Abstract
Cell-specific promoters allow only spatial control of transgene expression in Caenorhabditis elegans. We describe a method, using cell-specific rescue of heat-shock factor-1 (hsf-1) mutants, that allows spatial and temporal regulation of transgene expression. We demonstrate the utility of this method for timed reporter gene expression and for temporal studies of gene function.
THE ability to manipulate transgene expression in animals has facilitated the study of many biological processes. While global expression of transgenes can be used as an effective tool for studying the consequences of gene activation or inactivation, in many instances it is desirable to control both spatial and temporal aspects of transgene expression. For example, restriction of transgene expression to a specific cell type can allow determination of whether gene function is cell autonomous or nonautonomous. Likewise, temporal control of transgene expression is critical for determining whether the function of a gene of interest is required continuously or only during specific times.
Several strategies that allow control of both where and when transgenes are expressed have been developed. Spatial resolution is often achieved by the use of tissue- or cell-specific promoters, and timing of transgene expression can be controlled by introduction, or withdrawal, of small-molecule inducers, or inhibitors, of gene expression. For example, a fusion protein consisting of the tetracycline repressor fused to the transcription activation domain of VP16 (TetR-VP16), and expressed using a tissue-specific promoter, can promote tissue-specific expression of genes bearing tetO operator sequences. Addition of the cell-permeable ligand tetracycline, which binds to and prevents TetR-VP16 from binding tetO (Gossen and Bujard 1992; Gossen et al. 1995), can be used to extinguish expression at specific times. Similarly, Cre recombinase fused to the estrogen receptor, and expressed in specific tissues, can be temporally activated by the addition of tamoxifen (Feil et al. 1996).
An alternative to using small molecules for temporal control is afforded by proteins whose functions are regulated by temperature. For example, in Drosophila, the GAL4/UAS system has been used to control cell-type-specific transgene expression (Brand and Perrimon 1993). McGuire et al. (2003) modified this system to achieve temporal control of GAL4-driven expression by introducing a temperature-sensitive form of GAL80, a GAL4 inhibitor, into transgenic flies. At permissive temperatures, animals fail to express the transgene because GAL80 inhibits GAL4. Shifting to nonpermissive temperatures relieves GAL80 inhibition and allows transgene expression to proceed.
Another strategy for temporal control of transgene expression exploits the heat-shock response, a temperature-dependent stress defense mechanism. The heat-shock response is mediated by heat-shock factor (HSF), a transcription factor that is synthesized constitutively, but remains latent during unstressed conditions (Lis and Wu 1993). In response to heat stress, HSF trimerizes and binds with high affinity to promoters containing specific binding elements, leading to the transcription of heat-shock proteins (Pelham 1982; Westwood et al. 1991). Thus, transgenes containing HSF-binding elements can be induced, albeit with little cellular specificity, following a temperature shift. Attempts to lend spatial resolution to the heat-shock response by using heated needles (Monsma et al. 1988; Vekris et al. 2000) or focused laser microbeams (Stringham and Candido 1993; Halfon et al. 1997) to trigger the response in specific cells have been described. However, these physical methods for spatially restricted heat-shock delivery are labor intensive and potentially damaging to cells, explaining, in part, why they are not in common use.
