Abstract
Several recent publications have explored cap-independent translation through an internal ribosome entry site (IRES) in the 5′-UTR of the mRNA encoding the cyclin-dependent kinase inhibitor p27. The major experimental tool used in these reports was the use of bicistronic reporter constructs in which the 5′-UTR was inserted between the upstream and downstream cistrons. None of these reports has completely ruled out the possibility that the 5′-UTR has either cryptic promoter activity or a cryptic splice acceptor site. Either of these possibilities could result in expression of a monocistronic mRNA encoding the downstream cistron and false identification of an IRES. Indeed, Liu et al. recently published data suggesting that the p27 5′-UTR harbors cryptic promoter activity which accounts for its putative IRES activity. In this report, we have explored this potential problem further using promoterless bicistronic constructs coupled with RNase protection assays, siRNA knockdown of individual cistrons, RT-PCR to detect mRNA encoded by the bicistronic reporter with high sensitivity, direct transfection of bicistronic mRNAs, and insertion of an iron response element into the bicistronic reporter. The results do not support the conclusion that the p27 5′-UTR has significant functional promoter activity or cryptic splice sites, but rather that it is able to support cap-independent initiation of translation.
INTRODUCTION
Translation of most eukaryotic cellular mRNAs is initiated by the binding of the eIF4F complex to the 7-methylguanosine cap. This is followed by recruitment of the 43S small ribosomal preinitiation complex and then scanning of the complex to the start codon. An alternative mechanism of translational initiation involves an internal ribosome entry site (IRES). An IRES is an RNA element that can facilitate ribosome recruitment independent of the 5′ cap structure of an mRNA. IRESs were first characterized in viral RNAs (1,2). Viral infection often leads to inhibition of cellular mRNA translation by inactivating the cap-dependent mechanism. For example, polio virus infection leads to cleavage of eIF4G (3), an essential component of the cap-binding eIF4F complex, thereby inhibiting cap-dependent initiation. However, the presence of an IRES in the viral RNA allows efficient synthesis of viral proteins to proceed under these conditions.
A number of reports have described cellular mRNAs with IRES activity (4). In many cases, these IRESs are thought to allow continued translation under conditions of cellular stress in which cap-dependent initiation is repressed. The mechanisms by which translation is initiated through IRESs in cellular mRNAs have not yet been characterized in detail. Moreover, for many of the putative cellular IRESs, it has been questioned whether or not the analysis was stringent enough to prove their existence (5). For example, it is common to use a bicistronic vector system in which the putative IRES sequence is inserted between the upstream and downstream coding regions. Expression of the downstream cistron is indicative of an IRES encoded within the inserted sequence. However, possible artifacts could arise using this system if the insert has cryptic promoter activity or a cryptic 3′ splice site (5–7). In either case a monocistronic mRNA encoding the downstream coding region would be produced that would be translated in a cap-dependent manner. It is clear that stringent tests need to be performed in order to conclude that a cellular mRNA has a functional IRES.
p27 is an inhibitor of cyclin-dependent kinases (CDKs) and is best understood for its role in blocking the G1 to S transition through inhibition of CDK2. Its expression is often downregulated in tumor cells and for many types of cancer there is a correlation between loss of p27 expression and poor prognosis (8). Conversely, p27 is upregulated under conditions of growth factor deprivation and many other types of cellular stress. This is known to involve enhanced translation of the p27 mRNA (9–12). Therefore p27 translation is typically highest under conditions where cap-dependent translation rates are reduced. This suggested the possibility that the p27 mRNA contained an IRES that could mediate cap-independent translation initiation. We tested this using a variety of methods, including bicistronic vectors, strong upstream stem-loops to block scanning and inhibition of cap-dependent translation by active 4EBP (12). All of the results supported the conclusion that an IRES resides in the 5′-UTR of the p27 mRNA. These results have been independently verified by several other groups (13–15). However, none of these reports completely ruled out the possibility of a cryptic promoter or splice site residing within the p27 5′-UTR. In fact, a recent publication has suggested that the p27 5′-UTR does display cryptic promoter activity and that this accounts for the putative IRES activity (16). These authors used transfection of promoterless constructs and RNase protection assays to support their conclusions, which are clearly in disagreement with previous reports supporting cap-independent translation of the p27 mRNA. Given the conflicting conclusions reached by this report and previous reports, we have performed further experiments to examine the possibility of cap-independent translation through the p27 5′-UTR. The results do not support the existence of cryptic promoter activity or splice sites in the region, indicating the presence of an authentic IRES capable of mediating cap-independent initiation of translation.
MATERIALS AND METHODS
Construction of plasmids
The dual luciferase bicistronic construct pTKLL was constructed by ligating an XbaI/BglII fragment from pRL-TK (Promega) with an AvrII/BglII fragment from pGL2CAT. pTKLL-472, carrying the human p27 5′-UTR between the Renilla and firefly luciferase coding regions, was constructed by ligating the XbaI/BglII fragment from pRL-TK (Promega) with an AvrII/BglII fragment from pGL2CAT/Luc-472 (17). To create the promoterless bicistronic constructs, pLL and pLL-472, pTKLL and pTKLL-472 were digested with MluI to remove the entire TK promoter from the constructs. The digested plasmids were then recircularized by ligation. To insert an IRE into pTKLL-472, a two step PCR strategy was used. First, two PCR reactions were performed using pTKLL-472 as a template. One reaction used primers TKLL-IRE1 and TKLL-9-5′ and the other reaction used primers TKLL-IRE2 and TKLL-1080-3′ (sequences available upon request). The products of these reactions were mixed as a template for the subsequent PCR amplification using primers TKLL-9-5′ and TKLL-1080-3′. The product of this reaction was digested with NheI and ligated with the large fragment resulting from NheI digestion of pTKLL-472. The final product (pTKLL-472-IRE) has an IRE 35 nt downstream of the transcriptional start site of the TK promoter. Monocistronic constructs based on the pGL4 backbone were prepared by ligating the p27 5′-UTR sequence, in either the forward or reverse orientation, into the HindIII site of PGL4.10[luc2] (Promega). Bicistronic constructs based on the pGL4 backbone were prepared by first ligating the p27 5′-UTR sequence, in either the forward or reverse orientation, into the HindIII site of pGL4.70[hRluc] creating pGL4.70[hRluc]-472 and pGL4.70[hRluc]-472R. The NheI/XbaI fragments from these two constructs were then ligated into the XbaI site of pGL4.13[luc2/SV40] creating pGL4.13-F/R-472 and pGL4.13-F/R-472R. Promoterless bicistronic constructs, pGL4.10-F/R-472 and pGL4.10-F/R-472R, were prepared by ligating the NheI/XbaI fragments from pGL4.70[hRluc]-472 and pGL4.70[hRluc]-472R into the XbaI site of pGL4.10[luc2].
