Abstract
Background
Strongyloidiasis infects hundreds of millions of people worldwide and is an important cause of mortality from intestinal helminth infection in developed countries. The persistence of infection, increasing international travel, lack of familiarity by healthcare providers, and potential for iatrogenic hyperinfection, all make strongyloidiasis an important emerging infection.
Design & Methods
Two studies were performed. A retrospective chart review of Strongyloides stercoralis cases identified through microbiology laboratory records from 1993–2002 was conducted. Subsequently, 363 resident physicians in 15 training programs worldwide were queried with a case scenario of strongyloidiasis presenting an immigrant with wheezing and eosinophilia. The evaluation focused on resident recognition and diagnostic recommendations.
Results
In 151 strongyloidiasis cases, stool ova and parasite sensitivity is poor (51%), and eosinophilia (>5% or >400 cells/μL) commonly present (84%). Diagnosis averaged 56 months (Intra-quartile range: 4 to 72 months) after immigration. Presenting complaints were non-specific, although 10% presented with wheezing. Hyperinfection occurred in five patients prescribed corticosteroids with two deaths. Treatment errors occurred more often among providers unfamiliar with immigrant health (Relative Risk of Error: 8.4; 95% CI: 3.4 to 21.0; P<0.001).
When presented a hypothetical case scenario, U.S. physicians-in-training had poor recognition (9%) of the need for parasite screening and frequently advocated empiric corticosteroids (23%). International trainees had superior recognition at 56% (P<0.001). Among U.S. trainees, 41% were unable to choose any parasite causing pulmonary symptoms.
Conclusions
Strongyloidiasis is present in U.S. patients. Diagnostic consideration should occur with appropriate exposure, non-specific symptoms including wheezing, or eosinophilia (>5% relative or >400 eosinophils/μL). U.S. residents’ helminth knowledge is limited and places immigrants in iatrogenic danger. Strongyloides should be included in U.S. training and continuing medical education programs.
Keywords: strongyloides, parasites, immigrant, refugee, eosinophil, sensitivity, wheezing, graduate medical education
Introduction
Strongyloidiasis is an extremely common cause of morbidity and mortality worldwide. This disease is endemic in many tropical and subtropical areas, particularly Southeast Asia, but also including Latin America and sub-Saharan Africa, as well as temperate areas such as Spain and the Appalachian region of the U.S.1,2,3 Intestinal parasites are common in immigrants and refugees from developing countries, and the prevalence in some Southeast Asian refugees approaches 50%.4,5,6 Refugees from Cambodia, Laos, and Thailand are historically at high risk of strongyloidiasis.7,8,9,10 Among over 17,000 refugees arriving in Minnesota from 1993–1999, 22% harbored an intestinal helminth and 2.4% had detectable Strongyloides stercoralis in stool.11
Infection in non-endemic settings is predominately in immigrants or expatriates.12,13 By 2000 census data, 11% of the U.S. population is foreign-born. In Appalachia, the Strongyloides stercoralis prevalance by general screening is 4%.14,15,16 In developed countries, almost all deaths attributable to helminths occur secondary to strongylodiaisis hyperinfection or dissemination.13 Although once thought rare, disseminated strongyloidiasis may be relatively common in high risk populations and frequently misdiagnosed as isolated gram negative sepsis or acute respiratory distress syndrome.17,18 Even though this feared iatrogenic complication from corticosteroid therapy does occur, strongyloidiasis generally presents with diffuse, non-specific gastrointestinal, dermatologic, or respiratory symptoms.10,19,20 Most chronically infected are asymptomatic.1,21,22
Minimal data exists regarding strongyloides in non-endemic settings. This study was conducted to review demographic and clinical data. When unrecognized strongyloidiasis may have devastating iatrogenic complications, a goal of this project was to assess future physicians’ knowledge. Therefore, an assessment among U.S. and international physcians’-in-training basic knowledge of strongyloiodiasis was performed.
Methods
A retrospective clinical descriptive study was conducted of all cases of Strongyloides stercoralis from January 1, 1993 through December 31, 2002 at Regions Hospital/HealthPartners in St. Paul, MN, a 427-bed hospital with a Center for International Health serving an immigrant and refugee population. Indeed, 13% of St. Paul, MN is of Southeast Asian descent.23 Cases were identified through a microbiology database of parasitology specimens from the hospital laboratory. The charts of patients with positive ova and parasite (O&P) specimens for S. stercoralis (rhabditiform or filariform larvae) were reviewed. Reviews were conducted by three physicians knowledgeable in tropical medicine. A standardized data form collected: demographic information, U.S. arrival time, clinical manifestations, complete blood counts with differential (CBC), eosinophil count at diagnosis and at follow up, other organisms detected, total number of stool samples positive, treatment prescribed, treatment failures detected by recurrent positive samples or persistent eosinophilia, occurrence of hyperinfection, and appropriate management including follow-up examination.
