Abstract
The Rho GTPases interact with multiple downstream effectors to exert their biological functions, which include important roles in tissue morphogenesis during the development of multicellular organisms. Among the Rho effectors are the protein kinase N (PKN) proteins, which are protein kinase C (PKC)-like kinases that bind activated Rho GTPases. The PKN proteins are well conserved evolutionarily, but their biological role in any organism is poorly understood. We previously determined that the single Drosophila ortholog of mammalian PKN proteins, Pkn, is a Rho/Rac-binding kinase essential for Drosophila development. By performing “rescue” studies with various Pkn mutant constructs, we have defined the domains of Pkn required for its role during Drosophila development. These studies suggested that Rho, but not Rac binding is important for Pkn function in development. In addition, we determined that the kinase domain of PKC53E, a PKC family kinase, can functionally substitute for the kinase domain of Pkn during development, thereby exemplifying the evolutionary strategy of “combining” functional domains to produce proteins with distinct biological activities. Interestingly, we also identified a requirement for Pkn in wing morphogenesis, thereby revealing the first postembryonic function for Pkn.
THE Rho family small GTPases play a fundamental role in the regulation of cell shape and tissue morphogenesis in multicellular organisms. Activated, GTP-bound Rho GTPases interact directly with a variety of effector proteins that mediate their cellular functions (Bishop and Hall 2000). One such effector is protein kinase N (PKN), also known as protein kinase C-related kinase-1 (PRK1), which binds specifically to the activated Rho GTPase (Mukai 2003). PKN contains a serine/threonine kinase domain at its carboxyl terminus that is most closely related to the kinase domains of the protein kinase C (PKC) family kinases (Mukai and Ono 1994). Its amino terminus contains three tandem motifs of ∼70 amino acids, each composed of a charged region followed by a leucine-zipper-like region. Structural analysis of the first repeat showed that it consists of two helices that form an anti-parallel coiled coil (ACC finger) (Maesaki et al. 1999). Hence, these repeats are called the ACC domains. In the “middle” of PKN there is a region of unknown function with weak homology to the C2 domain of PKCs ε and η (Mukai 2003).
Two additional PKN isoforms have been identified in mammals: PKN-γ/PRK 2 and PKN-β (Palmer et al. 1995; Quilliam et al. 1996; Vincent and Settleman 1997; Oishi et al. 1999). These proteins share a similar domain structure to PKN but also have one or two proline-rich regions, respectively, between the C2-like domain and the kinase domain. PKN and PRK 2 are expressed ubiquitously, whereas expression of PKN-β mRNA has been detected only in a few cancer cell lines (Mukai and Ono 1994; Palmer et al. 1995; Quilliam et al. 1996; Oishi et al. 1999). Closely related PKN orthologs have been identified in Drosophila, Xenopus, and starfish (Mukai et al. 1995; Ueno et al. 1997; Stapleton et al. 1998; Lu and Settleman 1999).
PKN kinase activity is regulated in a variety of ways. Evidence indicates that the amino-terminal region of PKN serves to autoinhibit the activity of the kinase domain (Mukai et al. 1994; Kitagawa et al. 1996; Yoshinaga et al. 1999). Consistent with a role for the amino-terminal half of PKN in regulating the kinase domain, active Rho was shown to bind to the ACC domains of PKN and stimulate its kinase activity, possibly by relieving autoinhibition (Amano et al. 1996; Watanabe et al. 1996). Activated Rho as well as Rac can also bind and activate the PKN-related PRK2 protein (Quilliam et al. 1996; Vincent and Settleman 1997). PKN is also activated by unsaturated fatty acids such as arachadonic acid and by autophosphorylation (Mukai et al. 1994; Peng et al. 1996). Phosphorylation on amino acid S377 is required for PKN localization to the plasma membrane and is essential for PKN to function as a Rho effector in mammalian cells (Zhu et al. 2004). Activation loop phosphorylation of PKN and PRK2 by PDK1 is important for their activation in vitro and in vivo and is required for PKN to transduce signals from the insulin receptor to the actin cytoskeleton (Dong et al. 2000; Flynn et al. 2000).
In addition to the Rho GTPases, several proteins have been identified that interact with PKN family members. These include signaling proteins such as p38 MAP kinases, MKK3, MKK6, MLTKα, PDK1, MEKK2, Akt, phospholipase D, NCK, Graf/Graf2, and cyclin T2a (Quilliam et al. 1996; Flynn et al. 2000; Koh et al. 2000; Sun et al. 2000; Oishi et al. 2001; Shibata et al. 2001; Takahashi et al. 2003 Cottone et al. 2006); transcription factors such as the androgen receptor, NRDF/NeuroD2, and PCD17 (Takanaga et al. 1998; Shibata et al. 1999; Metzger et al. 2003); and cytoskeletal proteins such as neurofilament protein, vimentin, and α-actinin (Mukai et al. 1996b, 1997; Matsuzawa et al., 1997). However, in most cases, the physiological significance of these interactions has not been determined. In addition, several potential substrates for the kinase activity of PKN or PRK2 have been reported. These include phospholipase D, Graf/Graf2, neurofilaments, vimentin, tau, glial fibrillary acidic protein, and MLTKα (Mukai et al. 1996b; Matsuzawa et al. 1997; Oishi et al. 2001; Shibata et al. 2001;; Taniguchi et al. 2001; Takahashi et al. 2003). But again, the in vivo relevance of these phosphorylations is not clear.
Various cellular functions have been attributed to PKN family members, including potential roles in cell cycle regulation and apoptosis (Cryns et al. 1997; Stapleton et al. 1998; Takahashi et al. 1998; Koh et al. 2000; Misaki et al. 2001; Isagawa et al. 2005; Schmidt et al. 2007; Su et al. 2007). Consistent with their putative role as Rho effectors, PKN and PRK2 have also been implicated in regulation of the actin cytoskeleton (Vincent and Settleman 1997; Dong et al. 2000; Bourguignon et al. 2004; Lim et al. 2004; Darenfed et al. 2007). Several studies have also suggested a role for PKN proteins in transcriptional regulation. For example, PKN can regulate SRF-dependent transcription and has been implicated in regulating gene expression via the p38 MAP kinase pathway (Marinissen et al. 2001; Gudi et al. 2002; Deaton et al. 2005). In addition, PKN has also been shown to bind to the androgen receptor and stimulate its transcriptional activity (Metzger et al. 2003).
At the organismal level, the functions of PKN proteins are poorly understood. There is a single PKN ortholog, Pkn, encoded by the Drosophila genome (Ueno et al. 1997; Lu and Settleman 1999). Pkn binds specifically to the activated forms of Rho and Rac GTPases in vitro (Lu and Settleman 1999). We determined previously that homozygous mutants in the Pkn gene are lethal and exhibit specific defects in dorsal closure, a developmental process in which Rho and Rac GTPases have been directly implicated (Lu and Settleman 1999). The Pkn gene product appears to be required for the changes in epidermal cell shape that take place during this morphogenetic process (Lu and Settleman 1999). Here, we report studies in which we have used Drosophila as a model system to perform a structure-function analysis of Pkn in vivo. We specifically set out to determine whether Rho and/or Rac binding is required for Pkn function in vivo and to establish the functional relationship between Pkn and the other closely related kinases of the PKC family.
MATERIALS AND METHODS
Molecular biology:
The PknG58A and PknKD mutants have been described previously (Lu and Settleman 1999). These cDNAs were amplified by PCR using Pfu turbo (Stratagene, La Jolla, CA) and subcloned into the pCMV5-flag vector for transient expression in mammalian cells and into pCaSpeR-hs for generation of transgenic flies. Chimeras and deletions of Pkn and PKC53E were generated by PCR using overlapping primers. The cDNA templates used are as follows: Pkn (Lu and Settleman 1999), DPak (DGRC, Indiana), PKC53E-B (Drosophila Gene Collection at BDGP), Drok (Verdier et al. 2006), and the mouse Rhotekin Rho-binding domain (Ren and Schwartz 2000). The PCR products were subcloned into the pCMV5-flag vector and into pCaSpeR-hs. In addition, the kinase domains of Pkn and PKC53E were amplified by PCR and subcloned into the pUAS-T vector. All constructs were sequenced before use.