Cell culture, DNA transfections and reporter gene assays
Breast cancer cell lines were grown in Dulbecco's modified Eagle Medium (DMEM) containing 10% fetal calf serum, 100 U/ml penicillin and 100 μg/ml streptomycin. NIH3T3 cells were grown in the same medium but with newborn calf serum. All cells were incubated in a humidified atmosphere containing 5% CO2. DNA constructs were transfected using GenePORTER (Gene Therapy Systems) or Dreamfect (Boca Scientific) according to the manufacturers’ instructions. Renilla and firefly luciferase levels were tested using the Dual-Glo™ Luciferase assay system (Promega). Where indicated, DFO was added to the cultures at a final concentration of 100 μM.
In vitro transcription and mRNA transfection
DNA templates for in vitro transcription were made by PCR using the Advantage 2 PCR kit (BD Biosciences). Vectors containing the human p27 472 nt 5′-UTR inserted in the forward and reverse orientations between the Renilla and firefly coding regions were used as templates for PCR. The 5′ primer contained a T7 5′ overhang and the 3′ primer contained a 30 nt polyA 3′ overhang. The templates were gel purified after PCR.
Messenger RNA (G-capped, polyA-tailed) was made in vitro using the mMessage Machine kit (Ambion) using 1 μg of the gel-purified templates as per kit protocol. The mRNA was checked on a formaldehyde gel and the concentration calculated. The mRNA was then transfected into the different cell lines using DMRIE-C transfection reagent (Invitrogen). Cells in 35 mm dishes were rinsed to remove any antibiotics. DMEM (1 ml/dish) was mixed with the DMRIE-C and then the RNA was added at a ratio of 2 μl DMRIE-C per 1 μg RNA. The mixture was vortexed and immediately applied to the cells. After a 4h incubation at 37°C with 5% CO2, the medium was replaced with DMEM containing 10% FCS. The cells were incubated six additional hours and then harvested in 150 μl 1× Reporter Lysis Buffer (Promega).
RT-PCR
MCF7 cells were transfected with pTKLL or pTKLL-472 one day before harvesting. Cells were lysed in RLN buffer containing 50 mM Tris-HCl pH 8.0, 140 mM NaCl, 1.5 mM MgCl2, 0.5% NP-40, 1 mM dithiothreitol and SUPERase.in (Ambion). The cell lysates were centrifuged at ∼600 × g for 10 min and the supernatants were used to extract RNA using the RNeasy Mini Kit (Qiagen). To remove DNA contamination, RNA samples were further treated with DNase and then DNase was inactivated using TURBO DNA-free (Ambion). The isolated RNA was then used as a template for reverse transcription using AMV reverse transcriptase and a 3′-specific primer and amplified by PCR using specific primers. The PCR product spans the entire Renilla luciferase coding region and the first 70 bp of the firefly luciferase coding region (see Figure 5).
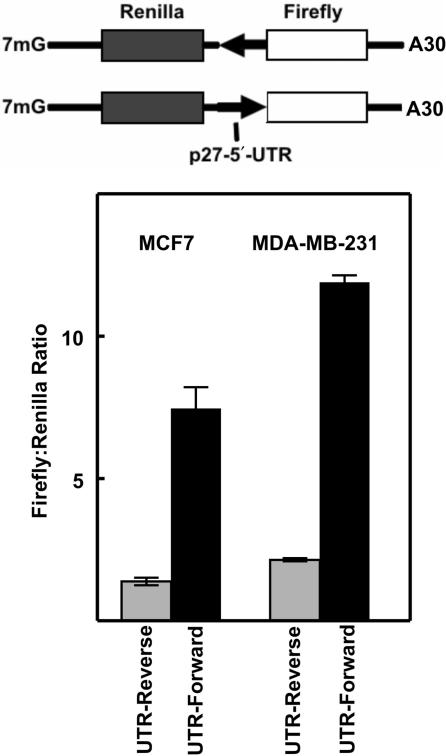
Figure 5.
Transfection of bicistronic mRNAs into human breast cancer cells. Top: Diagram of mRNA molecules that were synthesized by in vitro transcription. The arrows indicate the direction of the p27 5′-UTR sequence (reverse is the antisense orientation and forward is the sense orientation). Bottom: The in vitro synthesized mRNAs were transfected into two human breast cancer cell lines (MCF7 and MDA-MB-231) and Renilla and firefly luciferase activities were determined. For each cell line, the ratio of firefly to Renilla luciferase activity is shown for the antisense 5′-UTR (Reverse, gray bars) and the sense 5′-UTR (Forward, black bars).
RNase protection assays
DNA templates for preparing radioactive RNA probes were prepared by PCR using the Advantage PCR system (Clontech). The 5′ primer for each reaction included a T7 promoter sequence. The regions amplified for each probe are shown in Figure 2. The templates were gel purified by agarose gel electrophoresis, ethanol precipitated, and then redissolved in sterile water at a concentration of 0.5 μg/μl. Labeled probes were synthesized using the T7 Maxiscript kit (Promega) by including 32P-UTP in the reaction. Probes were purified by electrophoresis using denaturing 4% polyacrylamide gels. Probes were estimated to have a specific activity of ∼5 × 108 c.p.m./μg.
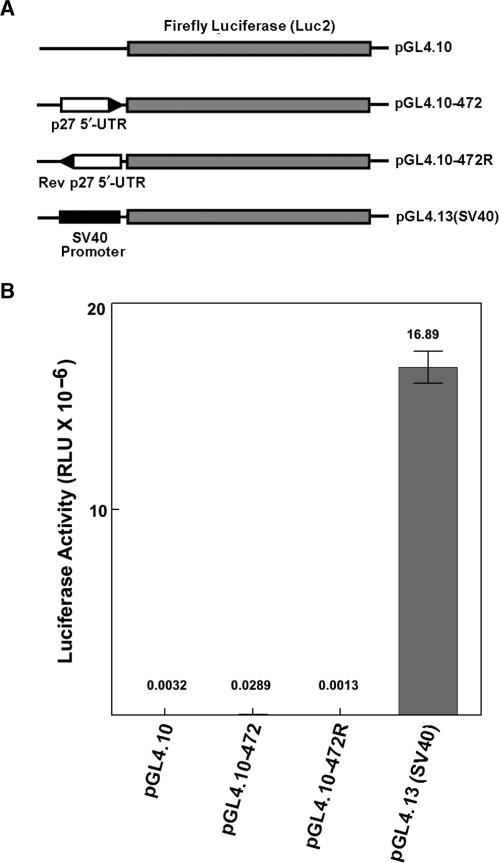
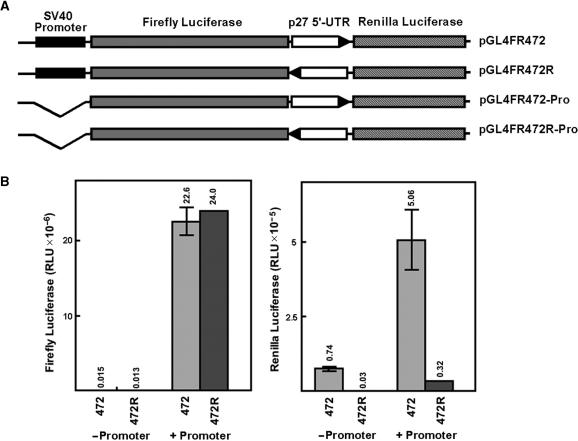
Figure 2.
