Abstract
Fabry disease, OMIM 301500, is a progressive multisystem storage disorder due to the deficiency of α-galactosidase A (GALA). Neurological and vascular manifestations of this disorder with regard to hearing loss have not been analysed quantitatively in large cohorts. We conducted a retrospective cross sectional analysis of hearing loss in 109 male and female patients with Fabry disease who were referred to and seen at the Clinical Center of the National Institutes of Health, Bethesda, MD, USA on natural history and enzyme replacement study protocols. There were 85 males aged 6–58 years (mean 31 years, SD 13) and 24 females aged 22–72 years (mean 42 years, SD 12). All patients underwent a comprehensive audiological evaluation. In addition, cerebral white matter lesions, peripheral neuropathy, and kidney function were quantitatively assessed. HL95, defined as a hearing threshold above the 95th percentile for age and gender matched normal controls, was present in 56% [95% CI (42.2–67.2)] of the males. Prevalence of HL95 was lower in the group of patients with residual GALA enzyme activity compared with those without detectable activity (33% versus 63%) HL95 was present in the low-, mid- and high-frequency ranges for all ages. Male patients with HL95 had a higher microvascular cerebral white matter lesion load [1.4, interquartile range (IQR) 0–30.1 ± versus 0, IQR 0–0], more pronounced cold perception deficit [19.4 ± 5.5 versus 13.5 ± 5.5 of just noticeable difference (JND) units] and lower kidney function [creatinine: 1.6 ± 1.2 versus 0.77 ± 0.2 mg/dl; blood urea nitrogen (BUN): 20.1 ± 14.1 versus 10.3 ± 3.28 mg/dl] than those without HL95 (P < 0.001). Of the females, 38% had HL95. There was no significant association with cold perception deficit, creatinine or BUN in the females. Word recognition and acoustic reflexes analyses suggested a predominant cochlear involvement. We conclude that hearing loss involving all frequency regions significantly contributes to morbidity in patients with Fabry disease. Our quantitative analysis suggests a correlation of neuropathic and vascular damage with hearing loss in the males. Residual GALA activity appears to have a protective effect against hearing loss.
Keywords: Fabry disease, stroke, hearing impairment, peripheral neuropathy, X-linked disorder
Introduction
Fabry disease, OMIM 301500, is due to the deficiency of ceramidtrihexosidase, commonly referred to as α-galactosidase A (GALA) (Brady et al., 1967). In this X-linked disorder, globotriaosylceramide (Gb3), digalactosylceramide, blood group B, B1, and P1 glycolipids are stored in a wide variety of different cell types (Klug et al., 1979; O’Brien et al., 1982; Gadoth and Sandbank, 1983; Sessa et al., 2002). Fabry disease is a progressive debilitating disorder affecting a multitude of organ systems. Its classical form includes signs and symptoms such as angiokeratoma, lymphoedema, cornea verticillata, hypohydrosis, neuropathic pain, cardiac hypertrophy, proteinuria, progressive kidney failure, abdominal pain, diarrhoea, fatigue, vertigo and stroke (Altarescu et al., 2001a; MacDermot et al., 2001b; Branton et al., 2002; Ries et al., 2005, 2006). Most patients with Fabry disease have a complete loss of GALA enzyme activity, but some individuals maintain residual enzyme function. The presence of residual enzyme is associated with a milder form of disease as shown before for pain, kidney involvement or as an overall effect in the pediatric population (Altarescu et al., 2001b; Branton et al., 2002; Ries et al., 2005).
The first auditory symptoms of Fabry disease often include tinnitus in childhood (Ries et al., 2003). The association of the disease and sensorineural hearing loss (SNHL) has been reported previously in a number of individual case studies and descriptive reports, but the rarity of Fabry disease has made it difficult to evaluate large groups of patients over a significant period of observation (Bird and Lagunoff, 1978; MacDermot et al., 2001a, b; Germain et al., 2002). Data on the extent and progression of hearing loss and the associated morbidities are not available for large cohorts.
We conducted this retrospective cross-sectional study to describe the point prevalence of hearing loss and its association with (i) cerebral white matter vascular lesion load (ii) peripheral neuropathy as assessed by cold perception threshold in the feet, (iii) kidney function as assessed by creatinine and blood urea nitrogen (BUN) measurements and (iv) residual GALA enzyme activity in the males.
Material and methods
Subjects
The medical records of 109 patients were reviewed. There were 85 males aged 6–58 years (mean 31 years, SD 13) and 24 females aged 22–72 years (mean 42 years, SD 12) (Table 1). All of these patients were enrolled in clinical protocols approved by the local institutional review board.
Table 1.