GTPase binding assays:
293-T cells were transfected with Pkn and Pkn mutants or PKC53E and PKC53E chimeras that had been subcloned into the CMV5-flag vector. Cell extracts were prepared 48 hr after transfection in lysis buffer [50 mm HEPES (pH 7.4), 150 mm NaCl, 1.5 mm MgCl2, 5 mm EGTA, 10% glycerol, 1% Triton X-100]. Lysates were precleared twice for 30 min at 4° with protein A-Sepharose beads before use in binding assays. Drosophila Rho1 and Rac1 GST fusion proteins were expressed in Escherichia coli bacteria and purified according to standard methods. Equal amounts of GST fusion proteins and GST (∼10 μg/assay) were incubated at 30° for 30 min in 50 μl of nucleotide exchange buffer [50 mm HEPES (pH 7.08), 5 mm EDTA, 0.1 mm EGTA, 50 mm NaCl, 0.1 mm DTT, 0.5 mm GTPγS] to load the proteins with GTPγS. The reaction was terminated by addition of MgCl2 to a final concentration of 20 mm. Precleared lysates were incubated with the GST–GTPases and glutathione agarose beads at 4° for 1 hr. The beads were then washed four times in wash buffer [20 mm HEPES (pH 7.5), 150 mm NaCl, 10% glycerol, 0.1% Triton X-100] and analyzed by SDS–PAGE, followed by immunoblotting with anti-Flag M2 monoclonal antibody (Sigma, St. Louis). One-percent samples of the lysates (1% of the input) were also resolved by SDS–PAGE and immunoblotted with the M2 antibody.
Germline transformation and heat-shock rescue:
All Drosophila stocks were maintained at 25°. Transgenic flies were generated by co-injection of the transgene with the Δ2–3 transposase into w1118 embryos prior to cellularization. At least four transgenic lines for each pCaSpeR-hs construct were introduced into the PknP mutant background (Lu and Settleman 1999). To test the ability of the transgenes to rescue the lethality associated with the P-element insertion in the Pkn gene, ∼40 PknP/CyO virgins were crossed to 15–20 males of each transgenic line in the PknP mutant background. The F1 progeny growing at 25° were subjected to 30-min heat shock at 37° once a day beginning 48 hr after egg laying until eclosion. For the Pkn-PKC53E transgene, 15-min heat shocks were performed, since longer heat shocks caused lethality. For each cross, the number of Cyo-balanced and nonbalanced flies was determined. On the basis of expected Mendelian ratios, the percentage of flies that could be rescued to adulthood was calculated for each line.
RT–PCR:
To test expression of the transgenes induced by heat shock, RT–PCR was performed on RNA extracted from heat-shocked larvae. Wandering third instar larvae were heat-shocked at 37° and incubated at 25° for 30–40 min to recover. Fifteen heat-shocked and 15 non-heat-shocked larvae were picked from each cross and lysed in 1 ml Trizol (Invitrogen, Carlsbad, CA). After centrifugation to remove the larval cuticles, chloroform was added to extract the RNA. The RNA was precipitated using isopropanol. RNA was then treated with DNAse using the DNA-free kit (Ambion, Austin, TX) according to the manufacturer's instructions. First-strand cDNA synthesis was performed with 1 μg RNA using random primers and the First-Strand DNA synthesis kit (Amersham/GE Healthcare, Piscataway, NJ) or the Protoscript II RT–PCR kit (New England Biolabs, Beverly, MA) according to the manufacturer's instructions. PCR was then performed using primers specific to the transgene. PCR products were resolved on an ethidium bromide-agarose gel.
Wing morphology:
Virgin females carrying the UAS-Pkn and UAS-PKC53E transgenes were crossed to w1118 males or to the following GAL4 driver lines: Act88f-GAL4, engrailed-GAL4 (en-GAL4), patched-GAL4 (ptc-GAL4), and Cy6-GAL4. The progeny were allowed to develop to adulthood. Wings were examined for changes in morphology, veination pattern, and wing hairs. For documentation, wings were removed and mounted on slides in Gary's Magic mounting medium.
COS cell transfection and immunofluoresence:
COS cells were seeded onto glass coverslips in six-well plates at 1–2 × 105 cells per well. When cells reached 50% confluence, they were transfected with Pkn, Pkn mutants, PKC53E, or PKC53E chimeras in the CMV5-flag vector using Lipofectamine 2000 (Invitrogen). At 24 hr post-transfection, cells were fixed in 3% paraformaldehyde. Cells were then permeabilized with 0.1% Triton and stained with the M2 mouse anti-flag monoclonal (Sigma), followed by Cy2-conjugated goat anti-mouse IgG (Jackson, West Grove, PA) and finally with DAPI. Coverslips were mounted onto glass slides using ProLong Gold anti-fade reagent (Invitrogen).
RESULTS
Rho GTPase binding is required for efficient Pkn function during Drosophila development:
In previous studies, we have generated a null mutation of Pkn that exhibits a lethal phenotype. This lethality is associated with defective dorsal closure (Lu and Settleman 1999) but Pkn mutants that were able to progress through embryogenesis die at larval or pupal stages, suggesting additional roles for Pkn later in development (data not shown). We were able to rescue the lethality of the Pkn mutant by expressing a full-length Pkn cDNA in transgenic flies under the control of a heat-shock promoter (Lu and Settleman 1999). We also determined that the rescued flies (males and females) do not exhibit any detectable defects in fertility (data not shown). To determine which of the various domains of the Pkn protein are important for its developmental function in vivo we made use of this system in structure-function studies.
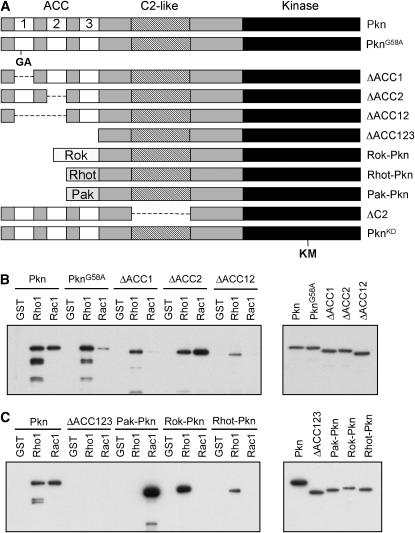
We first examined the requirement for Rho and Rac binding for Pkn function. Studies in mammalian cells have mapped the Rho- and Rac-binding region of Pkn to three tandem leucine- zipper repeats situated near the amino terminus of the protein (the ACC domains) (Flynn et al. 1998; Maesaki et al. 1999). We previously identified a missense mutation (G58A) in the first leucine-zipper repeat of Pkn (ACC1) that substantially reduces binding to Rac but not to Rho in in vitro protein interaction studies (Lu and Settleman 1999), suggesting that this domain is critical for Rac binding. In an effort to map more precisely the binding sites for Rho and Rac on Pkn, we generated specific deletions of the ACC1 domain (ΔACC1), the ACC2 domain (ΔACC2), and both domains together (ΔACC12; see Figure 1A). These various deletion constructs were expressed in transfected cells and binding to Drosophila Rho and Rac1 was assayed by “pull-down” using GST-tagged recombinant Rho and Rac GTPases. As shown in Figure 1B, deletion of ACC1 reduces Rho binding and practically abolishes Rac binding. Deletion of the ACC2 domain does not affect Rho and Rac binding. Upon deletion of both domains a residual amount of Rho binding remains, but no Rac binding can be observed. This suggests that Rac1 binds to the ACC1 domain, whereas Rho binds to both the ACC1 and ACC2 domains. In addition, a construct that lacks the amino terminus of the protein, as well as all three AC domains (ΔACC123), exhibits no detectable binding to Rho1 and Rac1 (Figure 1C), suggesting that Rho may also interact with the ACC3 domain.
Figure 1.—
Pkn mutations, deletions, and chimeras used in rescue experiments. (A) A diagram showing the structure of Pkn and the Pkn mutations and deletions used in this study. For the Rok-Pkn, Rhot-Pkn, and Pak-Pkn chimeras, the amino terminus of Pkn (up to and including the three ACC domains) was replaced with the Rho-binding domains of Rok or Rhotekin or the Rac-binding domain of Pak. (B) Binding of Pkn, PknG58A mutation, and ΔACC deletions to Rho1 and Rac1. Flag-tagged Pkn and the various deletions were transfected into 293-T cells. Pull-down assays were performed using GTPγS-loaded recombinant GST-Rho1, GST-Rac1, or GST. Pull downs (left) and samples of the lysates (right) were resolved on SDS–PAGE gels, blotted to PVDF, and probed with an anti-flag antibody. (C) Binding of Pkn, the ΔACC123 deletion, and the Pak-Pkn, Rok-Pkn, and Rhotekin-Pkn chimeras to Rho1 and Rac1. Pull-down binding assays were performed as described in B. The pull downs are shown on the left and samples of the lysates are shown on the right.
