.1128/mcb.16.6.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grbavec D, Stifani S. Molecular interaction between TLE1 and the carboxyl-terminal domain of HES-1 containing the WRPW motif. Biochem. Biophys. Res. Commun. 1996;223:701–705. doi: 10.1006/bbrc.1996.0959. [DOI] [PubMed] [Google Scholar]
- 61.Grbavec D, Lo R, Liu Y, Stifani S. Transducin-like Enhancer of split 2, a mammalian homologue of Drosophila Groucho, acts as a transcriptional repressor, interacts with Hairy/Enhancer of split proteins, and is expressed during neuronal development. Eur. J. Biochem. 1998;258:339–349. doi: 10.1046/j.1432-1327.1998.2580339.x. [DOI] [PubMed] [Google Scholar]
- 62.Gao X, Chandra T, Gratton MO, Quelo I, Prud'homme J, Stifani S, St-Arnaud R. HES6 acts as a transcriptional repressor in myoblasts and can induce the myogenic differentiation program. J. Cell. Biol. 2001;154:1161–1171. doi: 10.1083/jcb.200104058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McLarren KW, Theriault FM, Stifani S. Association with the nuclear matrix and interaction with Groucho and RUNX proteins regulate the transcription repression activity of the basic helix loop helix factor Hes1. J. Biol. Chem. 2001;276:1578–1584. doi: 10.1074/jbc.M007629200. [DOI] [PubMed] [Google Scholar]
- 64.Nuthall HN, Husain J, McLarren KW, Stifani S. Role for Hes1-induced phosphorylation in Groucho-mediated transcriptional repression. Mol. Cell. Biol. 2002;22:389–399. doi: 10.1128/MCB.22.2.389-399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gratton MO, Torban E, Jasmin SB, Theriault FM, German MS, Stifani S. Hes6 promotes cortical neurogenesis and inhibits Hes1 transcription repression activity by multiple mechanisms. Mol. Cell. Biol. 2003;23:6922–6935. doi: 10.1128/MCB.23.19.6922-6935.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen G, Fernandez J, Mische S, Courey AJ. A functional interaction between the histone deacetylase Rpd3 and the corepressor groucho in Drosophila development. Genes Dev. 1999;13:2218–2230. doi: 10.1101/gad.13.17.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Choi CY, Kim YH, Kwon HJ, Kim Y. The homeodomain protein NK-3 recruits Groucho and a histone deacetylase complex to repress transcription. J. Biol. Chem. 1999;274:33194–33197. doi: 10.1074/jbc.274.47.33194. [DOI] [PubMed] [Google Scholar]
- 68.Ju BG, Solum D, Song EJ, Lee KJ, Rose DW, Glass CK, Rosenfeld MG. Activating the PARP-1 sensor component of the groucho/TLE1 corepressor complex mediates a CaMKinase IIdelta-dependent neurogenic gene activation pathway. Cell. 2004;119:815–829. doi: 10.1016/j.cell.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 69.Bianchi-Frias D, Orian A, Delrow JJ, Vazquez J, Rosales-Nieves AE, Parkhurst SM. Hairy transcriptional repression targets and cofactor recruitment in Drosophila. PLoS Biol. 2004;2:E178. doi: 10.1371/journal.pbio.0020178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jennings BH, Pickles LM, Wainwright SM, Roe SM, Pearl LH, Ish-Horowicz D. Molecular recognition of transcriptional repressor motifs by the WD domain of the Groucho/TLE corepressor. Mol. Cell. 2006;22:645–655. doi: 10.1016/j.molcel.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 71.Pichon B, Taelman V, Bellefroid EJ, Christophe D. Transcriptional repression by the bHLH-Orange factor XHRT1 does not involve the C-terminal YRPW motif. Biochim. Biophys. Acta. 2004;1680:46–52. doi: 10.1016/j.bbaexp.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 72.Kathiriya IS, King IN, Murakami M, Nakagawa M, Astle JM, Gardner KA, Gerard RD, Olson EN, Srivastava D, et al. Hairy-related transcription factors inhibit GATA-dependent cardiac gene expression through a signal-responsive mechanism. J. Biol. Chem. 2004;279:54937–54943. doi: 10.1074/jbc.M409879200. [DOI] [PubMed] [Google Scholar]
