Abstract
Background
The purposes of this study were to examine the accuracy of fetal hemoglobin (HbF) as quickly measured by the hemoximeter,verified by the high-performance liquid chromatography method, and to examine related oxygen saturation (SO2) measurements in neonates.
Methods
Thirty-nine neonates with gestational ages ranging from 25 to 38 weeks were investigated (n=280 blood samples). Twenty younger premature neonates had blood transfusions (n=188 blood samples, 72 before and 116 after transfusions), and 19 older neonates did not.
Results
The bias of the hemoximeter was 23% (±9.1) against the HPLC; 25% (±7.9) before, and 19% (±8.6) after blood transfusions (all P < 0.001), for HbF measurements. A regression line (HbFt by the HPLC = 8.46+ 0.7 × HbF by the hemoximeter) has been provided for the prediction. Oxyhemoglobin dissociation curves with the status of (before and after) blood transfusions were presented. In relation to oxygen tension values of 50–75 mm Hg, in addition to the right-shifted oxyhemoglobin dissociation curves, pulse oximeter ranged from 95 to 98% before the transfusions, but decreased to 94 to 96% after the blood transfusions.
Conclusions
Accurate HbF and related oxygen saturation measurements need to be determined, especially for premature neonates, to minimize the risk of oxygen toxicity.
Keywords: Hemoglobin, Transfusion, Fetal hemoglobin, Acetylated fetal hemoglobin, Hb oxygen saturation
1. Introduction
Fetal hemoglobin (HbF) differs from adult hemoglobin (HbA) in that it has 2 γ chains in place of the 2 α chains, and has a higher oxygen (O2) affinity thus presenting a left-shifted oxyhemoglobin (HbO2) dissociation curve in neonates [1,2].When HbF levels were not adjusted, carbon-monoxide hemoglobin (HbCO) and oxygen saturation (SO2) measurements were arbitrarily increased for 6% in neonates [1–4]. The differences (bias) between the blood SO2 and pulse oximeter SO2 (SpO2) were reported to increase as the SO2 decreased [5–7], however, HbF levels did not change the difference between the blood and pulse oximeter SO2 [5,6]. To date, however, limited data is available regarding the changes of HbF levels of neonates on the accurate measurements of HbO2 and SO2.
Although the hemoximeter method is available for the quick estimate of HbF levels in clinical settings; a high-performance liquid chromatography method remains as the most sensitive, accurate, and practical method to determine HbF and HbF variations [8–11]. Acetylated HbF (HbF1) is a variation of HbF, a posttranslational modification of HbF by acetylation of the N-terminus of γ globin chains [12,13]. Its O2 carrying capacity was reported as dysfunctional, irresponsive to 2,3 diphosphoglycerate (2,3 DPG) binding to facilitate O2 release at tissue level [13,14]. Therefore, accurate HbF measurement is essential to assess adequate oxygenation and to prevent O2 related complications in these neonates [7,15].
Blood transfusion using HbA could improve a neonate’s tissue oxygenation [16,17]; however, when the blood was transfused in larger volume in a short period, a right-shifted HbO2 dissociation curve may cause O2 toxicity [18] and add cardiac load [19]. Thus, HbA blood should be transfused slowly and SO2 measurements should be monitored closely following blood transfusions in neonates [18,19]. For functional SO2 measurements in relation to the HbF changes in neonates, 2 previous small studies (n=10, and 11) have reported the right-shifting HbO2 dissociation curves following blood transfusions using P50 measurements [17,20]. Widespread clinical pulse oximeter (SpO2) readings had been reported in relation to the PaO2 values without providing a precise HbO2 dissociation curve for neonates [21] in relation to the clinical PaO2 ranges of 50–75 mm Hg [22], related SO2 measurements need to be established for neonates, accounting for the effects of HbF levels. The purposes of this study were, therefore, to examine: 1) the accuracy of HbF measurements using the hemoximeter against the HPLC method; and, 2) the changes HbO2 dissociation curves and SO2 measurements, following blood transfusions.
