Abstract
The major circulating form of vitamin D is 25-hydroxycholecalciferol [25(OH)D3], which is delivered to target tissues in complex with the serum vitamin D binding protein (DBP). We recently observed that mammary cells can metabolize 25(OH)D3 to 1,25-dihydroxycholecalciferol [1,25(OH)2D3], the vitamin D receptor (VDR) ligand, and the objective of our study was to elucidate the mechanisms by which the 25(OH)D3-DBP complex is internalized by mammary cells prior to metabolism. Using fluorescent microscopy and temperature-shift techniques, we found that T-47D breast cancer cells rapidly internalize DBP via endocytosis, which is blunted by receptor-associated protein, a specific inhibitor of megalin-mediated endocytosis. Endocytosis of DBP was associated with activation of VDR by 25(OH)D3 but not 1,25(OH)2D3 (as measured by induction of the VDR target gene, CYP24). We also found that megalin and its endocytic partner, cubilin, are coexpressed in normal murine mammary tissue, in nontransformed human mammary epithelial cell lines, and in some established human breast cancer cell lines. To our knowledge, our studies are the first to demonstrate that mammary-derived cells express megalin and cubilin, which contribute to the endocytic uptake of 25(OH)D3-DBP and activation of the VDR pathway.
Introduction
Fat soluble vitamins such as the calciferols (ergocalciferol and cholecalciferol) and the retinoids (vitamin A) circulate bound to specific carrier proteins that facilitate their delivery to sites of metabolism, storage, and action. Emerging data indicates that the protein-bound vitamin complexes can be internalized intact via interactions with specific plasma membrane localized receptors (1). In the case of calciferols, as much as 99% of 25-hydroxycholecalciferol [25(OH)D3,2 the major circulating form] is bound to the vitamin D binding protein (DBP), and cellular uptake of this complex in the kidney via receptor-mediated endocytosis is essential for vitamin D homeostasis in vivo (2,3). Specifically, the epithelial cells of the proximal tubule express the proteins megalin and cubilin, which bind and internalize the intact 25(OH)D3–DBP complex (2,3). Once internalized by megalin-mediated endocytosis, 25(OH)D3 is thought to dissociate from DBP for delivery to the renal mitochondria where it can be metabolized to 1,25-dihydroxycholecalciferol [1,25(OH)2D3], the ligand for the vitamin D receptor (VDR). The essentiality of the endocytic process for vitamin D action has been confirmed in vivo, insofar as mice lacking megalin (2), as well as humans and dogs with loss of function mutations in cubilin (3), exhibit vitamin D deficiency secondary to impaired renal cellular uptake of 25(OH)D3–DBP and the inability to generate 1,25(OH)2D3.
It is now recognized that the 25(OH)D3 1α-hydroxylase enzyme (CYP27B1) responsible for converting 25(OH)D3 to 1,25(OH)2D3 is expressed in many tissues, including mammary epithelium (4–9). However, the mechanisms by which the substrate 25(OH)D3 becomes available to the enzyme in nonrenal tissues have yet to be defined. Given the importance of endocytosis in renal cell uptake of 25(OH)D3–DBP, we hypothesized that megalin and cubilin might mediate uptake of protein-bound circulating 25(OH)D3 in extra-renal tissues as well. We chose to test this hypothesis using mammary epithelial cells because they express metabolizing enzymes and receptors for vitamin D steroids and are growth inhibited by 1,25(OH)2D3 (10). Furthermore, 25(OH)D3 and DBP are present in milk, suggesting that both compounds can enter and traverse mammary epithelium in a physiological context (11,12). In this study, we specifically examined whether megalin and cubilin play a role in the uptake of the 25(OH)D3-DBP complex and subsequent activation of the VDR pathway in mammary cells.
Materials and Methods
Cell lines and culture conditions
T-47D and MCF-7 breast cancer cells were obtained from the American Type Culture Collection. Telomerase immortalized, nontumorigenic human mammary epithelial cells (HME cells) were kindly provided by Dr. Robert Weinberg, Massachusetts Institute of Technology. All cell lines were cultured in a humidified incubator with 5% CO2 and a temperature of 37°C. T-47D cells were cultured in RPMI medium containing 10% fetal bovine serum (FBS), penicillin (100 kU/L), and streptomycin (0.1 g/L). MCF-7 cells were routinely cultured in α-MEM containing 5% FBS and penicillin/streptomycin, but, for some comparative experiments, MCF-7 cells were cultured in RPMI containing 10% FBS as described for T-47D cells. HME cells were maintained in culture medium 171 (Cascade Biologics), supplemented with bovine pituitary extract (0.4% v:v), bovine insulin (5 mg/L), hydrocortisone (0.5 mg/L), and recombinant human epithelial growth factor (3 μg/L).