Analysis is primarily descriptive. Results are reported as mean ±SD. The intra-quartile range (IQR) is reported when data have non-normal distribution. Comparisons are made with SPSS 13.0 (Chicago, IL) presenting relative risk (RR) and 95% confidence intervals (95% C.I.).
Trainee Survey
An internet based survey was conducted of 363 internal medicine, medicine-pediatric, and pediatric residents in 15 different residency training programs across the U.S., Brazil, Singapore, and Thailand. Nine U.S. residency programs participated in regions being endemic for strongyloides, with immigrants, and without either. The survey consisted of a case presentation of a Southeast Asian immigrant without prior asthma history presenting with new onset wheezing and respiratory distress. All values presented were typical of the cases reviewed in this study. For example, the case scenario presented a 38 year-old patient who immigrated 5 years prior. The patient’s peripheral eosinophil count was 900 cells/μL (9%) with reportedly normal chest radiograph. For surveys in Brazil and Thailand, the patient was from a “rural region” rather than an immigrant. The Brazilian survey was administered in Portuguese. Residents were queried as to their “work up and initial therapy.” Additional queries addressed residents’ helminth knowledge including: the “level of absolute eosinophilia considered abnormal,” “which parasites routinely cause chronic pulmonary symptoms,” and “which helminth causes the most mortality”. Answers were multiple-choice with individualized computer randomization of answer ordering to eliminate response-order bias. Institutional review board approvals occurred.
Results
During the 10 year period, 151 people were diagnosed with strongyloidiasis. Of 1291 positive stool specimens collected, Strongyloides stercoralis was the third most common parasite identified accounting for 11% of potentially pathogenic gastrointestinal parasites (Table 1). Countries of origin were diverse, but Southeast Asian patients predominated (Table 2). Stool specimens were positive for S. stercoralis in 149 patients while sputum samples were positive in 3 persons. The mean (±SD) age of infected individuals was 39 ±18 years. The sample consisted of 83 men and 68 women. The mean time of diagnosis from arrival in the United States was 45 ±52 months [IQR: 4 to 72 months]. If cases of newly arrived refugees detected at routine screening (n=28) are eliminated, the mean time to diagnosis increases to 55 months. There were five cases of documented hyperinfection or dissemination which occurred as an iatrogenic complication of corticosteroid therapy initiated in chronically infected persons. Three of the cases of hyperinfection had resided in the U.S. for greater than five years. These iatrogenic complications resulted in prolonged stays in an intensive care unit (ICU) in all five patients and led to two deaths.
Table 1.
Number of patients infected with individual parasite species 1996-2002
| Potentially Pathogenic Species | No. | (%) of positive specimens |
|---|---|---|
| Giardia intestinalis | 363 | (28.1) |
| Hookworm species | 297 | (23.0) |
| Strongyloides stercoralis | 139 | (10.8) |
| Trichuris trichiura | 132 | (10.2) |
| Ascaris lumbricoides | 115 | (8.9) |
| Entamoeba histolytica/dispar | 82 | (6.4) |
| Hymenolepis nana | 60 | (4.6) |
| Taenia species | 31 | (2.4) |
| Enterobius vermicularis | 23 | (1.8) |
| Opisthorchis spp and Clonorchiasis sinesis | 17 | (1.3) |
| Schistosoma mansoni | 13 | (1.0) |
| Othera | 19 | (1.5) |
|
| ||
| Total | 1291 | (100) |
Schistosoma haematobium (n=6), Diphyllobothrium latum (n=4), Fascioloidea spp. (n=4), Paragonimus westermani (n=3), Toxocara (n=1)
Table 2.
Country of origin/exposure
| Country | Number | Percent |
|---|---|---|
| Cambodia | 57 | 30% |
| Hmong (ethnicity) | 34 | 18% |
| Vietnam | 33 | 17% |
| Thailand | 28 | 15% |
| Laos | 16 | 8.4% |
| Ethiopia | 7 | 3.7% |
| Liberia | 4 | 2.1% |
| Philippines | 3 | 1.6% |
Eritrea, Guatemala, Mexico, Nigeria, Somalia, Sudan, and Uganda all contributed ≤ 2 persons each.
Note: Totals are >151 due to multiple countries of exposure and overlapping locales of the Hmong people who originated in Laos.