- 73.Fischer A, Klattig J, Kneitz B, Diez H, Maier M, Holtmann B, Englert C, Gessler M. Hey basic helix-loop-helix transcription factors are repressors of GATA4 and GATA6 and restrict expression of the GATA target gene ANF in fetal hearts. Mol. Cell. Biol. 2005;25:8960–8970. doi: 10.1128/MCB.25.20.8960-8970.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Belandia B, Powell SM, Garcia-Pedrero JM, Walker MM, Bevan CL, Parker MG. Hey1, a mediator of notch signaling, is an androgen receptor corepressor. Mol. Cell. Biol. 2005;25:1425–1436. doi: 10.1128/MCB.25.4.1425-1436.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.King IN, Kathiriya IS, Murakami M, Nakagawa M, Gardner KA, Srivastava D, Nakagawa O. Hrt and Hes negatively regulate Notch signaling through interactions with RBP-Jkappa. Biochem. Biophys. Res. Commun. 2006;345:446–452. doi: 10.1016/j.bbrc.2006.04.097. [DOI] [PubMed] [Google Scholar]
- 76.Shirvani S, Xiang F, Koibuchi N, Chin MT. CHF1/Hey2 suppresses SM-MHC promoter activity through an interaction with GATA-6. Biochem. Biophys. Res. Commun. 2006;339:151–156. doi: 10.1016/j.bbrc.2005.10.190. [DOI] [PubMed] [Google Scholar]
- 77.Takata T, Ishikawa F. Human Sir2-related protein SIRT1 associates with the bHLH repressors HES1 and HEY2 and is involved in HES1- and HEY2-mediated transcriptional repression. Biochem. Biophys. Res. Commun. 2003;301:250–257. doi: 10.1016/s0006-291x(02)03020-6. [DOI] [PubMed] [Google Scholar]
- 78.Longo VD, Kennedy BK. Sirtuins in aging and age-related disease. Cell. 2006;126:257–268. doi: 10.1016/j.cell.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 79.Rosenberg MI, Parkhurst SM. Drosophila Sir2 is required for heterochromatic silencing and by euchromatic Hairy/E(Spl) bHLH repressors in segmentation and sex determination. Cell. 2002;109:447–458. doi: 10.1016/s0092-8674(02)00732-8. [DOI] [PubMed] [Google Scholar]
- 80.Poortinga G, Watanabe M, Parkhurst SM. Drosophila CtBP: a Hairy-interacting protein required for embryonic segmentation and hairy-mediated transcriptional repression. EMBO J. 1998;17:2067–2078. doi: 10.1093/emboj/17.7.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang H, Levine M. Groucho and dCtBP mediate separate pathways of transcriptional repression in the Drosophila embryo. Proc. Natl Acad. Sci. USA. 1999;96:535–540. doi: 10.1073/pnas.96.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Quinlan KG, Verger A, Kwok A, Lee SH, Perdomo J, Nardini M, Bolognesi M, Crossley M. Role of the C-terminal binding protein PXDLS motif binding cleft in protein interactions and transcriptional repression. Mol. Cell. Biol. 2006;26:8202–8213. doi: 10.1128/MCB.00445-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Elagib KE, Xiao M, Hussaini IM, Delehanty LL, Palmer LA, Racke FK, Birrer MJ, Shanmugasundaram G, McDevitt MA, et al. Jun blockade of erythropoiesis: role for repression of GATA-1 by HERP2. Mol. Cell. Biol. 2004;24:7779–7794. doi: 10.1128/MCB.24.17.7779-7794.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Proweller A, Pear WS, Parmacek MS. Notch signaling represses myocardin-induced smooth muscle cell differentiation. J. Biol. Chem. 2005;280:8994–9004. doi: 10.1074/jbc.M413316200. [DOI] [PubMed] [Google Scholar]