The G58A mutant and the ACC1, ACC2, ACC12, and ACC123 deletions were subcloned into the pCaSpeR-hs (heat-shock) vector and injected into Drosophila embryos to generate transgenic animals. Four independent lines for each transgene were introduced into the PknP mutant background. The flies thus generated were crossed to PknP mutant flies. The F1 progeny of the cross were heat-shocked for 30 min every day from 48 hr after egg laying to eclosion to induce expression of the transgene. This heat-shock regimen was chosen because it produces the greatest rescue of the lethality of the PknP mutant when the full-length, wild-type Pkn transgene is used (data not shown). The adults were examined to determine whether expression of the transgenes could rescue lethality of the Pkn mutant flies. The percentage of rescue was determined for each line and compared to that observed upon expression of full-length, wild-type Pkn (Table 1). We found that expression of the G58A mutant was able to rescue the Pkn loss-of-function phenotype to a similar degree as wild-type Pkn, indicating that Rac binding is not required for Pkn function in vivo, at least in terms of maintaining viability. This is supported by the fact that the ΔACC1 mutant was also able to rescue lethality of Pkn mutant flies (Table 1). The ability of the various independently generated ΔACC1 mutant lines to rescue the Pkn phenotype appears to be correlated with the level of expression of the transgene, as shown by RT–PCR (supplemental Figure 1 at http://www.genetics.org/supplemental/).
TABLE 1.
Rho binding but not Rac binding is required for efficient Pkn function in vivo
| Transgenic line | % rescue (n) | Relative mRNA expression |
|---|---|---|
| PknP/CyO | 0 (646) | − |
| Pkn(11)/CyO (37) | 100 (883) | +++ |
| Pkn/TM2 (9A) | 69.2 (451) | +++ |
| PknG58A/Y (6) | 58.4 (332) | ++ |
| PknG58A/TM2 (15) | 80.4 (401) | +++ |
| PknG58A/CyO (24-R1L) | 81.0 (791) | +++ |
| PknG58A/TM2 (R2B) | 80.4 (628) | +++ |
| ΔACC1/TM2 (1a) | 6.7 (553) | + |
| ΔACC1/CyO (3c-A) | 13.9 (475) | ++ |
| ΔACC1/TM2 (3b) | 97.0 (539) | +++ |
| ΔACC1/TM6B (4a) | 2.1 (286) | + |
| ΔACC2/TM2 (3M) | 79.2 (437) | +++ |
| ΔACC2/TM2 (4b) | 84.4 (573) | + |
| ΔACC2/TM2 (2b) | 83.1 (620) | ++ |
| ΔACC2/CyO (2f) | 96.8 (914) | + |
| ΔACC12/TM2 (4a) | 19.4 (680) | ++ |
| ΔACC12/TM6B (2b) | 17.7 (480) | +++ |
| ΔACC12/CyO (2a-JJ) | 9.3 (632) | + |
| ΔACC12/CyO (3GN) | 24.5 (650) | ++ |
| ΔACC123/TM2 (1) | 0 (449) | +++ |
| ΔACC123/TM2 (2a) | 0 (476) | +++ |
| ΔACC123/TM2 (4b) | 1.3 (299) | ++ |
Percentage of rescue was determined by counting the number of CyO-balanced and nonbalanced flies and calculating the percentage of flies that could be rescued to adulthood on the basis of expected Mendelian ratios. The total number (n) of flies counted is shown in parentheses. Relative mRNA expression of the transgenes was estimated from ethidium bromide-agarose gels of RT–PCR products. For each transgene: +++, the line that gave the strongest PCR band; ++, the line with the second strongest bands; +, the weakest; −, no expression.
The ΔACC2 deletion construct rescued the PknP lethality with an efficiency similar to that of wild-type Pkn (Table 1). In contrast, the ΔACC12 transgene carrying a deletion of both the ACC1 and the ACC2 domains was able to rescue lethality only with low efficiency (Table 1). These findings suggest that Rho1 binding is required for full Pkn function, but that residual in vivo function is preserved with little Rho1 binding. The ΔACC123 construct, in contrast, exhibited virtually no ability to rescue the lethality of the PknP mutant (Table 1). Attempts to generate an antibody that specifically recognizes Pkn in Drosophila have so far been unsuccessful. Thus, it was not possible to determine whether the transgenes were expressed at the protein level. However, the fact that expression of all transgenes at the RNA level was confirmed by RT–PCR (supplemental Figure 1 at http://www.genetics.org/supplemental/), together with the observed efficient expression of these mutant proteins in transfected cells and their ability to bind activated Rho1 and Rac1 in vitro, suggests that the observed phenotypes do not reflect defects in transgene expression.
To further explore the role of Rho and Rac binding in the function of Pkn in vivo, chimeric constructs were generated in which the ACC domain of Pkn was replaced with the Rho-binding domains of Drosophila Rho-kinase (Rok) or mouse Rhotekin, or the Rac-binding domain of DPak (see Figure 1A). These mutant proteins exhibited the expected Rho/Rac-binding properties when examined in pull-down experiments. Thus, the Rok-Pkn and Rhotekin-Pkn chimeric proteins bind efficiently to Rho1, but not to Rac1, whereas Pak-Pkn binds only to Rac1 (Figure 1C). Transgenic flies were generated that expressed the Pak-Pkn, Rok-Pkn, and Rhotekin-Pkn chimeras under control of the heat-shock promoter. The transgenes were introduced into the PknP mutant background and tested for their ability to rescue the lethality of this mutant. Four lines were tested for each transgene and the expression of the transgenes upon heat shock was confirmed by RT–PCR (supplemental Figure 1 at http://www.genetics.org/supplemental/). Interestingly, none of the lines was able to rescue the PknP mutant (supplemental Table 1), suggesting that another function of the amino-terminal region of Pkn, in addition to Rho/Rac GTPase binding, is required for its role in development.
One potential explanation for the inability of the ΔACC123 deletion and the Pak-Pkn, Rok-Pkn, and Rhotekin-Pkn chimeras to rescue lethality in the Pkn mutant background is that they may exhibit aberrant subcellular localization. We determined that flag-tagged versions of Pkn, PknG58A, ΔACC1, ΔACC2, and ΔACC12 show a diffuse localization throughout the cytoplasm and are excluded from the nucleus when expressed in mammalian COS cells (supplemental Figure 2, A, B, and J, at http://www.genetics.org/supplemental/). In contrast, in 17–32% of cells expressing ΔACC123, Pak-Pkn, Rok-Pkn, or Rhotekin-Pkn, the tagged proteins localize to vesicular structures that are either distributed throughout the cell or clustered around the nucleus (supplemental Figure 2, C, D, and J).
The C2-like domain is dispensable for Pkn function during development:
In the central region of Pkn, there is a domain of unknown function that exhibits homology to the C2 domains of PKC ε and η, two novel PKCs (Mukai 2003). To determine whether this domain is important for Pkn function in vivo, a construct was generated in which this domain is deleted (ΔC2). This was cloned into the pCaSpeR-hs vector, transgenic flies were generated, and the ability of the ΔC2 transgene to rescue the lethality of the Pkn mutant was determined. Each of the four lines tested rescued the Pkn mutant lethality (Table 2). One line exhibited substantial rescue (39%), though less than that seen with wild-type Pkn. The other lines rescued to a lesser extent (Table 2). These results suggest that the C2 domain is not absolutely required for Pkn function during development. In 18% of COS cells expressing the transfected ΔC2 mutant, the protein localizes to vesicular structures (supplemental Figure 2I at http://www.genetics.org/supplemental/), which may explain the reduced ability to rescue the lethality of the PknP mutant in comparison to wild-type Pkn.
TABLE 2.