2. Methods
2.1. Setting
This study is part of a larger clinical study in 2 neonatal intensive care units (NICUs) [23]. The appropriate institutional human subjects review boards approved the study protocols. Informed consents were obtained from the parents and guardians of newborn subjects prior to or immediately following birth. For this study, all neonates had successful insertions of umbilical arterial catheters (UAC) and/or umbilical venous catheters (UVC) to monitor SaO2 or SvO2.
2.2. Sample
Immediately after birth, neonates who were diagnosed with respiratory distress syndrome requiring mechanical ventilation were randomly selected into the study from a possible pool of 100 consecutive newborn infants. Those with major congenital defects (cardiac, brain and neurological, or gastro-intestinal defects) were excluded, as interpretation of accurate SaO2 and SvO2, would be problematic in these cases. Neonates who had life-threatening persistent pulmonary hypertension requiring inhaled nitric oxide or extra-corporeal membrane oxygenation were also excluded for feasibility concerns. The sample included 39 neonates, and provided 280 blood samples (ranging from 1 to 19, at least 1 sample per day). Gestational ages of these newborns ranged from 25 to 38 weeks, and birth weights from 665 to 3696 g. Neonates with lower birth weights were not included because of their small size limiting the insertion of the 4F monitoring catheter and amount of blood that could be drawn.
2.3. Instruments
2.3.1. Hemoximeter
HbF and SO2 measurements [including total Hb (tHb), HbO2, SO2, HbCO, and met-Hb (MetHb)] were analyzed by using the hemoximeter model OSM3 (Radiometer Corp, Cleveland, OH), using 6-wavelength fiber optic reflectance oximetry (535, 560, 577, 622, 636, and 670 nm). The accuracy of this co-oximeter, as reported by the manufacturer, is a test–retest variability of <0.1% for normal Hb level, and −0.2% to +0.4% for extreme anemia and polycythemia (tHb ranges: 3.2 to 28 g/dl). The instrument allowed in-vitro measurements of HbF concentrations through determination of P50 on the HbO2 dissociation curve based on a linear assumption of maximum oxygenation status by fully-oxygenating the sample [24]. To follow the recommended guidelines from a consensus meeting for SO2 measurements [25], the cap for the restrictions of 100% maximum for HbF, SO2 and HbO2 measurements was removed, so that the test results exceeding 100% could be reported by the equipment. Validity was assured by 0-point calibration using the manufacturer’s rinse solution before and after each test. Quality control procedures included the reference method every 8 h, cleansing the internals of tubing with appropriate solutions every week, and changing maintenance tubing and tHb calibration every quarter, to assure test accuracy.
2.3.2. HPLC
Analyses of Hb and its variants were performed by using a Varian cation-exchange HPLC system (Varian Instruments, Walnut Creek, CA; Varian 5500 HPLC interfaced with a Vista 654 Data system, a Waters Model 440 detector and a Perkin Elmer ISS 100 auto-sampler). A 200S × 4.6 mm Poly CAT A column packed with 5 μm particle, porous silica coated with polyaspartic acid was obtained from Poly LC Inc. (Columbia, MD). The sample preparation, mobile phase composition, and chromatographic conditions have been described before [8,9]. Briefly, hemolysates were prepared by mixing the washed packed red cells with 2 volumes of water for 30 s. After lysis, 50 μl of hemolysate were added to 1 ml of mobile phase A. The mixture was then centrifuged at 3000 ×g for 5 min and 10 μl of supernate were injected onto the column. The chromatographic separation of all Hb was achieved by gradient elution with 2 mobile phases at 2 different pH (6.57 and 6.87) levels with flow rate set at 0.6 ml/min. The peak areas were measured at 436 nm. This method was developed and optimized for the high resolution of more than 45 commonly encountered Hb variants (including HbF, HbA2, HbF1, HbS, HbC etc. in addition to HbA) within 12 min [4].
Clinical monitor readings included pulse oximeter readings, respiratory rate and heart rate readings, and incubator temperature and skin temperature readings. SpO2 readings were recorded by using a pulse oximeter (Nellcor NPB 290, Tyco HealthCare, Mansfield, MA). This instrument was capable of measuring the percentage of peripheral SO2, detected transcutaneously by a probe positioned on either side of a pulsating arterial bed around the neonate’s foot. The transmittance sensor was configured so that the light-emitting diodes transmit infrared and red light through the pulsating vascular bed to a photo-detector positioned on the opposite site. For reading consistency purposes, the sensors for pulse oximeter were all placed on post-ductal sites. The pulse oximeter has shown moderate to good correlations (r=0.5, 0.88) with accounting for the HbF effects [3,26]. Inter-rater agreement on data coding reached 100% agreement between the 2 raters.