Analysis of megalin and cubilin mRNA expression in mammary cells
For initial screening of megalin and cubilin expression in mammary epithelium, total RNA was isolated with Trizol Reagent (Invitrogen) from surgically dissected glands of C57Bl6 mice at various stages of development (5). Total RNA was isolated from cell lines and isolated mammary ducts with the RNeasy Mini Kit (Qiagen). For isolation of mammary epithelial ducts, whole thoracic and inguinal mammary glands were removed, washed twice with PBS, minced, and incubated in RPMI containing 2 g/L collagenase A (Calbiochem) and 1 g/L hyaluronidase (Sigma). After 2 h digestion at 37°C in a shaking water bath, the mixture was centrifuged at 500 × g for 5 min and the supernatant was discarded. Following 2 successive washes with PBS, the pellet was resuspended in RPMI and the mammary ducts were collected under a stereoscope. Ducts were pooled, washed with PBS, and used for RNA isolation.
Total RNA from tissues, ducts, and cell lines was used for first-strand cDNA synthesis using TaqMan Reverse Transcription Reagents (N808–0234, Applied Biosystems) as previously described (13), generating three 2.0 μg cDNA stocks for each sample. Each of the cDNA stocks were independently analyzed in duplicate (200 ng/well) for megalin and cubilin expression via real-time PCR using SYBR Green Detection reagents (4309155, Applied Biosystems). Data with primer sets specific for human megalin (forward primer: AAATTGAGCACAGCACCTTTGA, reverse primer: TCTGCTTTCCTGACTCGAATAATG) and human cubilin (forward primer: GGTTCCCTGCCAATTATCCAA, reverse primer: CCGCCATCCAAAATTTCTACA) were normalized against GAPDH RNA (forward primer: CCACCCATGGCAAATTCC, reverse primer: TGATGGGATTTCCATTGATGAC). cDNA stocks generated from whole mammary gland and purified mammary ducts were analyzed using primer sets specific for mouse megalin (forward primer:AGGCCACCAGTTCACTTGCT, reverse primer: AGGACACGCCCATTCTCTTG) and mouse cubilin (forward primer: GGGATCCTCTCAGGGACACA, reverse primer: TGCTGGCCGATTCTAAATCAA) and were normalized against 18s RNA (forward primer: AGTCCCTGCCCTTTGTACACA, reverse primer: GATCCGAGGGCCTCACTAAAC). Relative gene expression was graded on a scale of 0 (nondetectable) to +++++ (strong expression). The expression scale indicates relative SYBR Green signal intensity in cell and tissue samples compared with a standard curve generated for megalin and cubilin. Expression data are representative of multiple experiments (usually 5 mice/group or 3 independent cell platings).
Western blotting
T-47D and MCF-7 cell lysates were separated on SDS-PAGE, transferred to nitrocellulose and immunoblotted with a polyclonal sheep anti-CYP27B1 antibody (The Binding Site) as previously described (8). Specific binding was detected by chemiluminescence using products from Pierce and exposure to autoradiography film (Kodak Biomax).
Analysis of CYP24 mRNA and promoter induction
CYP24, a VDR target gene that is highly induced by 1,25(OH)2D3, was used as a marker of VDR transcriptional activity. CYP24 mRNA was measured in MCF-7 and T-47D cells plated in 6-well plates (2.0 × 105 cells/well) and treated with 100 nmol/L 1,25(OH)2D3, 100 nmol/L 25(OH)D3 or vehicle for 48 h. Total RNAwas isolated as described above and PCR was performed using the TaqMan PCR Core Reagent Kit (Applied Biosystems) using primer sets and a probe specific for human CYP24 (forward primer: CAAACCGTGGAAGGCCTATC, reverse primer: AGTCTTCCCCTTCCAGGATCA, probe: ACTACCGCAAAGAAGGCTACGGGCTG). Data were normalized against 18 s expression. To assess direct induction of the CYP24 promoter, cells were cotransfected with a 300 bp region of the human CYP24 promoter linked to firefly luciferase (obtained from the late Jack Omdahl, University of New Mexico) and a thymidine kinase driven renilla luciferase normalization construct (Promega). Cells were treated for 48 h with 25(OH)D3, 1,25(OH)2D3 or vehicle, in the presence or absence of DBP or FBS as indicated. Luciferase activity was measured with the Dual Luciferase Kit (Promega) and expressed as fold increase relative to control cells after normalization for transfection efficiency.