The C2-like domain is not absolutely required for Pkn function in vivo
| Transgenic line | % rescue (n) | Relative mRNA expression |
|---|---|---|
| PknP/CyO | 0 (729) | − |
| Pkn(11)/CyO (37) | 74.5 (1514) | ++ |
| ΔC2/TM2 (3A) | 38.7 (475) | ++ |
| ΔC2/TM2 (3B) | 9.7 (582) | ++ |
| ΔC2/TM2 (3C) | 4.0 (506) | + |
| ΔC2/TM6B (1W) | 7.4 (530) | +++ |
Percentage of rescue was determined by counting the number of CyO-balanced and nonbalanced flies and calculating the percentage of flies that could be rescued to adulthood on the basis of expected Mendelian ratios. The total number (n) of flies counted is shown in parentheses. Relative mRNA expression of the transgenes was estimated from ethidium bromide-agarose gels of RT–PCR products. For each transgene: +++, the line that gave the strongest PCR band; ++, the line with the second stongest bands; +, the weakest; −, no expression.
Distinct properties of the closely related kinase domains of Pkn and PKC53E:
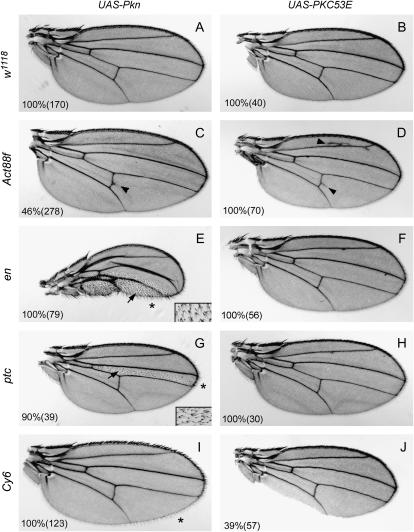
The kinase domain of Pkn most closely resembles the kinase domain of the PKC family kinases. In fact, evolutionary studies indicate that Pkns and PKCs have a common evolutionary ancestor (Herold et al. 2002). In yeast, a single PKN/PKC-like molecule exists that has domains similar to the ACC domains of Pkn and to the C1 and C2 domains of PKCs. In Drosophila, there are five PKCs: PKC53E, PKC98E, aPKC, PKCδ, and inaC (Shieh et al. 2002). Of these, the kinase domains of PKC53E and PKC98E, two PKCs of unknown function, show the highest similarity to the kinase domain of Pkn (65 and 69% similarity, respectively). Therefore, we chose these two PKC kinases for further study. To begin to address the role of the PKN kinase domain in vivo, we first compared the activity of the kinase domains of Pkn and PKC53E. The isolated kinase domains of Pkn and PKC53E were cloned into the pUAS-T vector to allow expression under control of the UAS promoter. Transgenic flies were generated carrying each UAS-kinase domain and these flies were crossed to a variety of GAL4 driver lines to drive transgene expression in the wing. Distinct phenotypes were observed for each kinase domain. The Act88f driver caused subtle ectopic wing veination from the posterior cross vein (pcv) in a subset of wings expressing the Pkn kinase domain but much more substantial ectopic veination from the pcv and L2 vein in wings expressing the PKC53E kinase domain (Figure 2C and D). Overexpression of the Pkn kinase domain using the en and ptc drivers produced an alteration in wing hair polarity and a multiple wing hair phenotype, as well as a reduction in wing material in the expression domains (Figure 2, E and G). Interestingly, Rho1 has been implicated in regulation of wing hair polarity (Strutt et al. 1997). In contrast, overexpression of the PKC53E kinase domain using the same drivers produced no obvious phenotype (Figure 2, F and H). Expression of the Pkn kinase domain in the wing margin using the Cy6 driver led to missing bristles at the anterior wing margin and missing hairs at the posterior wing margin, as well as longer posterior margin hairs (Figure 2I), whereas expression of PKC53E with this driver produced an occasional loss of material at the wing margin but no other defects (Figure 2J). Overall, we observed considerable differences in the phenotypes caused by overexpression of the Pkn kinase domain vs. the PKC53E kinase domain. This suggests that there are intrinsic differences in the signaling capacity of Pkn and PKC kinase domains in vivo.
Figure 2.—
Wing phenotypes generated upon overexpression of the Pkn or PKC53E kinase domain. Transgenic flies carrying the UAS-Pkn kinase domain or UAS-PKC53E kinase domain were crossed to w1118 flies (A and B) or to the following GAL4 drivers: Act88f (C and D), engrailed (en) (E and F), patched (ptc) (G and H), or Cy6 (I and J), and the wings of the progeny were examined for visible phenotypes. The insets in E and G represent higher-magnification images of areas indicated by the arrows to show the planar polarity defect. The penetrance for each phenotype is indicated as a percentage. The number of flies scored in each case is shown in parentheses. Arrows indicate alterations in wing hair polarity, arrowheads indicate ectopic wing veination, and asterisks indicate increased hair length at the posterior wing margin.
The kinase domain of PKC53E can functionally substitute for the kinase domain of Pkn:
To confirm that the kinase domain of Pkn is indeed required for Pkn function in vivo, the ability of a kinase-defective mutant of Pkn to rescue the lethality of the Pkn mutant was tested. The kinase-defective Pkn mutant contains a lysine-to-methionine substitution at amino acid 892 (Figure 1A). Four independent transgenic lines were tested and none of them was able to rescue, despite induction of transgene expression upon heat shock, as demonstrated by RT–PCR (supplemental Figure 1 at http://www.genetics.org/supplemental/). This strongly suggests that the kinase activity of Pkn is absolutely required for its in vivo function. Interestingly, PknKD exhibits some nuclear localization when expressed in transfected COS cells, which suggests that kinase activity may be important for correct compartmentalization of Pkn (supplemental Figure 2J).
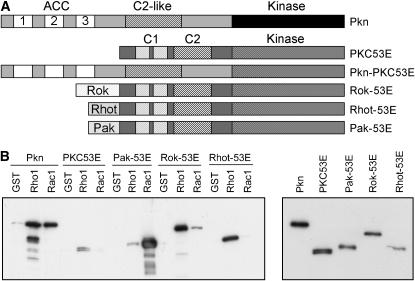
As described above, the kinase domain of Pkn is highly related to that of PKCs. To determine whether the kinase domain of Pkn could be functionally substituted by that of a PKC, chimeras were constructed in which the kinase domain of Pkn was replaced with that of PKC98E or PKC53E (Figure 3A). Transgenic lines were generated with these constructs under control of the heat-shock promoter and the ability of these chimeras to rescue the lethality of the Pkn mutant was tested. Using the standard heat-shock regimen (30 min heat shock once a day), these chimeras caused substantial lethality. If the length of heat shock was reduced to 15 min per day, the ability of the Pkn-PKC53E chimera to cause lethality was greatly reduced, and in fact, the Pkn-PKC53E chimera was able to rescue the lethality of the Pkn mutant (Table 3). In contrast, the Pkn-PKC98E chimera still caused substantial lethality with a 15-min heat shock. If the length of heat shock was reduced to 5 min, the Pkn-PKC98E transgene caused less lethality, but was unable to rescue to the Pkn mutant. However, since the ability of wild-type Pkn to rescue was substantially reduced under these heat-shock conditions, the Pkn-PKC98E chimera may not have been expressed at sufficient levels using 5-min heat shocks to rescue the lethality of the Pkn mutant. Both chimeras induce dramatic changes in cell shape when expressed in transfected COS cells (supplemental Figure 2, G and H, at http://www.genetics.org/supplemental/). The cells exhibit long protrusions and often become thin and spindly. The shape changes are more extreme with the Pkn-PKC98E chimera than with the Pkn-PKC53E chimera. This phenotypic effect of overexpression may contribute to the lethality associated with these chimeras when expressed in flies.
Figure 3.—
PKC53E chimeras used in rescue experiments. (A) A diagram showing the structure of PKC53E and the PKC53E chimeras used in this study. For the Rok-53E, Rhot-53E, and Pak-53E chimeras, the Rho-binding domains of Rok or Rhotekin or the Rac-binding domain of Pak were fused to the amino terminus of PKC53E. For the Pkn-PKC53E chimera, the kinase domain of Pkn was replaced with that of PKC53E. (B) Binding of Pkn, PKC53E, and the Pak-53E, Rok-53E, and Rhot-53E chimeras to Rho1 and Rac1. Pull-down binding assays were performed as described in Figure 1B. The pull downs are shown on the left and samples of the lysates are shown on the right.