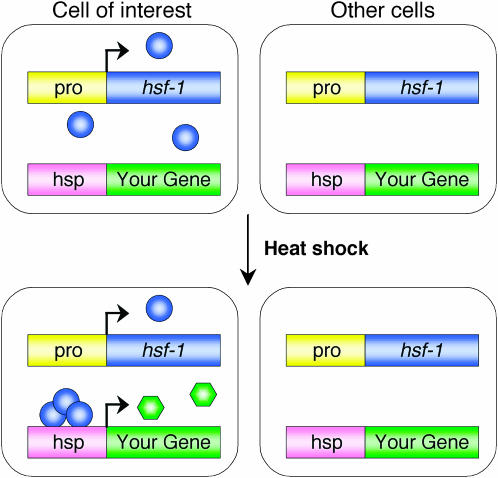
Currently, no facile approach is available for combined spatial and temporal regulation of transgene expression in Caenorhabditis elegans. To develop such a method, we reasoned that cell-specific rescue of mutants defective in the heat-shock response should allow expression of transgenes, following a heat shock, only in rescued cells. The C. elegans genome contains a single gene encoding the heat-shock factor hsf-1. An allele of hsf-1, sy441, was previously isolated in a screen for suppressors of an activated Gα protein expressed under the control of a heat-shock responsive promoter (Hajdu-Cronin et al. 2004). hsf-1(sy441) animals display decreased life span, egg-laying defects, and larval arrest at 25°; however, apart from the presence of a few thin, opaque larvae, they are otherwise healthy at 20° (Hajdu-Cronin et al. 2004). Importantly, however, following a brief heat shock at 34°, hsf-1(sy441) animals show a 100-fold reduction in expression of endogenous heat-shock protein mRNAs compared to wild-type animals (Hajdu-Cronin et al. 2004). Thus, cell-specific expression of hsf-1 in hsf-1(sy441) mutants should provide a normal heat-shock response only in targeted cells (Figure 1).
Figure 1.—
Temporal control of cell-specific transgene expression. In an animal lacking the normal heat-shock response due to loss of HSF-1 activity, hsf-1 is introduced in a desired subset of cells using a cell-specific promoter (pro). Since HSF-1 is present only in targeted cells, heat shock results in cell-specific transcription from heat-shock-responsive promoters (hsp). Therefore, any transgene under the control of a heat-shock promoter will be selectively expressed in the desired cells following a heat shock. Blue circles, HSF-1 protein. Green hexagons, protein of interest.
To test this idea, we sought to develop transgenic strains in which we could induce green fluorescent protein (GFP) expression in specific cells following a heat shock. To this end, we transformed hsf-1(sy441) mutants with two transgenes simultaneously. The first transgene, referred to as the driver, consisted of the hsf-1 cDNA under the control of the 5-kb promoter region of the gene vap-1, which is expressed specifically within the amphid sheath cell, an easily identifiable glial cell in the head of the animal (Perens and Shaham 2005). The second transgene, termed the responder, consisted of the gene encoding GFP under the control of the 400-bp promoter region of the hsp-16.2 gene (Jones et al. 1986; Fire et al. 1990). In C. elegans, the hsp-16.2 and hsp-16.41 heat-shock responsive genes are divergently transcribed from a shared 346-bp region that contains three heat-shock-factor binding elements (Jones et al. 1986). The hsp-16.2 promoter promotes expression most strongly in hypodermal cells and neurons, while the hsp-16.41 promoter is more efficient in directing expression in the intestine and pharyngeal tissue (Fire et al. 1990; Stringham et al. 1992).
hsf-1(sy441) animals carrying both transgenes as an extrachromosomal array, and raised at 20°, did not display detectable GFP expression. However, following administration of a heat shock at 34° for 30 min, GFP expression was observed specifically within the amphid sheath cells (Figure 2, A and B). GFP fluorescence was visible within 1 hr following the temperature shift and was still evident after 24 hr.