Fluorescein conjugation of DBP and cell uptake studies
DBP (Calbiochem) was conjugated to Alexa-488 using a commercial kit (A10235, Molecular Probes). For in vitro DBP uptake studies, subconfluent T-47D and MCF-7 cells plated on 4-well Lab-Tek II CC2 chamber slides (5000 cells/well) were incubated overnight in RPMI medium containing 10% FBS. For endocytosis assays, cells were washed with PBS, switched to serum-free medium and incubated with 0.02 g/L Alexa-DBP at either 37°C (the optimal temperature for endocytosis) or 4°C (a temperature that inhibits endocytosis) for periods of up to 60 min. To determine whether endocytosis of DBP requires megalin, uptake experiments were conducted for 30 min in the absence or presence of 1 μmol/L receptor associated protein (RAP) (Innovative Research), a known inhibitor of megalin-mediated endocytosis. In some experiments, cells were incubated with Hoechst (1 mg/L in PBS) for visualization of nuclei or Lysotracker (75 nmol/L, Molecular Probes) for identification of lysosomes. After incubations, cells were washed thoroughly in PBS, fixed with ice-cold methanol, mounted with antifade medium and examined under phase contrast and fluorescent filters. Comparative experiments on T-47D and MCF-7 cells were conducted in parallel, and images were acquired with a constant exposure time for both cell lines.
Statistical methods
Data were analyzed by Student’s t test or 1-way ANOVA and Dunn’s or Student-Neuman-Keuls post-tests, as appropriate, using InStat software (version 3.0 for Windows, GraphPad Software). Differences between means were considered significant when P <0.05 was obtained.
Results
Mammary cells that express both CYP27B1 and VDR are differentially responsive to 25(OH)D3
The actions of 25(OH)D3 were examined in an immortalized HME cell line, and in 2 breast cancer cell lines (MCF-7 and T-47D). All 3 cell lines express CYP27B1, the enzyme that converts the inactive precursor metabolite 25(OH)D3 to 1,25(OH)2D3 (7,8). The presence of CYP27B1 should enable qualitatively similar cellular responses to 25(OH)D3 and 1,25(OH)2D3. Indeed, treatment of VDR-positive HME cells with either 1,25(OH)2D3 or 25(OH)D3 significantly induced the CYP24 reporter gene construct derived from a known VDR target (Fig. 1A). In MCF-7 cells, 1,25(OH)2D3 highly induced CYP24 promoter activity, indicating functional VDR, but no induction was observed with 25(OH)D3 (Fig. 1B). These data suggest that, despite expression of CYP27B1 protein in both cell lines, functionally significant amounts of 1,25(OH)2D3 are generated from 25(OH)D3 in HME cells but not in MCF-7 cells.
Figure 1.
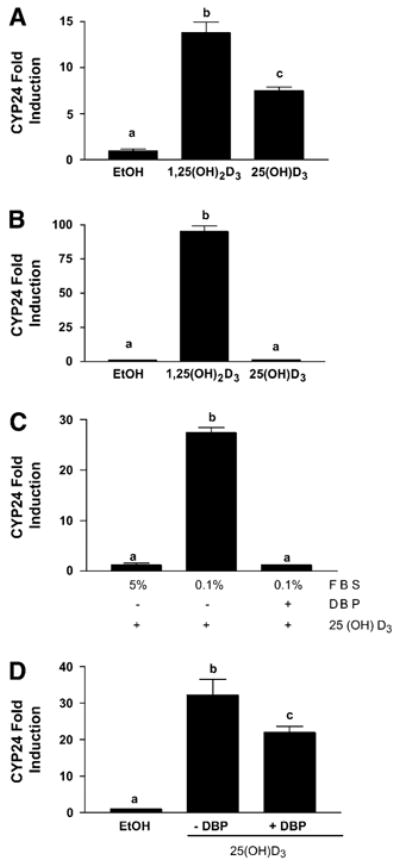
Induction of VDR responsive reporter gene by 25(OH)D3 in human mammary epithelial cells expressing both CYP27B1 and VDR. Human mammary epithelial cells were cotransfected with a 300 bp region of the VDR responsive human CYP24 promoter linked to firefly luciferase and a thymidine kinase driven renilla luciferase normalization construct. Luciferase reporter activity for treated cells was normalized and expressed as fold of the vehicle control, which was set to 1. (A) HME cells treated with 100 nmol/L 25-hydroxycholecalciferol [25(OH)D3], 100 nmol/L 1,25-dihydroxycholecalciferol [1,25(OH)2D3], or vehicle (ethanol) in serum-free medium for 48 h. (B) MCF-7 cells treated with 100 nmol/L 25(OH)D3, 100 nmol/L 1,25(OH)2D3, or vehicle in 5% FBS for 48 h. (C) MCF-7 cells treated with 100 nmol/L 25(OH)D3 in the presence of 5% FBS or 0.1% FBS with or without 0.02 g/L DBP. (D) HME cells treated with 100 nmol/L 25(OH)D3 in the presence or absence of 0.02 g/L DBP. Data were analyzed with ANOVA and Tukey’s post-test. Data are means ± SEM, n = 3. Bars with different letters differ, P < 0.001.