TABLE 3.
The kinase domain of PKC53E can functionally substitute for that of Pkn
| Transgenic line | % rescue (n) | Relative mRNA expression |
|---|---|---|
| PknP/CyO | 0 (896) | − |
| Pkn(11)/CyO (37) | 74.1 (1069) | ND |
| Pkn-PKC53E/TM6B (K1AA) | 20.4 (464) | ++ |
| Pkn-PKC53E/TM2 (L4-AD) | 1.2 (681) | +++ |
| Pkn-PKC53E/TM2 (M1) | 20.0 (451) | ++ |
| Pkn-PKC53E/CyO (3B-M2) | 13.6 (725) | ++ |
| Pkn-PKC53E/CyO (32-L2) | 23.2 (914) | ++ |
Percentage of rescue was determined by counting the number of CyO-balanced and nonbalanced flies and calculating the percentage of flies that could be rescued to adulthood on the basis of expected Mendelian ratios. The total number (n) of flies counted is shown in parentheses. Relative mRNA expression of the transgenes was estimated from ethidium bromide-agarose gels of RT–PCR products. For each transgene: +++, the line that gave the strongest PCR band; ++, the line with the second stongest bands; +, the weakest; −, no expression. ND, not determined.
In contrast to the rescue observed with the Pkn-PKC53E chimera, full-length, wild-type PKC53E was unable to rescue the Pkn mutant phenotype, suggesting that domains other than the kinase domain are important for Pkn function (data not shown). Therefore, we generated and tested additional chimeras in which the Rho-binding domains of DRok or mouse Rhotekin or the Rac-binding domain of DPak were fused to the amino terminus of PKC53E (Figure 3A). Binding to Rho and Rac1 was determined by GST pull-down assay. The Pak-53E chimera bound strongly to Rac1 and very weakly to Rho1. The Rok-53E chimera bound strongly to Rho1 and weakly to Rac1. In contrast, the Rhot-53E chimera bound only to Rho1 (Figure 3B). These chimeras were introduced into flies and four independent transgenic lines of each were tested for the ability to rescue the Pkn mutant. However, none of these chimeras was able to rescue the lethality associated with Pkn disruption. The expression of the transgenes in each line was confirmed by RT–PCR (supplemental Figure 1 at http://www.genetics.org/supplemental/). These data indicate that the Pkn amino-terminal half provides a function in addition to GTPase binding that is important during Drosophila development. Taken together, these findings also suggest that the closely related PKC53E catalytic domain can be imparted with context-dependent biological function via regulatory elements in the nonkinase domains of Pkn.
A defect in wing morphogenesis cannot be rescued with transgenic Pkn:
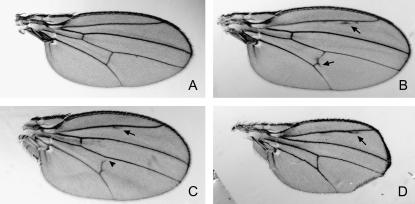
During the course of conducting the structure-function rescue experiments, we observed that a substantial proportion of the rescued adult flies exhibited abnormalities associated with wing vein formation—either extra or missing wing vein material, particularly at L2 and the pcv (Figure 4, A–C). In addition, some flies exhibited substantially smaller wings in comparison to those of wild-type flies (see Figure 4D). This was seen for each of the transgenes that was capable of rescuing the lethality of the Pkn mutant. When the percentage of flies with wing vein alterations or reduced wing size was determined for each line that rescued, it was observed that there was a significant variation among transgenic lines with respect to the percentage of flies that exhibit these wing phenotypes. Similar proportions of flies rescued with the G58A mutant and the ΔACC1 transgenes exhibited vein defects in comparison to flies rescued with the wild-type Pkn transgene (Table 4). However, a larger proportion of flies rescued with the ΔACC2 and ΔACC12 constructs displayed vein phenotypes. This was even more marked for flies rescued with the ΔC2 construct where virtually all the flies exhibited wing vein alterations (Table 4).
Figure 4.—
Wing phenotypes observed in “rescued” flies. (A) Wing from a PknP /+ fly that shows no phenotype. (B) Example of a wing from PknP flies rescued with wild-type Pkn showing ectopic wing veination. (C) Example of a wing from PknP flies rescued with the Pkn-PKC53E chimera showing missing wing veination. (D) Example of a wing from PknP flies rescued with the Pkn-PKC53E chimera showing a reduction in wing size. Ectopic veination is indicated with an arrow. Missing vein material is indicated with an arrowhead.
TABLE 4.
Wing phenotypes observed in rescue experiments
| Transgenic line | % flies with vein defects | % flies with small wings | Total no. |
|---|---|---|---|
| Pkn(11)/CyO (37) | 37.4 | 0 | 115 |
| Pkn/TM2 (9A) | 10.2 | 8.5 | 59 |
| PknG58A/Y (6) | 41.7 | 5.0 | 60 |
| PknG58A/TM2 (15) | 21.2 | 6.1 | 33 |
| PknG58A/CyO (24-R1L) | 10.6 | 8.5 | 94 |
| ΔACC1/CyO (3cA) | 22.2 | 22.2 | 9 |
| ΔACC1/TM2 (3B) | 32.3 | 3.2 | 124 |
| ΔACC2/TM2 (3M) | 47.9 | 2.1 | 96 |
| ΔACC2/TM2 (4b) | 55.7 | 10.0 | 70 |
| ΔACC2/TM2 (2b) | 57.5 | 2.7 | 73 |
| ΔACC2/CyO (2f) | 35.2 | 0 | 91 |
| ΔACC12/TM2 (4a) | 33.3 | 8.3 | 12 |
| ΔACC12/TM2 (2b) | 100 | 0 | 6 |
| ΔACC12/CyO (2a-JJ) | 75.0 | 0 | 4 |
| ΔACC12/CyO (3GN) | 66.7 | 0 | 24 |
| ΔC2/TM2 (3A) | 97.0 | 3.0 | 33 |
| ΔC2/TM2 (3B) | 86.7 | 13.3 | 15 |
| ΔC2/TM2 (3C) | 71.4 | 28.6 | 7 |
| ΔC2/TM6B (1W) | 100 | 0 | 13 |
| Pkn(II)/CyO (37)a | 38.4 | 10 | 99 |
| Pkn-53E/TM6B (K1AA)a | 38.1 | 4.8 | 21 |
| Pkn-53E/TM2 (L4-AD)a | 100 | 0 | 3 |
| Pkn-53E/TM2 (M1)a | 50.0 | 12.5 | 16 |
| Pkn-53E/CyO (3B-M2)a | 20.0 | 20.0 | 25 |
| Pkn-53E/CyO (32-L2)a | 31.5 | 9.3 | 54 |
Fifteen-minute heat shock.
There are three possible explanations for these wing phenotypes. First, they could result from overexpression of the Pkn transgenes upon heat shock. To determine whether this was the case, the two independent lines carrying the wild-type Pkn transgene under control of the heat-shock promoter were crossed to w1118 flies. The progeny were heat-shocked from 48 hr after egg laying until eclosion and adults were examined for wing phenotypes. At least 200 adults were scored for each transgene and none of them exhibited any wing defects. This suggests that the observed wing phenotypes are not a consequence of transgene overexpression. A second possible explanation is that the wing phenotype is a dominant phenotype caused by the Pkn mutation or a mutation in another gene carried on the same chromosome, which may go undetected in most crosses because the chromosomes were balanced over a CyO balancer. To test this possibility, PknP/CyO flies were crossed to w1118 flies and the wings of PknP/+ progeny were examined. None of the 200 flies scored showed any wing phenotypes, suggesting that the wing phenotypes observed in the rescued flies are not the result of a dominant mutation on the PknP chromosome (Figure 4). This is consistent with the fact that the penetrance of the phenotypes varies from one transgenic line to another. Thus, the most likely explanation for the observed wing phenotypes is that Pkn plays a role in normal wing morphogenesis and vein development and that this function for Pkn cannot be fully rescued in the Pkn mutant background by heat-shock-induced expression of a Pkn transgene. The observed wing phenotypes reveal the only specific developmental requirement, other than dorsal closure, that we have been able to identify for Pkn thus far.
