Abstract
Antisense c-fos oligonucleotides injected into the neostriatum of conscious rats selectively inhibited c-fos expression associated with compensatory increases in striatal c-fos mRNA levels and also with increased expression of junB and NGFI-A mRNA, probably as a result of regulatory phenomena. Dual probe in vivo microdialysis was used to investigate γ-aminobutyric acid (GABA) release in the substantia nigra and the globus pallidus, which represent the terminal sites of the dopamine D1 receptor regulated striatonigral and the dopamine D2 receptor regulated striatopallidal GABA pathways, respectively. Intrastriatal infusion of the c-fos antisense oligonucleotide profoundly decreased dialysate GABA levels in the ipsilateral substantia nigra within 60 min but did not influence the dialysate GABA levels in the globus pallidus compared with the sham and control oligonucleotide treated groups. The site of action of the antisense oligonucleotides was mainly restricted to striatal neurons as shown by the distribution of locally injected fluoresceine isothiocyanate and radiolabeled oligonucleotides. The findings demonstrate a facilitatory role for c-fos mediated gene regulation in striatonigral GABA transmission and strengthen the evidence that the regulation of neurotransmission is different in the striatonigral and striatopallidal GABA pathways.
Keywords: phosphorothioate oligonucleotides, microdialysis, dopamine receptors, motor circuit
Neuronal plasticity is mediated by alterations in gene expression patterns. The first stage of this genomic reaction to environmental cues culminates in the selective short-term expression of transactivating proteins that influence the transcription of downstream genes involved in long-term changes in the cellular phenotype. This stimulus–transcription coupling in the brain is particularly well established for the c-fos gene and other members of the fos and jun gene families (1, 2, 3).
Dopamine (DA) releasing drugs (indirect agonists) like amphetamine and cocaine but also high doses of the DA agonist apomorphine stimulate locomotion and induce c-fos in the medium sized spiny striatonigral γ-aminobutyric acid (GABA) neurons (4, 5, 6, 7, 8, 9, 10). Suppression of striatal c-fos expression by antisense oligonucleotides has recently been demonstrated to attenuate the effects of DA agonists on motor function. We reported earlier that rats which received an unilateral c-fos antisense infusion in the neostriatum developed a strong ipsilateral turning behavior within 15–20 min after systemic amphetamine administration (11). Others have found similar inhibitory effects on DA agonist stimulated motor behavior after intrastriatal infusion of c-fos antisense oligonucleotides (12, 13, 14). While both the induction of c-fos expression in the neostriatum and the locomotor behavior are mediated by the DA D1 receptors, no functional link between them has been established earlier and the mechanisms underlying the c-fos antisense-induced reduction in motor function is unclear. Since in the above-mentioned studies the behavioral consequences occurred very rapidly after the rats had been treated with the psychostimulants, a low level of striatal c-fos expression regulating a number of genes has been proposed (13) and that the antisense oligonucleotide acts on this steady state long before the drug is given.
In the present study we investigated the effect of intrastriatal c-fos antisense oligonucleotide injections on the activity of the two major output pathways of the neostriatum, namely the D1 receptor-regulated striatonigral and the D2 receptor-regulated striatopallidal pathway, which have opposing influences on the basal ganglia output nuclei within the so-called motor circuit (15, see Fig. 7). Toward this end, we employed dual probe in vivo microdialysis to monitor GABA release in the substantia nigra (SN) pars reticulata (SNr) and the globus pallidus (GP).
Figure 7.
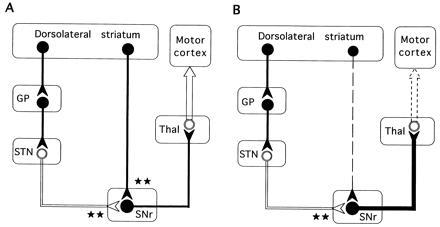
Simplified schematic representation of the basal ganglia–thalamocortical neuronal circuitry indicating the possible functional relationships underlying intrastriatal c-fos antisense induced akinesia-like symptoms. Inhibitory neurons (GABAergic) are shown as solid symbols, and excitatory neurons (glutamatergic) are shown as open symbols. ★, The degree of excitatory or inhibitory influence on the inhibitory nigrothalamic GABA pathway. (A) Untreated control. Two parallel and opposing GABAergic striatonigral (direct) and striatopallidal (indirect) pathways project from the striatum to the basal ganglia output nuclei and differentially regulate the inhibitory nigral projection to the thalamus. Striatal DA exerts contrasting effects on both of these pathways. DA has a net excitatory effect on striatal neurons that send GABA projections to the SN (the direct pathway) mediated by activation of D1 receptors, and a net inhibitory effect on those that send GABA projections to the GP (the indirect pathway) mediated by activation of D2 receptors (15, 34). Under normal conditions (A), activation of the striatonigral pathway facilitates movement by providing a positive feedback to the movement related neurons in the motor cortex through a reduced inhibitory GABA nigrothalamic influence, while activation of the opposing striatopallidal GABA pathway inhibits movements by providing negative feedback to the precentral motor fields by increasing the inhibitory GABA nigrothalamic influence. (B) Mechanism for the akinesia-like symptoms following acute intrastriatal injections of c-fos antisense oligonucleotide. The rapid decrease in nigral GABA release observed after the c-fos antisense oligonucleotide injection probably reflects reduction of impulse flow in the striatonigral GABAergic neurons (dashed line) leading to an increase in the inhibitory drive of the nigrothalamic GABA projections (thick black line) with a subsequent decrease in thalamocortical (motor cortex) glutamate transmission (dashed arrow). STN, subthalamic nucleus; Thal, thalamus.