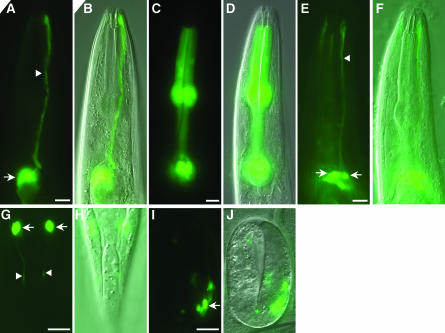
Figure 2.—
Spatiotemporal control of GFP expression using a heat-shock promoter. (A) Amphid sheath cell-specific labeling. Arrow, sheath cell body. Arrowhead, sheath cell process. Sheath cell-specific hsf-1 rescue was achieved by driving hsf-1 cDNA expression using a 5-kb promoter region of vap-1. GFP was under the control of the hsp-16.2 promoter. Transgenic animals with the genotype hsf-1(sy441); nsEx992 [Pvap-1hsf-1; Phsp-16.2GFP; pRF4] were obtained by germline injection. The plasmid pRF4 encodes rol-6(su1006), a dominant marker used for selection of transgenic animals (Mello et al. 1991). Animals were incubated for 30 min at 34° and allowed to recover at 20° for 1 hr before imaging; an adult animal is shown. (B) DIC image of the animal in A. (C) Pharynx GFP expression. An adult animal expressing GFP in pharyngeal muscles after a 30-min heat shock. The genotype of the animal shown is hsf-1(sy441); nsEx1730 [Pmyo-2hsf-1; Phsp-16.2GFP; Phsp-16.41GFP; pRF4]. (D) DIC image of the animal in C. (E) Ciliated neuron-specific GFP expression following heat shock. Animals in E–J have the genotype hsf-1(sy441); nsEx1129 [Posm-6hsf-1; Phsp-16.2GFP; pRF4]. In the animal in E, labeling is seen in four amphid neurons. (F) DIC image of the animal in E. (G) Heat-shock-induced GFP expression in ciliated neurons of the phasmid, a sensory organ in the tail, in an adult animal. (H) DIC image of the animal in G. (I) Neuronal GFP labeling in a twofold embryo. (J) DIC image of the animal in I. In all cases, anterior is at the top. Bar, 10 μm.
To determine whether this method is applicable to other C. elegans cells, we established new hsf-1(sy441) transgenic lines in which the HSF driver transgene was under the control of the 1.1-kb promoter region of myo-2, a gene expressed in pharyngeal muscle (Okkema et al. 1993). These lines also contained both the hsp-16.2 promoter∷GFP and the hsp-16.41 promoter∷GFP responder transgenes. Following heat shock, GFP expression was observed only in the pharynx (Figure 2, C and D). We found that GFP expression was fainter in lines containing only the hsp-16.2 promoter∷GFP responder transgene (data not shown), consistent with the reported poorer expression of hsp-16.2 in pharyngeal muscles.
To extend this method to neurons, hsf-1(sy441) transgenic lines in which the HSF driver transgene was under the control of the 2.4-kb promoter region of osm-6, a gene expressed in all ciliated neurons (Collet et al. 1998), were established. Whereas no GFP was detectable at 20°, heat shock resulted in specific GFP expression within all ciliated neuron classes (Figure 2, E–J). Importantly, all developmental stages of hsf-1(sy441) animals could tolerate at least 60 min of 34° heat shock, and neuronal staining was observed in larvae and embryos alike (Figure 2, I and J), suggesting that this method of cell-specific timed expression can be used in most developmental stages. Similar results were obtained when the hsp-16.2 promoter∷GFP and hsp-16.41 promoter∷GFP responder transgenes were cotransformed (data not shown). However, in some of the animals in which both heat-shock promoters were used, GFP was also variably expressed in the intestine. Intestinal expression was also occasionally observed with the hsp-16.2 promoter alone. We suspect that this expression might reflect cryptic regulatory elements driving intestinal expression within the vectors that we used. Indeed, such intestinal misexpression is common to several transcriptional reporter transgenes that we have generated in the laboratory (data not shown).