Transfection of pGL4-based monocistronic vectors into MCF7 cells. (A) Diagram of monocistronic constructs. (B) Firefly luciferase activity after transfection of each of the constructs as shown in A. The number above each bar represents the mean of three separate transfections (in RLU × 10−6). The error bars represent SD.
Total cellular RNA was isolated from transfected cells using the RNeasy Mini kit (Qiagen). RNA samples were treated with Turbo DNA-Free (Ambion) to remove any traces of DNA. RNase protection assays were performed using the RPA II kit (Ambion) following the manufacturer's protocol. Protected fragments were separated by electrophoresis on denaturing polyacrylamide gels and bands were visualized by autoradiography.
RESULTS
Analysis of a promoterless bicistronic construct containing the sequence encoding the p27 5′-UTR
One of the most common methods of testing for IRES activity is to use a bicistronic vector in which the putative IRES is inserted between two protein coding regions. We (12,17) and others (13–15) have used this assay for analyzing the activity of the p27 5′-UTR and the results have supported the conclusion that this region contains an IRES. Transfection of the bicistronic construct into cells should result in the transcription of a single mRNA that encodes both the upstream and downstream cistrons. Transcription of this message is dependent on the promoter sequence upstream of the first cistron. However, if the putative IRES harbors a sequence that can function as a promoter, then a monocistronic mRNA encoding only the downstream cistron will be produced. Since the reporter gene encoded by the downstream cistron is monitored by a very sensitive assay, even a low level of expression through a cryptic promoter in the putative IRES-containing sequence will be detectable.
One way to test for cryptic promoter activity in the putative IRES-containing sequence is to delete the authentic promoter upstream of the first cistron. This should eliminate or greatly reduce expression of the bicistronic message. Expression of both the upstream cistron, which is translated in a cap-dependent manner and the downstream cistron, which is translated in an IRES-dependent manner, should be affected. However, if expression of the downstream cistron is dependent on a cryptic promoter, then its expression should not be affected. Indeed, Liu et al. (16), based on the use of promoterless bicistronic constructs together with RNase protection assays, have concluded that the sequence encoding the p27 5′-UTR has promoter activity. This conclusion is inconsistent with a substantial body of work suggesting the presence of an IRES in the 5′-UTR of the p27 mRNA.
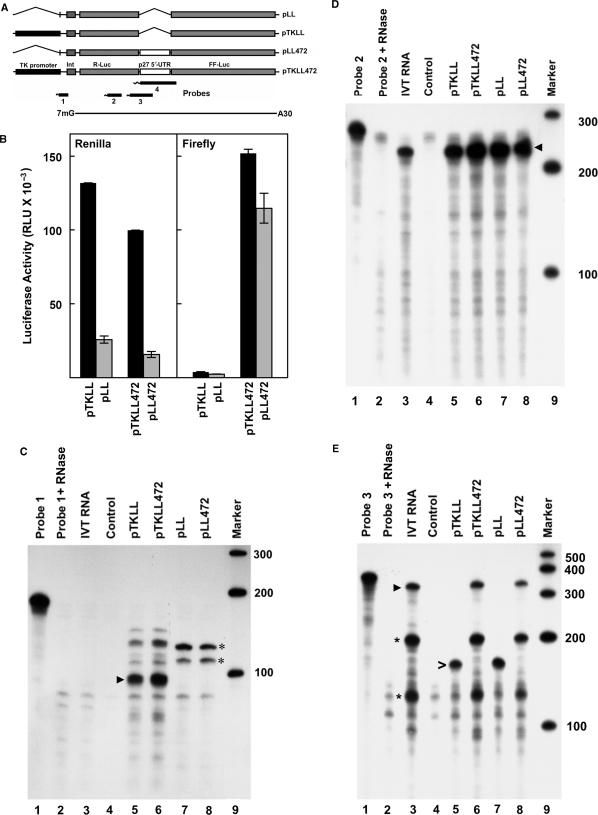
To further explore the possibility of cryptic promoter activity, the sequence encoding the full-length p27 5′-UTR (18) was inserted into a bicistronic construct between an upstream cistron encoding Renilla luciferase and a downstream cistron encoding firefly luciferase (Figure 1A). Expression of this construct (pTKLL472) is driven by the viral thymidine kinase (TK) promoter. A second construct was prepared in which the TK promoter was deleted (pLL472). Similar constructs lacking the p27 5′-UTR sequence were also prepared (pTKLL and pLL). These constructs were transfected into the breast cancer cell line MCF7 (Figure 1B). Deletion of the TK promoter resulted in considerable loss of Renilla luciferase expression (compare pTKLL to pLL and pTKLL472 to pLL472). However, there was not a complete loss of activity, with both promoterless constructs retaining 15–20% of the activity of the upstream cistron relative to the construct containing the TK promoter.
Figure 1.
