Abstract
Eight members of a recently identified family of tetrahydrofuran-diols (THFDs), originating from epoxyeicosatrienoic acids (EETs), were prepared stereospecifically from D-(+)-glucose. The THFDs potently induced relaxation of pre-contracted bovine arteries.
Arachidonic acid is metabolized by the cytochrome P450 epoxygenase pathway into four regioisomeric epoxyeicosatrienoic acids (EETs),1 whose varied contributions to homeostasis and pathophysiology have attracted considerable attention.2 Secondary metabolism results in even greater structural diversity by converting the EETs into vic-diols,3 S-glutathione adducts,4 or more highly oxygenated products5 including a family of bioactive tetrahydrofuran-diols (THFDs).6–8 It is unclear, at present, if the THFDs originate from completely enzymatic processes9 or from spontaneous, nonenzymatic epoxide annulations (e.g., eq 1).10 To expedite current structural and pharmacological investigations, we report herein the synthesis of eight isomers of defined stereochemistry starting from an inexpensive member of the chiral pool and their evaluations as vasomodulators. A structurally similar, but biosynthetically distinct class of endogenous arachidonate tetrahydrofuran-diols,11 known collectively as isofurans,12 has also been described and representative members prepared by chemical synthesis.13
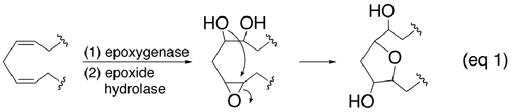
Since the “mid-chain” THFDs, i.e., those derived from transannular cyclizations between epoxides at the original Δ8,9- and Δ11,12-olefins, were found to be the most efficacious for increasing intracellular free Ca2+ in rat pulmonary alveolar cells,6b our initial synthetic efforts focused on this system. Our strategy (Scheme 1) utilized furanoside 1,14 readily obtained from D-(+)-glucose, as a convenient starting point.15 Alkynylation of 1 using the dianion of commercial 5-hexynoic acid and esterification of the adduct with diazomethane provided the known homopropargylic alcohol 2.14 Benzoylation of 2 followed by reductive allylation at the anomeric center induced with BF3·Et2O according to the method of García-Tellado16 afforded a chromatographically separable mixture (5:1) of 3 and its α-isomer 4 in good combined yield.17 Sequential silylation of the newly liberated alcohol, OsO4 dihydroxylation of the terminal olefin, and cleavage of the resultant vic-diol converted 3 into the somewhat labile aldehyde 5 which was immediately subjected to Wittig cis-olefination using n-hexylidenetriphenylphosphorane in a non-polar solvent to minimize β-elimination. Semi-hydrogenation of the acetylene over P-2 nickel18 and fluoride-induced desilylation gave 6. Saponification of the esters in 6 delivered 8(R),9(S),11(R),12(S)-THFD (7) without incident whereas Mitsunobu inversion19 at C(11) prior to saponification led to 8(R),9(S),11(S),12(S)-THFD (8).
Scheme 1.
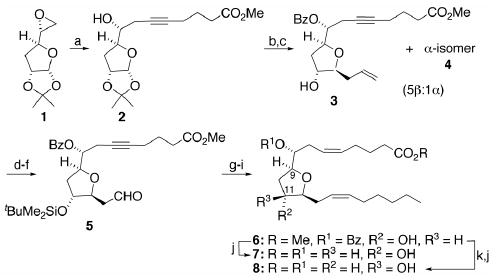
Reagents and conditions: (a) 5-hexynoic acid, n-BuLi (2 equiv), HMPA, 5°C for 1 h, then 23°C for 12 h; CH2N2, 5% MeOH/Et2O, 23°C, 1 h, 60–65%; (b) PhC(O)Cl, DMAP, Et3N, CH2Cl2, 23°C, 12 h, 97%; (c) Me3SiCH2CH=CH2/BF3·Et2O, CH2Cl2, 23°C, 18 h, 82% (α-/β-isomers combined); (d) t-BuMe2SiCl, ImH, DMF, 50°C, 12 h, 95%; (e) OsO4 (2 mol %), NMO, t-BuOH, 23°C, 12 h; (f) NaIO4/SiO2, CH2Cl2, 23°C, 1.5 h; (g) H3C(CH2)5PPh3Br, NaN(SiMe3)2, PhCH3/THF (1:1), −90°C for 0.5 h, then warm to 23°C overnight, 70% over three steps; (h) Ni(OAc)2, NaBH4, (H2NCH2)2, H2 (1 atm), EtOH, 23°C, 5 h, 85%; (i) n-Bu4NF, THF, 23°C, 12 h, 70%; (j) LiOH, THF/H2O (3:1), 92%; (k) DEAD/PPh3/PhCO2H, THF, 1 h, 0°C, 93%.
