Abstract
The properties of left ventricular cardiac myocytes vary transmurally. This may be related to the gradients of stress and strain experienced in vivo across the ventricular wall. We tested the hypothesis that within the rat left ventricle there are transmural differences in the expression of genes for proteins that are involved in mechanosensitive pathways and in associated physiological responses. Real time reverse transcription polymerase chain reaction was used to measure messenger RNA (mRNA) levels of selected targets in sub-epicardial (EPI) and sub-endocardial (ENDO) myocardium. Carbon fibres were attached to single myocytes to stretch them and to record contractility. We observed that the slow positive inotropic response to stretch was not different between EPI and ENDO myocytes and consistent with this, that the mRNA expression of two proteins implicated in the slow response, non-specific cationic mechanosensitive channels (TRPC-1) and Na/H exchanger, were not different. However, mRNA levels of other targets, e.g. the mechanosensitive K+ channel TREK-1, Brain Natriuretic Peptide and Endothelin-1 receptor B, were significantly greater in ENDO than EPI. No targets had significantly greater mRNA levels in EPI than ENDO. On the basis of these findings, we suggest that the response of the ventricle to stretch will depend upon both the regional differences in stimuli and the relative expression of the mechanosensitive targets and that generally, stretch sensitivity is predicted to be greater in ENDO.
Keywords: Stretch, Cardiac myocytes, Gene expression, TRP channels, Stretch-activated channel
Introduction
It is known that mechanical stimulation of the myocardium (stress and strain) can lead to both acute and chronic changes in the electrical and mechanical activity of the heart and contribute to the development of cardiac hypertrophy [7, 11]. It is acknowledged that the ventricular myocardium is not homogenous and that there are regional differences in the basic properties of ventricular myocytes, e.g. [1, 10]. In the whole heart, transmural gradients in ventricular wall stress and strain exist [6], which may influence the expression of genes for proteins that are sensitive to mechanical stimuli. This may contribute to regional variations in physiological characteristics and thereby to transmural differences in the response to stimuli such as stretch.
Therefore, regional variations in wall stress and strain, in conjunction with regional differences in gene expression, may regulate the response of the heart. To date, relatively little is known about these possibilities, although it has been shown that the messenger RNA (mRNA) expression and current density of the mechanosensitive twin-pore K+ channel TREK-1 is greater in the rat sub-endocardium (ENDO) than the sub-epicardium (EPI) [12, 8, 6]. The purpose of this study was to test the hypothesis that the mRNA expression levels of several proteins, which are thought to modulate the mechanical and electrical response to stretch, vary across the rat left ventricular wall. Additionally, we wished to test whether the slow contractile response to axial stretch [2], which is thought to be modulated by some of the chosen targets, differs between myocytes from different regions of the left ventricular free wall, thus potentially linking gene expression to function.
Materials and methods
All animal experimentation was carried out in accordance with the Animals (Scientific Procedures) Act 1986 and the local Institutional ethical review panel.
Isolation of RNA
Ten Sprague–Dawley rats (194–240 g) were killed and the hearts removed. The left ventricular free wall was excised and snap frozen in liquid nitrogen between the large flat ends of a pair of tongs. The thickness of the left ventricular wall was measured with pre-cooled callipers, and 25-μm sections, equivalent to one third of the wall thickness, were collected on a cryostat from the EPI and ENDO surfaces, leaving a distinct mid-myocardial region. All samples were stored at −80°C until RNA extraction. Total RNA extraction was performed following the Qiagen mini-kit protocol for striated muscle, with the exception that an additional volume of RLT was added to the Proteinase K solution before binding of the RNA onto the mini-columns. Integrity and concentration of the isolated RNA were assessed by electrophoresis through 0.6% formaldehyde/1% agarose gels with ethidium bromide and the concentration of RNA adjusted to 1 g/l. Complementary DNA (cDNA) was prepared from 4 μg of total RNA with random priming using Superscript III first-strand synthesis system (Invitrogen, Life Technologies, Rockville, MD) and diluted 1:10 in TE (10 mM Tris-HCl [pH 7.5], 1 mM ethylenediamine tetraacetic acid) before use in real-time polymerase chain reaction (PCR).