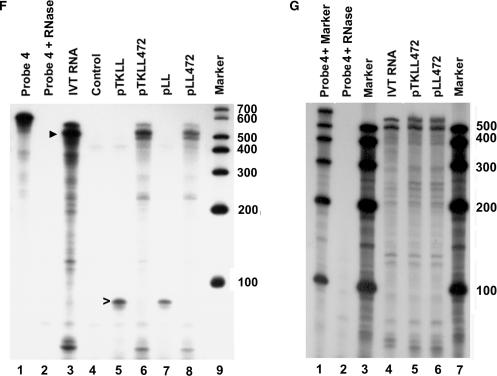
Transfection of bicistronic reporter constructs into MCF7 cells. (A) Diagram of constructs used in the transfections and probes used for RNase protection assays. Construct names are indicated on the right. The p27 5′-UTR is a 472 nt sequence upstream of the p27 start codon. It is inserted between the coding regions for Renilla luciferase (R-Luc) and firefly luciferase (FF-Luc). The region spanned by the in vitro synthesized mRNA used as a control in RNase protection assays is shown at the bottom. (B) The bicistronic constructs were transfected into MCF7 human breast cancer cells. One day after transfection, the cells were harvested and the activity of both Renilla luciferase (left) and firefly luciferase (right) was determined. Black bars represent enzyme activity in the constructs carrying the TK promoter (pTKLL and pTKLL472) and gray bars represent activity in the promoterless constructs (pLL and pLL472). RLU indicates relative light units. Error bars represent SD. (C) RNase protection assay using Probe 1 (see panel A). Lane 1 is undigested probe. Lane 2 is probe treated with RNase. Lane 3 is probe hybridized with in vitro synthesized mRNA. Lane 4 is probe hybridized with RNA isolated from untransfected cells. In lanes 5–8, probe was hybridized with RNA from cells transfected with the constructs indicated at the top of each lane. Lane 9 is labeled marker RNAs of the size indicated on the right. The arrowhead indicates a band of ∼100 nt and is the size expected for accurate transcription initiation from the TK promoter. The asterisks mark major bands observed after transfecting cells with constructs lacking the TK promoter. (D) RNase protection assay using Probe 2. Lanes are the same as described for C. The arrowhead indicates a band of ∼227 nt, which is the size expected if transcription is initiated at any point upstream of the probe sequence. (E) RNase protection assay using Probe 3. Lanes are as describe for C. The arrowhead in lane 3 indicates a band of ∼367 nt which is the expected size for cells transfected with the constructs carrying the p27 5′-UTR. The asterisks indicate bands of ∼210 and ∼125 nt which are also observed in lanes 6 and 8. The open arrowhead in lane 5 indicates a band of ∼166 which is the expected size for cells transfected with the constructs lacking the p27 5′-UTR. (F) RNase protection assay using Probe 4. Lanes are as described for C. Arrowhead in lane 3 indicates a band of ∼530 nt and is the major band observed for in vitro transcribed mRNA as well as in cells transfected with constructs carrying the p27 5′-UTR. The open arrowhead in lane 5 indicates a band of the expected size for cells transfected with constructs lacking the p27 5′-UTR. (G) The RNase protection assay with Probe 4 was repeated using less in vitro synthesized mRNA (lane 4) in order to obtain bands of similar intensity to those observed using RNA from cells transfected with constructs containing the p27 5′-UTR (lanes 5 and 6).
As expected, neither construct lacking the sequence encoding the p27 5′-UTR (pTKLL and pLL) had substantial levels of firefly luciferase expression. In agreement with previous publications (12–15,17), the TK promoter-driven construct containing the p27 5′-UTR sequence (pTKLL472) showed high-level expression of the downstream cistron. However, deletion of the TK promoter resulted in only a ∼24% decrease in expression of firefly luciferase. This indicates that a major portion of the expression of the downstream cistron is dependent on the p27 5′-UTR but not on the TK promoter.
One possibility for the results above is that the p27 5′-UTR has promoter activity resulting in production of a monocistronic message encoding firefly luciferase. To examine this possibility we performed RNase protection assays. Four different probes, each complimentary to a different region of the mRNAs encoded by the bicistronic constructs, were utilized (Figure 1A). Probe 1 is 180 nt in length and protects a fragment of ∼100 nt from cells transfected with pTKLL (Figure 1C, lane 5) and pTKLL472 (Figure 1C, lane 6). This corresponds to the size expected if the TK promoter is used to initiate transcription. A second major band of ∼130 nt is also detected in these lanes. This fragment could only result from transcriptional initiation in the vector somewhere upstream of the TK start site. Interestingly, there are also protected fragments from cells transfected with the promoterless constructs pLL (Figure 1C, lane 7) and pLL472 (Figure 1C, lane 8). These bands indicate a substantial level of transcription initiation within upstream vector sequences, even in the absence of any known promoter.
Probe 2 is 256 nt in length and protects a fragment of 227 nt within the upstream cistron encoding Renilla luciferase. This probe hybridizes with RNAs from cells transfected with each of the bicistronic reporter constructs, including those in which the TK promoter has been deleted (Figure 1D, lanes 5–8). The intensity of the protected band is similar in all four samples. An identical band is observed in the control sample using in vitro transcribed bicistronic RNA (IVT RNA, lane 3), while no band is observed in the control lane using RNA from untransfected cells (lane 4). In titration experiments, ∼0.024 fmol of the in vitro transcribed RNA was easily detectable, indicating the high sensitivity of this assay. The results again support the conclusion that substantial transcription initiation occurs in vector sequences independent of the TK promoter.
Probe 3 is 390 nt in length and encompasses the 3′ end of the Renilla luciferase gene and 200 nt of the p27 5′-UTR. It protects a band of 166 nt in cells transfected with bicistronic constructs lacking the p27 5′-UTR (pTKLL or pLL, Figure 1E, lanes 5 and 7). A band of approximately equal intensity is observed with both constructs, indicating a significant level of transcription independent of the TK promoter. Probe 3 should protect a fragment of 367 nt from cells transfected with bicistronic constructs carrying the p27 5′-UTR and a band of this size is detected both with (lane 6) and without (lane 8) the TK promoter. However, two additional strong bands of ∼210 and 125 nt are detected in these samples. Importantly, exactly the same pattern of bands is observed in the control sample using in vitro transcribed RNA. Thus, these bands do not arise from transcriptional initiation at a cryptic promoter within this region and are most likely to be the result of strong secondary structures in the RNA.
Probe 4 is 632 nt in length and spans the entire p27 5′-UTR and the 5′ end of the firefly luciferase coding region. This probe is expected to protect a 95 nt fragment from cells transfected with the bicistronic reporters lacking the p27 5′-UTR. A band of approximately this size is detected for both pTKLL and pLL (Figure 1F, lanes 5 and 7). For full-length transcripts from the bicistronic constructs containing the p27 5′-UTR, the probe should protect a fragment of 584 nt. For cells transfected with either pTKLL472 or pLL472 a faint band with a calculated size of 588 nt is observed along with two stronger bands with calculated sizes of ∼531 and ∼494 nt and a series of minor bands of smaller size (Figure 1F, lanes 6 and 8). The identical pattern of bands is observed when in vitro transcribed bicistronic RNA is used for the hybridization reaction (Figure 1F, lane 3 and Figure 1G, lane 4). Thus, the observed bands cannot be the result of alternative transcription initiation sites within the sequence encoding the p27 5′-UTR and there is no evidence of significant cryptic promoter activity within this region.