To assess whether our method could be used to study gene function, we sought to rescue a mutant phenotype in a temporally and spatially restricted manner. CHE-2, a conserved WD40 repeat protein, is required for sensory neuron cilium formation and function in C. elegans (Fujiwara et al. 1999). In che-2(e1033) mutants, neurons of the amphid sensory organ fail to take up lipophilic dyes, such as 1,1-dioctadecyl-3,3,3,3-tetramethylindocarbocyanine perchlorate (DiI), presumably because they lack functional cilia. We examined whether che-2(e1033) mutants could be rescued by expression of the che-2 cDNA exclusively within ciliated neurons in L4 larvae. Amphid neurons of hsf-1(sy441); che-2(e1033) double mutants, carrying both osm-6 promoter∷hsf-1 driver and hsp-16.2 promoter∷che-2 responder transgenes, were unable to take up DiI at 20° (Table 1). However, transgenic animals exposed to a 30-min heat shock at 34° and examined 8 hr later displayed robust neuronal dye filling (Table 1; Figure 3). These results suggest that che-2 may be continuously required for sensory cilia function, consistent with previous studies (Fujiwara et al. 1999). Less efficient, but reproducible rescue was observed when animals were examined 1 hr after heat shock (data not shown). These results suggest that the time required to assemble a functional cilium from its components may be as little as 1 hr (see also Fujiwara et al. 1999). No dye filling after heat shock was observed in animals carrying only the hsp-16.2 promoter∷che-2 responder transgene (six lines examined, n > 100 for each line), consistent with a lack of the heat-shock response in hsf-1(sy441) mutants.
TABLE 1.
Neuron-specific rescue of che-2(e1033) mutants
| % dye fillingb
|
|||
|---|---|---|---|
| Promoter driving hsf-1 | Straina | −HS | +HS |
| No transgene | 0 | 0 | |
| osm-6 | Line 1 | 0 | 73 |
| Line 2 | 0 | 53 | |
| Line 3 | 0 | 90 | |
| sra-6 | Line 1 | 0 | 83 |
| Line 2 | 0 | 37 | |
| Line 3 | 0 | 53 | |
All animals contained the hsf-1(sy441) and che-2(e1033) alleles. Three independent lines driving hsf-1 cDNA expression from the osm-6 or the sra-6 promoter were tested. The extrachromosomal arrays used were Ex[Posm-6hsf-1; Phsp-16.2che-2; pRF4(rol-6)] and Ex[Psra-6hsf-1; Phsp-16.2che-2; pRF4(rol-6)].
Heat-shocked animals were dye filled 8 hr after heat shock by soaking for 20 min in 5 μg/ml DiI. Adult or L4 animals exhibiting dye filling in any neuron of either the left or the right amphid were scored as positive. n = 30 in all cases. HS, heat-shock.
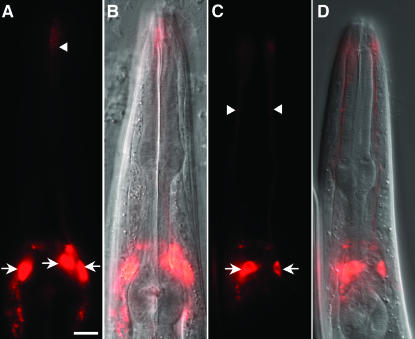
Figure 3.—
Cell-specific rescue of che-2(e1033) neuronal dye-filling defects. (A) Dye filling in an L4 animal of the genotype hsf-1(sy441); che-2(e1033); nsEx1555 [Posm-6hsf-1; Phsp-16.2che-2; pRF4] after a 30-min heat shock. Dye filling was performed 8 hr after heat shock by soaking for 20 min in 5 μg/ml DiI. Rescue was seen in all dye-filling amphid neurons. (B) DIC image of the animal in A. (C) Same as A, except that dye filling is restricted to neurons expressing sra-6. The genotype of the L4 animal shown is hsf-1(sy441); che-2(e1033); nsEx1552 [Psra-6hsf-1; Phsp-16.2che-2; pRF4]. (D) DIC image of the animal in C. Anterior, top. Arrows, neuronal cell bodies. Arrowheads, dendritic processes. Bar, 10 μm.