- 85.Ishiko E, Matsumura I, Ezoe S, Gale K, Ishiko J, Satoh Y, Tanaka H, Shibayama H, Mizuki M, et al. Notch signals inhibit the development of erythroid/megakaryocytic cells by suppressing GATA-1 activity through the induction of HES1. J. Biol. Chem. 2005;280:4929–4939. doi: 10.1074/jbc.M406788200. [DOI] [PubMed] [Google Scholar]
- 86.Crispino JD. GATA1 in normal and malignant hematopoiesis. Semin. Cell Dev. Biol. 2005;16:137–147. doi: 10.1016/j.semcdb.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 87.Sakata Y, Xiang F, Chen Z, Kiriyama Y, Kamei CN, Simon DI, Chin MT. Transcription factor CHF1/Hey2 regulates neointimal formation in vivo and vascular smooth muscle proliferation and migration in vitro. Arterioscler. Thromb. Vasc. Biol. 2004;24:2069–2074. doi: 10.1161/01.ATV.0000143936.77094.a4. [DOI] [PubMed] [Google Scholar]
- 88.Xiang F, Sakata Y, Cui L, Youngblood JM, Nakagami H, Liao JK, Liao R, Chin MT. Transcription factor CHF1/Hey2 suppresses cardiac hypertrophy through an inhibitory interaction with GATA4. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H1997–H2006. doi: 10.1152/ajpheart.01106.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Doi H, Iso T, Yamazaki M, Akiyama H, Kanai H, Sato H, Kawai-Kowase K, Tanaka T, Maeno T, et al. HERP1 inhibits myocardin-induced vascular smooth muscle cell differentiation by interfering with SRF binding to CArG box. Arterioscler. Thromb. Vasc. Biol. 2005;25:2328–2334. doi: 10.1161/01.ATV.0000185829.47163.32. [DOI] [PubMed] [Google Scholar]
- 90.Wang D, Chang PS, Wang Z, Sutherland L, Richardson JA, Small E, Krieg PA, Olson EN. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell. 2001;105:851–862. doi: 10.1016/s0092-8674(01)00404-4. [DOI] [PubMed] [Google Scholar]
- 91.Zamurovic N, Cappellen D, Rohner D, Susa M. Coordinated activation of notch, Wnt, and transforming growth factor-beta signaling pathways in bone morphogenic protein 2- induced osteogenesis. Notch target gene Hey1 inhibits mineralization and Runx2 transcriptional activity. J. Biol. Chem. 2004;279:37704–37715. doi: 10.1074/jbc.M403813200. [DOI] [PubMed] [Google Scholar]
- 92.McLarren KW, Lo R, Grbavec D, Thirunavukkarasu K, Karsenty G, Stifani S. The mammalian basic helix loop helix protein HES-1 binds to and modulates the transactivating function of the runt-related factor Cbfa1. J. Biol. Chem. 2000;275:530–538. doi: 10.1074/jbc.275.1.530. [DOI] [PubMed] [Google Scholar]
- 93.Shen Q, Christakos S. The vitamin D receptor, Runx2, and the Notch signaling pathway cooperate in the transcriptional regulation of osteopontin. J. Biol. Chem. 2005;280:40589–40598. doi: 10.1074/jbc.M504166200. [DOI] [PubMed] [Google Scholar]
- 94.Allen RD, III, Kim HK, Sarafova SD, Siu G. Negative regulation of CD4 gene expression by a HES-1-c-Myb complex. Mol. Cell. Biol. 2001;21:3071–3082. doi: 10.1128/MCB.21.9.3071-3082.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Holderfield MT, Henderson Anderson AM, Kokubo H, Chin MT, Johnson RL, Hughes CC. HESR1/CHF2 suppresses VEGFR2 transcription independent of binding to E-boxes. Biochem. Biophys. Res. Commun. 2006;346:637–648. doi: 10.1016/j.bbrc.2006.05.177. [DOI] [PubMed] [Google Scholar]
- 96.Henderson AM, Wang SJ, Taylor AC, Aitkenhead M, Hughes CC. The basic helix-loop-helix transcription factor HESR1 regulates endothelial cell tube formation. J. Biol. Chem. 2001;276:6169–6176. doi: 10.1074/jbc.M008506200. [DOI] [PubMed] [Google Scholar]