Stratification of 109 patients with Fabry disease according to gender, year of enrolment and study protocol
| Gender | Year of enrolment | Natural history protocol (number of subjects) | Enzyme replacement protocol (number of subjects) |
|---|---|---|---|
| Male | 1996 | 1 | 1 |
| 1997 | 1 | 0 | |
| 1998 | 2 | 1 | |
| 1999 | 2 | 19 | |
| 2000 | 18 | 6 | |
| 2001 | 5 | 4 | |
| 2002 | 9 | 4 | |
| 2003 | 11 | 1 | |
| Total | 49 | 36 | |
| Female | 1999 | 1 | — |
| 2000 | 6 | — | |
| 2001 | 9 | — | |
| 2002 | 6 | — | |
| 2003 | 2 | — | |
| Total | 24 | — |
Thirty-six males were enrolled in enzyme replacement therapy studies where they were recruited because of neuropathic pain. The remaining 73 patients were followed on natural history protocols studying phenotypic heterogeneity of the disease. All patients were seen at the National Institutes of Health (NIH) between 1996 and 2003 after having provided written informed consent.
Patients were referred to NIH by their local physician or contacted the institution directly for self-referral. Information about available study protocols was publicly available on www.clinicaltrials.gov.
As part of the otological and audiological evaluations at the time of visit, subjects were screened for a history of head trauma, otological surgery other than ventilation tube placement, exposure to ototoxic agents, genetic hearing loss not related to Fabry disease, and acoustic trauma. None of the investigated patients had a positive history for any of these conditions.
Diagnosis of Fabry disease
The diagnosis of Fabry disease was based on clinical presentation, demonstration of GALA enzyme deficiency, and/or mutation analysis of the GALA gene in heterozygotes. Fluorometric assay of residual GALA activity was performed as previously described (Kusiak et al., 1978). Patients were divided into those with residual enzyme activity (>1.5% of normal) and those without (≤1.5% of normal). DNA for genetic analysis was obtained from fibroblasts or whole blood. All seven exons of the GALA gene were sequenced as previously described (Altarescu et al., 2001b).
Audiology evaluation
Complete audiological assessments included pure-tone air and bone conduction thresholds for 250–8000 Hz, speech audiometry, tympanometry and middle ear muscle reflex evaluation. Audiological testing was conducted with clinical audiometers in sound treated test suites meeting American National Standards Institute (ANSI) approved conditions for audiological testing (ANSI, 1989, 1991).
Definitions
Type of hearing loss
The classification of the hearing loss type into conductive, mixed or sensorineural was based on the three-frequency air conduction pure-tone average (500, 1000 and 2000 Hz) using the recommendations of the GENDEAF study group (Mazzoli et al., 2003). Conductive hearing loss was defined as an average bone conduction threshold of ≤20 dB HL and an average air–bone gap of ≥15 dB HL. Hearing loss was classified as sensorineural when the average bone conduction threshold was >20 dB HL and the average air–bone gap was <15 dB HL. A mixed hearing loss comprised an average bone conduction threshold >20 dB HL and an average air–bone gap of ≥15 dB HL.
Clinical hearing loss
Degree of clinical hearing loss was classified according to the recommendations of the GENDEAF study group and was based on a four-frequency pure-tone average (500, 1000500, 2000 and 4000 Hz) (Mazzoli et al., 2003). Hearing thresholds ≤20 dB HL were considered within age-independent normal limits. A mild hearing loss ranged from >20 to ≤40 dB HL. Thresholds ranging from >40 to ≤70 dB HL were classified as moderate. Hearing loss was classified as severe for thresholds >70 to <95 dB HL, and hearing loss that was ≥95 dB HL was classified as profound.
Hearing loss based on age and gender-specific percentiles (HL95)
HL95 was based on 95th percentiles elaborated by Morrell et al. (1996) for 500, 1000, 2000 and 4000 Hz. These percentiles account for differences in the distribution of hearing levels by age and gender. These normative hearing data were derived from the Baltimore Longitudinal Study of Aging, and were collected from subjects who had been systematically screened to rule out otological disorders and noise induced hearing loss. Percentiles for 8000 Hz were obtained from the International Organization for Standardization standards (ISO, 1984). Norms for subjects below 25 years of age were extrapolated. We defined the 95th percentile as critical threshold, i.e. a hearing threshold exceeding this cut-off at any one or more octave frequencies was considered abnormal. Test frequency regions were also categorized into low (500 Hz), mid (1000–2000 Hz) and high (4000–8000 Hz).
Word-recognition ability
Suprathreshold word-recognition ability for phonetically balanced monosyllabic words was reported as percent correct. Word recognition scores were evaluated in the context of the degree of hearing loss as represented by the three-frequency pure-tone average (500, 1000 and 2000 Hz) using confidence limits previously established (Dubno et al., 1995). Word-recognition scores of 88% or better were classified as normal. Word-recognition scores <88% but within the confidence interval for degree of hearing loss were consistent with cochlear pathology. Word-recognition scores poorer than the 95% confidence limit (95% CI) were consistent with retrocochlear pathology.
Middle ear acoustic immittance measurement
Middle ear pressure and peak compensated static compliance were quantified from the tympanograms to evaluate middle ear function. Middle ear pressure between −100 and +50 daPa and compliance between 0.3 and 1.4 ml were defined as normal. Middle ear pressures and compliance values outside of these ranges were classified as abnormal (Margolis and Heller, 1987). Both ipsilateral and contralateral acoustic reflexes were measured. Acoustic reflex thresholds were categorized as normal, elevated or absent with consideration for degree of SNHL according to confidence intervals previously reported (Silman and Gelfand, 1981).