Real-time RT-PCR
Real-time reverse transcription PCR (RT-PCR) was performed using TaqMan low-density arrays (Micro Fluidic Cards, Applied Biosystems, Foster City, CA). Each card consisted of 384 wells, preloaded with pre-designed fluorogenic TaqMan probes and primers, configured to allow detection of 48 transcripts for 7 experimental and 1 calibrator samples. Our calibrator sample consisted of a mixture of cDNA from EPI and ENDO regions. Each of the eight sample lanes in a card was loaded with 100 μl of a 1:1 mixture of cDNA (equivalent to 77 ng input RNA) and TaqMan Universial PCR master mix (Applied Biosystems). PCR was done according to the recommended protocol (50°C for 2 min and 94.5°C for 10 min, followed by 40 cycles at 97°C for 30 s and 59.7°C for 1 min) on an ABI RISM 7900HT Sequence detection system (Applied Biosystems). Data were collected with instrument spectral compensations by the Applied Biosystems SDS 2.2 software and analysed using the threshold cycle relative quantification method. Relative transcript expression was normalised to the housekeeper gene GAPDH.
Mechanosensitive targets
mRNA targets chosen for this investigation were: TREK-1 (a two-pore-domain K+-selective channel) and TRPC-1 (transient receptor potential canonical, a cationic non-selective mechanosensitive channel [MSC]); the Na/H exchanger, thought to be central to the slow increase in force that occurs upon axial stretch; endothelin-1 and its receptor types A and B and the angiotensin II receptor A, known to regulate the hypertrophic response to mechanical stimulation and possibly the slow inotropic response; caveolin-3 a critical component of caveolae, which are potential mechanosensors; caveolin-1, a major component of caveolae in non-cardiac muscle and recently identified in cardiac myocytes; brain natruiretic peptide (BNP) and the α and β sub-types of myosin heavy chain (MHC), the expression of both BNP and β-MHC are known to be increased by stretch [11].
Isolation of left ventricular myocytes
The isolation of single rat left ventricular myocytes and the measurement of the rapid and slow responses to axial stretch by attached carbon fibres was performed as previously described [2]. In brief, excised hearts were perfused on a Langendorff apparatus with a collagenase/protease containing physiological solution. EPI and ENDO strips, each one third of the wall thickness, were manually cut from the left ventricular free wall with scissors leaving a distinct and intact mid-myocardial region. Further incubation with enzyme solution resulted in the isolation of separate populations of EPI and ENDO myocytes.
Myocytes were attached to carbon fibres to record auxotonic force development in response to external stimulation and to axially stretch myocytes. Force was calculated from fibre motion, and stretch was calculated from the increase in sarcomere length. The rapid response to stretch was measured within 20 s of an increase in sacromere length, whereas the slow response was calculated as the further increase in force 5 min after the stretch. These experiments were carried out at a stimulation frequency of 1 Hz at a temperature of 22–24°C (to improve the experimental success rate) using a bicarbonate-based buffer containing (in mM): NaCl 118.5; NaHCO3 14.5; KCl 4.2; KH2PO4 1.2; MgSO4 1.2; glucose 11.1; CaCl2 1, equilibrated with 95% O2/5% CO2 (pH 7.4).
Statistics
Data were analysed using paired t tests to compare relative mRNA expression from the EPI vs ENDO region of each heart and unpaired t tests to compare the magnitude of the rapid and slow inotropic responses. Statistical significance was assumed when P < 0.05.
Results
There was some consistency in the pattern of regional expression of mechanosensitive genes (Table 1). Although the expression levels of some targets were unchanged, others were significantly greater in ENDO but never so in EPI. This observation was not due to a systematic error of the technique as levels of mRNA of targets known to be greater in EPI, e.g. the potassium channel component, Kv4.2, were significantly greater in EPI than ENDO (results not presented). Indeed, if α-MHC is excluded (being chosen as a counterpoint to β-MHC), 9 of the 11 targets had a larger mean mRNA expression in ENDO than EPI; this trend is statistically significant (P < 0.05, sum of ranks test).