MATERIALS AND METHODS
Oligonucleotides.
All oligonucleotides were synthesized as deoxyoligo-phosphorothioates on an Applied Biosystems model 380B synthesizer and HPLC purified. The partial phosphorothioate antisense (5′-GAA CAT CAT GGT CGT-3′) oligonucleotide was designed to span the region around the start codon of the rat c-fos mRNA (16). Also the corresponding sense (5′-ACG ACC ATG ATG TTC-3′) and scrambled (5′-GTA CCA ATC GGG ATT-3′) oligonucleotides were synthesized. The modification was carried out in a way that every second orthophosphate group was replaced by a phosphorothioate. As an additional control, a random sequence (5′-TCT GGG CTG GAG CTA AAG-3′) was synthesized as a completely phosphorothioate modified oligonucleotide. This oligonucleotide would not be expected to hybridize to the mRNA for any sequence in the European Molecular Biology Laboratory data base. For labeling with a fluoresceine isothiocyanate reporter group, c-fos antisense phosphorothioate modified oligonucleotides were synthesized with a six carbon spacer attached to a 3′-amino group. Unincorporated fluoresceine isothiocyanate was removed by gel filtration followed by HPLC. Custom synthesis of all oligodeoxynucleotides was made by BIOTEZ (Berlin). Radioactive labeling of phosphorothioate modified antisense c-fos oligonucleotides was made by end labeling with [35S]ATPγS and T4 polynucleotide kinase, followed by PAGE for purification. Aliquots of 5 × 10E5 dpm were dried under vacuum and then mixed for injection with 1 nmol of cold oligonucleotides. All oligonucleotides were infused at 1 mM concentration in Ringer solution at 1 μl/min.
Animals and Treatment.
Male Sprague–Dawley rats (Alab, Stockholm) weighing 350–400 g were maintained under a standard light/dark cycle and allowed free access to food and water. For surgery rats were anesthetized with halothane (1.5% in an air flow of 1.5 liters/min) and placed in a Kopf stereotaxic frame. In one study radioactive or fluorescence labeled oligonucleotides were injected in the neostriatum via a 32-gauge syringe (Hamilton). In the other studies rats were implanted with a 23-gauge stainless steel indwelling cannula guide in the neostriatum at the coordinates: Bregma A +0.5, L 3.1, V −5.0 according to the atlas of Paxinos and Watson (17). Additionally, in the microdialysis study implantation of two microdialysis probes (CMA 12, o.d. 0.5 mm; Carnegie Medicin, Stockholm) was made. One probe (1 mm dialyzing membrane) was implanted into the ipsilateral SN and a second probe (2-mm dialyzing membrane) was implanted into the ipsilateral GP using standard stereotaxic procedures (Fig. 1).
Figure 1.
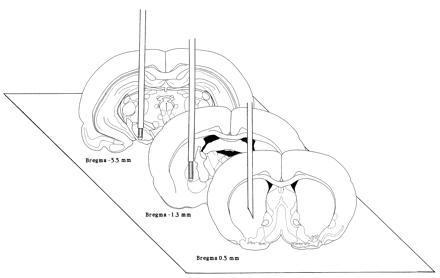
Experimental design of the dual probe in vivo microdialysis approach. The location of the cannula in the striatum was Bregma A +0.5, L 3.1, V −5 and of the two microdialysis probes, one in the globus pallidus (2 mm probe) and the other in the SN (1 mm probe), were Bregma A −1.3, L 3.1, V −8.0 and A −5.5, L 2.2, V −9.5, respectively. The coordinates used are based on the atlas of Paxinos and Watson (17).
Histological Evaluation.
At the end of the experiments the rats were killed with an overdose of pentobarbital. To verify the placement of the cannula and probes the brains were cut on a Leitz cryomicrotome and examined in a microscope. For fluorescence microscopy the rats were perfused through the left cardiac ventricle using 300 ml of a fixative containing 4% paraformaldehyde and 0.25% (vol/vol) glutaraldehyde in 0.1 M phosphate-buffered saline (PBS). The brains were kept in the fixative for 2 h and then transferred to 10% phosphate-buffered sucrose for 1–2 days after which they were frozen and sectioned (14 μm) in a cryostat. For immunohistochemistry the animals were perfused through the left cardiac ventricle using 200 ml of fixative [4% paraformaldehyde/0.2% (vol/vol) picric acid/0.1 M PBS]. The brains were cut in 40-μm coronal sections and stained for Fos-like immunoreactivity (Fos-LI) with an anti-Fos sheep polyclonal antiserum (Affinity Research Products, Nottingham, U.K.) using the avidin-biotin-peroxidase kit (Vector Laboratories) as described (11). Animals that had received radiolabeled material were decapitated, and the brains were removed and frozen in isopentane at −40°C. For autoradiography, cryosections (14 μm) were exposed on Hyperfilm-3H (Amersham) for 2 days.