An analogous series of transformations as described in Scheme 1, when applied to α-isomer 4, yielded 8(R),9(S),11(R),12(R)-THFD (10) and 8(R),9(S),11(S),12(R)-THFD (11) by way of methyl ester 9 (Scheme 2).
Scheme 2.
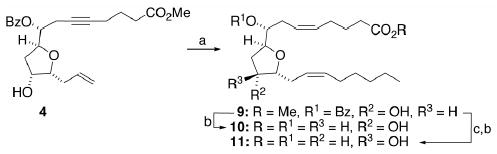
Reagents and conditions: (a) steps d-i from Scheme 1; (b) LiOH, THF/H2O (3:1), 23°C, 10 h, 92–96%; (c) DEAD/PPh3/PhCO2H, THF, 1 h, 0°C, 90–93%.
Regrettably, all attempts to access the 8(S)-series of THFDs via Mitsunobu inversion19 of alcohol 2 or the derived 5(Z)-olefin were discouraged by a facile dehydration. Only minor amounts of the desired 8(S)-ester could be obtained. Oxidation/reduction sequences through the corresponding ketone as a means of establishing the S-alcohol were also stymied by poor yields and/or migration of the adjacent olefin. Consequently, the known20 epimeric epoxide 12 (Scheme 3) was used to prepare benzoates 13 and 17 following the now well-established protocols from Scheme 1. After chromatographic separation, 13 and 17 were elaborated into 14 and 18, respectively. In turn, these intermediates were advanced to 8(S),9(S),11(R),12(S)-THFD (15)/8(S),9(S),11(S),12(S)-THFD (16) and 8(S),9(S),11(R),12(R)-THFD (19)/8(S),9(S),11(S),12(R)-THFD (20), accordingly.
Scheme 3.
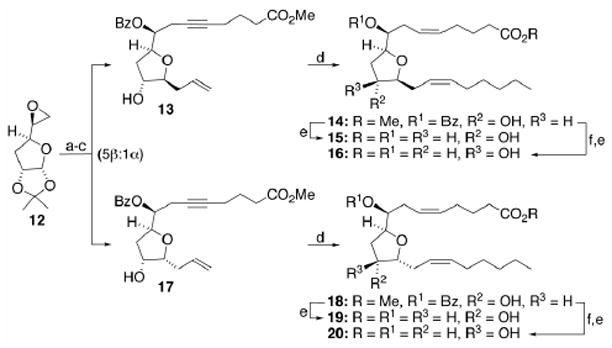
Reagents and conditions: (a) 5-hexynoic acid, n-BuLi (2 equiv), HMPA, 5°C for 1 h, then 23°C for 12 h; CH2N2, 5% MeOH/Et2O, 23°C, 1 h, 60–65%; (b) PhC(O)Cl, DMAP, Et3N, CH2Cl2, 23°C, 12 h, 93%; (c) Me3SiCH2CH=CH2/BF3·Et2O, CH2Cl2, 23°C, 18 h, 80–82% (α-/β-isomers combined); (d) steps d-i in Scheme 1; (e) LiOH, THF/H2O (3:1), 23°C, 10 h, 92–96%; (f) DEAD/PPh3/PhCO2H, THF, 1 h, 0°C, 90–93%.
The THFDs were tested for vasodilatory activity using bovine coronary arteries preconstricted with the thromboxane-mimetic U-46619 (10–20 nM).21 All caused relaxation of the arteries when used from 10−8–10−5 M (Figure 1A and 1B). In the same assay, the endogenous dilator 14,15-epoxyeicosatrienoic acid (14,15-EET) also relaxed coronary arteries over a similar concentration range.22 Notably, THFD 10 (ED50 = 3.0 ± 0.11 μM) and 14,15-EET (ED50 = 2.5 ± 0.10 μM) were equally active while the other THFDs were less efficacious. These studies indicate that, in this series, a trans-tetrahydrofuran skeleton and 11(R)-hydroxyl are necessary for full agonist activity with respect to the parent EETs. The physiological significance of these secondary metabolites and their utility as EET mimetics are under investigation and the results will be published in due course.