Peripheral neuropathy
Peripheral neuropathy was quantified by the Computer Evaluated Sensory Evaluator as described before (Dyck and O’Brien, 1999; Schiffmann et al., 2003). Cold perception in the feet is the predominant marker for Fabry disease neuropathy and was quantified in units of just noticeable difference (JND) on a scale from 1 to 25 (Table 2).
Table 2.
Synopsis of 109 patients with Fabry disease and continuous measure of their renal, neuropathic, and cerebrovascular status
| Characteristics | Mean | SD | Median | n |
|---|---|---|---|---|
| Age (years) | 33.1 | 13.5 | 35 | 109 |
| Creatinine (mg/dl) | 1.2 | 1.0 | 0.9 | 109 |
| BUN (mg/dl) | 15.7 | 11.0 | 13 | 109 |
| Cold detection threshold (foot) (JND) | 16.1 | 6.6 | 17 | 75 |
| White matter microvascular lesion load | Median = 0 | 0–14* | 0 | 49 |
IQR.
Kidney function
Kidney function was assessed by serum creatinine and BUN determined at the Department of Laboratory Medicine of the NIH Clinical Center (Table 2).
Vascular white matter lesion load
The quantification of cerebral white matter lesions in fluid attenuated inversion recovery images (TR 10 000, TE 148, slice thickness 5 mm, FOV 24.0 cm), representing a continuous measure for microvascular brain damage, was conducted according to the algorithm described by Moore et al. (2003).
Statistics
Methods of descriptive statistics were applied for cross-sectional data. Measures of central tendency were compared by t-tests or ANOVA for normally distributed data or non-parametric statistics (Mann–Whitney test) for data of non-Gaussian distribution. The analyses were two-tailed at a significance level of 0.05 and were performed using SPSS for Windows (version 10.1, SPSS Inc., Chicago, IL). Data are presented as mean ± SD or median and interquartile range (IQR).
Results
Type and degree of clinical hearing loss
Types of hearing loss, classified according to the clinical definitions and based on the three-frequency pure-tone average of 500, 1000 and 2000 Hz, are summarized in Table 3 for each tested ear. Sensorineural was the most common type, occurring in 72% of male and 86% of female ears with clinical hearing loss. In addition, conductive hearing loss was observed in a small number for both genders. Degree of clinical hearing loss (Table 4), based on the four-frequency pure-tone average of 500, 1000, 2000 and 4000 Hz, was classified as normal for the majority of subjects. The degree of clinical hearing loss ranged from mild to profound in the males and was mild in the females.
Table 3.
Type of hearing loss based on the three-frequency pure-tone average (500, 1000 and 2000 Hz) in abnormally hearing ears of male and female patients with Fabry disease
| Type of hearing loss | Males ears N (%)(n = 32) | Females ears N (%)(n = 7) |
|---|---|---|
| Sensorineural | 23 (72) | 6 (86) |
| Conductive | 9 (28) | 1 (14) |
Table 4.
Degree of clinical hearing loss based on the 4-frequency pure-tone average (500, 1000500, 2000 and 4000 Hz) in the worse hearing ear of male and female subjects with Fabry disease (Mazzoli et al., 2003)
| Clinical hearing loss | Males patients (n = 85) n (%) | Females patients (n = 24) n (%) |
|---|---|---|
| Normal | 56 (65.9) | 19 (79.2) |
| Mild | 19 (22,4) | 5 (20.8) |
| Moderate | 8 (9.4) | — (0) |
| Severe | — (0) | — (0) |
| Profound | 2 (2.4) | — (0) |
HL95 males
HL95 was present at one or more of the octave frequencies from 500 to 8000 Hz in 55% (95% CI = 42.2–67.2) of the males. HL95 was observed for all ages and at all frequencies, most commonly in high frequencies (Fig. 1, Table 5). Male patients with HL95 had a higher microvascular cerebral white matter lesion load (1.4, IQR 0–30.1 versus 0, IQR 0–0), more pronounced cold perception deficit (19.4 ± 5.5 versus 13.5 ± 5.5 JND units), and lower kidney function (creatinine: 1.7 ± 1.2 versus 0.8 ± 0.2 mg/dl; BUN: 20.1 ± 14.1 versus 10.3 ± 3.3 mg/dl) than those individuals without hearing loss (Table 6).
Fig. 1.