The results presented above indicate that the reporter constructs have significant transcriptional activity that is independent of the authentic TK promoter. Cryptic transcriptional regulatory sequences and promoter elements in vector sequences have been observed previously and are a common problem in reporter gene assays (19–23). In an attempt to minimize this problem we generated a series of reporter constructs base on the vector pGL4. This vector (Promega Corporation) has been extensively modified to remove cryptic regulatory sequences and promoter elements in the vector backbone as well as in the luciferase coding region (24). An upstream synthetic poly(A) signal/transcription pause site was also incorporated to impede read through from any transcripts initiated in vector sequences. We prepared both monocistronic and bicistronic reporters based on the pGL4 backbone. For the monocistronic constructs, the p27 5′-UTR was cloned upstream of firefly luciferase either in the forward or reverse orientation (Figure 2A). The forward 5′-UTR construct expressed slightly higher luciferase activity than either the reverse 5′-UTR construct or the parent vector (pGL4.10, Figure 2B). However, the activity of the forward 5′-UTR construct was expressed at a level nearly 600-fold less than a similar construct in which luciferase expression is driven by the SV40 promoter [pGL4.13(SV40)]. These results indicate that the p27 5′-UTR has minimal, if any, promoter activity in this setting.
pGL4-based bicistronic constructs were prepared in which the p27 5′-UTR, either forward or reverse orientation, was cloned between the firefly luciferase (upstream cistron) and Renilla luciferase (downstream cistron) coding regions. Constructs with and without the SV40 promoter upstream of firefly luciferase were prepared (Figure 3A). Expression of the upstream cistron was almost entirely dependent on the presence of the SV40 promoter regardless of the orientation of the p27 5′-UTR (Figure 3B). For the downstream cistron, elevated expression was only observed for the construct carrying the SV40 promoter and the p27 5′-UTR in the forward orientation. There was some expression of the downstream cistron in the construct carrying the p27 5′-UTR in the absence of the promoter, but this was only ∼15% of that observed with the promoter. With the promoter-driven construct carrying the 5′-UTR in the reverse orientation the downstream cistron was expressed at ∼6% of the level of same construct with the 5′-UTR in the forward orientation. Thus expression of the downstream cistron is dependent on the orientation of the p27 5′-UTR and the presence of the SV40 promoter upstream of the first cistron.
Figure 3.
Transfection of pGL4-based bicistronic vectors into MCF7 cells. (A) Diagram of bicistronic constructs. (B) The left panel shows activity of the upstream cistron (Firefly luciferase) and the right panel shows activity of the downstream cistron (Renilla luciferase). Bars labeled 472 indicate constructs with the p27 5′-UTR in the forward orientation and bars labeled 472R indicate constructs with the p27 5′-UTR in the reverse orientation. For bars labeled −Promoter cells were transfected with constructs lacking a promoter upstream of the first cistron and for bars labeled +Promoter cells were transfected with constructs carrying the SV40 promoter. The number above each bar indicates the mean of three separate transfections. The error bars represent SD.
Transfection of siRNAs targeted to the upstream and downstream cistrons of bicistronic RNAs
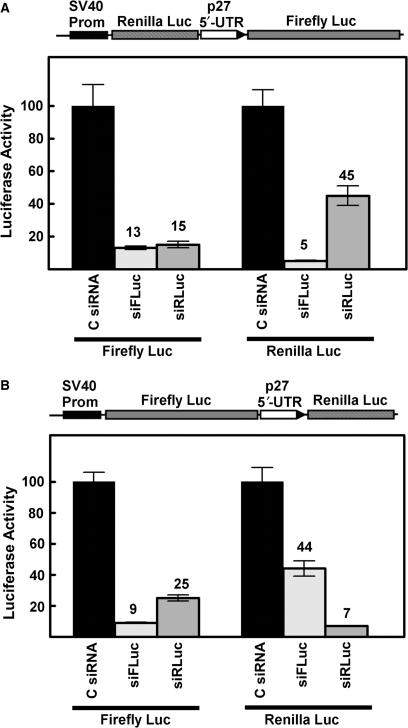
To further support the conclusion that both cistrons can be expressed from a single mRNA molecule, bicistronic vectors containing the p27 5′-UTR were cotransfected with siRNAs targeted to either the Renilla luciferase sequence or the firefly luciferase sequence. Two different pGL4-based bicistronic vectors were used in these experiments. One construct has the Renilla luciferase sequence as the upstream cistron and the firefly luciferase sequence as the downstream cistron (Figure 4A) while the other has the firefly luciferase sequence upstream and the Renilla luciferase sequence downstream (Figure 4B). With both constructs, siRNAs targeting either of the luciferase genes substantially reduced expression of both enzymes. It is especially important to note that siRNA inhibition of the upstream cistron also led to inhibition of the downstream cistron, further supporting the conclusion that the p27 5′-UTR mediates translation in a cap-independent manner.
Figure 4.
siRNA knockdown of luciferase expressed from bicistronic constructs. (A) The pGL4-based bicistronic reporter shown at the top of the diagram was cotransfected into MCF7 cells with a control scrambled siRNA, an siRNA targeted to firefly luciferase (siFLuc), or an siRNA targeted to Renilla luciferase (siRLuc). Firefly and Renilla luciferase activities were determined and are expressed as percent of the control with the scrambled siRNA. The number above each bar represents the relative luciferase activity (% of control). (B) Same as in A except that firefly luciferase is the upstream cistron and Renilla luciferase is the downstream cistron.
Transfection of in vitro transcribed bicistonic mRNAs
Another means of testing for IRES activity is to directly transfect bicistonic mRNAs into cells. In this way, the processes of transcription and RNA processing are bypassed and there is no possibility of cryptic promoter activity or cryptic splicing through the putative IRES encoding region. Two bicistronic mRNAs were produced by in vitro transcription (Figure 5, top). One mRNA included the p27 5′-UTR in the forward direction and the other mRNA included the complement of the p27 5′-UTR. Thus both mRNAs were of equal length and the distance between the two protein coding regions was identical. In addition, a 7-methylguanosine cap and a polyA tail of 30 nt was incorporated into both mRNAs. The mRNAs were transfected into two different breast cancer cell lines (Figure 5, bottom). In both lines expression of the downstream cistron (firefly luciferase) was enhanced by the p27 5′-UTR in the forward direction, supporting the conclusion that this sequence is able to promote cap-independent translation.