The average absolute eosinophil count was 981 ± 978 [IQR: 480 to 1130 cells/μL], and the average percent eosinophilia was 11.8% ±7.6% [IQR: 6.3% to 16.4%]. Eosinophilia varied dramatically with age. Children had higher eosinophil counts (Figure 1). Persons harboring multiple parasites (n=80) had similar eosinophilia (1015 ± 950; P=0.7). The sensitivity of an absolute eosinophil cutoff of >500 cells/μL for predicting ≥1 positive stool sample was 73% [94/128]. For a cutoff of >400 cells/μL, the sensitivity improved to 84% [107/128]. Using a 5% relative differential as the abnormal cutoff for distinguishing eosinophilia had equivalent sensitivity 84% with good, but not identical, agreement (Kappa = 0.829). Consideration of both absolute and relative eosinophilia only minimally increased detection sensitivity (86%).
Figure 1. Absolute eosinophil count versus age.
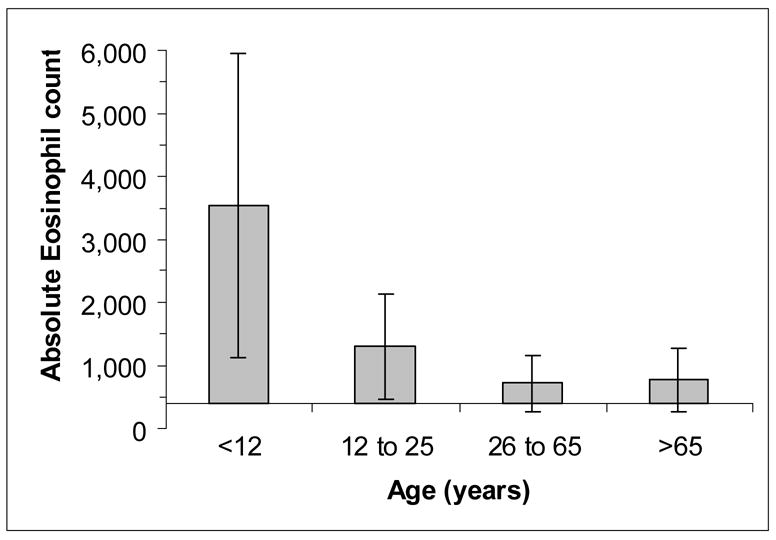
Data represent mean ±SD of absolute eosinophil counts (cells/μL). Intercept is at 400 cells/μL.
Stool ova and parasite examination is a relatively insensitive technique for diagnosis of strongyloidiasis with larvae detected in 51% [262/517] of all collected specimens of patients with at least one positive sample. Further, 16% [24/151] of patients had at least 3 prior negative specimens for Strongyloides [mean 3.6 ± 2.1; max 9] confirming the poor sensitivity of stool examination alone. Interestingly, 46% [11/24] of those antecedent specimens did harbor other parasites indicating a history of fecal-oral contamination. Prior to the correct diagnosis of strongyloidiasis, patients with previously negative stool specimens were subjected to numerous invasive procedures and labeled with an array of misdiagnoses including irritable bowel syndrome, somatization disorder, and psychogenic pruritis.
Presenting symptoms were diverse with 12% being asymptomatic and identified through routine arrival medical screening. Abdominal complaints (40%) and pulmonary complaints (22%) were common, including wheezing (10%).
Follow up laboratory investigations were performed in 93 persons (62%) and included: three stool samples (n=53) alone, CBC with differential (n=3), or both stool and CBC (n=37) (Figure 2). Treatment failures occurred in 7.6% [11/145] with eight documented by positive stool specimens and three by persistent symptoms and eosinophilia. All patients with primary drug failure by stool examination also had persistently elevated eosinophil counts [absolute: 975±425 cells/μL (Min 510); relative: 13.6 ± 7.3% (Min 5.4%)]. In the 37 persons with both follow up tests, no person with an eosinophil count <400 cells/μL, had positive strongyloides specimens (0/28; NPV 100%). However, two persons did harbor giardia. Medication failures occurred with thiabendazole 6% [6/97], albendazole 14% [3/22], and mebendazole 50% [2/4]. No ivermectin failures occurred.
Figure 2.