DISCUSSION
The PKN family kinases are established biochemical effectors of Rho/Rac GTPase signaling; however, their functional role in the context of cellular and developmental biology is poorly understood. To begin to model the biological function of PKN in vivo, we had previously cloned the single PKN ortholog in Drosophila, Pkn, and generated a loss-of-function mutant (Lu and Settleman 1999). The Pkn gene is essential for normal development, and homozygous mutant animals exhibit specific defects in dorsal closure, reflecting an apparent requirement for Pkn in tissue morphogenesis during gastrulation.
In the studies described here, we have utilized this experimental system to begin to elucidate the structure-function relationship of Pkn in the context of Drosophila larval and pupal development. Our observations lead to the following conclusions: (1) Rac GTPase binding is completely dispensable for the developmental function of Pkn; (2) Rho GTPase binding to Pkn appears to be important for the developmental function of Pkn; (3) the kinase function of Pkn is essential for its role in development; (4) the C2-like domain of Pkn is not absolutely required for its role in development; (5) the PKC53E catalytic kinase domain can functionally substitute for the Pkn kinase domain during development; and (6) Pkn is required for normal wing morphogenesis.
The structural organization of domains within the PKN proteins suggests that these proteins might simply function as Rho/Rac-binding PKC-like kinases. In such a scenario, the interaction of PKNs with activated Rho and Rac GTPases might serve to localize their PKC-like kinase activity to a particular membrane region. This would be analogous to the documented roles of other small GTPases with their kinase effectors. For example, the activated Ras GTPase recruits the Raf kinase to the plasma membrane, and the oncogenic function of Raf can be realized by adding the C-terminal “CAAX” domain from Ras to the Raf protein (Leevers et al. 1994). However, our findings suggest a more complex picture for the regulation of PKN activity in vivo. For example, the Rok-Pkn or Rhot-Pkn chimeras, which can efficiently bind the activated Rho GTPase, are unable to rescue the lethality associated with disruption of Pkn, suggesting that the Pkn amino terminus and ACC domains provide an essential function beyond Rho binding, although it is also possible that these chimeras were not correctly folded or properly localized in the cell. Notably, the fact that Rac binding is dispensable for Pkn's developmental function does not necessarily rule out the possibility that this interaction is required for a very subtle aspect of development that was not detected or for a physiological role of Rac-Pkn binding in the adult fly.
The cellular functions of PKN proteins may be controlled, in part, through regulation of their subcellular localization. Endogenous mammalian PKN has been detected in the cytoplasm and is associated with membranes, but in response to stresses such as heat shock, PKN translocates to the nucleus (Mukai et al. 1996a). In contrast, in response to hyperosmotic stress, PKN translocates to a vesicular compartment (Torbett et al. 2003). Similarly, overexpressed PKN has also been detected in endosomes (Mellor et al. 1998). In keratinocytes that lack cell–cell adhesions, the PKN-related PRK2 kinase is diffusely distributed in the cytoplasm and membrane; however, when cell–cell adhesion is stimulated, PRK2 is recruited to the plasma membrane (Bourguignon et al. 2004). Thus, the regulation of PKN subcellular localization may be critical for its biological activity, and this appears to be determined by more than simply the ability to bind activated Rho GTPases. Consistent with this, our analysis of the subcellular localization of the various constructs used for rescue in COS cells suggests that there is some correlation between localization and ability to rescue. For example, ΔACC123, ΔC2, and the Pak-, Rok-, and Rhotekin-Pkn chimeras all localize to vesicles in a subset of expressing cells (supplemental Figure 2 at http://www.genetics.org/supplemental/). Some other mutants exhibit a partial nuclear localization, which is not observed with full-length, wild-type Pkn.
The Drosophila Pkn kinase domain is most closely related to that of the PKC family kinases, PKC53E and PKC98E, whose function in Drosophila has yet to be reported. Our observation that the PKC53E kinase domain can functionally substitute for that of Pkn during development suggests that these two kinases largely overlap in their spectrum of potential phosphorylation substrates in vivo. Interestingly, however, our finding that expressing the isolated catalytic domains of each of these kinases leads to a very distinct set of phenotypes in the developing wing, suggests that additional regulatory domains of these proteins play an important role in establishing the functional context of their activities. This also highlights how the evolutionary “strategy” of combining functional domains can produce functionally distinct proteins that would otherwise appear to be highly related on the basis of shared motifs. We note that the observed differences between PKC53E and Pkn do not appear to reflect differences in their expression levels since multiple different transgenic lines of each, with varying levels of expression, were associated with distinct phenotypes.
With regard to the Pkn-PKC53 chimera that we generated, it is possible that the Pkn amino terminus localizes the PKC53E kinase domain to a particular subcellular region where a subset of biologically relevant “PKC substrates” resides. Thus, these results may reflect “compartmentalized signaling,” a theme that is emerging within the signal transduction literature (Mor and Philips 2006). Notably, another closely related PKC-like kinase, PKC98E, is unable to rescue Pkn-associated developmental defects, when expressed as a chimera with the amino terminus of Pkn. This might reflect a small but important difference in the substrate specificity of the various PKC-like kinases. Similarly, the diminished efficiency with which the Pkn-PKC53E chimera rescues Pkn-associated lethality (relative to wild-type Pkn) could also reflect a suboptimal interaction between a critical Pkn phosphorylation substrate and the PKC53E kinase domain. However, it is also possible that such chimeras are not optimally folded and consequently exhibit reduced biological activity.
The wing defects observed in a large fraction of the rescued Pkn mutant flies indicate an essential role for Pkn in wing morphogenesis. This is the first specific role identified for Pkn in postembryonic fly development and is consistent with several reports indicating a requirement for Rho GTPase signaling in several aspects of wing morphogenesis (Bayer et al. 2003; Chen et al. 2005; Denholm et al. 2005). Notably, several additional Rho pathway effectors have been implicated in wing morphogenesis, including Rho-kinase, lim-kinase, and diaphanous (Winter et al. 2001; Chen et al. 2004; Verdier et al. 2006). The fact that a heat-shock protocol for expressing transgenic Pkn in these studies fails to rescue this particular developmental phenotype might reflect a requirement for especially high levels of expression in a subset of developing wing cells. A substantial proportion of flies rescued with the ACC2 and ACC12 domain-deleted transgenes exhibited vein alterations, suggesting the ACC2 domain, which is entirely dispensable for development, may play a specific role in wing morphogenesis and that Rho binding may be important for this process. In contrast, rescue with the constructs that show perturbed Rac binding (the G58A mutant and ΔACC1 deletion) did not produce enhanced wing vein phenotypes in comparison to rescue with wild-type Pkn, suggesting that Rac binding is not involved in wing morphogenesis. Most strikingly, the vast majority of flies rescued with the C2 domain-deleted Pkn mutant exhibited vein defects. This was true for four independently generated transgenic lines and suggests that this domain, which does not appear to be absolutely required for Drosophila development, may provide a critical regulatory component for Pkn function during wing morphogenesis.
In summary, we have used a transgene-rescue strategy to begin to dissect the functional domains of the multidomain Rho effector kinase, Pkn, in the context of its biological role in development. These studies have revealed several new aspects of Pkn regulation and function that could potentially be extended to the mammalian PKNs, which share a closely related arrangement of structural domains. They also exemplify the evolutionary strategy of combining functional domains to produce proteins with distinct biological activities.
Acknowledgments
We gratefully acknowledge Douglas Rennie and the CBRC Fly Core at Massachusetts General Hospital for injection of Drosophila embryos to generate transgenic lines. We thank members of the Settleman and Bernards labs for helpful discussions, Anabel Herr and Wei Jiang for critical reading of the manuscript, and James Walker for comments on the manuscript and for valuable advice during the course of the studies. M.B. was supported by a Department of Defense Prostate Cancer Postdoctoral Traineeship Award. This work was supported by National Institutes of Health grant RO1-CA62142 to J.S.