Figure 1.
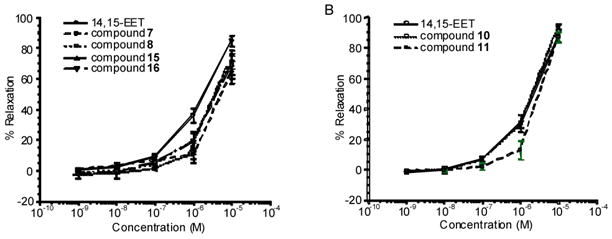
Relaxation of pre-contracted bovine arteries by THFDs and 14,15-EET.
Acknowledgments
Financial support provide by the Robert A. Welch Foundation and NIH (GM31278, DK38226, HL-51055). Dr. Elaine R. Fogel is thanked for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Review: Capdevila JH, Falck JR. Prostag Other Lipid Mediat. 2002;68–69:325. doi: 10.1016/s0090-6980(02)00038-2.
- 2.(a) Inceoglu B, Schmelzer KR, Morisseau C, Jinks SL, Hammock BD. Prostag Other Lipid Mediat. 2007;82:42. doi: 10.1016/j.prostaglandins.2006.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Olearczyk JJ, Imig JD. Curr Hypertens Rev. 2005;1:235. [Google Scholar]; (c) Fleming I. Pharmacol Res. 2004;49:525. doi: 10.1016/j.phrs.2003.11.016. [DOI] [PubMed] [Google Scholar]; (d) Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Nature. 2003;424:434. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]; (e) Jiang JG, Chen CL, Card JW, Yang S, Chen JX, Fu XN, Ning YG, Xiao X, Zeldin DC, Wang DW. Cancer Res. 2005;65:4707. doi: 10.1158/0008-5472.CAN-04-4173. [DOI] [PubMed] [Google Scholar]
- 3.Zeldin DC, Shouzou W, Falck JR, Hammock BD, Snapper JR, Capdevila JH. Arch Biochem Biophys. 1995;316:443. doi: 10.1006/abbi.1995.1059. [DOI] [PubMed] [Google Scholar]
- 4.Spearman ME, Prough RA, Estabrook RW, Falck JR, Manna S, Leibman KC, Murphy RC, Capdevila J. Arch Biochem Biophys. 1985;242:225. doi: 10.1016/0003-9861(85)90496-5. [DOI] [PubMed] [Google Scholar]
- 5.Capdevila JH, Mosset P, Yadagiri P, Lumin S, Falck JR. Arch Biochem Biophys. 1988;261:122. doi: 10.1016/0003-9861(88)90111-7.Oliw EH. J Biol Chem. 1984;259:2716.Zhang JY, Prakash C, Yamashita K, Blair IA. Biochem Biophys Res Commun. 1992;183:138. doi: 10.1016/0006-291x(92)91619-2.le Quéré V, Plée-Gautier E, Potin P, Madec S, Salaün J-P. J Lipid Res. 2004;45:1446. doi: 10.1194/jlr.M300463-JLR200.
- 6.(a) Moghaddam MF, Motoba K, Borhan B, Pinot F, Hammock BD. Biochim Biophys Acta. 1996;1290:327. doi: 10.1016/0304-4165(96)00037-2. [DOI] [PubMed] [Google Scholar]; (b) Hammock BD, Moghaddam MF, Cheek JM, Borhan B, Fergusson J, Grant DF, Greene JF, Matoba K, Zheng J, Sisemore MF. US 5,955,496 (A1) CAN. 1998;128:201056. [Google Scholar]
- 7.THFDs from other fatty acids: (a) Hosokawa M, Hou CT, Weisleder D. Appl Environ Microb. 2003;69:3868. doi: 10.1128/AEM.69.7.3868-3873.2003.Nourooz-Zadeh J, Uematsu T, Borhan B, Kurth MJ, Hammock BD. Arch Biochem Biophys. 1992;294:675. doi: 10.1016/0003-9861(92)90741-e.Halarnkar PP, Nourooz-Zadeh J, Kuwano E, Jones AD, Hammock BD. Arch Biochem Biophys. 1992;294:586. doi: 10.1016/0003-9861(92)90729-g.