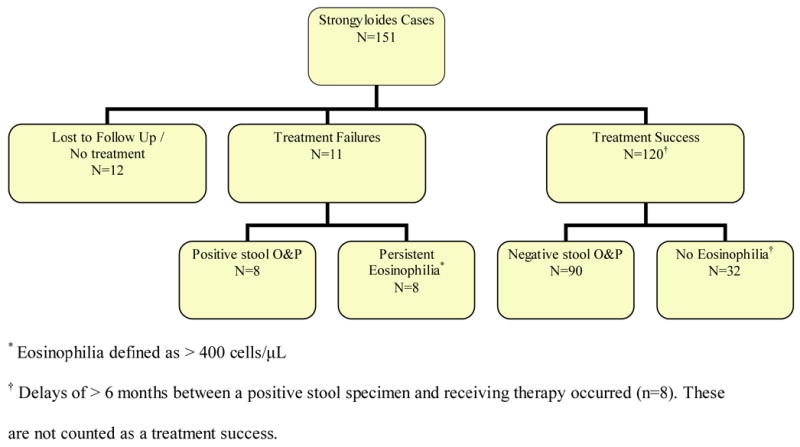
Patient outcomes
Persons with strongyloidiasis frequently harbored other parasites (56%). (Table 3) Potentially pathogenic parasites accounted for 63% of positive samples with hookworm being the most common co-infection (n=46). In persons with hookworm co-infection, no difference was observed in age, hemoglobin, or eosinophil count [947 ± 660 cells/μL]. Of hookworm diagnoses, 79% occurred within 3 years of immigration and 90% within 6 years from arrival (Max: 12 years). As hookworm species have a finite 3–5 year average lifespan, either 10–20% of hookworm greatly exceeded the average lifespan or were acquired by post-immigration visiting friends and relatives (VFR) travel. Post treatment stool specimens detected persistent parasites in 30% [27/90] of patients; 52% [23/44] of detected species were potential pathogens.
Table 3.
Other parasites discovered in strongyloidiasis patients
| Parasite | Number of Patients (n=151) | Percent of positive stool specimens [positive/collected*] | |
|---|---|---|---|
| Strongyloides stercoralis | 151 | 51% | [262/517] |
|
| |||
| Co-infections of Pathogenic Parasites | |||
|
| |||
| Hookworm | 46 | 76% | [105/139] |
| Giardia intestinalis | 14 | 78% | [28/36] |
| Blastocystis hominis† | 12 | 71% | [25/35] |
| Trichuris trichuria | 5 | 73% | [11/15] |
| Clonorchis sinensis | 5 | 57% | [10/18] |
| Ascaris lumbricoides | 4 | 100% | [12/12] |
| Hymenolepis nana | 4 | 83% | [10/12] |
| Paragonimus westermani | 4 | 50% | [4/6] |
| Entamoeba histolytica | 3 | 33% | [3/9] |
|
| |||
| Additional Non-pathogenic parasites | |||
|
| |||
| Endolimax nana | 25 | 66% | [53/78] |
| Entamoeba coli | 12 | 86% | [36/42] |
| Entamoeba hartmanni | 10 | 78% | [28/36] |
| Iodamoeba butschlii | 3 | 67% | [6/9] |
collected stool specimens for ova and parasite examination among infected individuals
Controversial as to the pathogenicity of Blastocystis hominis.
Fascioloidea, Schistosoma japonicum, Schistosoma mansoni, Taenia species, and trichostrongylus co-infections were present in one patient each with a detection rate of 67%.
Treatment Errors
Initial diagnostic evaluations were with non-international health or travel-clinic providers in 24% [36/151] of cases. Three patients presenting with wheezing to primary care physicians were treated with corticosteroids for “asthma” with subsequent development of hyperinfection. Two patients died. All patients presenting with wheezing to clinicians familiar with immigrant health or travel medicine (n=12) had an evaluation for strongyloidiasis, appropriate treatment, and confirmatory follow-up evaluation.
Delays of >6 months in treatment from a positive sample or highly elevated eosinophilia (>1000 cells/μL) occurred in 5% [8/151] of cases with an average delay of 2.8 ± 3.2 years [IQR: 1 to 3 years, Max. 10 years]. In several cases, multiple referrals to specialists such as dermatology and gastroenterology occurred with invasive procedures performed without consideration of strongyloidiasis. One patient underwent multiple procedures for six months following a positive strongyloides stool sample. Errors in treatment, either prescribing an ineffective medication (e.g. mebendazole, metronidazole) or no medication, occurred in 6% [9/151]. Delays or errors in treatment occurred more often with healthcare providers not familiar with immigrant/travel medicine (RR 8.4; 95% CI: 3.4 to 21.0; P<0.001). In 66% of treatment errors, multiple parasites were present [mean 1.5 ± 0.8 other species] perhaps causing confusion. Twelve patients (8%) were never effectively treated.