Impaired responsiveness to 25(OH)D3 but not 1,25(OH)2D3 in the setting of transcriptionally active VDR could arise from multiple mechanisms including inactive CYP27B1 or poor substrate availability to the enzyme. In support of the latter mechanism, HME cells are cultured in serum-free medium, whereas MCF-7 cells are cultured in medium containing 5% FBS, a rich source of DBP. We therefore hypothesized that DBP (which binds tightly to 25(OH)D3 and less avidly to 1,25(OH)2D3) present in the serum-containing medium might sequester 25(OH)D3 and prevent its uptake and subsequent metabolism to 1,25(OH)2D3 in MCF-7 cells. To test this hypothesis, we manipulated the concentration of FBS in the medium and compared VDR activation of the CYP24 reporter gene in MCF-7 cells treated with 25(OH)D3 in the presence or absence of DBP (Fig. 1C). When the medium FBS concentration was reduced to 0.1%, 25(OH)D3 induced the CYP24 promoter in MCF-7 cells, demonstrating that CYP27B1 is indeed capable of converting 25(OH)D3 into 1,25(OH)2D3 in these cells. However, when MCF-7 cells in 0.1% serum were treated with 25(OH)D3 in the presence of 0.02 g/L DBP (an amount equivalent to that present in 5% human serum), no induction of CYP24 was observed, indicating that physiological concentrations of DBP block the activation of 25(OH)D3, most likely by binding and sequestering it outside of the cell. Surprisingly, and in contrast to MCF-7 breast cancer cells, 25(OH)D3 induced the CYP24 promoter in the nontransformed HME cells in both the presence and absence of DBP (Fig. 1D). Of note, the presence of DBP did not impair responsiveness to 1,25(OH)2D3 (as measured by induction of CYP24) in either cell line (data not shown).
To determine whether the differential responses to 25(OH)D3 might be related to transformation, we compared the ability of 25(OH)D3 to induce CYP24 in MCF-7 cells and in T-47D cells, another human breast cancer cell line. MCF-7 and T-47D cells express CYP27B1 mRNA (6) and protein (Fig. 2) at equivalent levels. The ability of 1,25(OH)2D3 and 25(OH)D3 to induce endogenous CYP24 gene expression, as measured by real time PCR, was compared in these cell lines grown under identical medium conditions (RPMI containing 10% FBS). Consistent with the CYP24 reporter gene data (Fig. 1C), 25(OH)D3 did not induce endogenous CYP24 gene expression in MCF-7 cells under these conditions, although 1,25(OH)2D3 robustly induced this VDR target gene (Table 1). In contrast, both 25(OH)D3 and 1,25(OH)2D3 induced CYP24 gene expression in T-47D cells (464- and 982-fold, respectively). Collectively, these data suggest that serum and DBP interfere with 25(OH)D3 actions in MCF-7 cells, but that some cells (HME, T-47D) are capable of responding to 25(OH)D3 even in the presence of DBP.
Figure 2.
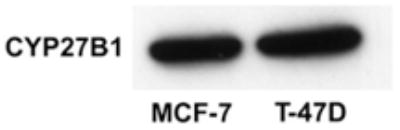
CYP27B1 protein expression in mammary breast cancer cell lines. CYP27B1 protein levels in MCF-7 and T-47D cells were detected by western blot using a polyclonal anti-CYP27B1 antibody. Equal loading was confirmed by Ponceau staining (not shown).