2.4. Procedures
Blood was drawn through the UAC or UVC from the day of admission to 5 days old, coordinated to occur simultaneously about every 8 h with routine blood gas tests when neonates were resting quietly to obtain stable measurements. For the hemoximeter analyses, an extra 0.1 ml blood was split into 2 syringes for the HbF test and the SO2 tests. HbF determination required oxygenating blood samples with 100% O2 and twirling the syringe between the hands for 90 s to yield fully oxygenated samples. The oxygenation status was confirmed by SO2 readings close to 100%. HbF was then determined by using a linear assumption model. Since SO2 using fully oxygenated blood can only reach a maximum of 100% (with 0% HbF), a linear formula programmed in the hemoximeter would yield 106% SO2 with 100% HbF, and this program would thus estimate the HbF levels based on this formula [24]. Study personnel completed the training and execution of blood-handling protocol [3].
Once the hemoximeter analyses were completed, the residual blood was stored in a refrigerator, and the samples were transported in an icebox and sent for HPLC analyses within a 2-day period during weekdays daytime hours. To obtain the most accurate HbF measurements by the hemoximeter and the corresponding HPLC method, the daily samples with the highest HbF levels determined by the hemoximeter were sent for HPLC analyses. Medical records were prospectively reviewed to obtain a subject’s demographic data, medical history, and routine laboratory tests and monitoring parameters.
2.5. Data analysis
Data were analyzed by using the Statistical Packages for Social Sciences (SPSS ver. 13.0, Chicago, IL). The technique of Bland and Altman [27] was used to calculate bias, precision, and the limits of agreement. The bias was defined as the mean difference (paired t test) between the hemoximeter and the HPLC measurements for HbF levels, and the precision as the SE of the mean difference. The limit of agreement was defined as ± 2 SD for the differences between 2 measurements. In addition, multivariate regression analysis was performed to predict HbF levels for the hemoximeter using the data from the HPLC. Thereafter, HbO2 dissociations were examined by using multiple regression (R) curve-fitting analysis on Sigmoid (S) curve, for PO2 values and all SO2 measurements (HbO2, SO2, and SpO2).
3. Results
Table 1 presents the descriptive statistics for the demographic data of this study. There were 20 girls and 19 boys for the 39 subjects; while 19 subjects did not have any blood transfusions, 20 subjects had blood transfusions. The racial/ethnic compositions of the study sample included 15 African Americans, 2 Asian Americans, 4 Caucasians and 18 Hispanics. Those who had blood transfusions had lower gestational ages and birth weights, and had longer hospital stays compared to those who did not have blood transfusions (all P<0.01). The volume of blood transfusion for these neonates ranged from 3 to 5 ml kg/h, lasting 2–3 h.
Table 1.