- 97.Taylor KL, Henderson AM, Hughes CC. Notch activation during endothelial cell network formation in vitro targets the basic HLH transcription factor HESR-1 and downregulates VEGFR-2/KDR expression. Microvasc. Res. 2002;64:372–383. doi: 10.1006/mvre.2002.2443. [DOI] [PubMed] [Google Scholar]
- 98.Williams CK, Li JL, Murga M, Harris AL, Tosato G. Up-regulation of the Notch ligand Delta-like 4 inhibits VEGF-induced endothelial cell function. Blood. 2006;107:931–939. doi: 10.1182/blood-2005-03-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sun H, Taneja R. Stra13 expression is associated with growth arrest and represses transcription through histone deacetylase (HDAC)-dependent and HDAC-independent mechanisms. Proc. Natl Acad. Sci. USA. 2000;97:4058–4063. doi: 10.1073/pnas.070526297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Oellers N, Dehio M, Knust E. bHLH proteins encoded by the Enhancer of split complex of Drosophila negatively interfere with transcriptional activation mediated by proneural genes. Mol. Gen. Genet. 1994;244:465–473. doi: 10.1007/BF00583897. [DOI] [PubMed] [Google Scholar]
- 101.Tietze K, Oellers N, Knust E. Enhancer of splitD, a dominant mutation of Drosophila, and its use in the study of functional domains of a helix-loop-helix protein. Proc. Natl Acad. Sci. USA. 1992;89:6152–6156. doi: 10.1073/pnas.89.13.6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Van Doren M, Bailey AM, Esnayra J, Ede K, Posakony JW. Negative regulation of proneural gene activity: hairy is a direct transcriptional repressor of achaete. Genes Dev. 1994;8:2729–2742. doi: 10.1101/gad.8.22.2729. [DOI] [PubMed] [Google Scholar]
- 103.Jennings BH, Tyler DM, Bray SJ. Target specificities of Drosophila enhancer of split basic helix-loop-helix proteins. Mol. Cell. Biol. 1999;19:4600–4610. doi: 10.1128/mcb.19.7.4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hojo M, Ohtsuka T, Hashimoto N, Gradwohl G, Guillemot F, Kageyama R. Glial cell fate specification modulated by the bHLH gene Hes5 in mouse retina. Development. 2000;127:2515–2522. doi: 10.1242/dev.127.12.2515. [DOI] [PubMed] [Google Scholar]
- 105.Dubrulle J, Pourquie O. Coupling segmentation to axis formation. Development. 2004;131:5783–5793. doi: 10.1242/dev.01519. [DOI] [PubMed] [Google Scholar]
- 106.Palmeirim I, Henrique D, Ish-Horowicz D, Pourquie O. Avian hairy gene expression identifies a molecular clock linked to vertebrate segmentation and somitogenesis. Cell. 1997;91:639–648. doi: 10.1016/s0092-8674(00)80451-1. [DOI] [PubMed] [Google Scholar]
- 107.Jouve C, Palmeirim I, Henrique D, Beckers J, Gossler A, Ish-Horowicz D, Pourquie O. Notch signalling is required for cyclic expression of the hairy-like gene HES1 in the presomitic mesoderm. Development. 2000;127:1421–1429. doi: 10.1242/dev.127.7.1421. [DOI] [PubMed] [Google Scholar]
- 108.Bessho Y, Sakata R, Komatsu S, Shiota K, Yamada S, Kageyama R. Dynamic expression and essential functions of Hes7 in somite segmentation. Genes Dev. 2001;15:2642–2647. doi: 10.1101/gad.930601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Takebayashi K, Sasai Y, Sakai Y, Watanabe T, Nakanishi S, Kageyama R. Structure, chromosomal locus, and promoter analysis of the gene encoding the mouse helix-loop-helix factor HES-1. Negative autoregulation through the multiple N box elements. J. Biol. Chem. 1994;269:5150–5156. [PubMed] [Google Scholar]
- 110.Hirata H, Bessho Y, Kokubu H, Masamizu Y, Yamada S, Lewis J, Kageyama R. Instability of Hes7 protein is crucial for the somite segmentation clock. Nat. Genet. 2004;36:750–754. doi: 10.1038/ng1372. [DOI] [PubMed] [Google Scholar]