To test whether hsf-1 rescue was cell autonomous and whether dye uptake is a cell-autonomous process or a group property of exposed amphid cilia, we examined hsf-1(sy441); che-2(e1033) mutants carrying both sra-6 promoter∷hsf-1 driver and hsp-16.2 promoter∷CHE-2 responder transgenes. The 2.4-kb sra-6 promoter is primarily expressed in the two dye-filling amphid neurons ASI and ASH (Troemel et al. 1995). While transgenic animals grown at 20° failed to take up DiI, dye filling was observed exclusively in the two sra-6-expressing neurons 8 hr after heat shock (Figure 3), suggesting that hsf-1 and che-2 indeed function cell autonomously. A similar conclusion was reached for the cilia gene dyf-6 (Bell et al. 2006). In both osm-6 and sra-6 transgenic lines, rescue was very efficient, reaching nearly 90% in some lines (Table 1). Importantly, we never observed rescue in non-heat-shock-treated animals, indicating that any leaky expression from the hsp promoter is not only below detectable levels of GFP expression but also below detectable levels in this functional assay.
Temporal control of transgene expression in C. elegans has been limited to the use of heat-shock promoters, without spatial restriction, and to reliance on the inherent period of onset of defined cell-specific promoters. Here we have described a method using the heat-shock response to allow both temporal and spatial control of transgene expression. The technique requires that experiments be performed in hsf-1(sy441) mutants, restricting its use to temperatures <25°. However, since the majority of studies in C. elegans are performed at lower temperatures, the method is broadly applicable. A further limitation of the method may stem from the transient nature of the heat-shock response, which does not allow for sustained transgene expression. However, continual expression could be achieved by multiple heat-shock applications and, as we demonstrated above, proteins with similar stability to GFP should persist for at least 24 hr after heat-shock administration. Furthermore, for many applications, transient expression may be sufficient. In particular, this technique might useful for performing cell ablations in a desired larval stage by heat-shock induction of a toxin. In the same vein, adult expression of dominant-negative proteins or cell-specific expression of an RNAi hairpin in a sid-1 background (Winston et al. 2002) could yield tissue-specific knock-down of gene function.
Interestingly, a Drosophila melanogaster heat-shock factor mutant, hsf4, is also viable (Jedlicka et al. 1997). Therefore, our method could be extended to this organism as well.
Acknowledgments
We thank Paul Sternberg for providing us the hsf-1(sy441) animals and members of the Shaham laboratory for helpful comments on the project and manuscript. This work was supported, in part, by a grant from the National Institutes of Health to S.S.
References
- Bell, L. R., S. Stone, J. Yochem, J. E. Shaw and R. K. Herman, 2006. The molecular identities of the Caenorhabditis elegans intraflagellar transport genes dyf-6, daf-10 and osm-1. Genetics 173: 1275–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand, A. H., and N. Perrimon, 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415. [DOI] [PubMed] [Google Scholar]
- Collet, J., C. A. Spike, E. A. Lundquist, J. E. Shaw and R. K. Herman, 1998. Analysis of osm-6, a gene that affects sensory cilium structure and sensory neuron function in Caenorhabditis elegans. Genetics 148: 187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil, R., J. Brocard, B. Mascrez, M. LeMeur, D. Metzger et al., 1996. Ligand-activated site-specific recombination in mice. Proc. Natl. Acad. Sci. USA 93: 10887–10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire, A., S. W. Harrison and D. Dixon, 1990. A modular set of lacZ fusion vectors for studying gene expression in Caenorhabditis elegans. Gene 93: 189–198. [DOI] [PubMed] [Google Scholar]