Table 1.
Comparison of mRNA expression of mechanosensitive proteins from the EPI and ENDO region of rat left ventricle
| Assay ID reference | Common name | EPI | ENDO | Paired t test |
|---|---|---|---|---|
| Mean ± SEM | Mean ± SEM | |||
| Slow response and/or hypertrophic | ||||
| Agtr1a-Rn00578456_m1 | Angiotensin II receptor 1A | 1.64 ± 0.16 | 1.30 ± 0.22 | NS |
| Edn1-Rn00561129_m1 | Endothelin-1 | 1.17 ± 0.14 | 1.58 ± 0.24 | NS |
| Ednra-Rn00561137_m1 | Endothelin-1 receptor type A | 1.19 ± 0.14 | 1.38 ± 0.12 | NS |
| Ednrb-Rn00569139_m1 | Endothelin-1 receptor type B | 1.27 ± 0.17 | 1.93 ± 0.22 | 0.001 ENDO > EPI |
| Nppb-Rn00580641_m1 | Brain natriuretic peptide | 1.42 ± 0.15 | 2.77 ± 0.42 | 0.002 ENDO > EPI |
| Slc9a1-Rn00561924_m1 | Sodium–hydrogen exchanger | 1.33 ± 0.09 | 1.51 ± 0.17 | NS |
| Myofilaments | ||||
| Myh6-Rn00568304_m1 | α-myosin heavy chain | 1.56 ± 0.12 | 1.37 ± 0.17 | NS |
| Myh7-Rn00568328_m1 | β-myosin heavy chain | 1.20 ± 0.23 | 2.19 ± 0.36 | 0.006 ENDO > EPI |
| Mechanosensitive channels | ||||
| Kcnk2-Rn00597042_m1 | Kcnk2 (TREK-1) | 0.84 ± 0.04 | 1.04 ± 0.04 | 0.001 ENDO > EPI |
| Trpc1-Rn00585625_m1 | Transient receptor potential canonical-1 | 0.72 ± 0.04 | 0.72 ± 0.05 | NS |
| Mechanotransducers (caveolae) | ||||
| Cav-Rn00755834_m1 | Caveolin-1 | 0.90 ± 0.03 | 0.96 ± 0.03 | 0.015 ENDO > EPI |
| Cav3-Rn0055343_m1 | Caveolin-3 | 0.81 ± 0.03 | 0.85 ± 0.05 | NS |
Data shown are mean ± SEM, expression is relative to GAPDH. The probability of paired t tests is shown in the table. P > 0.05 (n = 10 pairs).
NS Not significantly different
When single myocytes were stretched from their resting sarcomere length (ENDO 1.83 ± 0.09 μm; EPI 1.82 ± 0.07 μm) by an equivalent amount (ENDO 7.8 ± 0.9 %; EPI 7.5 ± 0.5%, n = 11 ENDO, 18 EPI myocytes) as illustrated in Fig. 1a, we observed that the magnitude of neither the rapid nor slow inotropic response was dependent upon regional origin (Fig. 1b).
Fig. 1.
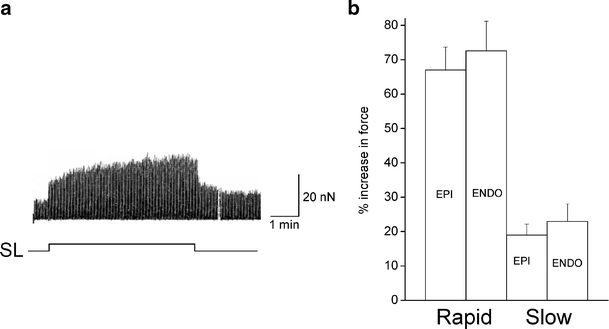
The inotropic response of left ventricular myocytes to axial stretch. a Representative trace showing the typical biphasic inotropic response of a myocyte to a stretch of 8% from a resting sacromere length (SL) of 1.85 μm. The trace shows changes in active force, resting force has been subtracted with a sample and hold device. The rapid increase in force is seen immediately upon an increase in SL, the slow response develops over the following minutes. b Mean data showing the rapid and slow response to stretch of ≅8% from a resting SL of ≅1.82 μm in sub-epicardial (EPI) and sub-endocardial (ENDO) left ventricular myocytes. The responses from EPI and ENDO cells were not significantly different from each other. P > 0.05, n = 18 EPI , n = 11 ENDO
Discussion
This investigation of transcripts for proteins sensitive to stretch, or involved in mechanosensitive pathways, has allowed us to detect a number of small but nevertheless significant differences in mRNA levels between ENDO and EPI samples.