TABLE 1.
Endogenous CYP24 gene expression in MCF-7 and T-47D cells treated with 1,25(OH)2D3 and 25(OH)D3 in the presence of 10% FBS1
| Cell line | Treatment | CYP24 induction |
|---|---|---|
| Fold of control | ||
| MCF-7 | Control | 1.0 ± 0.1 |
| 1,25(OH)2D3 | 251 ± 56.7** | |
| 25(OH)D3 | 2.7 ± 0.5 | |
| T-47D | Control | 1.0 ± 0.1 |
| 1,25(OH)2D3 | 982 ± 232.5** | |
| 25(OH)D3 | 464 ± 67.8* |
Values are means ± SEM, n = 5. Asterisks indicate different from control for each cell line,
P < 0.01,
P < 0.005.
HME and T-47D, but not MCF-7, cells internalize DBP
Because several cell types, including renal tubular epithelial cells, BN-MSV cells, and B-lymphocytes (2,14) internalize DBP via endocytosis, we examined whether uptake of DBP occurred in breast-derived cells. When DBP conjugated to Alexa-488 was incubated with HME (Fig. 3A) or T-47D (Fig. 3B) cells in serum-free medium, intracellular fluorescence was detected within 30 min in most, if not all, cells. When MCF-7 cells were incubated under the same conditions, no uptake of Alexa-DBP was detected after 30 min (Fig. 3B), or when examined at shorter (15 min) or longer (1 h) incubation times (not shown). These data clearly indicated that under identical conditions, HME and T-47D, but not MCF-7, mammary cells are capable of rapid internalization of DBP. Furthermore, internalized DBP was restricted to the cytosolic compartment, and did not colocalize with nuclei in either HME cells (Fig. 3A) or T47D cells (not shown).
Figure 3.
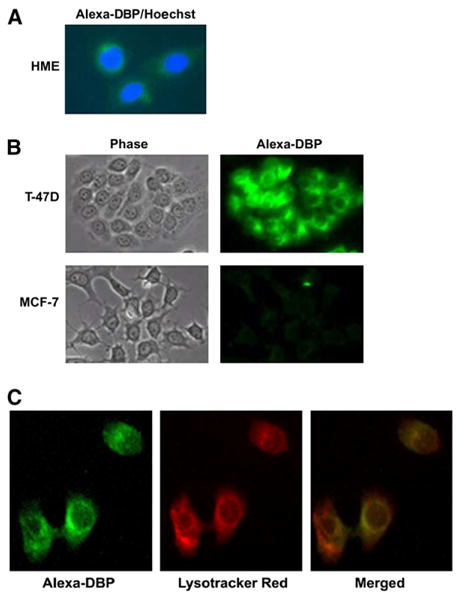
DBP uptake in human breast-derived cell lines. (A) HME cells were incubated for 30 min in serum-free medium containing 0.02 g/L DBP conjugated to Alexa-488 (green). Nuclei were counterstained with Hoechst dye (blue). Fluorescent images were captured on a Leica DMRXA2 microscope with a Hamamatsu Orca-ER camera and merged. (B) T-47D and MCF-7 cells were incubated for 30 min in serum-free medium containing 0.02 g/L DBP conjugated to Alexa-488. Phase contrast (left panels) and fluorescent images (right panels) were captured on an Olympus AX70 microscope with a Spot RT camera in parallel for both cell lines with identical exposure times. (C) T-47D cells were coincubated with 0.02 g/L DBP conjugated to Alexa-488 and 75 nmol/L lysotracker red for 30 min. Fluorescent images were captured on an Olympus AX70 microscope with a Spot RT digital camera. Left panel: Alexa-DBP fluorescence, middle panel: Lysotracker red fluorescence, right panel: merged image.
Megalin and cubilin are expressed in mammary cells and whole mammary gland
Because the coreceptors megalin and cubilin are essential mediators of endocytic uptake of DBP in renal cells (2,3), we used real time PCR to examine whether mammary cells express these genes. Megalin expression was detected in immortalized HME cells and in T-47D and MCF-7 breast cancer cell lines, whereas cubilin was detected only in HME and T-47D cells (Table 2). Both cubilin and megalin mRNA were detected in whole murine mammary gland samples obtained during the reproductive cycle. The expression was highest during pregnancy and lactation, when the epithelial compartment of the gland undergoes full secretory differentiation. Megalin and cubilin gene expression in the epithelial compartment of the gland was verified by analysis of RNA derived from mammary ducts isolated by collagenase digestion. These analyses indicated that mammary epithelial cells in vivo express both megalin and cubilin, and that coexpression of these genes are retained in some, but not all, established epithelial cell lines of mammary origin.