RT-PCR analysis of mRNA transcribed from the bicistronic constructs
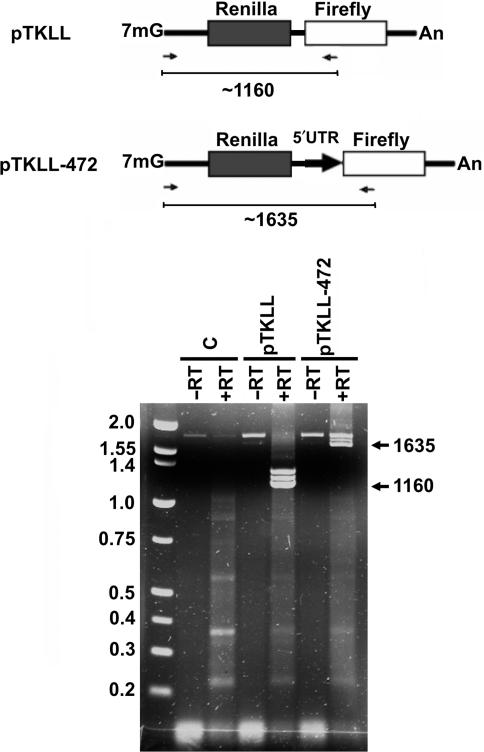
Using northern blotting, we (12) and others (13,14) have previously characterized the transcripts produced by transfecting cells with bicistronic constructs containing the p27 5′-UTR. These experiments did not reveal any small transcripts that might have resulted from a cryptic splice site in the 5′-UTR. However, northern blotting may not be sensitive enough to detect low levels of short transcripts. Van Eden et al. (7) have utilized an RT-PCR strategy for detecting transcripts produced from bicistronic vectors. We have employed a similar procedure using one primer from just downstream of the TK promoter transcriptional start site and the other primer from within the firefly luciferase coding region (Figure 6, top). Using this primer pair should result in the amplification of a ∼1635 bp fragment from the authentic bicistronic message from the reporter construct carrying the p27 5′-UTR (pTKLL-472) and a ∼1160 bp fragment from the control construct lacking the UTR (pTKLL). MCF7 cells were transfected with these bicistronic reporter constructs. As a control, another culture was left untransfected. Total RNA was isolated, and then RT-PCR was performed (Figure 6, bottom). To control for contaminating plasmid DNA, a separate reaction was performed in which the reverse transcriptase was left out. A major band of the expected size was observed only in the lanes which included reverse transcriptase. Also, slightly larger RT-PCR products, of unknown origin, were observed for both the pTKLL and pTKLL-472 transfected cells. Importantly, in cells transfected with the p27 5′-UTR-containing construct, we did not detect any amplification products of a smaller size that were also not detected in the untransfected cells and the cells transfected with pTKLL. These results do not support the existence of a cryptic splice site within the 5′-UTR.
Figure 6.
RT-PCR analysis of transcripts produced from bicistronic reporter plasmids. Top: Diagram of the transcripts that should be produced following transfection when transcription initiates from the TK promoter of the bicistronic reporter construct lacking an insert (pTKLL) and the construct carrying the p27 5′-UTR (pTKLL-472). Arrows below each diagram indicate the position of primers used for RT-PCR analysis. RT-PCR should produce a DNA fragment of ∼1635 nt if there is no splicing due to a cryptic splice acceptor site in the p27 5′-UTR. The expected size for the fragment from pTKLL transfected cells is ∼1160 nt. Bottom: The bicistronic reporters were transfected into MCF7 cells as indicated. The control reactions (C) were from untransfected cells. RNA was isolated and used for RT-PCR with the primer pair indicated in the diagram. Reactions in which all conditions were identical except that reverse transcriptase was omitted (−RT) were performed for each of the RNA templates.
An iron-response element (IRE) inhibits expression of only the upstream cistron
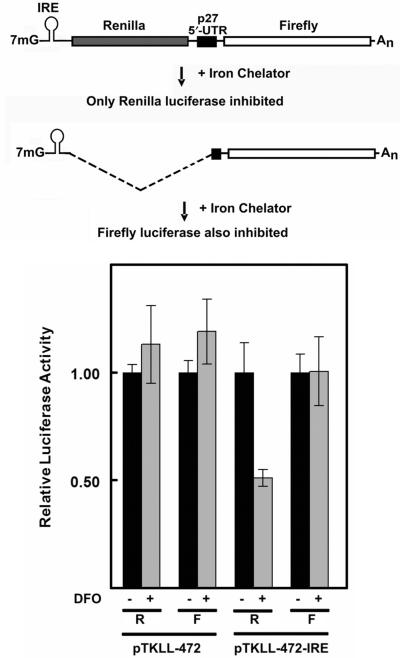
To further explore the possibility of cryptic splicing sites within the p27 5′-UTR we inserted an IRE 35 nt downstream of the transcriptional start site of the TK promoter in pTKLL-472 (Figure 7, top). There is no potential 5′ splice site within this 35 nt sequence. This, and the proximity of the IRE to the transcriptional start site, makes it extremely unlikely that any splicing event would occur within this region. Thus any splicing event involving a cryptic 3′ splice site in the p27 5′-UTR would have to use a 5′ splice site downstream of the IRE. This, in effect, would transfer the IRE to a position upstream of the firefly luciferase coding region. If this occurred, expression of firefly luciferase would become sensitive to iron chelation, which leads to activation of iron-response protein (IRP). Active IRP binds to IREs and blocks cap-dependent translation.
Figure 7.
Effect of an IRE on expression of bicistronic reporters carrying the p27 5′-UTR. Top: Diagram of potential mRNA products resulting from transcription of a bicistronic reporter in which an IRE has been inserted just downstream of the transcriptional start site within the TK promoter. The top diagram shows the expected transcript if there is no cryptic splice site within the 5′-UTR. In this case, only the upstream cistron (Renilla luciferase) would be affected by iron chelation. The bottom diagram shows the expected transcript if the 5′-UTR has a cryptic splice acceptor site. The IRE would be linked to a monocistronic mRNA encoding only firefly luciferase and iron chelation would inhibit expression of this enzyme. Bottom: NIH3T3 cells were transfected with bicistronic reporters pTKLL-472 and pTKLL-472-IRE. These constructs are identical except that pTKLL-472-IRE has an IRE inserted 35 nt downstream of the TK transcriptional start site. The transfected cells were treated with (+, gray bars) or without (−, black bars) the iron chelator DFO. The cells were harvested and Renilla (R) and firefly (F) luciferase activities were determined. For each transfection, the value of luciferase activity in the absence of DFO was set to 1.
The bicistronic construct containing the IRE, as well as the control construct lacking the IRE, were transfected into NIH3T3 cells (Figure 7, bottom). The cells were treated with or without deferoxamine (DFO), a cell permeable iron chelator, and then harvested for luciferase assays. For the control construct lacking an IRE, treatment with an iron chelator had no effect on either Renilla or firefly luciferase activity. In contrast, treatment of cells transfected with the IRE-containing construct caused ∼50% inhibition of Renilla luciferase expression while having no effect on firefly luciferase expression. These data provide further support for the conclusion that expression of the downstream cistron does not result from the presence of cryptic splice acceptor site within the p27 5′-UTR.
DISCUSSION
Bicistronic reporter constructs are commonly used to study cap-independent translation through IRESs. However, this approach has been criticized because of possible artifacts from cryptic promoter activity (5,6) or a cryptic splicing site (5,7) residing within the test sequence. At least five previous publications have analyzed p27 5′-UTR sequences using bicistronic vectors and all have concluded that an IRES is present within this region (12–15,17). However, these findings were recently contradicted by the experiments of Liu et al. (16). This group used promoterless bicistronic reporter constructs to support the conclusion the p27 5′-UTR has an absence of IRES activity, but rather that the DNA sequence encoding the p27 5′-UTR has cryptic promoter activity. This group further supported this conclusion using RNase protection assays to detect putative transcription start sites within the sequence encoding the p27 5′-UTR. In this report, we also used bicistronic constructs with and without a promoter upstream of the first cistron. Like Liu et al. (16) we found that significant expression of the downstream cistron was retained even after deletion of the TK promoter in the bicistronic construct carrying the p27 5′-UTR. However, our RNase protection assays demonstrated that there is considerable transcriptional initiation within vector sequences that is independent of the TK promoter. This vector-initiated transcription would be expected to inhibit expression of the upstream cistron because of numerous upstream AUG start codons and stop codons. The downstream cistron should still be actively expressed through cap-independent translational initiation mediated by the IRES. This is what was observed in our experiments.