References
- Amano, M., H. Mukai, Y. Ono, K. Chihara, T. Matsui et al., 1996. Identification of a putative target for Rho as the serine-threonine kinase protein kinase N. Science 271: 648–650. [DOI] [PubMed] [Google Scholar]
- Bayer, C. A., S. R. Halsell, J. W. Fristrom, D. P. Kiehart and L. von Kalm, 2003. Genetic interactions between the RhoA and Stubble-stubbloid loci suggest a role for a type II transmembrane serine protease in intracellular signaling during Drosophila imaginal disc morphogenesis. Genetics 165: 1417–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop, A. L., and A. Hall, 2000. Rho GTPases and their effector proteins. Biochem. J. 348(2): 241–255. [PMC free article] [PubMed] [Google Scholar]
- Bourguignon, L. Y., P. A. Singleton and F. Diedrich, 2004. Hyaluronan-CD44 interaction with Rac1-dependent protein kinase N-gamma promotes phospholipase Cgamma1 activation, Ca(2+) signaling, and cortactin-cytoskeleton function leading to keratinocyte adhesion and differentiation. J. Biol. Chem. 279: 29654–29669. [DOI] [PubMed] [Google Scholar]
- Chen, G. C., P. Gajowniczek and J. Settleman, 2004. Rho-LIM kinase signaling regulates ecdysone-induced gene expression and morphogenesis during Drosophila metamorphosis. Curr. Biol. 14: 309–313. [DOI] [PubMed] [Google Scholar]
- Chen, G. C., B. Turano, P. J. Ruest, M. Hagel, J. Settleman et al., 2005. Regulation of Rho and Rac signaling to the actin cytoskeleton by paxillin during Drosophila development. Mol. Cell. Biol. 25: 979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone, G., A. Baldi, E. Palescandolo, L. Manente, R. Penta et al., 2006. Pkn is a novel partner of cyclin T2a in muscle differentiation. J. Cell Physiol. 207: 232–237. [DOI] [PubMed] [Google Scholar]
- Cryns, V. L., Y. Byun, A. Rana, H. Mellor, K. D. Lustig et al., 1997. Specific proteolysis of the kinase protein kinase C-related kinase 2 by caspase-3 during apoptosis. Identification by a novel, small pool expression cloning strategy. J. Biol. Chem. 272: 29449–29453. [DOI] [PubMed] [Google Scholar]
- Darenfed, H., B. Dayanandan, T. Zhang, S. H. Hsieh, A. E. Fournier et al., 2007. Molecular characterization of the effects of Y-27632. Cell Motil. Cytoskeleton 64: 97–109. [DOI] [PubMed] [Google Scholar]
- Deaton, R. A., C. Su, T. G. Valencia and S. R. Grant, 2005. Transforming growth factor-beta1-induced expression of smooth muscle marker genes involves activation of PKN and p38 MAPK. J. Biol. Chem. 280: 31172–31181. [DOI] [PubMed] [Google Scholar]
- Denholm, B., S. Brown, R. P. Ray, M. Ruiz-Gomez, H. Skaer et al., 2005. Crossveinless-c is a RhoGAP required for actin reorganisation during morphogenesis. Development 132: 2389–2400. [DOI] [PubMed] [Google Scholar]
- Dong, L. Q., L. R. Landa, M. J. Wick, L. Zhu, H. Mukai et al., 2000. Phosphorylation of protein kinase N by phosphoinositide-dependent protein kinase-1 mediates insulin signals to the actin cytoskeleton. Proc. Natl. Acad. Sci. USA 97: 5089–5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn, P., H. Mellor, R. Palmer, G. Panayotou and P. J. Parker, 1998. Multiple interactions of PRK1 with RhoA. Functional assignment of the Hr1 repeat motif. J. Biol. Chem. 273: 2698–2705. [DOI] [PubMed] [Google Scholar]
- Flynn, P., H. Mellor, A. Casamassima and P. J. Parker, 2000. Rho GTPase control of protein kinase C-related protein kinase activation by 3-phosphoinositide-dependent protein kinase. J. Biol. Chem. 275: 11064–11070. [DOI] [PubMed] [Google Scholar]
- Gudi, T., J. C. Chen, D. E. Casteel, T. M. Seasholtz, G. R. Boss et al., 2002. cGMP-dependent protein kinase inhibits serum-response element-dependent transcription by inhibiting rho activation and functions. J. Biol. Chem. 277: 37382–37393. [DOI] [PubMed] [Google Scholar]
- Herold, M., M. Cikala, H. MacWilliams, C. N. David and A. Bottger, 2002. Cloning and characterisation of PKB and PRK homologs from Hydra and the evolution of the protein kinase family. Dev. Genes Evol. 212: 513–519. [DOI] [PubMed] [Google Scholar]
- Isagawa, T., M. Takahashi, T. Kato, Jr., H. Mukai and Y. Ono, 2005. Involvement of protein kinase PKN1 in G2/M delay caused by arsenite. Mol. Carcinogen. 43: 1–12. [DOI] [PubMed] [Google Scholar]
- Kitagawa, M., H. Shibata, M. Toshimori, H. Mukai and Y. Ono, 1996. The role of the unique motifs in the amino-terminal region of PKN on its enzymatic activity. Biochem. Biophys. Res. Commun. 220: 963–968. [DOI] [PubMed] [Google Scholar]
- Koh, H., K. H. Lee, D. Kim, S. Kim, J. W. Kim et al., 2000. Inhibition of Akt and its anti-apoptotic activities by tumor necrosis factor-induced protein kinase C-related kinase 2 (PRK2) cleavage. J. Biol. Chem. 275: 34451–34458. [DOI] [PubMed] [Google Scholar]
- Leevers, S. J., H. F. Paterson and C. J. Marshall, 1994. Requirement for Ras in Raf activation is overcome by targeting Raf to the plasma membrane. Nature 369: 411–414. [DOI] [PubMed] [Google Scholar]
- Lim, M. A., L. Yang, Y. Zheng, H. Wu, L. Q. Dong et al., 2004. Roles of PDK-1 and PKN in regulating cell migration and cortical actin formation of PTEN-knockout cells. Oncogene 23: 9348–9358. [DOI] [PubMed] [Google Scholar]
- Lu, Y., and J. Settleman, 1999. The Drosophila Pkn protein kinase is a Rho/Rac effector target required for dorsal closure during embryogenesis. Genes Dev.. 13: 1168–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maesaki, R., K. Ihara, T. Shimizu, S. Kuroda, K. Kaibuchi et al., 1999. The structural basis of Rho effector recognition revealed by the crystal structure of human RhoA complexed with the effector domain of PKN/PRK1. Mol. Cell 4: 793–803. [DOI] [PubMed] [Google Scholar]
- Marinissen, M. J., M. Chiariello and J. S. Gutkind, 2001. Regulation of gene expression by the small GTPase Rho through the ERK6 (p38 gamma) MAP kinase pathway. Genes Dev. 15: 535–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa, K., H. Kosako, N. Inagaki, H. Shibata, H. Mukai et al., 1997. Domain-specific phosphorylation of vimentin and glial fibrillary acidic protein by PKN. Biochem. Biophys. Res. Commun. 234: 621–625. [DOI] [PubMed] [Google Scholar]
- Mellor, H., P. Flynn, C. D. Nobes, A. Hall and P. J. Parker, 1998. PRK1 is targeted to endosomes by the small GTPase, RhoB. J. Biol. Chem. 273: 4811–4814. [DOI] [PubMed] [Google Scholar]
- Metzger, E., J. M. Muller, S. Ferrari, R. Buettner and R. Schule, 2003. A novel inducible transactivation domain in the androgen receptor: implications for PRK in prostate cancer. EMBO J. 22: 270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misaki, K., H. Mukai, C. Yoshinaga, K. Oishi, T. Isagawa et al., 2001. PKN delays mitotic timing by inhibition of Cdc25C: possible involvement of PKN in the regulation of cell division. Proc. Natl. Acad. Sci. USA 98: 125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor, A., and M. R. Philips, 2006. Compartmentalized Ras/MAPK signaling. Annu. Rev. Immunol. 24: 771–800. [DOI] [PubMed] [Google Scholar]
- Mukai, H., 2003. The structure and function of PKN, a protein kinase having a catalytic domain homologous to that of PKC. J. Biochem. 133: 17–27. [DOI] [PubMed] [Google Scholar]