Microdialysis Procedure.
In vivo microdialysis was performed on the second day (48 h) after probe implantation. During the experiment the rats were placed in a semispherical bowl and both probes were connected to a microinfusion pump via a two-channel swivel (CMA 120 System 4; Carnegie Medicin) and were perfused (2 μl/min) with Ringer solution (Karolinska Apoteket; Stockholm) containing 148 mM Na+, 4 mM K+, 2.4 mM Ca2+, and 156 mM Cl− (pH 6.0). After 60 min of stabilization, sample collection commenced and 60 μl perfusate were collected every 30 min over a 300-min period. The oligonucleotides were injected locally via a guided cannula into the striatum following the measurement of three stable basal levels. Each perfusate sample from the SNr was divided into 10 μl used for assay of DA and 20 μl for assay of GABA, while only GABA (20 μl) was assayed from the GP. The samples were placed in a separate automatic microsampler injector (CMA 200/240; Carnegie Medicin) and automatically injected into HPLC columns.
Neurotransmitter Analysis.
Reverse-phase HPLC coupled with electrochemical detection was used to assay DA and GABA as described (refs. 18 and 19, respectively). The limits of sensitivity for DA and GABA were 2 fmol/sample and 20 fmol/sample, respectively.
The results are expressed as mean ± SEM of percent changes from the respective basal values. To study the overall effect of each treatment on basal GABA levels, the values obtained before and after intrastriatal injections were analyzed by paired Student’s t test. To compare the different treatments a two-way ANOVA followed by Newman–Keuls test for multiple comparisons was used.
RNA Isolation and Protection Analysis.
For detection of basal c-fos expression, four rats were taken out from their normal environment and immediately killed by guillotine. Oligonucleotide-treated animals were decapitated 90 min after the infusion. Striatal tissue was obtained by gross dissection and total RNA was extracted using the Ultraspec RNA isolation kit (Biotecx Laboratories, Houston). The cDNA fragments used as antisense riboprobes were all cloned in Bluescript KS II+ (Stratagene). The c-fos antisense riboprobe was a 557-nt BglII/StuI fragment from rat c-fos cDNA (16), kindly provided by J. Morgan (St. Jude Children’s Research Hospital, Memphis). The junB antisense riboprobe was a 474-bp BamHI/SacI fragment from mouse junB cDNA (20), kindly provided by M. Yanif (Institut Pasteur, Paris). A single band at 360 nt was obtained with rat RNA. The NGFI-A antisense probe contains a 467-bp SphI/AvrII fragment from a NGFI-A cDNA clone (21), kindly provided by N. H. Neff (Ohio State University). The plasmid pSKrβac (22) containing 150 nt of the rat β-actin cDNA sequence was obtained from M. Bader (Max-Delbrück Centrum, Berlin). The probes were labeled using the MAXIscript in vitro transcription kit (Ambion, Austin, TX) according to the manufacturer’s protocol with 5 μl of [32P]UTP (800 Ci/mmol; 1 Ci = 37 GBq) except for the β-actin probe which was generated with 2 μl of [32P]UTP in the presence of 0.5 mM cold UTP. All probes were purified on a 8 M urea/5% acrylamide gel and eluted overnight at 37°C. Approximately 1.5 × 105 cpm for the c-fos, junB, and NGFI-A probes and 9 × 104 cpm for the β-actin probe were used per protection reaction (RPA II; Ambion). The samples were loaded on a 8 M urea/5% acrylamide gel, detected by autoradiography at −80°C for 72 h, and quantitated with a phosphoimager (BAS-1500; Fuji).
The results are reported as a percentage of the mean value of the Ringer group. The data are calculated as means ± SEM of the ratio between the c-fos, junB, and NGFI-A signal to the rat β-actin standard. The statistical analysis was performed by one-way ANOVA followed by Fisher’s probable least-squares difference test.
RESULTS
Detection of Basal c-fos Expression in the Striatum by RNase Protection Analysis.
A c-fos-specific riboprobe corresponding to the sequence of exon 2, 3, and a part of exon 4 of the rat c-fos gene (16) was used in the RNase protection assays. A representative experiment is shown in Fig. 2. The riboprobe is clearly protected by 2 μg of total RNA isolated from rat striatum.
Figure 2.
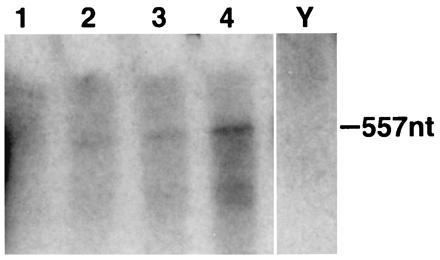
RNase protection analysis of the c-fos gene expression in the striatum of normal awake rats. Different amounts of total RNA from rat striatum were analyzed by using 32P-labeled antisense rat c-fos riboprobe (lanes 1–4: 1, 2, 5, and 10 μg, respectively). The 557-nt protected fragment is clearly visible with 2 μg of total RNA after autoradiography at −80°C for 72 h. The yeast RNA lane (Y) serves as a negative control.
Effects of Striatal c-fos Antisense Oligonucleotide Injection on Striatal c-fos, junB, and NGFI-A mRNA levels.