Descriptive statistics for demographic data by groups of neonates who had transfusions of adult blood within the first 5 days after birth (n = 39 subjects)
| Mean ± SD (ranges) | No transfusion
(n = 19) |
Transfusion
(n = 20) |
|---|---|---|
| Gestation weeks, at birth** | 32.7 ± 2.72 (28–38) | 29.5 ± 3.03 (25–37) |
| Gestation weeks mother admitted** | 32.2 ± 2.72 (28–38) | 28.4 ± 2.93 (24–32) |
| Birth weight, g** | 1798 ± 672 (992–3695) | 1178 ± 347 (665–1843) |
| NICU days*** | 26 ± 18.5 (3–79) | 65 ± 38.2 (8–139) |
| Hospital days** | 33 ± 27.0 (8–120) | 67 ± 36.6 (8–139) |
| Gender (boy/girl) | 8/11 | 11/9 |
| Race/ethnicity Hispanics | 8 | 10 |
| Black | 6 | 9 |
| White | 3 | 1 |
| Asian | 2 | 0 |
P<0.01
P<0.001
NICU = Neonatal Intensive Care Unit
Table 2 presents the descriptive statistics for the HbF analyses using the HPLC and the hemoximeter methods for 72 blood samples prior to blood transfusions and 116 blood samples following blood transfusions (presented with only the data of neonates who had blood transfusions for comparisons). The blood samples were drawn on an average at 22 h after birth, prior to blood transfusions, and 76 h after birth, following blood transfusions. Additional analyses using the hemoximeter analysis for SO2 measurements (including tHb, HbO2, SO2, HbCO, and MetHb), as well as the simultaneous recorded clinical monitor readings and blood gas parameters are presented. Before blood transfusions, there were 5 samples with elevated HbC (1.7–2.8%) from 2 neonates (both African Americans, 26 and 31 weeks gestation each), and 3 samples with elevated HbA2 from another neonate (African American, 38 weeks gestation). After blood transfusions, 98 samples (84%) had elevated HbA2 levels, and additional 2 subjects (5 samples) showed increased HbC levels. Following the blood transfusions, HbF levels decreased (HPLC: 94%± 3.0 before, and 64%± 11.3 after transfusions; hemoximeter: 119%± 9 before, and 82%± 16.4 after transfusions), and HbA levels increased (HPLC: 6.5%± 3.1 before, and 35%± 11.1 after transfusions) (all P <0.001). In addition, following the blood transfusions, bicarbonate levels increased (P<0.05) with lower PO2 in the blood, and SpO2 decreased (P <0.01); compared to those samples prior to the blood transfusions.
Table 2.
Descriptive statistics before and after the blood transfusions (n=188 blood samples)
| Mean±SD | No transfusions
(n=72) |
After transfusions
(n=116) |
|---|---|---|
| Hours after birth*** | 21.6 ± 17.35 | 75.6 ± 29.55 |
| HPLC | ||
| HbFt, %*** | 93.9 ± 3.02 | 63.9 ± 11.34 |
| HbF*, %*** | 83.3 ± 2.90 | 56.4 ± 9.97 |
| HbF1, %*** | 10.2 ± 2.29 | 7.54 ± 1.96 |
| HbA, %*** | 6.5 ± 3.14 | 35.4 ± 11.14 |
| HbA2, %*** | 0.01 ± 0.07 | 0.75 ± 0.45 |
| HbC, % | 0.18 ± 0.69 | 0.11 ± 0.38 |
| Hemoximeter | ||
| tHb, g/dl | 12.2 ± 2.45 | 12.6 ± 1.87 |
| HbF, %*** | 119 ± 9.02 | 82.4 ± 16.40 |
| HbO2, % | 94.1 ± 3.04 | 93.4 ± 2.76 |
| SO2, % | 97.2 ± 3.05 | 96.9 ± 2.87 |
| HbCO, %*** | 1.8 ± 0.76 | 2.4 ± 0.63 |
| MetHb, %** | 1.3 ± 0.21 | 1.2 ± 0.23 |
| Clinical monitor | ||
| Pulse oximeter SO2 %** | 97.6 ± 2.73 | 96.2 ± 2.79 |
| Pulse rate/min | 142 ± 14.67 | 146 ± 13.84 |
| Respiratory rate/min | 36 ± 17.77 | 43 ± 25.22 |
| Skin temperature °C | 36.4 ± 0.32 | 36.4 ± 0.30 |
| Blood gas | ||
| pH | 7.34 ± 0.09 | 7.33 ± 0.08 |
| PO2, mm Hg* | 87.9 ± 48.0 | 77.3 ± 22.7 |
| PCO2, mm Hg | 41.8 ± 11.27 | 44.3 ± 9.37 |
| Bicarbonate, mmol/l* | 21.5 ± 2.06 | 22.3 ± 2.58 |
| Base excess | -3.6 ± 2.30 | -3.0 ± 2.90 |
Note: HbF = fetal hemoglobin. HbF1 = acetylated fetal hemoglobin, HbA = adult hemoglobin, HbA2, adult hemoglobin A2.
P <0.05
P < 0.01
P<0.001.