- 111.Hirata H, Yoshiura S, Ohtsuka T, Bessho Y, Harada T, Yoshikawa K, Kageyama R. Oscillatory expression of the bHLH factor Hes1 regulated by a negative feedback loop. Science. 2002;298:840–843. doi: 10.1126/science.1074560. [DOI] [PubMed] [Google Scholar]
- 112.Masamizu Y, Ohtsuka T, Takashima Y, Nagahara H, Takenaka Y, Yoshikawa K, Okamura H, Kageyama R. Real-time imaging of the somite segmentation clock: revelation of unstable oscillators in the individual presomitic mesoderm cells. Proc. Natl Acad. Sci. USA. 2006;103:1313–1318. doi: 10.1073/pnas.0508658103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chi JT, Chang HY, Haraldsen G, Jahnsen FL, Troyanskaya OG, Chang DS, Wang Z, Rockson SG, van de Rijn M, et al. Endothelial cell diversity revealed by global expression profiling. Proc. Natl Acad. Sci. USA. 2003;100:10623–10628. doi: 10.1073/pnas.1434429100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen H, Thiagalingam A, Chopra H, Borges MW, Feder JN, Nelkin BD, Baylin SB, Ball DW. Conservation of the Drosophila lateral inhibition pathway in human lung cancer: a hairy-related protein (HES-1) directly represses achaete-scute homolog-1 expression. Proc. Natl Acad. Sci. USA. 1997;94:5355–5360. doi: 10.1073/pnas.94.10.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sriuranpong V, Borges MW, Strock CL, Nakakura EK, Watkins DN, Blaumueller CM, Nelkin BD, Ball DW. Notch signaling induces rapid degradation of achaete-scute homolog 1. Mol. Cell. Biol. 2002;22:3129–3139. doi: 10.1128/MCB.22.9.3129-3139.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Murata K, Hattori M, Hirai N, Shinozuka Y, Hirata H, Kageyama R, Sakai T, Minato N. Hes1 directly controls cell proliferation through the transcriptional repression of p27Kip1. Mol. Cell. Biol. 2005;25:4262–4271. doi: 10.1128/MCB.25.10.4262-4271.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu A, Li J, Marin-Husstege M, Kageyama R, Fan Y, Gelinas C, Casaccia-Bonnefil P. A molecular insight of Hes5-dependent inhibition of myelin gene expression: old partners and new players. EMBO J. 2006;25:4833–4842. doi: 10.1038/sj.emboj.7601352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Strom A, Castella P, Rockwood J, Wagner J, Caudy M. Mediation of NGF signaling by post-translational inhibition of HES-1, a basic helix-loop-helix repressor of neuronal differentiation. Genes Dev. 1997;11:3168–3181. doi: 10.1101/gad.11.23.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Itoh F, Itoh S, Goumans MJ, Valdimarsdottir G, Iso T, Dotto GP, Hamamori Y, Kedes L, Kato M, et al. Synergy and antagonism between Notch and BMP receptor signaling pathways in endothelial cells. EMBO J. 2004;23:541–551. doi: 10.1038/sj.emboj.7600065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ishibashi M, Ang SL, Shiota K, Nakanishi S, Kageyama R, Guillemot F. Targeted disruption of mammalian hairy and Enhancer of split homolog-1 (HES-1) leads to up-regulation of neural helix-loop-helix factors, premature neurogenesis, and severe neural tube defects. Genes Dev. 1995;9:3136–3148. doi: 10.1101/gad.9.24.3136. [DOI] [PubMed] [Google Scholar]
- 121.Cau E, Gradwohl G, Casarosa S, Kageyama R, Guillemot F. Hes genes regulate sequential stages of neurogenesis in the olfactory epithelium. Development. 2000;127:2323–2332. doi: 10.1242/dev.127.11.2323. [DOI] [PubMed] [Google Scholar]