RNase protection analysis demonstrated that after the antisense injection striatal c-fos, junB, and NGFI-A mRNA levels increased significantly (P < 0.05) compared with the sense, scrambled, and sham-treated groups (Table 1). A rat β-actin probe was used as a standard in all reactions. The levels of β-actin were not affected by the injections.
Table 1.
RNase protection analysis of striatal c-fos, jun B, an dNGFI-A mRNA levels after treatment with c-fos antisense, sense, and scrambled oligonucleotides
| Treatment | n | c-fos | junB | NGFI-A |
|---|---|---|---|---|
| Ringer | 3 | 100 ± 9.4 | 100 ± 10.5 | 100 ± 17.9 |
| Antisense | 6 | 202 ± 16.8* | 247 ± 41.2* | 214 ± 30.8* |
| Sense | 4 | 139 ± 6.5 | 152 ± 12.9 | 124 ± 13.0 |
| Scrambled | 4 | 145 ± 19.9 | 152 ± 14.3 | 123 ± 7.4 |
Oligonucleotides (2 nmol) were intrastriatally injected into conscious rats and striatal tissue was prepared 90 min later. Values represent the mean (±SEM) ratio of the signal obtained with a 32P-labeled c-fos-, junB-, or NGFI-A- specific riboprobe in relation to the rat β-actin standard and are expressed as percentage of the Ringer solution group. The ratios of signals for c-fos, junV, and NGFI-A to β-actin in the Ringer solution group were 0.56 ± 0.053, 0.20 ± 0.022, and 1.86 ± 0.34, respectively.
P < 0.05 using one-way ANOVA and Fisher’s partial least-squares difference post-hoc test for significance.
Effect of Striatal c-fos Antisense Oligonucleotide Injection on Amphetamine Induced Fos-LI in the Neostriatum.
The results of the in vivo experiments to test the performance of partially phosphorothioate modified c-fos antisense oligonucleotides are illustrated in Fig. 3. Levels of amphetamine induced striatal Fos-LI were strongly reduced in the antisense-infused striatum (2 nmol oligonucleotide) 1 h after d-amphetamine was administered (5 mg/kg, i.p.). In the sense, random, and sham-infused control animals the amphetamine induced Fos-LI was unaffected.
Figure 3.
Photomicrographs illustrating the effectiveness of the partially phosphorothioate modified c-fos antisense oligonucleotides on amphetamine-induced neostriatal c-fos expression. The rats were unilaterally infused with 2 nmol oligonucleotide into the neostriatum, 4 h later injected with d-amphetamine (5 mg/kg, i.p.), and sacrificed for immunohistochemistry after an additional 1 h. The sections were cut in the coronal plane and stained with the anti-Fos antiserum (see text). Intense Fos-LI nuclear neuronal profiles are present on the untreated side of the striatal section (Lower). Staining is strongly reduced in the antisense infused neostriatum (Left), whereas in the neostriatum infused with the sense oligonucleotide Fos-LI is not affected (Right). Bregma level +0.5, according to the atlas of Paxinos and Watson (17).
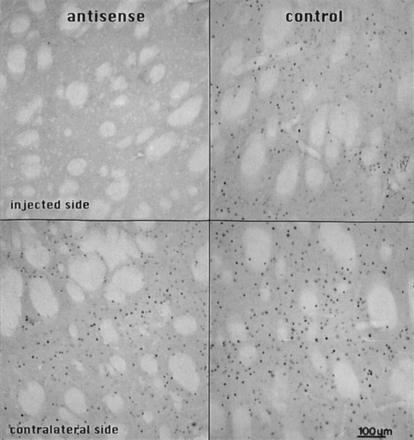
Uptake and Diffusion of Labeled Oligonucleotides After Intrastriatal Injection.
To estimate the distribution of oligonucleotides after a single intrastriatal injection, a small amount of radioactively labeled phosphorothioate modified oligonucleotide was added to 1 nmol of cold oligonucleotide. Fig. 4 demonstrates that the oligonucleotides diffuse throughout the dorsal part of the striatum within 20 min after the injection and that the entire striatum is labeled within 4 h. After the injection of 1 nmol of fluorescence-labeled oligonucleotides in the same location these compounds appear to be taken up mostly by cells with a neuronal morphology or with a perivascular location (pericytes) throughout the injected area. Within 30 min of the injection a strong cytoplasmatic and nuclear staining of nerve cell bodies and their dendrites was observed (Fig. 5A). In addition, a large amount of labeling was also observed in the extracellular space seen as a diffuse staining. Six hours following the injection the pattern of the neuronal intracellular staining had become more punctate in appearance and the extracellular fluorescence had largely disappeared (Fig. 5B). No signs of lesions could be detected except for the mechanical injury made by the cannula.
Figure 4.
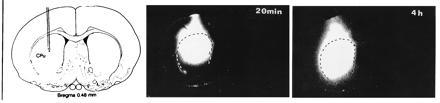
Autoradiographs showing the diffusion of [35S]ATPγS labeled c-fos antisense phosphorothioate oligonucleotides 20 min and 4 h after a single injection (1 μl) into the striatum, all taken from coronal sections of the striatal region. The border of the striatum is outlined on the autoradiograms (hatched line). The injection cannula was placed at the coordinates: Bregma A +0.5, L 3.1, V −5.0 according to the atlas of Paxinos and Watson (17).