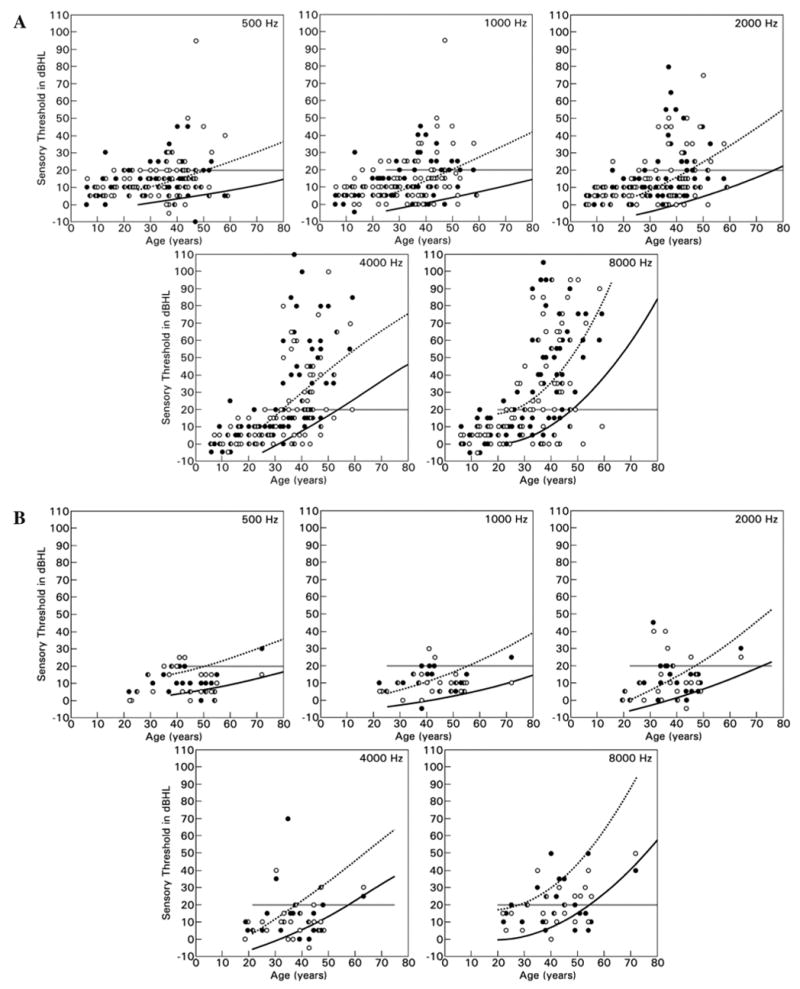
Age- and gender-adjusted determination of hearing loss (HL95) in (A) male and (B) female patients with Fabry disease at various frequencies. The solid line indicates the 95th percentile, the interrupted line indicates the 50th percentile. The cut-off for clinical hearing loss is 20 dB HL (grey line), which does not take age and gender into consideration. Open circles represent right ear and closed circles left ear.
Table 5.
Distribution of hearing loss (HL95) in the worse hearing ear in male and female patients with Fabry disease given in absolute numbers per age category and row percent
| Age category (years) | Males
|
Females
|
||||
|---|---|---|---|---|---|---|
| No hearing loss (HL95) n (%) | Hearing loss (HL95) n (%) | Total (100%) | No hearing loss (HL95) n (%) | Hearing loss (HL95) n (%) | Total (100%) | |
| Below 21 | 19 (95) | 1 (5) | 20 | — | — | — |
| 21–30 | 12 (75) | 4 (25) | 16 | 4 (100) | 0 (0) | 4 |
| 31–40 | 3 (14.3) | 18 (85.7) | 21 | 3 (50) | 3 (50) | 6 |
| Above 40 | 4 (14.3) | 24 (85.7) | 28 | 8 (57.1) | 6 (42.9) | 14 |
| All categories | 38 (44.7) | 47 (55.3) | 85 | 15 (62.5) | 9 (37.5) | 24 |
Table 6.
Association of hearing loss (HL95) with peripheral neuropathy, kidney function, and cerebrovascular lesions
| Males
|
Females
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No hearing loss
|
Hearing loss
|
P | No hearing loss
|
Hearing loss
|
P | |||||||||
| n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | |||
| Age (years) | 38 | 21.1 | 10.4 | 47 | 38.2 | 9.1 | <0.001 | 15 | 34.7 | 11.5 | 9 | 46.8 | 11.4 | 0.19 |
| Creatinine (mg/dl) | 38 | 0.8 | 0.2 | 47 | 1.7 | 1.2 | <0.001 | 15 | 0.8 | 0.1 | 9 | 1.8 | 1.9 | 0.056 |
| BUN (mg/dl) | 38 | 10.3 | 3.3 | 47 | 20.1 | 14.1 | <0.001 | 15 | 14.8 | 4.3 | 9 | 17.1 | 12.6 | 0.52 |
| Cold perception feet (JND) | 19 | 13.5 | 5.5 | 39 | 19.4 | 5.5 | <0.001 | 12 | 11.6 | 3.7 | 5 | 11.0 | 3.9 | 0.78 |
| White matter lesion load | 17 | Median 0 | IQR 0–0 | 32 | Median 1.4 | IQR 0–30.1 | 0.011* | — | — | — | — | — | — | — |
An asterisk (*) denotes a Mann–Whitney test, which was applied because of non-parametric distribution of the data. The other comparisons are based on ANOVA. White matter lesion load was not performed in the females. P-values were not adjusted for multiple comparison.
Severity of neuropathy, as determined by cold perception in feet, was associated with an increased number of affected frequency regions, i.e. none, or any or a combination of low-, middle- or high-frequency regions (Fig. 2).
Fig. 2.