4. Accurate HbF measurements
For the summary statistics between the HPLC and the hemoximeter methods (n=280 blood samples), the bias for the hemoximeter was 23% (±9.05, higher for the hemoximeter) (25.3% ± 7.85 and 18.7% ± 8.62, before and after the transfusions). The precision was 0.54 (0.64 and 0.80, before and after the transfusions). The correlation for the HbF measurements using the 2 methods was 0.93 (0.56 and 0.87 for the data before and after the transfusions, all P<0.001). The limit of agreement (+2SD) was 18.1%, and the disagreement (outside or exceeding the limit of ±2SD) was 0%.
Using the multivariate regression approach, HbF levels were predicted for the hemoximeter measurements, against the HPLC measurements (n=280 blood samples). Fig. 1 presents the scatter plots and a regression line comparing HbF measurements using the 2 methods (HbFt=8.46+0.7×HbF by hemoximeter, R2 = 0.87, all P<0.001). Using this formula, if the HbF levels determined by the hemoximeter method is 120%, the HbFt level predicted for the HPLC method would be 92.5% (rounded to 93%); and if the HbF level by the hemoximeter is 90%, the HbFt predicted would be 72%.
Fig. 1.
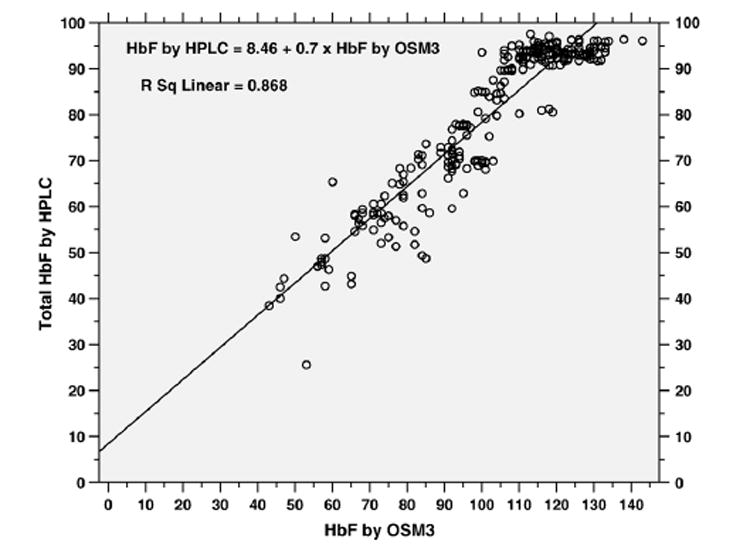
Scatter plots of HbF measured by OSM3 hemoximeter (X-axis) and HbF (%) measured by HPLC (Y-axis) with a regression line (n = 280 blood samples).
5. HbO2 dissociation curves
Fig. 2 presents the fitted S-curves, before and after the blood transfusions, using the arterial blood samples for those who had blood transfusions (n = 188 blood samples). For the ease of visualization, the ranges of PO2 values were focused close to the critical ranges of 50 to 75 mm Hg. In relation to the critical ranges of 50 to 75 mm Hg PaO2, arterial HbO2 ranged from 91 to 94% before transfusions (R2 = 0.45, P < 0.001, n= 72), and 90 to 93.5% after transfusions (R2 = 0.65, P= 0.001, n= 116). SaO2 ranged from 94 to 97% before transfusions (R2 = 0.38, P < 0.001, n = 72), and 93.5 to 97% after transfusions (R2 = 0.63, P<0.001, n= 116). And, SpO2 ranged from 95 to 98% before transfusions (R2 = 0.43, P <0.001, n = 72), and 94 to 96% after transfusions (R2 = 0.23, P <0.001, n= 116).
Fig. 2.