- 8.Markaverich BM, Alejandro MA, Markaverich D, Zitzow L, Casajuna N, Camarao N, Hill J, Bhirdo K, Faith R, Turk J, Crowley JR. Biochem Biophys Res Commun. 2002;291:692. doi: 10.1006/bbrc.2002.6499.Murray LM, Barrow RA, Capon RJ. Aust J Chem. 1991;44:843.Yoda H, Maruyama K, Takabe K. Tetrahedron Asym. 2001;12:1403.
- 9.Glueck SM, Fabian WMF, Faber K, Mayer SF. Chem-Eur J. 2004;10:3467. doi: 10.1002/chem.200400061. [DOI] [PubMed] [Google Scholar]
- 10.(a) Piazza GJ, Nunez A, Foglia TA. J Am Oil Chem Soc. 2003;80:901. [Google Scholar]; (b) Williams DR, Grote J, Harigaya Y. Tetrahedron Lett. 1984;25:5231. [Google Scholar]; (c) Orru RVA, Groenendaal B, van Heyst J, Hunting M, Wesseling C, Schmitz RF, Mayer SF, Faber K. Pure Appl Chem. 2003;75:259. [Google Scholar]; (d) Jan ST, Li K, Vig S, Rudolph A, Uckun FM. Tetrahedron Lett. 1999;40:193. [Google Scholar]
- 11.Fessel JP, Porter NA, Moore KP, Sheller JR, Roberts LJ., II Proc Natl Acad Sci USA. 2002;99:16713. doi: 10.1073/pnas.252649099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isofuran nomenclature: Taber DF, Fessel JP, Roberts LJ., II Prostag Other Lipid Mediat. 2004;73:47. doi: 10.1016/j.prostaglandins.2003.11.004.
- 13.Taber DF, Zhang Z. J Org Chem. 2006;71:926. doi: 10.1021/jo051889a. [DOI] [PubMed] [Google Scholar]
- 14.(a) Just G, Luthe C, Oh H. Tetrahedron Lett. 1980;21:1001. [Google Scholar]; (b) Just G, Luthe C. Can J Chem. 1980;58:1799. [Google Scholar]
-
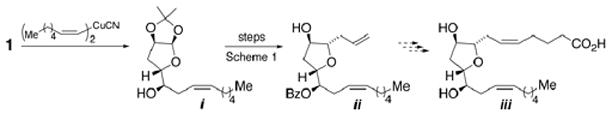
15.As proof of principle that our strategy could be used to prepare other members of this family, e.g., iii, 1-(Z)-heptene cuprate was added to epoxide 1 to give i which ultimately evolved ii following the procedures in Scheme 1.
- 16.García-Tellado F, de Armas P, Marrero-Tellado JJ. Angew Chem Int Ed. 2000;39:2727. doi: 10.1002/1521-3773(20000804)39:15<2727::aid-anie2727>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 17.Compound 3: TLC, EtOAc/hexane (1:1), Rf ~ 0.40; [α]D23 - 34.2 (c 1.00, CHCl3); 1H NMR (400 MHz, CDCl3) δ 1.74 (apparent p, J ~ 7.2 Hz, 2H), 1.91 (bs, 1H), 2.01 (ddd, J ~ 3.2, 6.4, 13.2 Hz, 1H), 2.11–2.23 (m, 5H), 2.37 (t, 2H, J ~ 7.6 Hz), 2.60–2.70 (m, 2H), 3.64 (s, 3H), 3.87 (td, J ~ 3.2, 6.4 Hz, 1H), 4.15 (t, J ~ 2.4 Hz, 1H), 4.70 (dt, J ~ 6.0, 6.4 Hz, 1H), 4.99–5.06 (m, 2H), 