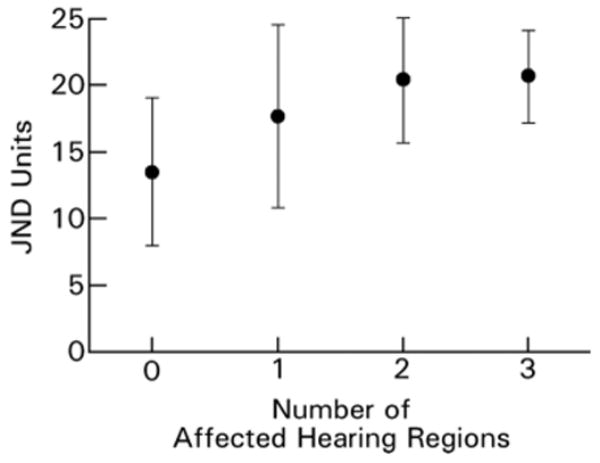
The association of the cold perception threshold, as expressed by JND units (just noticeable difference), and the number of frequency regions with HL95 in males with Fabry disease. n = 58, P = 0.001 (ANOVA).
Although not statistically significant, the mean lesion load tended to increase with the number of affected frequency regions. Males with HL95 in none of the frequency regions had a mean lesion load of 2.8 ± 83.1 (n = 17), those with one affected region had 114.4 ± 95.1 (n = 13), those with two affected regions had 68.3 ± 11.2 (n = 9), and those with hearing loss for all three regions had 350.1 ± 108.4 (n = 10), P = 0.53 (ANOVA).
In order to address concerns that younger patients might potentially bias the sample towards overall more healthy individuals and that the hearing loss percentile curves for patients <25 years of age were extrapolated, we analysed data of male patients ≥25 years of age separately. All differences in microvascular cerebral white matter lesion load, cold perception deficit, and kidney function (creatinine, BUN) between males with and without HL95 >25 years of age remained statistically significant (P < 0.05).
HL95 females
Thirty-eight per cent (95% CI = 15.7–61.2) of the females had HL95 (Table 5). There was no significant association with cold perception deficit. Although not significant, there was a trend for female patients with Fabry disease and HL95 to have higher blood creatinine (1.8 ± 1.9 versus 0.8 ± 0.1 mg/dl) and BUN concentrations (17.1 ± 12.6 versus 14.8 ± 4.3 mg/dl) than female patients without HL95.
Word recognition
Word-recognition ability was normal for 94% of the males and 96% of the females. Abnormal word recognition ranged from 0 to 70% correct in five (6%) of the males and in one (4%) of the females. In each of these cases, reduced word-recognition scores could be accounted for by the significant degree of hearing loss and was consistent with cochlear deficits. In no case were word-recognition scores reduced to a level that suggested retrocochlear involvement.
Middle ear function
Normal tympanograms, characterized by both normal middle ear pressure and compliance, occurred in 74% of the male ears and 98% of the female ears. Acoustic reflexes were present at normal levels for 84% of the male ears and 98% of the female ears. Absent or elevated acoustic reflexes occurred in 15% of the male ears and 2% of the female ears who had abnormal tympanometry and/or a conductive hearing loss, or a severe to profound SNHL. Only one male (1%) with normal tympanometry and mild SNHL had absent acoustic reflexes, which may suggest a retrocochlear process involving the auditory nerve and the brainstem in this single case.
Tinnitus
Tinnitus was reported for one ear in 14.5% and in two ears in 36.8% of the male patients (n = 76). In the females, 20.8% had tinnitus in one ear and 41.7% in both ears (n = 24).
Sudden hearing loss
Ten (11.8%) of the male patients had sudden SNHL. This was documented in one case by serial audiograms and in the remaining cases by anamnestic information. No female reported sudden hearing loss.
Residual GALA enzyme activity in the group of male patients
The prevalence of HL95 was lower in the group of patients with residual GALA enzyme activity >1.5% of normal (n = 9) compared with those without detectable activity (33 versus 63%) There was no statistically significant difference in age between the patients with and without residual enzyme activity.
Discussion
We have found that hearing loss contributed in a significant way to the morbidity of Fabry disease. Using age and gender-adjusted criteria for hearing loss (HL95), which is more sensitive and specific than the age and gender independent 20 dB HL threshold criterion for normal hearing, we found that Fabry disease was associated with hearing loss at all frequencies and was not limited to high frequencies. Hearing loss was more severe in males with white matter lesions, in those with peripheral neuropathy as assessed by quantitative measurement of cold perception in feet, and in those with decreased kidney function. Hearing loss in women with Fabry disease was observed later in life than in male patients (Fig. 1, Table 5). Our cross-sectional findings suggest that the progression of hearing loss may be insidious or, less often, may be marked. The onset of subclinical changes in hearing can occur early in life. There was no Fabry specific configuration in the audiograms. Sensori-neural was the most common type of hearing loss. Word recognition and acoustic reflex findings were consistent with cochlear rather than retrocochlear involvement of the auditory system. Thus far, this is the largest and only cohort of patients with Fabry disease whose audiological status, peripheral neuropathy, cerebral white matter lesions and kidney function have been assessed in a quantitative manner.