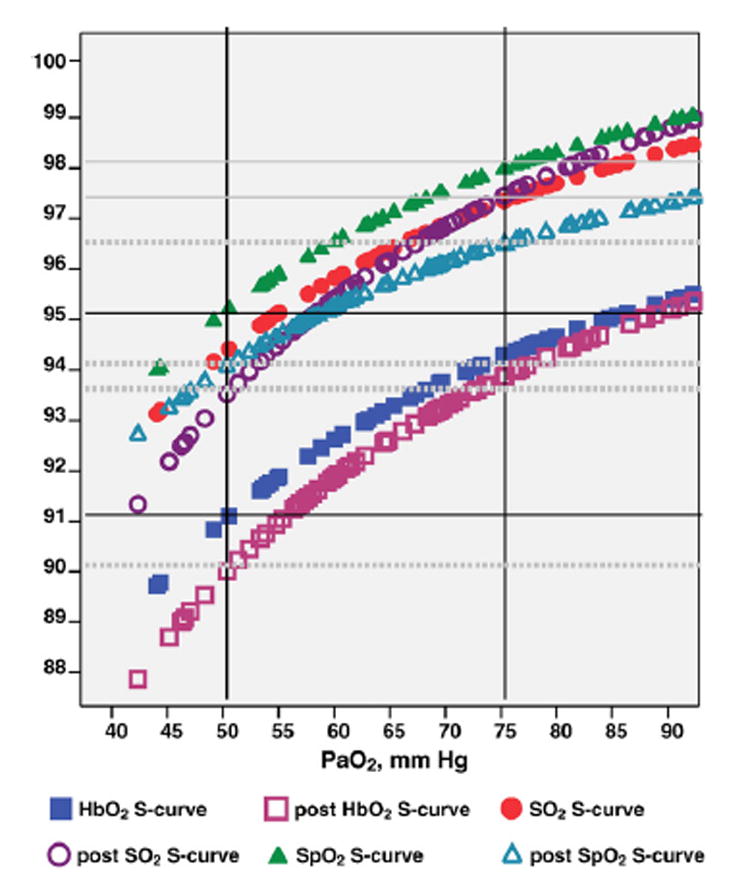
Fitted Sigmoid curves for arterial HbO2, SaO2, and SpO2 before (n =72 blood samples) and after (n=116 blood samples) blood transfusions of HbA (solid markings = before transfusions, hollow markings = after transfusion); X-axis: arterial blood gas PO2 (mm Hg); Y-axis: arterial oxygen saturation measurements (%). Note: HbO2 = oxyhemoglobin, SO2 = arterial oxygen saturation, SpO2 = oxygen saturation by pulse oximetry, PaO2 = arterial oxygen tension.
6. Discussion
To continue a previous report on predicting the HbF levels of HPLC method using the hemoximeter method [28], we reported a linear regression model with a much better correlation coefficient with blood transfusions for neonates. This linear regression line might be useful in clinical settings for the measurements of the hemoximeter, to predict the most accurate HbF levels. The HbF levels >100% determined by the hemoximeter were actually biased and represented inaccurate measurements. Thus, accurate HbF measurements using the HPLC method are needed for neonates in clinical settings [9,10].
Wimberly et al. examined HbF effects on SO2 measurements using 11 cord blood samples of healthy term neonates [1,2]. This study included more blood samples and data points for HbF and related SO2 measurements to compare the hemoximeter readings against the HPLC in neonates. Following the blood transfusions, HbO2 dissociation curves were right-shifted in these neonates [17,20], and we substantiated these right-shifted S-curves with decreased blood and clinical oximeter oxygen saturation readings in relation to the same ranges of PaO2 values. In relation to the critical PaO2 value of 50 mm Hg (for the critical value for hypoxemia), following the blood transfusions, arterial HbO2 was 1% lower (91% to 90%), SaO2 was 0.5% lower (94 to 93.5%), and SpO2 was 1% lower (95% to 94%), when compared to the measurements prior to the blood transfusions. In addition, following the blood transfusions, PO2 was lower in the blood with increased bicarbonate levels, and pulse oximetry was lower compared to those prior to the blood transfusions. For these premature neonates who had blood transfusions, there was a tendency of increased heart rate and respiratory rate following the transfusions (but not statistically significant). Thus, HbF levels and oxygenation status of these premature neonates need to be monitored closely following the blood transfusions, to prevent potential oxygen toxicity [18–20].