TABLE 2.
Megalin and cubilin expression in mammary cells and tissues1
| Cell/Tissue | Megalin | Cubilin |
|---|---|---|
| MCF-7 | +++++ | Undetectable |
| T-47D | +++ | +++ |
| HME | ++ | ++ |
| Mammary ducts2 | ++++ | ++++ |
| Murine gland3 | + to +++ | + to +++ |
Measured by real time PCR and expressed as signal intensity relative to standard curve (n = 3–5); graded from low (+) to high (+++++) expression.
Epithelial cells purified from virgin murine mammary glad (n = 5).
Assessed during reproductive cycle (n = 3–5); lowest levels detected during puberty; highest levels detected during pregnancy and lactation.
DBP internalization occurs by endocytosis and is blocked by megalin inhibition
The demonstration that T-47D cells expressed the endocytic coreceptors megalin and cubilin suggested that DBP uptake in these cells could be mediated by megalin-mediated endocytosis. To test this suggestion, we first examined whether newly internalized DBP in T-47D cells colocalized with lysotracker, a probe for the late endosomal pathway. Alexa-DBP (Fig. 3C, left panel, green) localized to cytosol and peri-nuclear regions, similar to that reported for receptor-mediated endocytosis of DBP in B-lymphocytes and BN/MSV cells (2,3,14). Lysotracker (middle panel, red) localized predominantly to peri-nuclear regions, and the merged image (right panel, yellow) indicated substantial (but not complete) colocalization. Under identical conditions, DBP also colocalized with rhodamine dextran, which labels the entire endocytic pathway (data not shown). These data indicate that, like other megalin ligands, DBP traffics through endosomes and lysosomes after internalization in T-47D cells.
The requirement for endocytosis during DBP uptake in T-47D cells was confirmed with temperature-shift techniques. Alexa-DBP uptake into the cytoplasm and perinuclear regions of T-47D cells (Fig. 4, top panels) was readily detected in cells incubated at 37°C, a temperature conducive to endocytosis. As expected for an endocytic process, intracellular DBP did not colocalize with the nuclear stain Hoechst. When cells were incubated with Alexa-DBP at 4°C, a temperature that disrupts microtubules and blocks endocytosis, DBP uptake was completely blocked (Fig. 4, middle panels). To provide evidence that megalin and cubilin are required for DBP uptake, we utilized an inhibitor of megalin-mediated endocytosis, RAP, which binds to the extracellular domain of megalin and inhibits ligand binding and internalization (2). When T-47D cells were coincubated at 37°C with Alexa-DBP and RAP, Alexa-DBP uptake was markedly blunted, with low levels of fluorescence detected in only 1–2 cells per field (Fig. 4, bottom panels). Collectively, this series of studies supports the concept that internalization of DBP in T-47D breast cancer cells occurs via endocytosis and is facilitated by the membrane localized co-receptors megalin and cubilin.
Figure 4.
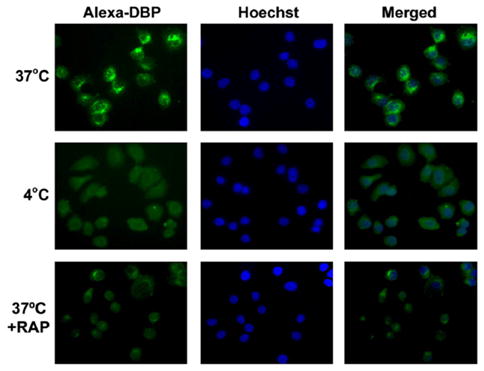
Effect of temperature shift and the megalin inhibitor RAP on DBP uptake in T-47D cells. T-47D cells were incubated at 37°C (top row) or 4°C (middle row) in serum-free medium with 0.02 g/L Alexa-DBP in the absence (top, middle rows) or presence (bottom row) of 1 μmol/L RAP for 30 min. Cells were fixed and counterstained with Hoechst for visualization of nuclei. Alexa-DBP (green fluorescence, left panels) and Hoechst (blue fluorescence, center panels) images were captured in parallel with identical exposure times for all conditions.