Medical Resident Trainee Survey
The survey response rate was 70% [363/520]. When presented with a Southeast Asian immigrant* with new onset wheezing and 9% eosinophilia (absolute 900 eosinophils/μL), only 9% of U.S. trainees recommended further evaluation for abnormal eosinophilia or parasites as compared to 56% [53/94] of international trainees. Empiric corticosteroid use without further evaluation was recommended by 23% [61/269] of U.S. versus 7% [7/94] of international trainees (RR 3.0; 95% CI: 1.4 to 6.4; P=0.005). There was no performance difference between upper level residents and first or second year residents (P=0.2). Internal medicine and medicine-pediatric residents significantly outperformed their pediatric colleagues in recognition (RR 9.6; 95% CI: 2.1 to 42; P=0.004), but the recommendation for empiric steroids without evaluation or strongyloidiasis treatment was similar (RR 0.7; 95% CI: 0.5 to 1.1; P=0.11).
General helminth knowledge was poor with 51% of residents unable to identify the absolute level of eosinophilia considered abnormal. Most experts agree that eosinophilia above 400–500 cells/μL is considered abnormal.24 Among respondents, 21% had a cutoff too low (250–300 cells/μL) and 30% had too high (>XX?) an abnormal threshold.
When presented a list of 16 parasites and asked “which two parasites routinely cause chronic pulmonary symptoms?” 62% of residents knew Strongyloides stercoralis causes pulmonary symptoms, but 20% also believed Blastocystis hominis caused pulmonary symptoms. Ascaris was listed by 43%, which can give pulmonary symptoms during initial larval migration, as can hookworm (12%), but neither parasite does so chronically. Only 18% correctly identified Paragonimus westermani (Oriental Lung Fluke) as associated with chronic pulmonary disease. Forty-one percent of U.S. trainees were unable to choose any parasite that caused any pulmonary symptoms. The performance of international trainees was superior with 65% correctly naming the two main parasitic causes of chronic pulmonary symptoms. When asked “which helminth causes the most mortality in the U.S.?” 34% correctly identified strongyloidiasis with other responses being: amebiasis (18%), schistosomiasis (11%), and Blastocystis hominis (9%). Blastocystis has minimal, if any, pathogenicity.
Discussion
The principal precept of the practice of medicine is “first, to do no harm” (primum non nocere). As 11% of the U.S. population is foreign-born, and annual immigration exceeds one million, basic helminth knowledge is important for physicians. Strongyloides is an important pathogen in refugee and immigrant populations.10,20 This study demonstrates, because the stool is not always positive, strongyloidiasis is frequently misdiagnosed (e.g. psychosomatic) or diagnosis significantly delayed. In this series, if not detected at new refugee arrival medical screening, the diagnosis was delayed by five years on average from U.S. arrival. Strongyloides’ unique auto-infective cycle creates indefinite infection. Among World War II British prisoners of war, the average time from leaving an endemic area to diagnosis was 37±6 years.25 Thus, potential iatrogenic maleficence remains life-long.
Strongyloidiasis diagnosis is difficult. The reported sensitivity of three routine stool specimens by direct examination is reported as 50%.1,19,26,27 In this study, three stool ova and parasite examinsations detected 83% of strongyloidiasis cases; however, 17% had ≥3 prior false negative specimens. Overall, 51% of all stool samples contained Strongyloides stercoralis. This higher yield may represent the burden of infection in a refugee population, but likely may be biased by even more undiscovered cases as there was no external gold standard utilized, such as agar plate methods of “parasite cultures” or serology.10,19 The CDC strongyloides enzyme immunoassay (ELISA) serology is 95% sensitive for IgG antibody detection and can be a useful adjuvant for diagnosis.10 Several reference labs also have serology available of unknown validity.
In many, a mild relative (>5%) and absolute eosinophilia (>400 cells/μL) were the only abnormal finding, predicting a positive stool specimen in 84%. This sensitivity is comparable to the 83% sensitivity of eosinophilia in immigrants screened by CDC serology.10 Similarly, among Spanish agricultural workers, Roman-Sanchez et al. found those with eosinophilia (>400 cells/μL), had 73-fold higher odds of strongylodiasis.22 However, when hyperinfection syndrome occurs, patients without eosinophilia have high mortality.3,18 In the largest hyperinfection series from Brazil, Canada, and Minnesota, presenting eosinophil counts <400 cells/μL had 100% mortality.17,18,28
Wheezing was the presenting symptom in 10%. Asthma has been previously reported,29,30 but this series represents a higher occurrence of wheezing. Strongyloides is not causitive for true asthma.30,31 Nevertheless, empiric corticosteroid therapy is problematic as empiric corticosteroids may cause life threatening hyperinfection. Immunosuppression allows the strongyloides larvae to penetrate the gut wall causing secondary gram negative sepsis.3,32 In our case series, those presenting with wheezing to practitioners unfamiliar with travel or tropical medicine uniformly received corticosteroids. In high risk persons from Southeast Asia or agricultural workers, we recommend screening (i.e. serology) when time allows or empiric treatment with ivermectin 200 mcg/kg prior to immunosuppressive therapy. Since the average time to diagnosis was five years from arrival in this series and hyperinfection has occured >50 years after immigration, we suggest the aforementioned management regardless of immigration timing.17,33 This is particularly true with new onset wheezing in adult immigrants.