Figure 5.
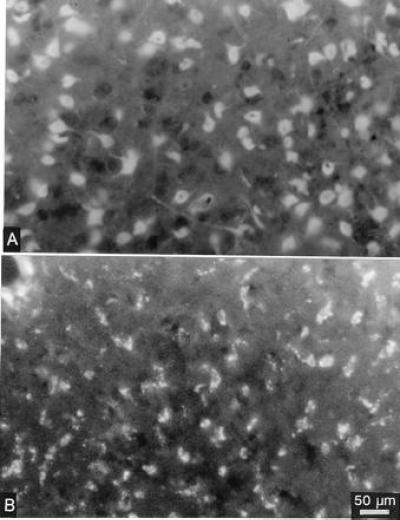
Uptake of fluorescence labeled phosphorothioate oligonucleotides is shown 30 min (A) and 6 h (B) after a single injection (1 μl) into the striatum. The diffuse staining of cytoplasm, nuclei of nerve cell bodies, and their dendrites early after the injection changes to a granular appearance of the fluorescence at the 6-h time point. For Bregma level, see Fig. 4.
Effects of Striatal c-fos Antisense Oligonucleotide Injection on Nigral and Pallidal GABA Release.
Basal dialysate GABA levels in the SNr and the GP measured in 30-min perfusate fraction were 7.9 ± 0.2 nM (22 rats) and 9.6 ± 0.7 nM (19 rats), respectively. As shown in Fig. 6, intrastriatal injection of 2 nmol c-fos antisense oligonucleotide (partially phosphorothioate modified) decreased GABA release in the ipsilateral SNr significantly and markedly compared with sense and sham-treated groups. The maximal effect was observed 120 min after the onset of the oligonucleotide injection. In contrast GABA release in the ipsilateral GP was left unaffected (Table 2). Intrastriatal injection of a random sequence oligonucleotide that was completely phosphorothioate modified did not affect GABA release in the SNr (Fig. 6) or in the GP (data not shown), clearly demonstrating absence of unspecific actions.
Figure 6.
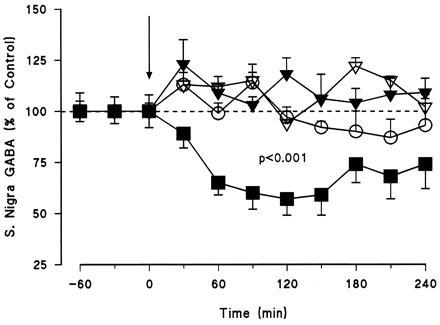
Effects of intrastriatally injected c-fos antisense (▪, n = 10), sense (▾, n = 5), and random (▴, n = 2) oligonucleotides (2 nmol) on GABA release in the SN of awake, freely moving rats. The sham group (○, n = 5) was infused with Ringer solution alone. The vertical arrow indicates the onset of the intrastriatal injection with the oligonucleotide. The results are expressed as a percentage of three basal values measured prior to the oligonucleotide injection. Basal nigral GABA levels measured in 30 min perfusate fractions were 7.9 ± 0.2 nM. Student’s paired t test, comparing the overall mean of pre- and the postinjection periods, showed that only the intrastriatally injected c-fos antisense oligonucleotide induced a significant decrease in nigral basal GABA levels (P < 0.001). Two-way ANOVA followed by Newman–Keuls test for multiple comparisons showed a significant difference between treatments (P < 0.001), with the antisense group being significantly different from all the other groups.
Table 2.
Effect of intrastriatal infusion with c-fos antisense and sense oligonucleotides on pallidal extracellular GABA levels in the awake freely moving rat
| Treatment, nmol | Baseline, nM | n | ↓Time, min
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −60 | −30 | 0 | 30 | 60 | 90 | 120 | 150 | 180 | 210 | |||
| Ringer | 9.5 ± 0.7 | 5 | 96 ± 2 | 98 ± 3 | 118 ± 13 | 95 ± 5 | 92 ± 11 | 88 ± 11 | 90 ± 13 | 100 ± 17 | 108 ± 16 | 109 ± 17 |
| Antisense | 9.8 ± 1 | 7 | 95 ± 7 | 98 ± 3 | 87 ± 3 | 103 ± 5 | 101 ± 7 | 107 ± 8 | 102 ± 6 | 102 ± 8 | 91 ± 5 | 101 ± 10 |
| Sense | 9.6 ± 0.2 | 5 | 110 ± 12 | 85 ± 7 | 102 ± 5 | 100 ± 7 | 99 ± 8 | 113 ± 9 | 114 ± 9 | 112 ± 8 | 106 ± 7 | 102 ± 7 |
Rats were injected with c-fos antisense or sense oligonucleotides. The sham group was injected with Ringer solution alone. The vertical arrow indicates the injection time. The results are expressed as a percentage of three basal values measured prior to the oligonucleotide infusion. Statistical analysis was performed by Student’s paired t test and two-way ANOVA followed by Newman–Keuls test for multiple comparisons.
Basal DA levels in the SN were 1.5 ± 0.3 nM (19 rats) in the same group of animals. Basal levels of the nigral DA metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanilic acid (HVA) were 47.6 ± 3.3 nM and 34.2 ± 2.2 nM, respectively. The extracellular levels of DA and its metabolites, DOPAC and HVA, in the SN were not affected by the intrastriatal injections (data not shown).