5.24 (apparent q, J ~ 6.0 Hz, 1H), 5.70–5.82 (m, 1H), 7.42 (apparent t, J ~ 7.6 Hz, 2H), 7.54–7.60 (tt, J ~ 1.2, 7.2 Hz, 1H), 8.03–8.06 (dd, J ~ 1.2, 8.4 Hz, 2H); 13C NMR (75 MHz, CDCl3) δ 18.20, 21.79, 23.97, 32.82, 36.23, 38.51, 51.64, 73.54, 75.04, 75.94, 78.06, 81.34, 85.95, 117.51, 128.47, 129.73, 130.17, 133.18, 134.09, 165.82, 173.93. TBDMS ether of 3: TLC, MeOH/CH2Cl2 (1:1), Rf ~ 0.87; [a]D23 -24.1 (c 0.87, CHCl3); 1H NMR (400 MHz, CDCl3) δ 0.01 (s, 3H), 0.02 (s, 3H), 0.83 (s, 9H), 1.68 (apparent p, J ~ 7.2 Hz, 2H), 1.85 (ddd, J ~ 3.6, 5.2, 12.8 Hz, 1H), 1.98–2.21 (m, 5H), 2.30 (t, J ~ 7.6 Hz, 2H), 2.51–2.63 (m, 2H), 3.59 (s, 3H), 3.74 (dt, J ~ 4.0, 6.4 Hz, 1H), 4.00 (dt, J ~ 3.6, 7.2 Hz, 1H), 4.37 (dt, J ~ 6.4, 8.0 Hz, 1H), 4.80–4.97 (m, 2H), 5.19 (apparent q, J ~ 6.0 Hz, 1H,), 5.63–5.74 (m, 1H), 7.45 (apparent t, J ~ 8.0 Hz, 2H), 7.57 (apparent t, J ~ 7.2 Hz, 1H), 8.02–8.05 (apparent dd, J ~ 1.6, 7.2 Hz, 2H); 13C NMR (75 MHz, CDCl3) δ-4.58, −4.41, 18.08, 18.29, 21.82, 24.06, 25.90, 32.84, 36.62, 38.18, 51.60, 73.76, 75.25, 76.02, 78.11, 81.35, 85.87, 117.35, 128.49, 129.83, 130.39, 133.15, 134.36, 165.80, 173.74. Compound 4: [α]D23 -19.6 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3) δ 1.71 (apparent p, J ~ 7.2 Hz, 2H), 2.02 (ddd, J ~ 1.6, 6.8, 13.2 Hz, 1H), 2.09 (td, J ~ 4.4, 8.4 Hz, 1H), 2.14–2.19 (m, 2H), 2.28–2.43 (m, 4H), 2.57–2.70 (m, 2H), 3.64 (s, 3H), 3.85 (td, J ~ 2.8, 6.4 Hz, 1H), 4.30 (br s, 1H), 4.50 (dt, J ~ 6.0, 8.8 Hz, 1H), 5.01–5.12 (m, 2H), 5.24 (q, J ~ 6.0 Hz, 1H,),5.78–5,81 (m, 1H), 7.45 (apparent t, J ~ 7.6 Hz, 2H), 7.57 (apparent tt, J ~ 1.2, 7.2 Hz, 1H), 8.02–8.05 (apparent dd, J ~ 1.2, 7.6 Hz, 2H); 13C NMR (75 MHz, CDCl3) δ 21.92, 24.09, 25.94, 32.88, 33.99, 37.80, 51.64, 73.30, 74.16, 76.08, 77.66, 81.32, 83.33, 116.67, 128.54, 129.85, 130.42, 133.18, 135.40, 165.95, 173.79. Compound 6: TLC, EtOAc/hexane (1:4), Rf ~ 0.40; [α]D23 -23.0 (c 1.18, CHCl3); 1H NMR (400 MHz, CDCl3) δ 0.87 (t, J ~ 7.2 Hz, 3H), 1.16–1.32 (m, 6H), 1.61–1.72 (m, 3H), 1.88–1.98 (m, 4H), 2.03–2.24 (m, 5H), 2.28 (t, J ~ 7.2 Hz, 2H), 2.41–2.55 (m, 2H), 3.65 (s, 3H), 3.76–3.81 (m, 1H), 4.10 (dt, J ~ 2.8, 5.6 Hz, 1H), 4.31 (dt, J ~ 6.0, 8.8 Hz, 1H), 5.27–5.51 (m, 5H), 7.44 (apparent t, J ~ 7.6 Hz, 2H), 7.56 (apparent t, J ~ 7.6 Hz, 1H), 8.01–8.04 (m, 