This study extends prior observations of MacDermot et al. (2001b) who conducted a questionnaire-based cross-sectional study. She described audiometrically confirmed mild-to-severe SNHL for 78% of 23 males with Fabry disease. The majority had hearing loss limited to the high frequencies (2000–3000 Hz). Self-reported hearing loss was present in 23.3% of six female obligate carriers, but was not confirmed with audiometric testing (MacDermot et al., 2001a). More recently, auditory characteristics of individuals with Fabry disease have received more detailed and increased attention. Mid- and high-frequency SNHL has been observed in affected males with an incidence of 47–80% in two studies of 22 and 15 male patients (Germain et al., 2002; Hajioff et al., 2003). We observed clinically normal hearing in 70.6% of 85 males and 87.5% of 24 females when this determination was based on a four-frequency pure-tone average which may obscure hearing loss limited to the high frequencies only. However, when individual frequencies were examined and age and gender corrections were taken into consideration (HL95), the prevalence of hearing loss in our group was as high as 86% in males aged 31–58 years and 45% in females aged 31–72 years.
Many individuals are unaware of their diminished hearing as evidenced by a self-report of hearing loss in 41% (n = 61) of a group with audiometrically confirmed hearing loss in 78% (MacDermot et al., 2001b). In other cases, individuals are acutely aware of auditory change, especially when the onset is abrupt. While sudden onset of SNHL was reported by 7 (32%) of 22 subjects reported by Germain et al. (2002), we observed sudden hearing loss in only 10 (11.8%) of 85 males of our group.
Evaluation of hearing ability in individuals with Fabry disease must take into consideration other systemic disease manifestations. In males with HL95, we observed decreased kidney function (determined as a continuous measurement of BUN and creatinine) as compared with those who did not have HL95. This extends the findings of two reports in which glomerular filtration rate correlated with hearing status in cohorts of 22 and 15 affected males (Germain et al., 2002; Hajioff et al., 2003).
In this study, males with HL95 had more severe cerebrovascular manifestations as quantified by white matter lesion load than those without HL95. This extends the report by Germain et al. (2002) in which males with evidence of cerebrovascular disease had higher average pure-tone thresholds than those without evidence of cerebrovascular disease.
Tinnitus is a frequent symptom in patients with Fabry disease and was reported by 27–38% of hemizygous males (MacDermot et al., 2001b; Germain et al., 2002) and 25% of female carriers (MacDermot et al., 2001a). Our data suggest that tinnitus may be present in a larger percentage (41.3% males, 62.5% females). This difference may reflect phenotypic heterogeneity, differences in age or differences in the query methods used to assess the presence of tinnitus. In an Australian population based sample which was older than our cohort (n = 2015, age 55–99 years), the prevalence of tinnitus was 30.3% (Sindhusake et al., 2003).
The mechanisms of hearing loss in Fabry disease are not precisely understood. Abnormal blood flow (Moore et al., 2002), altered blood vessel properties (Altarescu et al., 2001a) or abnormal blood composition (DeGraba et al., 2000) may contribute to microvascular damage resulting in cochlear dysfunction. In a temporal bone histopathological report of two Fabry patients with bilateral sloping SNHL, Schachern et al. (1989) observed cytoplasmic vacuolization of the endothelial cells of blood vessels in the modiolus, a reduced number of spiral ganglia of the auditory nerve, ballooning of Scarpa ganglia of the vestibular nerve, strial and spiral ligament atrophy, and hair cell loss in the cochlear basal turns. It is possible that progressive storage of Gb3 in the auditory sensory organ itself might contribute to the pathogenesis of hearing loss. However, these histopathological findings more likely suggest that in Fabry disease the accumulation of Gb3 in the audiovestibular nerve ganglia and vascular endothelium of the cochlear vessels results in chronic microvascular insufficiency and permanent progressive cochlear neuronal and sensory organ damage. Sudden hearing loss reported by >10% of our cohort of Fabry patients is most likely a manifestation of an acute microvascular event. In this study we relate the quantitative state of organs remote from the ear (renal, cerebrovascular and neuropathic) to hearing status suggesting a parallel impact of vascular and neuropathic dysfunction on these systems.
Our observations indicate that detectable hearing loss starts in the second and fourth decades of life for Fabry male and females, respectively. These auditory deficits affect all frequencies, but the high frequencies deteriorate more rapidly with increasing age. Because auditory deficits are common yet often underdiagnosed, we recommend that all patients with a diagnosis of Fabry disease should undergo annual audiological evaluation to monitor their hearing status starting their second decades for males and third decades for females, especially in the presence of renal dysfunction and cerebral vascular insufficiency. In cases of sudden hearing loss, the patients with Fabry disease should immediately undergo otolaryngological and audiological evaluation since a prompt medical intervention might be beneficial in restoring auditory function in some cases of sudden SNHL as suggested by a retrospective study in 75 patients with idiopathic hearing loss by Slattery (Slattery et al., 2005).
The conclusions of this study are limited by its retrospective design and the fact that patients were seen as adolescents or adults at various ages. We cannot exclude selection bias. Patients were seen on natural history and enzyme replacement therapy protocols that were not designed to study auditory involvement in Fabry disease. Some patients were first studied prior to the initiation of enzyme replacement and some subsequently. The patients described in this study were not randomly selected, but referred to the NIH by their physicians or themselves. Initially the audiological evaluations were not indicated for research but for clinical reasons after an increasing number of patients with auditory complaints raised our attention. We consequently directed our efforts towards a broad and thorough auditory examination of our available study population, which is based on the chart review of 189 audiograms in 109 patients. Last, the interpretation of findings in patients with residual enzyme activity above 1.5% is based on a small group of patients. A large, prospective study with both male and female patients with Fabry disease should be helpful to further elucidate the auditory manifestations of Fabry disease.