Another important observation of our experiments is the detection of ‘extra’ bands in RNase protection assays using probes complimentary to the p27 5′-UTR. These bands could be interpreted as being the result of transcriptional initiation through cryptic promoters in the 5′-UTR sequence, as suggested by Liu et al. (16). However, exactly the same pattern of bands was observed when in vitro transcribed RNA was used in the RNase protection assay. Thus, the bands cannot be due to promoter activity in this region and are most likely the result of very strong secondary structure in the p27 5′-UTR. The findings highlight the importance of including the in vitro transcribed RNA control when using RNase protection assays to map potential transcription initiation sites. This control was not included in the experiments described by Liu et al. (16) and it is likely that the putative transcription start sites in the p27 5′-UTR reported by this group are actually due to RNA secondary structure rather than transcriptional initiation.
Our experiments indicate a significant level of vector-initiated TK promoter-independent transcription in our original bicistronic constructs. A number of reports have previously described promoter activity in vector backbone sequences (19–23). To surmount this problem, Promega Corporation recently developed the pGL4 series of luciferase reporter vectors (24). This was done by removing dozens of consensus transcription factor binding sites and promoter modules. We used these redesigned reporter vectors to create monocistronic and bicistronic constructs carrying the p27 5′-UTR. With the p27 5′-UTR, monocistronic construct expression of luciferase was ∼600-fold less than that driven by the SV40 promoter. With the bicistronic constructs, expression of the downstream cistron was largely dependent on both the p27 5′-UTR and the presence of the SV40 promoter upstream of the first cistron. Thus, these experiments suggest that the sequence encoding the p27 5′-UTR has very minimal promoter activity and that the p27 5′-UTR is able to mediate cap-independent translation. This is further supported by the observation that siRNAs targeted to either the upstream or downstream cistron of these pGL4-based bicistronic reporter constructs leads to decreased expression of both cistrons.
A second potential problem associated with bicistronic vectors is the possibility of cryptic splice sites within the putative IRES sequence. Van Eden et al. (7) described an RT-PCR procedure that was able to detect products from cryptic splice sites with high sensitivity. In this report we used a similar strategy for analyzing the transcripts produced from the bicistronic reporter carrying the p27 5′-UTR. The results provided no indication that monocistronic mRNAs were produced by splicing within the 5′-UTR. Further support for a lack of cryptic splicing at sites within the p27 5′-UTR was provided by experiments in which an IRE was inserted just downstream of the transcription start site for the bicistronic mRNA. A splicing event involving the 5′-UTR and removing the upstream cistron (Renilla luciferase) would result in linking the IRE to the downstream cistron (firefly luciferase). If such an event was responsible for expression of the downstream cistron then this expression should be sensitive to treatment with iron chelators. However, we found that only the upstream cistron was inhibited by DFO.
Another test for IRES activity is to directly transfect bicistronic mRNAs rather than bicistronic DNA vectors. This approach completely avoids the processes of transcription and splicing. However, a potential problem with this approach is that the transfected RNAs are not processed in the nucleus and may not have access to all of the factors necessary to promote efficient translation. Nevertheless, we have observed significant expression of the downstream cistron that is dependent on the presence of the p27 5′-UTR (Figure 3). Similar RNA transfection experiments have been carried out by Cho et al. (13) and their results are in agreement with ours.
In addition to the results described above, several additional findings indicate that the p27 5′-UTR is able to mediate cap-independent translation. Both Cho et al. (13) and Shi et al. (15) have demonstrated p27 IRES-dependent expression in a cell-free in vitro translation system. Kullmann et al. (14) found that the presence of the p27 5′-UTR makes expression of the downstream cistron resistant to the effects of a phosphatidylinositol-3 kinase inhibitor that represses global cap-dependent translation. Similarly, we (12) have found that p27 IRES-driven expression is not sensitive to the effects of rapamycin while Shi et al. (15) have actually observed an increase in p27 IRES activity following treatment with rapamycin. Rapamycin blocks mTOR kinase activity and specifically inhibits cap-dependent translation because of accumulation of active, hypophosphorylated 4EBP, an inhibitor of the cap-binding protein eIF4E. Finally, we have demonstrated that overexpression of a constitutively active 4EBP enhances cap-independent expression through the p27 5′-UTR as well as expression of the endogenous p27 gene (17).
Finally, a highly pertinent finding was recently reported by Yoon et al. (25). They found that cells expressing a mutant form of the DKC1 gene had impaired translation of p27 mRNA and other mRNAs having IRESs. DKC1 is mutated in the X-linked disease dyskeratosis congenita which is associated with a number of abnormalities including increased susceptibility to cancer. The DKC1 protein is a pseudouridine synthase that modifies ribosomal RNA. Ribosomes derived from cells carrying the DKC1 mutation have lower levels of pseudouridine and are defective in translational initiation through cellular and viral IRESs but show no loss in global cap-dependent translation. Reduced expression of endogenous p27 protein and impaired ability of the p27 5′-UTR to mediate cap-independent translation, as shown by direct transfection of in vitro transcribed bicistronic mRNA, were observed in these cells (25).
In summary, our results uncover a shortcoming in the use of ‘promoterless’ bicistronic constructs as a means of demonstrating cryptic promoter activity in a 5′-UTR coding sequence. They also demonstrate the importance of using in vitro transcribed RNA as a control when mapping putative transcription start sites by RNase protection assays. The data presented here and other recent publications strongly support the conclusion that the p27 5′-UTR has an authentic IRES. It will be important to determine the molecular mechanism of translation initiation through this element, how its activity is controlled, and if it has a role in the downregulation of p27 that is often observed in tumor cells.
ACKNOWLEDGEMENTS
Funding was provided by National Institutes of Health (CA84325 to W.K.M.) and the National Multiple Sclerosis Society (RG 3293-B-7 to R.M.). Funding to pay the Open Access publication charges for this article was provided by Sanford Research/USD.
Conflict of interest statement. None declared.