Discussion
With the recognition that 1,25(OH)2D3 mediates antiproliferative and prodifferentiating signaling through the VDR (15), attention has focused on whether tissues other than the kidney can generate 1,25(OH)2D3 (7). In support of this concept, the mitochondrial CYP27B1 enzyme capable of converting 25(OH)D3 to 1,25(OH)2D3 is present in both normal and transformed mammary epithelial cells (5),(7). Although the presence of CYP27B1 in mammary cells suggests that local activation of 25(OH)D3 can occur, this suggestion presumes that circulating 25(OH)D3 is accessible to the intracellular enzyme. Despite the recognition that 99% of 25(OH)D3 is delivered to sites of metabolism and storage in complex with DBP, the mechanisms for cellular uptake of 25(OH)D3-DBP in extrarenal tissues have not been defined. In these studies, we have demonstrated that internalization of the 25(OH)D3-DBP complex occurs by megalin-mediated endocytosis in mammary cells. HME and T-47D cells were identified as established, mammary-derived cell lines that coexpress megalin and cubilin and internalize DBP by endocytosis. In contrast, MCF-7 breast cancer cells express megalin but no detectable cubilin and fail to internalize DBP. More importantly, activation of a VDR-dependent reporter gene by 25(OH)D3 in the presence of DBP was demonstrated in T-47D cells as well as nontransformed HME cells, indicating that internalization of the 25(OH)D3-DBP complex can be correlated to activation of the VDR pathway. In MCF-7 cells, 25(OH)D3 failed to activate the VDR-dependent reporter gene in the presence of DBP despite the presence of CYP27B1 and VDR. Because both megalin and cubilin are known to be essential for renal uptake of DBP (3), the lack of cubilin expression may underlie the inability of MCF-7 cells to internalize the 25(OH)D3-DBP complex. However, because other explanations have not been ruled out by our data, further studies will be required to clarify the basis for impaired DBP uptake in MCF-7 cells and to confirm the role of megalin in DBP internalization in HME and T47D cells.
Although our DBP uptake data were obtained with mammary cells in culture, they raise the possibility that the mammary gland in vivo may be capable of transporting vitamin D metabolites bound to DBP via receptor-mediated endocytosis. Both DBP and 25(OH)D3 are present in breast milk (16), but whether endocytosis in general, or the megalin-cubilin complex in particular, participates in transport of either molecule across the mammary epithelia is, at present, unknown. In support of this possibility, we detected megalin and cubilin gene expression in normal murine mammary gland as well as isolated mammary ducts, and megalin protein has been identified in human mammary gland (17). Furthermore, megalin expression was highest in glands removed during pregnancy and lactation, and supplementation of mice with estrogen and progesterone to simulate pregnancy enhanced megalin expression in mammary ductal epithelial cells 50-fold (M. Rowling and J. Welsh, unpublished data). These observations warrant further study on the impact of megalin-mediated endocytosis on 25(OH)D3 uptake, transcytosis, storage, and metabolism in mammary epithelia.
In addition to endocytosis of 25(OH)D3-DBP, megalin mediates the cellular uptake of many serum transport proteins, including those that act as carriers for the steroid hormones androgen and estrogen as well as lipophilic vitamins such as retinol (1,18–21). As these bioactive molecules have important roles in regulation of cell proliferation, differentiation, and survival, the coexpression of megalin and cubilin in mammary cells, reported here, to our knowledge, for the first time, could have important implications for both development and transformation in this tissue. Indeed, studies with megalin knockout mice have demonstrated that megalin-mediated endocytosis plays a functional role in several epithelial tissues, including the thyroid gland, gall bladder, and both male and female reproductive organs (19,22–27). Unfortunately, the limited viability of megalin knockout mice (2) precludes assessment of the impact of megalin on transport of vitamins in the mammary gland in vivo until a tissue-specific knockout is generated.
In summary, to our knowledge, these are the first studies to demonstrate that mammary epithelial cells coexpress megalin and cubilin, which contribute to the endocytic uptake of 25(OH)D3-DBP and activation of the VDR pathway. An important implication of these observations is that extra-renal hydroxylation of 25(OH)D3 and autocrine production of the VDR ligand 1,25(OH)2D3 may require the presence of functional megalin and cubilin for uptake of the circulating 25(OH)D3-DBP complex.