Treatment failure is common even with appropriate therapy. Although not a randomized trial, albendazole’s 14% failure rate reinforces its inferiority to thiabendazole or ivermectin.19,34,35 Ivermectin 200 mcg/kg orally and repeated at 2 weeks has 97% efficacy.34 Ivermectin is inactive against hookworm, a common co-infection. Thiabendazole is active against hookworm but has a high rate (>50%) of nausea.35 Post therapy stool examinations are recommended to verify strongyloides eradication and exclude other parasites as 15% harbored post-treatment pathogenic parasites. Patients with initial eosinophilia (>400 cells/μL) and subsequently normalized their post-treatment eosinophil counts had a 100% NPV for three follow up negative stool examinations, corresponding to other observations.19,26 When repeat stool testing is impractical or unobtainable, eosinophil count normalization is a reassuring surrogate marker for treatment success.
While strongyloidiasis may be considered an infectious disease, tropical medicine, or immigrant health issue, patients seek care from primary providers. Physicians’-in-training basic helminth knowledge is poor, particularly for U.S. resident-physicians. This lack of training was evident in the 151 person case series by the poorer quality of care given by physicians untrained in travel medicine. As the U.S. receives more immigrants than the rest of the world combined and immigrants constitute >25% of the Los Angeles, Miami, New York, and San Francisco metropolitan areas,23 this creates a special obligation for U.S. physicians. While comprehensive helminth knowledge among U.S. physicians is unrealistic, basic knowledge of iatrogenic danger posed by corticosteroids and strongyloides should exist.
Conclusion
Physicians should consider strongyloidiasis in patients with a potential exposure history in developing countries, non-specific symptoms, and >5% eosinophilia (or >400 eosinophils/μL), regardless of the time since immigration. A significant portion may present with pulmonary symptoms including wheezing. Screening in high risk individuals or empiric anti-helminth treatment in immigrants with eosinophilia is warranted prior to immunosuppression to prevent the severe morbidity and mortality associated with hyperinfection syndrome. Knowledge of this infection and steps to avoid iatrogenic complications should be included in training and continuing medical education programs to ensure physicians first do no harm.
Clinical Significance Bullets – FINAL
Physicians should consider strongyloidiasis in patients with a potential exposure history in developing countries, regardless of the time since immigration.
Clinical clues include wheezing, abdominal distress, and eosinophilia > 400 cells/μL or > 5% of differential count. Infection persists lifelong.
Strongyloides is unique in that deaths in the U.S. are nearly always iatrogenic. Steroids given to a person with chronic, asymptomatic strongyloides infection can lead to life threatening hyperinfection which has ~50% mortality due to gram negative sepsis.
Stool exams are insensitive (50% detection per exam). IgG serology is available.
Acknowledgments
We thank Guilherme Barroso for assistance. DRB, WMS received support from the NIH (T32-AI055433).
Appendix
The physicians-in-training survey may be taken at: http://www.tropical.umn.edu/strongyloides
Online Appendix 1.
Resident survey answers
| Parasite Species* | Chronic Pulmonary Symptoms | Greatest Mortality |
|---|---|---|
| % | % | |
| Ancylostoma duodenale (Hookworm) | 12 | 5 |
| Ascaris lumbricoides | 43 | 10 |
| Blastocystis hominis | 20 | 9 |
| Clonorchis sinensis (Oriental liver fluke) | 2.3 | 1 |
| Cryptosporidium parvum | 11 | -- |
| Entamoeba histolytica (Amebiasis) | 6 | 18 |
| Paragonimus westermani (Oriental lung fluke) | 18 | 4 |
| Schistosoma species | 14 | 11 |
| Strongyloides stercoralis | 62 | 35 |
| Taenia species (tapeworm) | 3.3 | 5 |
| Trichuris trichuria (whipworm) | 5 | 12 |
Endolimax nana, Entamoeba coli, Fasciolides, Giardia, Hymenolepis were also listed as possible answers but received <1% each. Cryptosporidium was not queried as to mortality.
Footnotes
In the case of surveys in Brazil and Thailand, the individual was reported to be from a “rural area.”