2H); 13C NMR (75 MHz, CDCl3) δ 14.25, 22.74, 24.84, 26.86, 27.52, 29.39, 29.58, 31.64, 32.12, 33.62, 36.16, 51.71, 74.88, 75.51, 78.67, 86.48, 124.24, 125.04, 128.56, 129.79, 130.53, 131.93, 133.02, 133.15, 166.02, 174.29. Compound 7: TLC, EtOAc, Rf 0.18; [α]D23 -26.5 (c 1.03, CHCl3); 1H NMR (400 MHz, CDCl3) δ 0.88 (t, J ~ 6.8 Hz, 3H), 1.22–1.39 (m, 6H), 1.70 (q, J ~ 7.2 Hz, 2H), 1.78 (ddd, J ~ 4.0, 6.0, 13.2 Hz, 1H), 2.03 (apparent q, J ~ 7.2 Hz, 2H), 2.08–2.16 (m, 3H), 2.17– 2.24 (m, 2H), 2.28 (t, J ~ 7.2 Hz, 2H), 2.35 (t, J ~ 7.2 Hz, 2H), 3.82–3.86 (m, 2H), 4.09–4.16 (m, 2H), 5.35–5.57 (m, 4H); 13C NMR (75 MHz, CDCl3) δ 14.26, 22.75, 24.65, 26.66, 27.63, 29.43, 31.02, 31.70, 31.96, 33.52, 33.76, 71.51, 75.73, 80.85, 86.31, 124.23, 126.32, 131.39, 133.41, 178.83; MS (AP-LC) m/z 354 (M+, 100 %). Compound 8: TLC, EtOAc, Rf ~ 0.29; [α]D23 15.3 (c 0.96, CHCl3); 1H NMR (400 MHz, CDCl3) δ 0.88 (t, J ~ 6.8 Hz, 3H), 1.24– 1.40 (m, 6H), 1.65–1.78 (m, 2H), 2.00 (dd, J ~ 3.2, 12.8 Hz, 1H), 2.04–2.26 (m, 7H), 2.34 (t, J ~ 7.2 Hz, 2H), 2.38–2.51 (m, 2H), 3.61(td, J ~ 2.4, 7.2 Hz, 1H), 3.88 (apparent t, J ~ 6.4 Hz, 1H), 5.40–5.55 (m, 4H); 13C NMR (75 MHz, CDCl3) δ 14.27, 22.79, 24.65, 26.66, 27.17, 27.55, 29.50, 31.73, 31.94, 33.46, 34.31, 71.21, 72.01, 80.07, 83.77, 125.12, 125.93, 132.21, 132.71, 178.56; MS (AP-LC) m/z 354 (M+, 100 %). Compound 9: TLC, EtOAc/hexane (1:1), Rf ~ 0.52; [α]D23 -26.5 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3) δ 0.87 (t, J ~ 10.0 Hz, 3H), 1.19–1.39 (m, 6H), 1.59–1.70 (m, 4H), 2.00–2.20 (m, 6H), 2.27 (t, J ~ 10.0 Hz, 2H), 2.31–2.58 (m, 4H), 3.64 (s, 3H), 3.82–3.86 (m, 1H), 4.30 (br s, 1H), 4.41 (dt, J ~ 9.2, 12.0 Hz, 1H), 5.24 (dt, J ~ 7.2, 9.2 Hz, 1H), 5.29–5.53 (m, 5H), 7.41–7.46 (m, 2H), 7.52–7.57 (m, 1H), 7.99–8.03 (m, 2H); 13C NMR (75 MHz, CDCl3) δ 14.22, 22.70, 24.81, 26.81, 27.44, 27.56, 29.36, 29.53, 31.65, 33.57, 37.35, 51.67, 72.97, 75.61, 77.86, 83.01, 124.55, 125.01, 128.56, 129.76, 130.45, 131.89, 132.90, 133.15, 166.09, 174.24. Compound 10: TLC, MeOH/CH2Cl2 (1:9), Rf ~ 0.28; [α]D23 -12.4 (c 1.10, CHCl3); 1H NMR (400 MHz, CDCl3) δ 0.88 (t, J ~ 6.8 Hz, 3H), 1.22–1.40 (m, 8H), 1.71 (apparent p, J ~ 7.2 Hz, 2H), 1.93 (dd, J ~ 6.0, 13.2 Hz, 1H), 2.24–2.22 (m, 7H), 2.35 (t, J ~ 7.2 Hz, 2H), 2.38–2.49 (m, 2H), 3.84–3.94 (m, 