- Fujiwara, M., T. Ishihara and I. Katsura, 1999. A novel WD40 protein, CHE-2, acts cell-autonomously in the formation of C. elegans sensory cilia. Development 126: 4839–4848. [DOI] [PubMed] [Google Scholar]
- Gossen, M., and H. Bujard, 1992. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. USA 89: 5547–5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen, M., S. Freundlieb, G. Bender, G. Muller, W. Hillen et al., 1995. Transcriptional activation by tetracyclines in mammalian cells. Science 268: 1766–1769. [DOI] [PubMed] [Google Scholar]
- Hajdu-Cronin, Y. M., W. J. Chen and P. W. Sternberg, 2004. The L-type cyclin CYL-1 and the heat-shock-factor HSF-1 are required for heat-shock-induced protein expression in Caenorhabditis elegans. Genetics 168: 1937–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfon, M. S., H. Kose, A. Chiba and H. Keshishian, 1997. Targeted gene expression without a tissue-specific promoter: creating mosaic embryos using laser-induced single-cell heat shock. Proc. Natl. Acad. Sci. USA 94: 6255–6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedlicka, P., M. A. Mortin and C. Wu, 1997. Multiple functions of Drosophila heat shock transcription factor in vivo. EMBO J. 16: 2452–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, D., R. H. Russnak, R. J. Kay and E. P. Candido, 1986. Structure, expression, and evolution of a heat shock gene locus in Caenorhabditis elegans that is flanked by repetitive elements. J. Biol. Chem. 261: 12006–12015. [PubMed] [Google Scholar]
- Lis, J., and C. Wu, 1993. Protein traffic on the heat shock promoter: parking, stalling, and trucking along. Cell 74: 1–4. [DOI] [PubMed] [Google Scholar]
- McGuire, S. E., P. T. Le, A. J. Osborn, K. Matsumoto and R. L. Davis, 2003. Spatiotemporal rescue of memory dysfunction in Drosophila. Science 302: 1765–1768. [DOI] [PubMed] [Google Scholar]
- Mello, C. C., J. M. Kramer, D. Stinchcomb and V. Ambros, 1991. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10: 3959–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsma, S. A., R. Ard, J. T. Lis and M. F. Wolfner, 1988. Localized heat-shock induction in Drosophila melanogaster. J. Exp. Zool. 247: 279–284. [DOI] [PubMed] [Google Scholar]
- Okkema, P. G., S. W. Harrison, V. Plunger, A. Aryana and A. Fire, 1993. Sequence requirements for myosin gene expression and regulation in Caenorhabditis elegans. Genetics 135: 385–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham, H. R., 1982. A regulatory upstream promoter element in the Drosophila hsp 70 heat-shock gene. Cell 30: 517–528. [DOI] [PubMed] [Google Scholar]
- Perens, E. A., and S. Shaham, 2005. C. elegans daf-6 encodes a patched-related protein required for lumen formation. Dev. Cell 8: 893–906. [DOI] [PubMed] [Google Scholar]
- Stringham, E. G., and E. P. Candido, 1993. Targeted single-cell induction of gene products in Caenorhabditis elegans: a new tool for developmental studies. J. Exp. Zool. 266: 227–233. [DOI] [PubMed] [Google Scholar]
- Stringham, E. G., D. K. Dixon, D. Jones and E. P. Candido, 1992. Temporal and spatial expression patterns of the small heat shock (hsp16) genes in transgenic Caenorhabditis elegans. Mol. Biol. Cell 3: 221–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troemel, E. R., J. H. Chou, N. D. Dwyer, H. A. Colbert and C. I. Bargmann, 1995. Divergent seven transmembrane receptors are candidate chemosensory receptors in C. elegans. Cell 83: 207–218. [DOI] [PubMed] [Google Scholar]
- Vekris, A., C. Maurange, C. Moonen, F. Mazurier, H. De Verneuil et al., 2000. Control of transgene expression using local hyperthermia in combination with a heat-sensitive promoter. J. Gene Med. 2: 89–96. [DOI] [PubMed] [Google Scholar]
- Westwood, J. T., J. Clos and C. Wu, 1991. Stress-induced oligomerization and chromosomal relocalization of heat-shock factor. Nature 353: 822–827. [DOI] [PubMed] [Google Scholar]
- Winston, W. M., C. Molodowitch and C. P. Hunter, 2002. Systemic RNAi in C. elegans requires the putative transmembrane protein SID-1. Science 295: 2456–2459. [DOI] [PubMed] [Google Scholar]