REFERENCES
- 1.Jang SK, Krausslich HG, Nicklin MJ, Duke GM, Palmenberg AC, Wimmer E. A segment of the 5′ nontranslated region of encephalomyocarditis virus RNA directs internal entry of ribosomes during in vitro translation. J. Virol. 1988;62:2636–2643. doi: 10.1128/jvi.62.8.2636-2643.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pelletier J, Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature. 1988;334:320–325. doi: 10.1038/334320a0. [DOI] [PubMed] [Google Scholar]
- 3.Gradi A, Svitkin YV, Imataka H, Sonenberg N. Proteolysis of human eukaryotic translation initiation factor eIF4GII, but not eIF4GI, coincides with the shutoff of host protein synthesis after poliovirus infection. Proc. Natl Acad. Sci. USA. 1998;95:11089–11094. doi: 10.1073/pnas.95.19.11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stoneley M, Willis AE. Cellular internal ribosome entry segments: structures, trans-acting factors and regulation of gene expression. Oncogene. 2004;23:3200–3207. doi: 10.1038/sj.onc.1207551. [DOI] [PubMed] [Google Scholar]
- 5.Kozak M. New ways of initiating translation in eukaryotes? Mol. Cell. Biol. 2001;21:1899–1907. doi: 10.1128/MCB.21.6.1899-1907.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han B, Zhang JT. Regulation of gene expression by internal ribosome entry sites or cryptic promoters: the eIF4G story. Mol. Cell. Biol. 2002;22:7372–7384. doi: 10.1128/MCB.22.21.7372-7384.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Eden ME, Byrd MP, Sherrill KW, Lloyd RE. Demonstrating internal ribosome entry sites in eukaryotic mRNAs using stringent RNA test procedures. RNA. 2004;10:720–730. doi: 10.1261/rna.5225204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bloom J, Pagano M. Deregulated degradation of the cdk inhibitor p27 and malignant transformation. Semin. in cancer biol. 2003;13:41–47. doi: 10.1016/s1044-579x(02)00098-6. [DOI] [PubMed] [Google Scholar]
- 9.Agrawal D, Hauser P, McPherson F, Dong F, Garcia A, Pledger WJ. Repression of p27kip1 synthesis by platelet-derived growth factor in BALB/c 3T3 cells. Mol. Cell. Biol. 1996;16:4327–4336. doi: 10.1128/mcb.16.8.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hengst L, Reed SI. Translational control of p27Kip1 accumulation during the cell cycle. Science. 1996;271:1861–1864. doi: 10.1126/science.271.5257.1861. [DOI] [PubMed] [Google Scholar]
- 11.Millard SS, Yan JS, Nguyen H, Pagano M, Kiyokawa H, Koff A. Enhanced ribosomal association of p27(Kip1) mRNA is a mechanism contributing to accumulation during growth arrest. J. Biol. Chem. 1997;272:7093–7098. doi: 10.1074/jbc.272.11.7093. [DOI] [PubMed] [Google Scholar]
- 12.Miskimins WK, Wang G, Hawkinson M, Miskimins R. Control of cyclin-dependent kinase inhibitor p27 expression by cap-independent translation. Mol. Cell. Biol. 2001;21:4960–4967. doi: 10.1128/MCB.21.15.4960-4967.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho S, Kim JH, Back SH, Jang SK. Polypyrimidine tract-binding protein enhances the internal ribosomal entry site-dependent translation of p27Kip1 mRNA and modulates transition from G1 to S phase. Mol. Cell. Biol. 2005;25:1283–1297. doi: 10.1128/MCB.25.4.1283-1297.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kullmann M, Gopfert U, Siewe B, Hengst L. ELAV/Hu proteins inhibit p27 translation via an IRES element in the p27 5′UTR. Genes Dev. 2002;16:3087–3099. doi: 10.1101/gad.248902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi Y, Sharma A, Wu H, Lichtenstein A, Gera J. Cyclin D1 and c-myc internal ribosome entry site (IRES)-dependent translation is regulated by AKT activity and enhanced by rapamycin through a p38 MAPK- and ERK-dependent pathway. J. Biol. Chem. 2005;280:10964–10973. doi: 10.1074/jbc.M407874200. [DOI] [PubMed] [Google Scholar]
- 16.Liu Z, Dong Z, Han B, Yang Y, Liu Y, Zhang JT. Regulation of expression by promoters versus internal ribosome entry site in the 5′-untranslated sequence of the human cyclin-dependent kinase inhibitor p27kip1. Nucleic Acids Res. 2005;33:3763–3771. doi: 10.1093/nar/gki680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang H, Coleman J, Miskimins R, Miskimins WK. Expression of constitutively active 4EBP-1 enhances p27Kip1 expression and inhibits proliferation of MCF7 breast cancer cells. Cancer Cell Int. 2003;3:2. doi: 10.1186/1475-2867-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coleman J, Hawkinson M, Miskimins R, Miskimins WK. The major transcription initiation site of the p27Kip1 gene is conserved in human and mouse and produces a long 5′-UTR. BMC Mol. Biol. 2001;2:12. doi: 10.1186/1471-2199-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Annicotte JS, Schoonjans K, Haby C, Auwerx J. An E-box in pGL3 reporter vectors precludes their use for the study of sterol regulatory element-binding proteins. BioTechniques. 2001;31:993–994. 996. [PubMed] [Google Scholar]
- 20.Bert AG, Burrows J, Osborne CS, Cockerill PN. Generation of an improved luciferase reporter gene plasmid that employs a novel mechanism for high-copy replication. Plasmid. 2000;44:173–182. doi: 10.1006/plas.2000.1474. [DOI] [PubMed] [Google Scholar]
- 21.Grimm SL, Nordeen SK. Luciferase reporter gene vectors that lack potential AP-1 sites. BioTechniques. 1999;27:220–222. doi: 10.2144/99272bm01. [DOI] [PubMed] [Google Scholar]
- 22.Ho CK, Strauss JF., III Activation of the control reporter plasmids pRL-TK and pRL-SV40 by multiple GATA transcription factors can lead to aberrant normalization of transfection efficiency. BMC Biotechnol. 2004;4:10. doi: 10.1186/1472-6750-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thirunavukkarasu K, Miles RR, Halladay DL, Onyia JE. Cryptic enhancer elements in luciferase reporter vectors respond to the osteoblast-specific transcription factor Osf2/Cbfa1. BioTechniques. 2000;28:506–510. doi: 10.2144/00283st09. [DOI] [PubMed] [Google Scholar]
- 24.Paguio A, Almond B, Fan F, Stecha P, Garvin D, Wood M, Wood K. pGL4 vectors: a new generation of luciferase reporter vectors. Promega Notes. 2005;89:7–10. [Google Scholar]
- 25.Yoon A, Peng G, Brandenburger Y, Zollo O, Xu W, Rego E, Ruggero D. Impaired control of IRES-mediated translation in X-linked dyskeratosis congenita. Science. 2006;312:902–906. doi: 10.1126/science.1123835. [DOI] [PubMed] [Google Scholar]