Rapid and slow inotropic responses to axial stretch
Following equivalent amounts of axial stretch, the rapid inotropic response was not significantly different in EPI and ENDO rat myocytes. This observation is consistent with findings in untrained intact myocytes [10]. However, a greater stretch-induced increase in myofilament Ca2+ sensitivity (δpCa50) has been reported in untrained skinned ENDO myocytes than EPI myocytes when larger stretches (from sarcomere lengths of 1.9 to 2.3 μm) are applied [3].
We observed that the slow response did not vary with myocyte regional origin. This is an important and novel observation because the multicellular cardiac preparations typically used to study the slow response (trabeculae and papillary muscles) are ENDO in nature. Additionally, previous whole heart studies recorded the aggregate response from the whole left ventricle while our own previous single cell experiments did not distinguish between myocyte origin. Consistent with the functional data, we observed that the mRNA expression levels for proteins implicated in the development of the slow inotropic response in rat (non-specific cationic MSCs, possibly TRPC-1) and the Na/H exchanger, see [2], are not regionally different. It should be noted however, that although TRPC-1 has been proposed as a non-specific cationic MSC [9], such a role has not yet been established in the myocardium.
MSCs and mechanotransducers
Although levels of TRPC-1 mRNA were not regionally different, we found that TREK-1 mRNA expression was greater in ENDO than EPI. This latter finding agrees with the observations of Tan et al. [12] and Kelly et al. [6], although the regional difference in our study was smaller than previously reported. We would therefore hypothesise that an equivalent mechanical stimulus that activated TRPC-1 and TREK-1 would produce a larger electrical response in ENDO tissue (see [6]). Whether increased TREK-1 in ENDO would stabilise ENDO membrane potential during the diastolic period and be potentially anti-arrhythmic or reduce the transmural gradient in action potential duration and be potentially pro-arrhythmic [1] remains to be resolved.
Although we found no evidence for a regionally different expression of caveolin-3, an essential component of cardiac caveolae, the role of caveolae may also be influenced by regional expression of the receptors and other signalling molecules that they aggregate. The observation that mRNA for caveolin-1 is greater in ENDO is intriguing as this isoform has only recently been identified in cardiac myocytes, and its role in myocyte caveolae is unknown.
Indicators of cardiac hypertrophy
The ATII/ET-1 signalling pathways are known to play a role in the hypertrophic response to mechanical stimulation and, in some species, the slow inotropic response, see [4] for review. We only observed significantly greater levels of mRNA for the ET-1B receptor whereas it is the ET-1 A receptor that Cingolani et al. report to be important for the slow response. We also saw increased mRNA levels of BNP and of β-MHC in ENDO. Previous studies have variously reported increased [5] or similar [3] levels of β-MHC protein in normal rat ENDO vs EPI myocardium. Both BNP and β-MHC are indicators of hypertrophy provoked by mechanical stimuli. It is therefore interesting that wall stress and, in some studies, wall strain, is thought to be greater in ENDO [6].