Neonates who need O2 supplementation are prone to develop oxygenation complications with HbF releasing less O2 (than HbA) to the tissue. HbF1 (acetylated HbF) is a variation of HbF; and, it is non-functional to release O2. Thus, the presence of HbF1 needs to be taken into considerations when assessing oxygenation status. Future studies are needed to examine the changes of HbF and HbF1 and their effects on the SO2 measurements in neonates, particularly for those who had hypoxia and had the history of maternal chronic diseases. Future studies also need to integrate the methods used in this and other studies, including P50 measurements, to assess HbF levels and related oxygen saturation measurements more accurately for premature neonates, to minimize the risks of oxygen toxicity with blood transfusions.
Acknowledgments
This study is supported in part by R01-NR04447 grant from the National Institutes of Health. The authors would also like to acknowledge the nurses, physicians, and respiratory therapists at the clinical settings who participated and helped with the blood sample collection.
References
- 1.Wimberly PD. Oxygen monitoring in the newborn. Scand J Clin Lab Invest. 1993;54(Suppl 214):127–30. [PubMed] [Google Scholar]
- 2.Wimberly PD, Siggaard-Anderson O, Fogh-Anderson N. Accurate measurement of hemoglobin oxygen saturation, and fraction of carboxyhemoglobin and methemoglobin in fetal blood using Radiometer OSM3: Corrections for fetal hemoglobin fraction and pH. Scand J Clin Lab Invest. 1990;50(Suppl 203):235–9. doi: 10.3109/00365519009087516. [DOI] [PubMed] [Google Scholar]
- 3.Shiao S-YPK. Functional versus fractional oxygen saturation readings: bias and agreement using simulated solutions and adult blood. Biosci Res Nur. 2002;3:210–21. doi: 10.1177/10900402003004006. [DOI] [PubMed] [Google Scholar]
- 4.Shiao S-YPK. Effects of fetal hemoglobin on accurate measurements of oxygen saturation in neonates. J Perinat Neonatal Nurs. 2005;19:348–61. doi: 10.1097/00005237-200510000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Praud JP, Carofilis A, Bridey F, Lacaille F, Dehan M, Gaultier CL. Accuracy of 2 wavelength pulse oximetry in neonates and infants. Pediatr Pulmonol. 1989;6:180–2. doi: 10.1002/ppul.1950060310. [DOI] [PubMed] [Google Scholar]
- 6.Lebecque P, Shango P, Stijns M, Vliers A, Coates AL. Pulse oximetry versus measured arterial oxygen saturation: a comparison of the Nellcor N100 and the Biox III. Pediatr Pulmonol. 1991;10:132–5. doi: 10.1002/ppul.1950100216. [DOI] [PubMed] [Google Scholar]
- 7.Shiao S-YPK. Desaturation events in neonates during mechanical ventilation. Crit Care Nurs Q. 2002;24(4):14–29. doi: 10.1097/00002727-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Ou CN. Diagnosis of hemoglobinopathies by high-performance liquid chromatography. J Biomed Sci. 1997;4:315–8. doi: 10.1007/BF02258356. [DOI] [PubMed] [Google Scholar]
- 9.Ou CN, Rognerud CL. Diagnosis of hemoglobinopathies: electrophoresis vs. HPLC. Clin Chim Acta. 2001;313:187–94. doi: 10.1016/s0009-8981(01)00672-6. [DOI] [PubMed] [Google Scholar]
- 10.Conti M, Gelfi C, Righetti PG. Screening of umbilical cord blood hemoglobin by isoelectric focusing in capillaries. Electrophoresis. 1995;16:1485–91. doi: 10.1002/elps.11501601246. [DOI] [PubMed] [Google Scholar]
- 11.Campbell TA, Ware RE, Mason M. Detection of hemoglobin variants in erythrocytes by flow cytometry. Cytometry. 1999;35:242–8. [PubMed] [Google Scholar]
- 12.Manning LR, Manning JM. The acetylation state of human fetal hemoglobin modulates the strength of its subunit interactions: longrange effects and implications for Histone interactions in the nucleosome. Biochemistry. 2001;40:1635–49. doi: 10.1021/bi002157+. [DOI] [PubMed] [Google Scholar]