All rats receiving c-fos antisense oligonucleotides showed a clear asymmetric posture characterized by the turning of the body toward the injected side (i.e., an ipsilateral akinesia) from about 1 h after the injection and still present at the end of the sampling period. This time course coincided with the detected reduction in GABA release in the SNr. Behavioral asymmetry was not observed in either the sham-injected or sense and random oligonucleotide injected control groups.
DISCUSSION
The suppression of GABA transmission in the striatonigral but not the striatopallidal projection neurons is reported using an antisense c-fos oligonucleotide injected into the striatum of conscious rats. In this study, we first tested the ability of the partially modified phosphorothioate c-fos antisense oligonucleotide to inhibit the in vivo translation of c-fos mRNA. Since under resting conditions basal c-fos protein levels in the neostriatum are only barely detectable with immunohistochemical methodology (4, 5, 23), amphetamine induced striatal Fos-LI was used to demonstrate a blockade of the in vivo translation by the c-fos antisense oligonucleotide and its absence with the control oligonucleotides. This result establishes the effectiveness of the c-fos antisense oligonucleotide and confirm earlier observations by other groups using c-fos antisense oligonucleotides (12, 13, 24, 25, 26, 27, 28). Data on c-fos mRNA levels after treatment with c-fos antisense oligonucleotides are rare. Robertson et al. (28), using in situ hybridization, observed a reduction of haloperidol-induced striatal c-fos expression after intrastriatal antisense oligonucleotide administration. On the other hand, Liu et al. (27) could not find any effect on c-fos mRNA after intraventricular antisense oligonucleotide treatment. Using immunohistochemistry no effects of the c-fos antisense oligonucleotides on the expression of fos B, c-jun, junB, and NGFI-A could be found in several studies (13, 24, 26, 28). However, Dragunow et al. (12, 25) reported inhibitory effects on NGFI-A and junB expression of intrastriatal c-fos antisense treatment after stimulation with apomorphine or amphetamine, respectively. Under the present experimental conditions with normal, unstimulated rats we noticed a weak but significant increase in mRNA levels for c-fos, junB, and NGFI-A by RNase protection analysis 90 min after the injection of the c-fos antisense oligonucleotides. In case of the c-fos gene, these findings were not unexpected considering the strong transcriptional autoregulation of the c-fos gene via the c-fos containing transcription factor complex AP-1 (29). Since the NGFI-A and the junB gene also contain AP-1 binding sites (30, 31) and are similar in their expression kinetics to c-fos, the present data give evidence that in the striatal neurons under physiological conditions all three immediate early genes are negatively regulated by AP-1. The discrepancy with the preceding findings on the expression of c-fos, junB and NGFI-A after c-fos antisense treatment may reflect complex regulatory interactions between c-fos and other immediate early genes, which might strongly depend on the time course and the conditions of the experiment.
In view of the rapid onset of the effect of the intrastriatal antisense oligonucleotide injection we investigated the fate of locally injected radiolabeled and fluoresceine isothiocyanate phosphorothioate oligonucleotides under the time course of the experiment by monitoring their local distribution and uptake. The results demonstrate that 20 min after the intrastriatal injection the labeled oligonucleotides are spread throughout the dorsal part of the striatum. Fluoresceine isothiocyanate-labeled oligonucleotides are taken up and accumulated within 30 min by the majority of medium-sized neurons in the injected striatum mainly representing the striatonigral and striatopallidal projection neurons (32). After 6 h the internalized oligonucleotides give rise to a granular appearance probably reflecting an entrapment of the compound in endosomal compartments in the neurons (33). The astroglia do not show any uptake of the fluoresceine isothiocyanate labeled oligonucleotides over the first hours after the injection (W.S. and C. Xia, unpublished data).
In this study we combined the antisense approach with in vivo microdialysis technology to further investigate the role of c-fos in the regulation of striatal GABA efferent neurons forming the two main striatofugal projections, the direct striatonigral pathway, and the indirect striatopallidal pathway, which are involved in the control of basal ganglia related motor functions (Fig. 7A). The major finding of the present study is that the intrastriatal injection of c-fos antisense oligonucleotides selectively decreased GABA release in the ipsilateral SNr without affecting DA release in this region or GABA release in the ipsilateral GP in the same animal. The striking blockade in striatonigral GABA transmission may result in a reduced excitatory thalamo-cortical drive for the initiation and maintenance of cortically induced movements probably reflected by the asymmetric posture observed in the antisense treated animals (Fig. 7B). The present data give clear evidence that in conscious rats neuronal transmission in the striatonigral D1 receptor regulated GABA neurons is tonically regulated by basal c-fos expression. To prove this hypothesis we applied RNase protection analysis. Low levels of c-fos mRNA could be demonstrated in the striatum of normal, unstressed rats. Earlier, Hooper et al. (13) had proposed a low level of striatal c-fos expression to explain the rapid onset of ipsilateral turning observed in rats following systemic amphetamine treatment (within 15 to 20 min), when the animals had been pretreated with an unilateral intrastriatal c-fos antisense injection (11, 12, 13). According to the present findings, these rotations are possibly caused by an inhibition of striatonigral GABA transmission.
