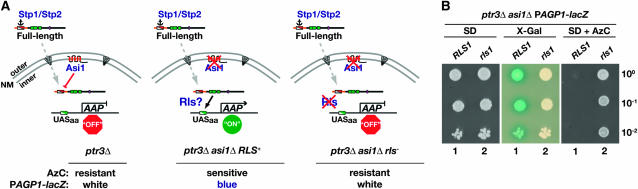
Figure 1.—
Mutations in RLS1 repress asi1Δ-induced constitutive SPS sensor-regulated gene expression. (A) Schematic of downstream events in the SPS-sensing pathway. In cells lacking a functional SPS sensor (left), the latent unprocessed forms of transcription factors Stp1 and Stp2 are primarily localized to the cytosol. The presence of cytoplasmic retention signals (anchor) restricts their entry to the nucleus (Andréasson and Ljungdahl 2004). The low levels of latent forms of Stp1 and Stp2 that enter the nucleus (dashed arrow) are prevented from binding SPS sensor-regulated promoters of amino acid permease genes (AAP) by the combined action of Asi proteins (Asi1, Asi2, and Asi3) localized to the inner nuclear membrane (NM) (Boban et al. 2006; Zargari et al. 2007). The ability of the Asi proteins to prevent transcription is dependent on the presence of sequences of Stp1 and Stp2 (Region I) in the N-terminal regulatory domain (red/white diagonal box). Consequently, there are low levels of AAP gene expression; cells are AzC resistant and remain white when incubated in the presence of X-Gal because of lack of PAGP1-lacZ expression. In cells lacking Asi1 (middle), the latent forms of Stp1 and Stp2 that enter the nucleus constitutively induce the expression of SPS-regulated AAP genes, and cells are AzC sensitive and turn blue in the presence of X-Gal (Andréasson and Ljungdahl 2004; Boban et al. 2006). Mutations in RLS genes (right) prevent the latent forms of Stp1 and Stp2 from gaining access to SPS sensor-regulated promoter; mutant cells are AzC resistant and remain white in the presence of X-Gal. (B) Strains MBY13 (ptr3Δasi1Δ) and MBY15 (ptr3Δasi1Δrls1−01) were grown on SD medium. Cells were resuspended in water to obtain identical cell densities. Aliquots of 10-fold serial dilutions were spotted on SD supplemented with glutamate, leucine, adenine, uracil, histidine (SD), and SD containing AzC (SD + AzC). The plates were grown at 30° for 2 days, after which the SD plate was overlaid with X-Gal substrate (materials and methods).