2H), 4.19–4.24 (m, 1H), 4.33 (t, J ~ 3.2 Hz, 1H), 5.36–5.55 (m, 4H); 13C NMR (75 MHz, CDCl3) δ 14.25, 22.75, 24.71, 26.71, 27.59, 29.44, 29.88, 31.01, 31.70, 33.65, 34.78, 72.33, 73.05, 80.09, 83.42, 124.65, 126.33, 131.48, 132.95, 179.00; MS (AP-LC) m/z 354 (M+, 100 %). Compound 11: TLC, MeOH/CH2Cl2 (1:9), Rf ~ 0.29; [α]D23 16.9 (c 1.00, CHCl3); 1H NMR (400 MHz, CDCl3) δ 0.89 (t, J ~ 6.8 Hz, 3H), 1.22–1.40 (m, 7H), 1.62– 1.78 (m, 2H) 1.92–2.30 (m, 10H), 2.35 (t, J ~ 6.8 Hz, 2H), 3.85–3.90 (m, 1H), 4.03 (t, J ~ 7.2 Hz, 1H), 4.07 (d, J ~ 5.6 Hz, 1H), 4.15 (dt, J ~ 2.8, 9.2 Hz, 1H), 5.34–5.55 (m, 4H); 13C NMR (75 MHz, CDCl3) δ 14.25, 22.74, 24.66, 26.68, 27.58, 29.42, 31.31, 31.69, 31.89, 32.82, 33.60, 72.20, 74.48, 80.54, 87.13, 124.47, 125.99, 131.89, 132.88, 178.69; MS (AP-LC) m/z 354 (M+, 100 %). Compound 13: TLC, 3% MeOH/CH2Cl2, Rf ~ 0.26; [α]D23 1.1 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3) δ 1.68 (s, 1H), 1.75 (apparent p, J ~ 7.2 Hz, 2H), 1.90–2.00 (m, 3H), 2.16–2.21 (m, 2H), 2.33– 2.41 (m, 3H), 2.59–2.74 (m, 2H), 3.65 (s, 3H), 3.86 (td, J ~ 2.8, 6.4 Hz, 1H), 4.12 (apparent q, J ~ 2.8 Hz, 1H), 4.70 (td, J ~ 4.0, 8.4 Hz, 1H), 5.07–5.14 (m, 2H), 5.19 (td, J ~ 4.0, 6.8 Hz, 1H), 5.82–5.94 (m, 1H), 7.42 (apparent t, J ~ 7.6 Hz, 2H), 7.55–7.60 (m, 1H), 8.06–8.09 (m, 2H); 13C NMR (75 MHz, CDCl3) δ 18.32, 21.81, 24.07, 32.95, 36.51, 38.64, 51.76, 73.58, 75.28, 76.24, 77.47, 81.38, 85.85, 117.62, 128.57, 129.94, 130.17, 133.29, 134.38, 166.17, 174.00. Compound 14: [α]D23 11.9 (c 0.9, CHCl3); 1H NMR (400 MHz, CDCl3) δ 0.87 (t, J ~ 7.1 Hz, 3H), 1.16–1.38 (m, 6H), 1.65 (quintet, J ~ 7.3 Hz, 2H), 1.88–2.18(m, 6H), 2.23–2.41 (m, 4H), 2.54 (t, J ~ 6.3, Hz, 2H), 3.65 (s, 3H), 3.81 (ddd, J ~ 3.2, 6.2, 7.6 Hz, 1H), 4.05–4.09 (m, 1H), 4.37 (dt, J ~ 3.9, 7.0 Hz, 1H), 5.17 (dt, J ~ 3.9, 6.6 Hz, 1H), 5.40–5.56 (m, 4H), 7.40–7.47 (m, 2H), 7.53–7.60 (m, 1H), 8.04–8.08 (m, 2H); 13C NMR (75 MHz, CDC13) δ 14.23, 22.73, 24.84, 26.81, 27.63, 29.45, 29.54, 31.67, 32.18, 33.63, 36.57, 51.69, 74.79, 75.46, 77.98, 86.26, 124.49, 125.20, 128.56, 129.91, 130.38, 132.08, 132.93, 133.18, 166.36, 174.31. Compound 15: [α]D23 -10.0 (c 0.7, CHCl3); 1H NMR (400 MHz, CDCl3) δ 0.88 (t, J ~ 7.0 Hz, 3H), 1.24–1.40 (m, 6H), 1.70 (quintet, J ~ 7.0 Hz, 2H), 1.85 (apparent ddd, J ~ 2.8, 