Conclusions
Hearing loss in Fabry disease extends throughout all frequency ranges and appears to be more rapidly progressive than the hearing loss associated with normal ageing. Hearing loss was present in females with Fabry disease, but to a lesser extent than in males. It occurred less often, was less severe and was later in onset. In male patients, residual enzyme activity >1.5% appears to have a protective function. In males, we found a positive association between HL95 and the degree of peripheral neuropathy, cerebrovascular and renal damage suggesting a combined neuropathic and vascular aetiology of the hearing loss.
Acknowledgments
This work was supported by The Intramural Research Program of the National Institute of Neurological Disorders and Stroke. This work was part of Dr Markus Ries’ thesis for a Master of Health Sciences in Clinical Research at Duke University and we would like to thank Dr E. Oddone and Dr W. Wilkinson for their critical comments.
Abbreviations
- GALA
α-galactosidase A
- JND
just noticeable difference
- IQR
interquartile range
References
- Altarescu G, Moore DF, Pursley R, Campia U, Goldstein S, Bryant M, et al. Enhanced endothelium-dependent vasodilation in Fabry disease. Stroke. 2001a;32:1559–62. doi: 10.1161/01.str.32.7.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarescu GM, Goldfarb LG, Park KY, Kaneski C, Jeffries N, Litvak S, et al. Identification of fifteen novel mutations and genotype-phenotype relationship in Fabry disease. Clin Genet. 2001b;60:46–51. doi: 10.1034/j.1399-0004.2001.600107.x. [DOI] [PubMed] [Google Scholar]
- ANSI S- American national standard specification for audiometers. New York, NY: American National Standards Institute; 1989. [Google Scholar]
- ANSI S- American national standard maximum permissible ambient noise levels for audiometric test rooms. New York, NY: American National Standards Institute; 1991. [Google Scholar]
- Bird TD, Lagunoff D. Neurological manifestations of Fabry disease in female carriers. Ann Neurol. 1978;4:537–40. doi: 10.1002/ana.410040610. [DOI] [PubMed] [Google Scholar]
- Brady RO, Gal AE, Bradley RM, Martensson E, Warshaw AL, Laster L. Enzymatic defect in Fabry’s disease. Ceramidetrihexosidase deficiency. N Engl J Med. 1967;276:1163–7. doi: 10.1056/NEJM196705252762101. [DOI] [PubMed] [Google Scholar]
- Branton MH, Schiffmann R, Sabnis SG, Murray GJ, Quirk JM, Altarescu G, et al. Natural history of Fabry renal disease: influence of alpha-galactosidase A activity and genetic mutations on clinical course. Medicine (Baltimore) 2002;81:122–38. doi: 10.1097/00005792-200203000-00003. [DOI] [PubMed] [Google Scholar]
- DeGraba T, Azhar S, Dignat-George F, Brown E, Boutiere B, Altarescu G, et al. Profile of endothelial and leukocyte activation in Fabry patients. Ann Neurol. 2000;47:229–33. [PubMed] [Google Scholar]
- Dubno JR, Lee FS, Klein AJ, Matthews LJ, Lam CF. Confidence limits for maximum word-recognition scores. J Speech Hear Res. 1995;38:490–502. doi: 10.1044/jshr.3802.490. [DOI] [PubMed] [Google Scholar]
- Dyck PJ, O’Brien PC. Quantitative sensation testing in epidemiological and therapeutic studies of peripheral neuropathy. Muscle Nerve. 1999;22:659–62. doi: 10.1002/(sici)1097-4598(199906)22:6<659::aid-mus1>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Gadoth N, Sandbank U. Involvement of dorsal root ganglia in Fabry’s disease. J Med Genet. 1983;20:309–12. doi: 10.1136/jmg.20.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain DP, Avan P, Chassaing A, Bonfils P. Patients affected with Fabry disease have an increased incidence of progressive hearing loss and sudden deafness: an investigation of twenty-two hemizygous male patients. BMC Med Genet. 2002;3:10. doi: 10.1186/1471-2350-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajioff D, Enever Y, Quiney R, Zuckerman J, Mackermot K, Mehta A. Hearing loss in Fabry disease: the effect of agalsidase alfa replacement therapy. J Inherit Metab Dis. 2003;26:787–94. doi: 10.1023/B:BOLI.0000009948.86528.72. [DOI] [PubMed] [Google Scholar]
- International Organization for Standardization. Acoustics—threshold of hearing by air conduction as a function of age and sex for otologically normal persons. Geneva: ISO; 1984. [Google Scholar]