The reduction in nigral extracellular GABA levels most probably reflects a reduced impulse flow in the striatonigral neurons, since the time course (onset about 30–60 min after the antisense oligonucleotide injection) and the distance from the site of the antisense action (5–6 mm) rules out a c-fos-regulated mechanism in the terminals of the SN at this time interval. Thus, the target of the tonic c-fos regulation appears to be a short-lived protein located in the cytoplasm and/or membrane of the nerve cell body and its dendrites where it may participate in the integration of input signals or in the control of initiation and maintenance of impulse flow.
The direct, striatonigral and indirect, striatopallidal GABA pathways arise from separate populations of striatal neurons containing primarily D1 and D2 receptors (7, 35, 36, 37). These two receptors activate and inhibit their respective neurons in response to DA release. The receptor-mediated transduction mechanism within the striatonigral and striatopallidal neurons is thought to be different and the present results strengthen the evidence for this concept. They provide direct in vivo evidence for a specific action of c-fos antisense oligonucleotides on striatonigral GABA neurons, since the striatopallidal pathway is unaffected by the c-fos antisense oligonucleotide. Recent microdialysis studies provided a positive control for this effect by demonstrating that pallidal GABA release decreases after intrastriatal perfusion with the DA D2 receptor agonist pergolide (34, 38).
Three hours after the antisense injection the decreased SN GABA levels began to return to pretreatment levels possibly reflecting a rapid degradation of the partially phosphorothioate-modified oligonucleotide. A partial modification was employed to reduce non-sequence-specific or toxic effects of fully phosphorothioate modified oligonucleotides but is known also to confer reduced nuclease resistance to the oligonucleotide (13, 39). However, no effect on GABA release was observed following the injection of a fully phosphorothioate modified random oligonucleotide suggesting absence of unspecific effects. Alternatively, compensatory or adaptive mechanisms to the antisense treatment might be involved in the reversal of the antisense effect.
In summary, the method of the antisense oligonucleotide treatment aimed to “knock down” the expression of the c-fos gene has proven to be useful in the study of basal ganglia function. The present results give in vivo evidence for a low level striatal c-fos expression in freely moving rats regulating a gene which in the striatonigral neurons plays a facilitatory role in the control of GABA transmission. These findings add a new element into the puzzle of understanding immediate early gene expression as a regulatory mechanism in brain function and point to a role of regulatory loops between membrane and nucleus in the control of neuronal transmission.
Acknowledgments
This work has been supported by a grant from the Swedish Medical Research Council. The fellowship to W.S. through Deutscher Akademischer Austauschdienst and the grants to W.O. through the Stanley Foundation and the Åke Wiberg Foundation are gratefully acknowledged.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. §1734 solely to indicate this fact.
Abbreviations: DA, dopamine; D1 or D2 receptor, dopamine D1 or D2 receptor, respectively; GABA, γ-aminobutyric acid; GP, globus pallidus; SN, substantia nigra; SNr, substantia nigra pars reticulata; Fos-LI, Fos-like immunoreactivity.
References
- 1.Morgan J I, Curran T. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- 2.Robertson H A. Biochem Cell Biol. 1992;70:729–737. doi: 10.1139/o92-112. [DOI] [PubMed] [Google Scholar]
- 3.Hughes P, Dragunow M. Pharmacol Rev. 1995;47:133–178. [PubMed] [Google Scholar]
- 4.Robertson H A, Peterson M R, Murphy K, Robertson G S. Brain Res. 1989;503:346–349. doi: 10.1016/0006-8993(89)91689-2. [DOI] [PubMed] [Google Scholar]
- 5.Graybiel A M, Moratalla R, Robertson H A. Proc Natl Acad Sci USA. 1990;87:6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robertson G S, Vincent S R, Fibiger H C. Brain Res. 1990;523:288–290. doi: 10.1016/0006-8993(90)91498-6. [DOI] [PubMed] [Google Scholar]
- 7.Cenci M A, Campell K, Wictorin K, Björklund A. Eur J Neurosci. 1992;4:376–380. doi: 10.1111/j.1460-9568.1992.tb00885.x. [DOI] [PubMed] [Google Scholar]
- 8.Cole A J, Bhat R V, Patt C, Worley P F, Baraban J M. J Neurochem. 1992;58:1420–1426. doi: 10.1111/j.1471-4159.1992.tb11358.x. [DOI] [PubMed] [Google Scholar]