- 122.Nakamura Y, Sakakibara S, Miyata T, Ogawa M, Shimazaki T, Weiss S, Kageyama R, Okano H. The bHLH gene hes1 as a repressor of the neuronal commitment of CNS stem cells. J. Neurosci. 2000;20:283–293. doi: 10.1523/JNEUROSCI.20-01-00283.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tomita K, Ishibashi M, Nakahara K, Ang SL, Nakanishi S, Guillemot F, Kageyama R. Mammalian hairy and Enhancer of split homolog 1 regulates differentiation of retinal neurons and is essential for eye morphogenesis. Neuron. 1996;16:723–734. doi: 10.1016/s0896-6273(00)80093-8. [DOI] [PubMed] [Google Scholar]
- 124.Lee HY, Wroblewski E, Philips GT, Stair CN, Conley K, Reedy M, Mastick GS, Brown NL. Multiple requirements for Hes 1 during early eye formation. Dev. Biol. 2005;284:464–478. doi: 10.1016/j.ydbio.2005.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hatakeyama J, Bessho Y, Katoh K, Ookawara S, Fujioka M, Guillemot F, Kageyama R. Hes genes regulate size, shape and histogenesis of the nervous system by control of the timing of neural stem cell differentiation. Development. 2004;131:5539–5550. doi: 10.1242/dev.01436. [DOI] [PubMed] [Google Scholar]
- 126.Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P, et al. Control of endodermal endocrine development by Hes-1. Nat. Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- 127.Tomita K, Hattori M, Nakamura E, Nakanishi S, Minato N, Kageyama R. The bHLH gene Hes1 is essential for expansion of early T cell precursors. Genes Dev. 1999;13:1203–1210. doi: 10.1101/gad.13.9.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hirata H, Tomita K, Bessho Y, Kageyama R. Hes1 and Hes3 regulate maintenance of the isthmic organizer and development of the mid/hindbrain. EMBO J. 2001;20:4454–4466. doi: 10.1093/emboj/20.16.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zine A, Aubert A, Qiu J, Therianos S, Guillemot F, Kageyama R, de Ribaupierre F. Hes1 and Hes5 activities are required for the normal development of the hair cells in the mammalian inner ear. J. Neurosci. 2001;21:4712–4720. doi: 10.1523/JNEUROSCI.21-13-04712.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ohtsuka T, Sakamoto M, Guillemot F, Kageyama R. Roles of the basic helix-loop-helix genes Hes1 and Hes5 in expansion of neural stem cells of the developing brain. J. Biol. Chem. 2001;276:30467–30474. doi: 10.1074/jbc.M102420200. [DOI] [PubMed] [Google Scholar]
- 131.Hatakeyama J, Sakamoto S, Kageyama R. Hes1 and Hes5 regulate the development of the cranial and spinal nerve systems. Dev. Neurosci. 2006;28:92–101. doi: 10.1159/000090756. [DOI] [PubMed] [Google Scholar]
- 132.Fischer A, Schumacher N, Maier M, Sendtner M, Gessler M. The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev. 2004;18:901–911. doi: 10.1101/gad.291004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kokubo H, Miyagawa-Tomita S, Nakazawa M, Saga Y, Johnson RL. Mouse hesr1 and hesr2 genes are redundantly required to mediate Notch signaling in the developing cardiovascular system. Dev. Biol. 2005;278:301–309. doi: 10.1016/j.ydbio.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 134.Gessler M, Knobeloch KP, Helisch A, Amann K, Schumacher N, Rohde E, Fischer A, Leimeister C. Mouse gridlock: no aortic coarctation or deficiency, but fatal cardiac defects in Hey2 -/- mice. Curr. Biol. 2002;12:1601–1604. doi: 10.1016/s0960-9822(02)01150-8. [DOI] [PubMed] [Google Scholar]
- 135.Donovan J, Kordylewska A, Jan YN, Utset MF. Tetralogy of fallot and other congenital heart defects in Hey2 mutant mice. Curr. Biol. 2002;12:1605–1610. doi: 10.1016/s0960-9822(02)01149-1. [DOI] [PubMed] [Google Scholar]