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liu LX, Weller PF. Strongyloides and other intestinal nematode infections. Infect Dis Clin North Am. 1993;7:655–82. [PubMed] [Google Scholar]
- 2.Genta RM. Global prevalence of strongyloidiasis: critical review with epidemiologic insights into the prevention of disseminated disease. Rev Infect Dis. 1989;11:755–67. doi: 10.1093/clinids/11.5.755. [DOI] [PubMed] [Google Scholar]
- 3.Keiser PB, Nutman TB. Strongyloides stercoralis in the immunocompromised population. Clin Microbiol Rev. 2004;17:208–17. doi: 10.1128/CMR.17.1.208-217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nutman TB, Ottesen EA, Ieng S, et al. Eosinophilia in Southeast Asian refugees: evaluation at a referral center. J Infect Dis. 1987;155:309–13. doi: 10.1093/infdis/155.2.309. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman SL, Barrett-Connor E, Norcross W, Nguyen D. Intestinal parasites in Indochinese immigrants. Am J Trop Med Hyg. 1981;30:340–43. doi: 10.4269/ajtmh.1981.30.340. [DOI] [PubMed] [Google Scholar]
- 6.Lindes C. Intestinal parasites in Laotian refugees. J Fam Pract. 1979;9:819–22. [PubMed] [Google Scholar]
- 7.Vannachone B, Kobayashi J, Nambanya S, Manivong K, Inthakone S, Sato Y. An epidemiological survey on intestinal parasite infection in Khammouane Province, Lao PDR, with special reference to Strongyloides infection. Southeast Asian J Trop Med Public Health. 1998;29:717–22. [PubMed] [Google Scholar]
- 8.Wiesenthal AM, Nickels MK, Hashimoto KG, Endo T, Ehrhard HB. Intestinal parasites in Southeast Asian refugees. Prevalence in a community of Laotians. JAMA. 1980;244:2543–44. doi: 10.1001/jama.1980.03310220041024. [DOI] [PubMed] [Google Scholar]
- 9.Jongsuksuntigul P, Intapan PM, Wongsaroj T, et al. Prevalence of Strongyloides stercoralis infection in northeastern Thailand (agar plate culture detection) J Med Assoc Thai. 2003;86:737–41. [PubMed] [Google Scholar]
- 10.Loutfy MR, Wilson M, Keystone JS, Kain KC. Serology and eosinophil count in the diagnosis and management of strongyloidiasis in a non-endemic area. Am J Trop Med Hyg. 2002;66:749–52. doi: 10.4269/ajtmh.2002.66.749. [DOI] [PubMed] [Google Scholar]
- 11.Swanson S, Lee B, Mamo B, Smith K, Stauffer WM. Changing prevalence of intestinal parasites among newly arrived Southeast Asian and African refugees after empiric predeparture albendazole treatment – Minnesota, 1993–2004 [abstract]. 55th Annual Epidemic Intelligence Service Conference; Atlanta, GA. April 24–28, 2006. [Google Scholar]
- 12.Hira PR, Al-Ali F, Shweiki HM, et al. Strongyloidiasis: challenges in diagnosis and management in non-endemic Kuwait. Annal Trop Med Parasitol. 2004;98:261–70. doi: 10.1179/000349804225003299. [DOI] [PubMed] [Google Scholar]
- 13.Muennig P, Pallin D, Sell RL, Chan The cost effectiveness of strategies for the treatment of intestinal parasites in immigrants. N Engl J Med. 1999;340:773–79. doi: 10.1056/NEJM199903113401006. [DOI] [PubMed] [Google Scholar]
- 14.Fulmer HS, Huempfner HR. Intestinal helminths in eastern Kentucky: a survey in three rural counties. Am J Trop Med Hyg. 1965;14:269–75. doi: 10.4269/ajtmh.1965.14.269. [DOI] [PubMed] [Google Scholar]
- 15.Walzer PD, Milder JE, Banwell JG, Kilgore G, Klein M, Parker R. Epidemiologic features of Strongyloides stercoralis infection in an endemic area of the United States. Am J Trop Med Hyg. 1982;31:313–19. doi: 10.4269/ajtmh.1982.31.313. [DOI] [PubMed] [Google Scholar]
- 16.Berk SL, Verghese A, Alvarez S, Hall K, Smith B. Clinical and epidemiologic features of strongyloidiasis. A prospective study in rural Tennessee. Arch Intern Med. 1987;147:1257–61. [PubMed] [Google Scholar]
- 17.Lim S, Katz K, Krajden S, et al. Complicated and fatal Strongyloides infection in Canadians: risk factors, diagnosis and management. Canadian Med Assoc J. 2004;171:479–84. doi: 10.1503/cmaj.1031698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newberry AM, Stauffer WM, Hendel-Paterson BR, et al. Strongyloides hyperinfection presenting as acute respiratory distress and gram negative sepsis. Chest. 2005;128:3681–4. doi: 10.1378/chest.128.5.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis. 2001;33:1040–47. doi: 10.1086/322707. [DOI] [PubMed] [Google Scholar]
- 20.de Silva S, Saykao P, Kelly H, et al. Chronic Strongyloides stercoralis infection in Laotian immigrants and refugees 7–20 years after resettlement in Australia. Epidemiol Infect. 2002;128:439–44. doi: 10.1017/s0950268801006677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gyorkos TW, Genta RM, Viens P, MacLean JD. Seroepidemiology of strongyloides infection in the South East Asian refugee population in Canada. Am J Epidemiol. 1990;132:257–64. doi: 10.1093/oxfordjournals.aje.a115655. [DOI] [PubMed] [Google Scholar]
- 22.Román-Sánchez P, Pastor-Guzmán A, Moreno-Guillén S, Igual-Adell R, Suñer-Generoso S, Tornero-Estébanez C. High prevalence of Strongyloides stercoralis among farm workers on the Spanish Mediterranean coast. Analysis of the predictive factors of infection in developed countries. Am J Trop Med Hyg. 2003;69:336–40. [PubMed] [Google Scholar]
- 23.U.S. Census Bureau, County and City Data Book. [Accessed Dec 18, 2005];2000 Available at: http://quickfacts.census.gov/qfd/states/00000.html.