Assumptions and limitations
In this study, we have made the common, implicit assumption that there is a link between levels of mRNA expression and protein activity. This assumption can only be validated by measuring in situ protein activity, a huge task given the diverse nature of our targets. However, in measuring the slow inotropic response to stretch and the mRNA expression of the targets thought to underlie this effect, we have attempted to address this problem. We chose the rat for consistency with our previous work on regional differences and on the slow response to stretch [10, 2] and because of the ready availability of mRNA sequences for this species. Given that regional variations exist in other species [1], it seems reasonable to expect that our observations on mechanosensitive targets are relevant to other mammalian species, although investigation of other species is required to confirm this.
Conclusions
The level of mRNA expression of some proteins that are modulated by mechanical stimuli vary across the rat left ventricular wall. In vivo, the response to mechanical stimulation is likely to be complex. Targets (e.g. in the present study caveolin-3 and TRPC-1) whose mRNA expression are not regionally different might still be differentially regulated by transmural variation in the mechanical stimuli, whereas other permutations of increased/decreased mRNA expression and protein activity with increased/decreased stimuli are possible. Based upon our present observations and those from the literature reporting increased stretch sensitivity of various responses in ENDO, e.g. [5, 10, 3, 6], it seems that in general, ENDO tissue is likely to be more influenced by, and be responsive to, mechanical stimulation than EPI tissue.
Acknowledgements
This work was supported by The British Heart Foundation and The Wellcome Trust
References
- 1.Antzelevitch C (2005) Modulation of transmural repolarization. Ann N Y Acad Sci 1047:314–323 [DOI] [PMC free article] [PubMed]
- 2.Calaghan S, White E (2004) Activation of Na+–H+ exchange and stretch-activated channels underlies the slow inotropic response to stretch in myocytes and muscle from the rat heart. J Physiol 559:205–214 [DOI] [PMC free article] [PubMed]
- 3.Cazorla O, Ait MY, Goret L, Vassort G, Dauzat M, Lacampagne A, Tanguy S, Obert P (2006) Effects of high-altitude exercise training on contractile function of rat skinned cardiomyocyte. Cardiovasc Res 71:652–660 [DOI] [PubMed]
- 4.Cingolani HE, Perez NG, Aiello EA, de Hurtado MC (2005) Intracellular signaling following myocardial stretch: an autocrine/paracrine loop. Regul Pept 128:211–220 [DOI] [PubMed]
- 5.Gorza L, Pauletto P, Pessina AC, Sartore S, Schiaffino S (1981) Isomyosin distribution in normal and pressure-overloaded rat ventricular myocardium. An immunohistochemical study. Circ Res 49:1003–1009 [DOI] [PubMed]
- 6.Kelly D, Mackenzie L, Hunter P, Smaill B, Saint DA (2006) Gene expression of stretch-activated channels and mechanoelectric feedback in the heart. Clin Exp Pharmacol Physiol 33:642–648 [DOI] [PubMed]
- 7.Kohl P, Sachs F, Franz MR (2005) Cardiac mechano-electric feedback & arrhythmias, from patient to pipette. Saunders, Philadelphia, pp 1–423
- 8.Liu W, Saint DA (2004) Heterogeneous expression of tandem-pore K+ channel genes in adult and embryonic rat heart quantified by real-time polymerase chain reaction. Clin Exp Pharmacol Physiol 31:174–178 [DOI] [PubMed]
- 9.Maroto R, Raso A, Wood TG, Kurosky A, Martinac B, Hamill OP (2005) TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat Cell Biol 7:179–185 [DOI] [PubMed]
- 10.Natali AJ, Wilson LA, Peckham M, Turner DL, Harrison SM, White E (2002) Different regional effects of voluntary exercise on the mechanical and electrical properties of rat ventricular myocytes. J Physiol 541:863–875 [DOI] [PMC free article] [PubMed]
- 11.Ruwhof C, van der Laarse A (2000) Mechanical stress-induced cardiac hypertrophy: mechanisms and signal transduction pathways. Cardiovasc Res 47:23–37 [DOI] [PubMed]
- 12.Tan JH, Liu W, Saint DA (2004) Differential expression of the mechanosensitive potassium channel TREK-1 in epicardial and endocardial myocytes in rat ventricle. Exp Physiol 89:237–242 [DOI] [PubMed]