- 136.Sakata Y, Kamei CN, Nakagami H, Bronson R, Liao JK, Chin MT. Ventricular septal defect and cardiomyopathy in mice lacking the transcription factor CHF1/Hey2. Proc. Natl Acad. Sci. USA. 2002;99:16197–16202. doi: 10.1073/pnas.252648999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fischer A, Klamt B, Schumacher N, Glaeser C, Hansmann I, Fenge H, Gessler M. Phenotypic variability in Hey2 -/- mice and absence of HEY2 mutations in patients with congenital heart defects or Alagille syndrome. Mamm. Genome. 2004;15:711–716. doi: 10.1007/s00335-004-2389-x. [DOI] [PubMed] [Google Scholar]
- 138.Kokubo H, Miyagawa-Tomita S, Tomimatsu H, Nakashima Y, Nakazawa M, Saga Y, Johnson RL. Targeted disruption of hesr2 results in atrioventricular valve anomalies that lead to heart dysfunction. Circ. Res. 2004;95:540–547. doi: 10.1161/01.RES.0000141136.85194.f0. [DOI] [PubMed] [Google Scholar]
- 139.Lee JS, Thomas DM, Gutierrez G, Carty SA, Yanagawa S, Hinds PW. HES1 cooperates with pRb to activate RUNX2-dependent transcription. J. Bone Miner. Res. 2006;21:921–933. doi: 10.1359/jbmr.060303. [DOI] [PubMed] [Google Scholar]
- 140.Persson P, Stockhausen MT, Pahlman S, Axelson H. Ubiquilin-1 is a novel HASH-1-complexing protein that regulates levels of neuronal bHLH transcription factors in human neuroblastoma cells. Int. J. Oncol. 2004;25:1213–1221. [PubMed] [Google Scholar]
- 141.Yan B, Heus J, Lu N, Nichols RC, Raben N, Plotz PH. Transcriptional regulation of the human acid alpha-glucosidase gene. Identification of a repressor element and its transcription factors Hes-1 and YY1. J. Biol. Chem. 2001;276:1789–1793. doi: 10.1074/jbc.M005959200. [DOI] [PubMed] [Google Scholar]
- 142.Yan B, Raben N, Plotz PH. Hes-1, a known transcriptional repressor, acts as a transcriptional activator for the human acid alpha-glucosidase gene in human fibroblast cells. Biochem. Biophys. Res. Commun. 2002;291:582–587. doi: 10.1006/bbrc.2002.6483. [DOI] [PubMed] [Google Scholar]
- 143.Mammucari C, Tommasi di Vignano A, Sharov AA, Neilson J, Havrda MC, Roop DR, Botchkarev VA, Crabtree GR, Dotto GP. Integration of Notch 1 and calcineurin/NFAT signaling pathways in keratinocyte growth and differentiation control. Dev. Cell. 2005;8:665–676. doi: 10.1016/j.devcel.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 144.Kim HK, Siu G. The notch pathway intermediate HES-1 silences CD4 gene expression. Mol. Cell. Biol. 1998;18:7166–7175. doi: 10.1128/mcb.18.12.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hartman J, Muller P, Foster JS, Wimalasena J, Gustafsson JA, Strom A. HES-1 inhibits 17beta-estradiol and heregulin-beta1-mediated upregulation of E2F-1. Oncogene. 2004;23:8826–8833. doi: 10.1038/sj.onc.1208139. [DOI] [PubMed] [Google Scholar]
- 146.Fryer CJ, White JB, Jones KA. Mastermind recruits CycC:CDK8 to phosphorylate the Notch ICD and coordinate activation with turnover. Mol. Cell. 2004;16:509–520. doi: 10.1016/j.molcel.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 147.Castella P, Sawai S, Nakao K, Wagner JA, Caudy M. HES-1 repression of differentiation and proliferation in PC12 cells: role for the helix 3-helix 4 domain in transcription repression. Mol. Cell. Biol. 2000;20:6170–6183. doi: 10.1128/mcb.20.16.6170-6183.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lee JC, Smith SB, Watada H, Lin J, Scheel D, Wang J, Mirmira RG, German MS. Regulation of the pancreatic pro-endocrine gene neurogenin3. Diabetes. 2001;50:928–936. doi: 10.2337/diabetes.50.5.928. [DOI] [PubMed] [Google Scholar]
