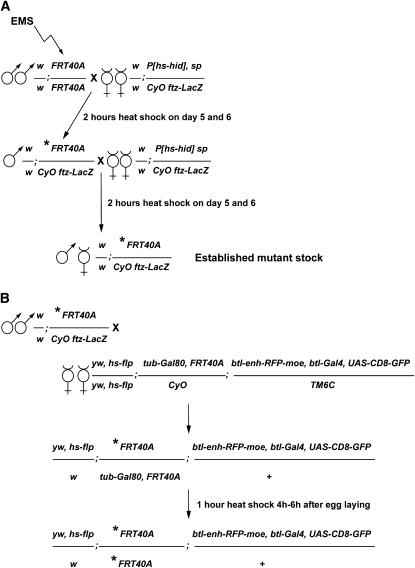
Figure 1.—
Mutagenesis and crossing scheme to generate MARCM clones of cells homozygous for mutations on chromosome 2L. (A) Scheme for the establishment of Drosophila stocks carrying mutations on the second chromosome. Ethyl methanesulfonate (EMS)-induced mutations were randomly generated in the genome of males bearing a FRT40A chromosome. EMS-treated males were subsequently crossed to females carrying an hs-hid construct and a balancer chromosome. The asterisk represents the induced mutation. Balanced mutant stocks were established in two generations. A heat-shock regime applied to the progeny of the F0 and F1 generation induced the expression of the hs-hid construct and the death of animals due to ectopic apoptosis. Therefore, establishment of the heterozygous mutant stocks did not require virgin female collection. (B) Crossing scheme to induce MARCM clones in the Drosophila larval tracheal system. F2 heterozygous mutant males were crossed to so-called MARCM females carrying a heat-shock-flipase (hs-flp) source, a FRT40A chromosome recombined to a tubulin-Gal80 (tub-Gal80) construct, and a third chromosome bearing the breathless-Gal4 (btl-Gal4), UAS-CD8-green fluorescent protein (UAS-CD8-GFP), and breathless enhancer-red fluorescent protein-moesin (btl-enh-RFP-moe) constructs. Heat-shock treatment of generation F3 induced the Flp-driven recombination at FRT sequences, which segregate the tub-Gal80 construct away from the induced mutation. Therefore, the btl-Gal4-dependent expression of CD8-GFP was possible only in clones of cells homozygous for the induced mutation (see also Figure 3A).