- Mukai, H., and Y. Ono, 1994. A novel protein kinase with leucine zipper-like sequences: its catalytic domain is highly homologous to that of protein kinase C. Biochem. Biophys. Res. Commun. 199: 897–904. [DOI] [PubMed] [Google Scholar]
- Mukai, H., M. Kitagawa, H. Shibata, H. Takanaga, K. Mori et al., 1994. Activation of PKN, a novel 120-kDa protein kinase with leucine zipper-like sequences, by unsaturated fatty acids and by limited proteolysis. Biochem. Biophys. Res. Commun. 204: 348–356. [DOI] [PubMed] [Google Scholar]
- Mukai, H., K. Mori, H. Takanaga, M. Kitagawa, H. Shibata et al., 1995. Xenopus PKN: cloning and sequencing of the cDNA and identification of conserved domains. Biochim. Biophys. Acta 1261: 296–300. [DOI] [PubMed] [Google Scholar]
- Mukai, H., M. Miyahara, H. Sunakawa, H. Shibata, M. Toshimori et al., 1996. a Translocation of PKN from the cytosol to the nucleus induced by stresses. Proc. Natl. Acad. Sci. USA 93: 10195–10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai, H., M. Toshimori, H. Shibata, M. Kitagawa, M. Shimakawa et al., 1996. b PKN associates and phosphorylates the head-rod domain of neurofilament protein. J. Biol. Chem. 271: 9816–9822. [DOI] [PubMed] [Google Scholar]
- Mukai, H., M. Toshimori, H. Shibata, H. Takanaga, M. Kitagawa et al., 1997. Interaction of PKN with alpha-actinin. J. Biol. Chem. 272: 4740–4746. [DOI] [PubMed] [Google Scholar]
- Oishi, K., H. Mukai, H. Shibata, M. Takahashi and Y. Ona, 1999. Identification and characterization of PKNbeta, a novel isoform of protein kinase PKN: expression and arachidonic acid dependency are different from those of PKNalpha. Biochem. Biophys. Res. Commun. 261: 808–814. [DOI] [PubMed] [Google Scholar]
- Oishi, K., M. Takahashi, H. Mukai, Y. Banno, S. Nakashima et al., 2001. PKN regulates phospholipase D1 through direct interaction. J. Biol. Chem. 276: 18096–18101. [DOI] [PubMed] [Google Scholar]
- Palmer, R. H., J. Ridden and P. J. Parker, 1995. Cloning and expression patterns of two members of a novel protein-kinase-C-related kinase family. Eur. J. Biochem. 227: 344–351. [DOI] [PubMed] [Google Scholar]
- Peng, B., N. A. Morrice, L. C. Groenen and R. E. Wettenhall, 1996. Phosphorylation events associated with different states of activation of a hepatic cardiolipin/protease-activated protein kinase. Structural identity to the protein kinase N-type protein kinases. J. Biol. Chem. 271: 32233–32240. [DOI] [PubMed] [Google Scholar]
- Quilliam, L. A., Q. T. Lambert, L. A. Mickelson-Young, J. K. Westwick, A. B. Sparks et al., 1996. Isolation of a NCK-associated kinase, PRK2, an SH3-binding protein and potential effector of Rho protein signaling. J. Biol. Chem. 271: 28772–28776. [DOI] [PubMed] [Google Scholar]
- Ren, X. D., and M. A. Schwartz, 2000. Determination of GTP loading on Rho. Methods Enzymol. 325: 264–272. [DOI] [PubMed] [Google Scholar]
- Schmidt, A., J. Durgan, A. Magalhaes and A. Hall, 2007. Rho GTPases regulate PRK2/PKN2 to control entry into mitosis and exit from cytokinesis. EMBO J. 26: 1624–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata, H., H. Oda, H. Mukai, K. Oishi, K. Misaki et al., 1999. Interaction of PKN with a neuron-specific basic helix-loop-helix transcription factor, NDRF/NeuroD2. Brain Res. Mol. Brain Res. 74: 126–134. [DOI] [PubMed] [Google Scholar]
- Shibata, H., K. Oishi, A. Yamagiwa, M. Matsumoto, H. Mukai et al., 2001. PKNbeta interacts with the SH3 domains of Graf and a novel Graf related protein, Graf2, which are GTPase activating proteins for Rho family. J. Biochem. 130: 23–31. [DOI] [PubMed] [Google Scholar]
- Shieh, B. H., L. Parker and D. Popescu, 2002. Protein kinase C (PKC) isoforms in Drosophila. J. Biochem. 132: 523–527. [DOI] [PubMed] [Google Scholar]
- Stapleton, G., C. P. Nguyen, K. A. Lease and M. B. Hille, 1998. Phosphorylation of protein kinase C-related kinase PRK2 during meiotic maturation of starfish oocytes. Dev. Biol. 193: 36–46. [DOI] [PubMed] [Google Scholar]
- Strutt, D. I., U. Weber and M. Mlodzik, 1997. The role of RhoA in tissue polarity and Frizzled signalling. Nature 387: 292–295. [DOI] [PubMed] [Google Scholar]
- Su, C., R. A. Deaton, M. A. Iglewsky, T. G. Valencia and S. R. Grant, 2007. PKN activation via transforming growth factor-beta1 (TGF-beta1) receptor signaling delays G2/M phase transition in vascular smooth muscle cells. Cell Cycle 6: 739–749. [DOI] [PubMed] [Google Scholar]
- Sun, W., S. Vincent, J. Settleman and G. L. Johnson, 2000. MEK kinase 2 binds and activates protein kinase C-related kinase 2. Bifurcation of kinase regulatory pathways at the level of an MAPK kinase kinase. J. Biol. Chem. 275: 24421–24428. [DOI] [PubMed] [Google Scholar]
- Takahashi, M., Y. Gotoh, T. Isagawa, T. Nishimura, E. Goyama et al., 2003. Regulation of a mitogen-activated protein kinase kinase kinase, MLTK by PKN. J. Biochem. 133: 181–187. [DOI] [PubMed] [Google Scholar]
- Takanaga, H., H. Mukai, H. Shibata, M. Toshimori and Y. Ono, 1998. PKN interacts with a paraneoplastic cerebellar degeneration-associated antigen, which is a potential transcription factor. Exp. Cell Res. 241: 363–372. [DOI] [PubMed] [Google Scholar]
- Taniguchi, T., T. Kawamata, H. Mukai, H. Hasegawa, T. Isagawa et al., 2001. Phosphorylation of tau is regulated by PKN. J. Biol. Chem. 276: 10025–10031. [DOI] [PubMed] [Google Scholar]
- Torbett, N. E., A. Casamassima and P. J. Parker, 2003. Hyperosmotic-induced protein kinase N 1 activation in a vesicular compartment is dependent upon Rac1 and 3-phosphoinositide-dependent kinase 1. J. Biol. Chem. 278: 32344–32351. [DOI] [PubMed] [Google Scholar]
- Ueno, N., I. Oishi, S. Sugiyama, Y. Nishida, Y. Minami et al., 1997. Identification of a novel Drosophila protein kinase highly homologous to protein kinase N (PKN). Biochem. Biophys. Res. Commun. 232: 126–129. [DOI] [PubMed] [Google Scholar]
- Verdier, V., G. C. Chen and J. Settleman, 2006. Rho-kinase regulates tissue morphogenesis via non-muscle myosin and LIM-kinase during Drosophila development. BMC Dev. Biol. 6: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent, S., and J. Settleman, 1997. The PRK2 kinase is a potential effector target of both Rho and Rac GTPases and regulates actin cytoskeletal organization. Mol. Cell. Biol. 17: 2247–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, G., Y. Saito, P. Madaule, T. Ishizaki, K. Fujisawa et al., 1996. Protein kinase N (PKN) and PKN-related protein rhophilin as targets of small GTPase Rho. Science 271: 645–648. [DOI] [PubMed] [Google Scholar]
- Winter, C. G., B. Wang, A. Ballew, A. Royou, R. Karess et al., 2001. Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell 105: 81–91. [DOI] [PubMed] [Google Scholar]
- Yoshinaga, C., H. Mukai, M. Toshimori, M. Miyamoto and Y. Ono, 1999. Mutational analysis of the regulatory mechanism of PKN: the regulatory region of PKN contains an arachidonic acid-sensitive autoinhibitory domain. J. Biochem. 126: 475–484. [DOI] [PubMed] [Google Scholar]
- Zhu, Y., D. B. Stolz, F. Guo, M. A. Ross, S. C. Watkins et al., 2004. Signaling via a novel integral plasma membrane pool of a serine/threonine protein kinase PRK1 in mammalian cells. FASEB J. 18: 1722–1724. [DOI] [PubMed] [Google Scholar]