- 149.Fujimori K, Fujitani Y, Kadoyama K, Kumanogoh H, Ishikawa K, Urade Y. Regulation of lipocalin-type prostaglandin D synthase gene expression by Hes-1 through E-box and interleukin-1 beta via two NF-kappa B elements in rat leptomeningeal cells. J. Biol. Chem. 2003;278:6018–6026. doi: 10.1074/jbc.M208288200. [DOI] [PubMed] [Google Scholar]
- 150.Matter-Sadzinski L, Puzianowska-Kuznicka M, Hernandez J, Ballivet M, Matter JM. A bHLH transcriptional network regulating the specification of retinal ganglion cells. Development. 2005;132:3907–3921. doi: 10.1242/dev.01960. [DOI] [PubMed] [Google Scholar]
- 151.Castella P, Wagner JA, Caudy M. Regulation of hippocampal neuronal differentiation by the basic helix-loop-helix transcription factors HES-1 and MASH-1. J. Neurosci. Res. 1999;56:229–240. doi: 10.1002/(SICI)1097-4547(19990501)56:3<229::AID-JNR2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 152.Esni F, Ghosh B, Biankin AV, Lin JW, Albert MA, Yu X, MacDonald RJ, Civin CI, Real FX, et al. Notch inhibits Ptf1 function and acinar cell differentiation in developing mouse and zebrafish pancreas. Development. 2004;131:4213–4224. doi: 10.1242/dev.01280. [DOI] [PubMed] [Google Scholar]
- 153.Ross DA, Rao PK, Kadesch T. Dual roles for the Notch target gene Hes-1 in the differentiation of 3T3-L1 preadipocytes. Mol. Cell. Biol. 2004;24:3505–3513. doi: 10.1128/MCB.24.8.3505-3513.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Huang Q, Raya A, DeJesus P, Chao SH, Quon KC, Caldwell JS, Chanda SK, Izpisua-Belmonte JC, Schultz PG. Identification of p53 regulators by genome-wide functional analysis. Proc. Natl Acad. Sci. USA. 2004;101:3456–3461. doi: 10.1073/pnas.0308562100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Georgia S, Soliz R, Li M, Zhang P, Bhushan A. p57 and Hes1 coordinate cell cycle exit with self-renewal of pancreatic progenitors. Dev. Biol. 2006;298:22–31. doi: 10.1016/j.ydbio.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 156.Fuke S, Sasagawa N, Ishiura S. Identification and characterization of the Hesr1/Hey1 as a candidate trans-acting factor on gene expression through the 3' non-coding polymorphic region of the human dopamine transporter (DAT1) gene. J. Biochem. (Tokyo) 2005;137:205–216. doi: 10.1093/jb/mvi020. [DOI] [PubMed] [Google Scholar]
- 157.Fuke S, Minami N, Kokubo H, Yoshikawa A, Yasumatsu H, Sasagawa N, Saga Y, Tsukahara T, Ishiura S. Hesr1 knockout mice exhibit behavioral alterations through the dopaminergic nervous system. J. Neurosci. Res. 2006;84:1555–1563. doi: 10.1002/jnr.21062. [DOI] [PubMed] [Google Scholar]
- 158.Sakamoto M, Hirata H, Ohtsuka T, Bessho Y, Kageyama R. The basic helix-loop-helix genes Hesr1/Hey1 and Hesr2/Hey2 regulate maintenance of neural precursor cells in the brain. J. Biol. Chem. 2003;278:44808–44815. doi: 10.1074/jbc.M300448200. [DOI] [PubMed] [Google Scholar]
- 159.Takahashi R, Kobayashi C, Kondo Y, Nakatani Y, Kudo I, Kunimoto M, Imura N, Hara S. Subcellular localization and regulation of hypoxia-inducible factor-2alpha in vascular endothelial cells. Biochem. Biophys. Res. Commun. 2004;317:84–91. doi: 10.1016/j.bbrc.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 160.Yada K, Do E, Sakakibara S, Ohsaki E, Ito E, Watanabe S, Ueda K. KSHV RTA induces a transcriptional repressor,HEY1 that represses rta promoter. Biochem. Biophys. Res. Commun. 2006;345:410–418. doi: 10.1016/j.bbrc.2006.04.092. [DOI] [PubMed] [Google Scholar]
- 161.Kokubo H, Tomita-Miyagawa S, Hamada Y, Saga Y. Hesr1 and Hesr2 regulate atrioventricular boundary formation in the developing heart through the repression of Tbx2. Development. 2007;134:747–755. doi: 10.1242/dev.02777. [DOI] [PubMed] [Google Scholar]