- 13.Yagami T, Ballard BT, Padovan JC, Chait BT, Popowicz AM, Manning J. N-terminal contributions of the r-subunit of fetal hemoglobin to its tetramer strength: remote effects at subunit contracts. Protein Sci. 2002;11:27–35. doi: 10.1110/ps.30602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huisman M, Egberts J, van Loon J. Derivated fetal haemoglobin as a marker for red cell age in the human fetus reflecting stimulated or impaired red blood cell production. Prenat Diagn. 2001;21:521–8. doi: 10.1002/pd.91. [DOI] [PubMed] [Google Scholar]
- 15.Shiao S-YPK, Andrews CM, Ahn CW. Predictors of intubation and oxygenation complications in neonates. Newborn Infant Nur Rev. 2002;2:128–37. [Google Scholar]
- 16.James L, Greenough A, Naik S. The effect of blood transfusion on oxygenation in premature ventilated neonates. Eur J Pediatr. 1997;156(2):139–1141. doi: 10.1007/s004310050572. [DOI] [PubMed] [Google Scholar]
- 17.Halleux VD, Truttmann A, Gagnon C, Bard H. The effect of blood transfusion on the hemoglobin oxygen dissociation curve of very early preterm infants during the first week of life. Sem Perinatol. 2002;26(6):411–5. doi: 10.1053/sper.2002.37313. [DOI] [PubMed] [Google Scholar]
- 18.DeHalleux V, Truttmann A, Gagnon C, Bard H. The effect of blood transfusion on the hemoglobin oxygen dissociation curve of very early preterm infants during the first week of life. Semin Perinatol. 2002;26(6):411–5. doi: 10.1053/sper.2002.37313. [DOI] [PubMed] [Google Scholar]
- 19.Nemeto S, Aoki M, Dehua C, Imai Y. Free hemoglobin impairs cardiac function in neonatal rabbit heart. Ann Thorac Surg. 2000;69:1484–9. doi: 10.1016/s0003-4975(00)01176-0. [DOI] [PubMed] [Google Scholar]
- 20.Brown MS, Phipps RH, Dallman PR. Postnatal changes in fetal hemoglobin, oxygen affinity and 2,3-diphosphoglycerate in previously transfused preterm infants. Biol Neonate. 1985;48(2):70–6. doi: 10.1159/000242156. [DOI] [PubMed] [Google Scholar]
- 21.Poets CF, Wilken M, Seidenberg J, Southall DP, van der Hardt H. Reliability of a pulse oximeter in the detection of hyperoxemia. J Pediatr. 1993;122:87–90. doi: 10.1016/s0022-3476(05)83494-8. [DOI] [PubMed] [Google Scholar]
- 22.Askin DF. Interpretation of neonatal blood gases, part II: disorders of acid–base balance. Neonatal Netw. 1997;16(6):23–8. [PubMed] [Google Scholar]
- 23.Shiao S-YPK, Andrews CM, Helmreich RJ. Maternal race/ethnicity and predictors of pregnancy and infant outcomes. Biosci Res Nur. 2005;7:55–66. doi: 10.1177/1099800405278265. [DOI] [PubMed] [Google Scholar]
- 24.Krzeminski A. How is fetal hemoglobin determined and corrected for in the OSM3, the ABL510, and the ABL 520? Copenhagen : Radiometer; May, 1992. pp. 1–4. Info, No:1992–4. [Google Scholar]
- 25.Ehrmeyer S, Burnett RW, Chatburn RL, Clausen JL, Durst RA. Fractional oxyhemoglobin, oxygen content and saturation, and related quantities in blood: terminology, measurement, and reporting; approved guidelines. NCCLS. 1997 January 3;:C25–A17. [Google Scholar]
- 26.Rajadurai VS, Walker AM, Yu VY, Oates A. Effect of fetal haemoglobin on the accuracy of pulse oximetry in preterm infants. J Paediatr Child Health. 1992;28:43–6. doi: 10.1111/j.1440-1754.1992.tb02615.x. [DOI] [PubMed] [Google Scholar]
- 27.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:161–79. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 28.Shiao S-YPK, Ou C-N. Accurate measurements of fetal hemoglobin for neonates with different gestational ages. Hemoglobin. 2006;30:251–64. doi: 10.1080/03630260600867883. [DOI] [PubMed] [Google Scholar]