- 9.Paul M L, Graybiel A M, David J C, Robertson H A. J Neurosci. 1992;12:3729–3742. doi: 10.1523/JNEUROSCI.12-10-03729.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cenci M A, Kalen P, Mandel R J, Wictorin K, Björklund A. Neuroscience. 1992;46:943–957. doi: 10.1016/0306-4522(92)90196-9. [DOI] [PubMed] [Google Scholar]
- 11.Sommer W, Bjelke B, Ganten D, Fuxe K. NeuroReport. 1993;5:277–280. doi: 10.1097/00001756-199312000-00024. [DOI] [PubMed] [Google Scholar]
- 12.Dragunow M, Lawlor P, Chiasson B, Robertson H. NeuroReport. 1993;5:305–306. doi: 10.1097/00001756-199312000-00031. [DOI] [PubMed] [Google Scholar]
- 13.Hooper M L, Chiasson B J, Robertson H A. Neuroscience. 1994;63:917–924. doi: 10.1016/0306-4522(94)90559-2. [DOI] [PubMed] [Google Scholar]
- 14.Heilig M, Engel J A, Soderpalm B. Eur J Pharmacol. 1993;236:339–340. doi: 10.1016/0014-2999(93)90610-t. [DOI] [PubMed] [Google Scholar]
- 15.Alexander G E, Crutcher M D. Trends Neurosci. 1990;13:226–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- 16.Curran T, Gordon M B, Rubino K L, Sambucetti L C. Oncogene. 1987;2:79–84. [PubMed] [Google Scholar]
- 17.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic; 1986. [DOI] [PubMed] [Google Scholar]
- 18.Morari M, O’Connor W T, Ungerstedt U, Fuxe K. J Neurochem. 1993;60:1884–1893. doi: 10.1111/j.1471-4159.1993.tb13416.x. [DOI] [PubMed] [Google Scholar]
- 19.Kehr J, Ungerstedt U. J Neurochem. 1988;51:1308–1310. doi: 10.1111/j.1471-4159.1988.tb03101.x. [DOI] [PubMed] [Google Scholar]
- 20.Ryder K, Lan L F, Nathans D. Proc Natl Acad Sci USA. 1988;85:1487–1491. doi: 10.1073/pnas.85.5.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Changelion P S, Feng P, King T C, Milbrandt J. Proc Natl Acad Sci USA. 1989;86:377–381. doi: 10.1073/pnas.86.1.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Djavidani B, Sander M, Kreutz R, Zeh K, Bader M, Mellon S H, Vecsei P, Peters J, Ganten D. J Hypertens. 1995;13:637–645. doi: 10.1097/00004872-199506000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Smeyne R J, Schilling K, Robertson L, Luk D, Oberdick J, Curran T, Morgan J I. Neuron. 1992;8:13–23. doi: 10.1016/0896-6273(92)90105-m. [DOI] [PubMed] [Google Scholar]
- 24.Chiasson B J, Hooper M L, Murphy P R, Robertson H A. Eur J Pharmacol. 1992;227:451–453. doi: 10.1016/0922-4106(92)90167-t. [DOI] [PubMed] [Google Scholar]
- 25.Dragunow M, Tse C, Glass M, Lawlor P. Cell Mol Neurobiol. 1994;14:395–405. doi: 10.1007/BF02088826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gillardon F, Beck H, Uhlmann E, Herdegen T, Sandkuhler J, Peyman A, Zimmermann M. Eur J Neurosci. 1994;6:880–884. doi: 10.1111/j.1460-9568.1994.tb00999.x. [DOI] [PubMed] [Google Scholar]
- 27.Liu P K, Salminen A, He Y Y, Jiang M H, Xue J J, Liu J S, Hsu C Y. Ann Neurol. 1994;36:566–576. doi: 10.1002/ana.410360405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robertson G S, Tetzlaff W, Bedard A, St. Jean M, Wigle N. Neuroscience. 1995;67:325–344. doi: 10.1016/0306-4522(95)00049-o. [DOI] [PubMed] [Google Scholar]
- 29.Sassone-Corsi P, Sisson J C, Verma I M. Nature (London) 1988;334:314–319. doi: 10.1038/334314a0. [DOI] [PubMed] [Google Scholar]
- 30.Tsai-Morris C H, Cao X M, Sukhatme V P. Nucleic Acids Res. 1988;16:8835–8846. doi: 10.1093/nar/16.18.8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phinney D G, Tseng S W, Ryder K. Genomics. 1995;28:228–234. doi: 10.1006/geno.1995.1135. [DOI] [PubMed] [Google Scholar]
- 32.Heimer L, Zahm D S, Alheid G F. In: The Rat Nervous System. Paxinos G, editor. New York: Academic; 1995. pp. 579–628. [Google Scholar]
- 33.Loke S L, Stein C A, Zhang X H, Mori K, Nakanishi M, Subasinghe C, Cohen J S, Neckers L M. Proc Natl Acad Sci USA. 1989;86:3474–3478. doi: 10.1073/pnas.86.10.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ferre S, O’Connor W T, Fuxe K, Ungerstedt U. J Neurosci. 1993;12:5402–5406. doi: 10.1523/JNEUROSCI.13-12-05402.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gerfen C R, Engber T M, Mahan L C, Susel Z, Chase T N, Monsma F J J, Sibley D R. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- 36.Harrison M B, Wiley R G, Wooten G F. Brain Res. 1990;528:317–322. doi: 10.1016/0006-8993(90)91674-6. [DOI] [PubMed] [Google Scholar]
- 37.Le Moine C, Normand E, Bloch B. Proc Natl Acad Sci USA. 1991;88:4205–4209. doi: 10.1073/pnas.88.10.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fuxe K, O’Connor W T, Antonelli T, Osborne P G, Tanganelli S, Agnati L, Ungerstedt U. Proc Natl Acad Sci USA. 1992;89:5591–5595. doi: 10.1073/pnas.89.12.5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stein C A, Cheng Y-C. Science. 1993;261:1004–1012. doi: 10.1126/science.8351515. [DOI] [PubMed] [Google Scholar]