5.9, 13.1 Hz, 1H), 1.93–2.06 (m, 3H), 2.12 (apparent q, J ~ 5.7 Hz, 2H), 2.22–2.30 (m, 4H), 2.36 (t, J ~ 7.3 Hz, 2H), 3.47 (apparent q, J ~ 6.1 Hz, 1H), 3.83 (dt, J ~ 10.4, 3.0 Hz, 1H), 4.04–4.13 (m 2H), 5.36–5.56 (m, 4H); 13C NMR (75 MHz, CDC13) δ 14.26, 22.75, 24.57, 26.64, 27.64, 29.43, 31.71, 32.11, 32.22, 33.31, 36.98, 73.72, 75.96, 80.56, 86.14, 124.20, 126.51, 131.23, 133.35, 178.66. Compound 16: [α]D23 25.0 (c 0.68, CHCl3); 1H NMR (400 MHz, CDCl3) δ 0.88 (t, J ~ 6.7 Hz, 3H), 1.25–1.40 (m, 6H), 1.72 (quintet, J ~ 7.4 Hz, 2H), 1.83 (apparent dd, J ~ 14.0, 3.0 Hz, 1H), 2.07 (q, J ~ 7.0 Hz, 2H), 2.14 (q, J ~ 6.7 Hz, 2H), 2.28–2.52 (m, 7H), 3.54 (ddd, J ~ 10.4, 5.5, 2.4 Hz, 1H), 3.65 (dt, J ~ 14.0, 2.8 Hz, 1H), 4.01 (td, J ~ 9.8, 2.8 Hz, 1H), 4.07 (dd, J ~ 5.5, 2.8 Hz, 1H), 5.40–5.55 (m, 4H); 13C NMR (75 MHz, CDC13) δ 14.28, 22.80, 24.62, 26.66, 27.30, 27.56, 29.55, 31.74, 32.28, 33.35, 38.33, 71.67, 73.51, 78.99, 84.13, 125.25, 126.61, 132.04, 132.62, 178.58. Compound 17: TLC, 3% MeOH/CH2Cl2, Rf ~ 0.32; [α]D23 10.5 (c 0.72, CHCl3); 1H NMR (400 MHz, CDCl3) δ 1.76–1.78 (m, 3H), 1.97 (ddd, J ~ 4.8, 9.2, 13.6 Hz, 1H), 2.12 (dd, J ~ 6.8, 13.2 Hz, 1H), 2.15–2.21 (m, 2H), 2.33–2.53 (m, 4H), 2.59–2.74 (m, 2H), 3.66 (s, 3H), 3.86 (td, J ~ 2.4, 6.8 Hz, 1H), 4.28 (apparent s, 1H), 4.59–4.64 (m, 1H), 5.07–5.21 (m, 3H), 5.81–5.94 (m, 1H), 7.45 (apparent t, J ~ 7.6 Hz, 2H), 7.55–7.60 (m, 1H), 8.04–8.07 (m, 2H); 13C NMR (75 MHz, CDCl3) δ 18.37, 21.92, 24.13, 29.91, 32.98, 33.77, 37.54, 51.78, 73.19, 74.34, 76.22, 81.51, 82.50, 117.30, 128.62, 129.97, 130.25, 133.30, 134.76, 166.27, 173.95.
- 18.Brown CA, Ahuja VK. J Chem Soc, Chem Comm. 1973:553. [Google Scholar]
- 19.Mohapatra S, Bandyopadhyay A, Barma DK, Capdevila JH, Falck JR. Org Lett. 2003;5:4759. doi: 10.1021/ol035458v. [DOI] [PubMed] [Google Scholar]
- 20.Durand T, Guy A, Vidal JP, Rossi JC. J Org Chem. 2002;67:3615. doi: 10.1021/jo0109624. [DOI] [PubMed] [Google Scholar]
- 21.Falck JR, Krishna UM, Reddy YK, Kumar PS, Reddy KM, Hittner SB, Deeter C, Sharma KK, Gauthier KM, Campbell WB. Am J Physiol. 2003;284:H337. doi: 10.1152/ajpheart.00831.2001. [DOI] [PubMed] [Google Scholar]
- 22.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Circ Res. 1996;78:415. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]