- 24.Rothenberg ME. Eosinophilia. N Eng J Med. 1998;338:1592–1600. doi: 10.1056/NEJM199805283382206. [DOI] [PubMed] [Google Scholar]
- 25.Gill GV, Welch E, Bailey JW, Bell DR, Beeching NJ. Chronic Strongyloides stercoralis infection in former British Far East prisoners of war. QJM. 2004;97:789–95. doi: 10.1093/qjmed/hch133. [DOI] [PubMed] [Google Scholar]
- 26.Grove DI. Strongyloidiasis in Allied ex-prisoners of war in South-East Asia. BMJ. 1980;1:598–601. doi: 10.1136/bmj.280.6214.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pelletier LL. Chronic strongyloidiasis in World War II Far East ex-prisoners of war. Am J Trop Med Hyg. 1984;33:55–61. doi: 10.4269/ajtmh.1984.33.55. [DOI] [PubMed] [Google Scholar]
- 28.Ferreira MS, Nishioka Sde A, Borges AS, et al. Strongyloidiasis and infection due to human immunodeficiency virus: 25 cases at a Brazilian teaching hospital, including seven cases of hyperinfection syndrome. Clin Infect Dis. 1999;28:154–5. doi: 10.1086/517188. [DOI] [PubMed] [Google Scholar]
- 29.Robinson J, Ahmed Z, Siddiqui A, Roy T, Berk S, Smith JK, Krishnaswamy G. A patient with persistent wheezing, sinusitis, elevated IgE, and eosinophilia. Annals Allergy Asthma Immunol. 1999;82:144–9. doi: 10.1016/S1081-1206(10)62588-4. [DOI] [PubMed] [Google Scholar]
- 30.Wehner JH, Kirsch CM, Kagawa FT, Jensen WA, Campagna AC, Wilson M. The prevalence and response to therapy of Strongyloides stercoralis in patients with asthma from endemic areas. Chest. 1994;106:762–6. doi: 10.1378/chest.106.3.762. [DOI] [PubMed] [Google Scholar]
- 31.Leeman BJ, Cabrera MR. No association found between Strongyloides infestation and asthma. J Asthma. 1995;32:57–62. doi: 10.3109/02770909509089500. [DOI] [PubMed] [Google Scholar]
- 32.Igra-Siegman Y, Kapila R, Sen P, et al. Syndrome of hyperinfection with Strongyloides stercoralis. Rev Infect Dis. 1981;3:397–407. doi: 10.1093/clinids/3.3.397. [DOI] [PubMed] [Google Scholar]
- 33.Gill V, Beeching NJ, Khoo S, et al. A British Second World War veteran with disseminated strongyloidiasis. Trans Roy Soc of Trop Med Hyg. 2004;98:382–6. doi: 10.1016/j.trstmh.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Toma H, Sato Y, Shiroma Y, Kobayashi Shimakukuro I, Takara M. Comparative studies on the efficacy of three antihelminthics on treatment of human strongyloidiasis in Okinawa, Japan. Southeast Asian Trop Med Public Health. 2000;31:147–51. [PubMed] [Google Scholar]
- 35.Marti H, Haji HJ, Savioli L, et al. A comparative trial of a single dose ivermectin versus three days of albendazole for treatment of Strongyloides stercoralis and other soil transmitted helminth infections in children. Am J Trop Med Hyg. 1996;55:477–81. doi: 10.4269/ajtmh.1996.55.477. [DOI] [PubMed] [Google Scholar]