- Klug H, Zabel R, Evers U. [Fabry disease: clinical, biochemical and electron microscopical studies (author’s translation)] Dermatol Monatsschr. 1979;165:46–54. [PubMed] [Google Scholar]
- Kusiak JW, Quirk JM, Brady RO. Purification and properties of the two major isozymes of alpha-galactosidase from human placenta. J Biol Chem. 1978;253:184–90. [PubMed] [Google Scholar]
- MacDermot KD, Holmes A, Miners AH. Anderson-Fabry disease: clinical manifestations and impact of disease in a cohort of 60 obligate carrier females. J Med Genet. 2001a;38:769–75. doi: 10.1136/jmg.38.11.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDermot KD, Holmes A, Miners AH. Anderson-Fabry disease: clinical manifestations, impact of disease in a cohort of 98 hemizygous males. J Med Genet. 2001b;38:750–60. doi: 10.1136/jmg.38.11.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis RH, Heller JW. Screening tympanometry: criteria for medical referral. Audiology. 1987;26:197–208. doi: 10.3109/00206098709081549. [DOI] [PubMed] [Google Scholar]
- Mazzoli M, van Camp G, Newton V, Giarbini N, Declau F, Parving A. Recommendations for the description of genetic and audiological data for families with nonsyndromic hereditary hearing impairment. Audiol Med. 2003;1:148–50. [Google Scholar]
- Morrell CH, Gordon-Salant S, Pearson JD, Brant LJ, Fozard JL. Age- and gender-specific reference ranges for hearing level and longitudinal changes in hearing level. J Acoust Soc Am. 1996;100:1949–67. doi: 10.1121/1.417906. [DOI] [PubMed] [Google Scholar]
- Moore DF, Altarescu G, Ling GS, Jeffries N, Frei KP, Weibel T, et al. Elevated cerebral blood flow velocities in Fabry disease with reversal after enzyme replacement. Stroke. 2002;33:525–31. doi: 10.1161/hs0202.102601. [DOI] [PubMed] [Google Scholar]
- Moore DF, Altarescu G, Barker WC, Patronas NJ, Herscovitch P, Schiffmann R. White matter lesions in Fabry disease occur in ‘prior’ selectively hypometabolic and hyperperfused brain regions. Brain Res Bull. 2003;62:231–40. doi: 10.1016/j.brainresbull.2003.09.021. [DOI] [PubMed] [Google Scholar]
- O’Brien BD, Shnitka TK, McDougall R, Walker K, Costopoulos L, Lentle B, et al. Pathophysiologic and ultrastructural basis for intestinal symptoms in Fabry’s disease. Gastroenterology. 1982;82:957–62. [PubMed] [Google Scholar]
- Ries M, Ramaswami U, Parini R, Lindblad B, Whybra C, Willers I, et al. The early clinical phenotype of Fabry disease: a study on 35 European children and adolescents. Eur J Pediatr. 2003;162:767–72. doi: 10.1007/s00431-003-1299-3. [DOI] [PubMed] [Google Scholar]
- Ries M, Gupta S, Moore DF, Sachdev V, Quirk JM, Murray GJ, et al. Pediatric Fabry disease. Pediatrics. 2005;115:e344–55. doi: 10.1542/peds.2004-1678. [DOI] [PubMed] [Google Scholar]
- Ries M, Clarke JTR, Whybra C, Timmons M, Robinson C, Schlaggar BL, et al. Enzyme replacement therapy with agalsidase alfa in children with Fabry disease. Pediatrics. 2006;118:924–32. doi: 10.1542/peds.2005-2895. [DOI] [PubMed] [Google Scholar]
- Schachern PA, Shea DA, Paparella MM, Yoon TH. Otologic histopathology of Fabry’s disease. Ann Otol Rhinol Laryngol. 1989;98:359–63. doi: 10.1177/000348948909800509. [DOI] [PubMed] [Google Scholar]
- Schiffmann R, Floeter MK, Dambrosia JM, Gupta S, Moore DF, Sharabi Y, et al. Enzyme replacement therapy improves peripheral nerve and sweat function in Fabry disease. Muscle Nerve. 2003;28:703–10. doi: 10.1002/mus.10497. [DOI] [PubMed] [Google Scholar]
- Sessa A, Toson A, Nebuloni M, Pallotti F, Giordano F, Battini G, et al. Renal ultrastructural findings in Anderson-Fabry disease. J Nephrol. 2002;15:109–12. [PubMed] [Google Scholar]
- Silman S, Gelfand SA. The relationship between magnitude of hearing loss and acoustic reflex threshold levels. J Speech Hear Disord. 1981;46:312–6. doi: 10.1044/jshd.4603.312. [DOI] [PubMed] [Google Scholar]
- Sindhusake D, Mitchell P, Newall P, Golding M, Rochtchina E, Rubin G. Prevalence and characteristics of tinnitus in older adults: the Blue Mountains Hearing Study. Int J Audiol. 2003;42:289–94. doi: 10.3109/14992020309078348. [DOI] [PubMed] [Google Scholar]
- Slattery WH, Fisher LM, Iqbal Z, Liu N. Oral steroid regimens for idiopathic sudden sensorineural hearing loss. Otolaryngol Head Neck Surg. 2005;132:5–10. doi: 10.1016/j.otohns.2004.09.072. [DOI] [PubMed] [Google Scholar]