Acknowledgments
The authors thank Jessica Hornick for assistance with fluorescent imaging of HME cells, Lydia Endel for assistance with initial FBS studies, and Dr. Glendon Zinser for procurement of mammary tissues during development.
Abbreviations used
- 25(OH)D3
25-hydroxycholecalciferol
- 1
25(OH)2D3, 1,25-dihydroxycholecalciferol
- CYP
cytochrome P450
- DBP
vitamin D binding protein
- HME
human mammary epithelial
- RAP
receptor-associated protein
- VDR
vitamin D receptor
Footnotes
Supported by NIH grants CA103018 and CA96700 to J.W. and a Susan G. Komen Foundation Post-Doctoral award to M.R.
Literature Cited
- 1.Moestrup SK, Verroust PJ. Megalin- and cubilin-mediated endocytosis of protein-bound vitamins, lipids, and hormones in polarized epithelia. Annu Rev Nutr. 2001;21:407–28. doi: 10.1146/annurev.nutr.21.1.407. [DOI] [PubMed] [Google Scholar]
- 2.Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, Melsen F, Christensen E, Willnow T. An endocytic pathway essential for renal uptake and activation of the steroid 25(OH)hydroxyvitamin D3. Cell. 1999;96:507–15. doi: 10.1016/s0092-8674(00)80655-8. [DOI] [PubMed] [Google Scholar]
- 3.Nykjaer A, Fyfe J, Kozyraki R, Leheste JR, Jacobsen C, Nielsen MS, Verroust PJ, Aminoff M, de la Chapelle, et al. Cubilin dysfunction causes abnormal metabolism of the steroid hormone 25(OH) vitamin D(3) Proc Natl Acad of Sci USA. 2001;98:13895–900. doi: 10.1073/pnas.241516998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, Hewison M. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab. 2001;86:888–94. doi: 10.1210/jcem.86.2.7220. [DOI] [PubMed] [Google Scholar]
- 5.Zinser GM, Welsh J. Accelerated mammary gland development during pregnancy and delayed post-lactational involution in vitamin D3 receptor null mice. Mol Endocrinol. 2004;18:2208–23. doi: 10.1210/me.2003-0469. [DOI] [PubMed] [Google Scholar]
- 6.Townsend K, Banwell CM, Guy M, Colston KW, Mansi JL, Stewart PM, Campbell MJ, Hewison M. Autocrine metabolism of vitamin D in normal and malignant breast tissue. Clin Cancer Res. 2005;11:3579–86. doi: 10.1158/1078-0432.CCR-04-2359. [DOI] [PubMed] [Google Scholar]
- 7.Townsend K, Evans KN, Campbell MJ, Colston KW, Adams JS, Hewison M. Biological actions of extra-renal 25-hydroxyvitamin D-1alpha-hydroxylase and implications for chemoprevention and treatment. J Steroid Biochem Mol Biol. 2005;97:103–9. doi: 10.1016/j.jsbmb.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Kemmis CK, Salvador SM, Smith KM, Welsh JE. Human mammary epithelial cells express CYP27B1 and are growth inhibited by 25-hydroxyvitamin D3, the major circulating form of vitamin D3. J Nutr. 2006;136:887–92. doi: 10.1093/jn/136.4.887. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz GG, Whitlatch LW, Chen TC, Lokeshwar BL, Holick MF. Human prostate cells synthesize 1,25-dihydroxyvitamin D3 from 25-hydroxyvitamin D3. Cancer Epidemiol Biomarkers Prev. 1998;7:391–5. [PubMed] [Google Scholar]
- 10.Welsh J, Wietzke JA, Zinser GM, Byrne B, Smith K, Narvaez CJ. Vitamin D-3 receptor as a target for breast cancer prevention. J Nutr. 2003;133:2425S–33S. doi: 10.1093/jn/133.7.2425S. [DOI] [PubMed] [Google Scholar]
- 11.Kunz C, Niesen M, von Lilienfeld-Toal H, Burmeister W. Vitamin D, 25-hydroxy-vitamin D and 1,25-dihydroxy-vitamin D in cow’s milk, infant formulas and breast milk during different stages of lactation. Int J Vitam Nutr Res. 1984;54:141–8. [PubMed] [Google Scholar]
- 12.Hollis BW, Pittard WB, 3rd, Reinhardt TA. Relationships among vitamin D, 25-hydroxyvitamin D, and vitamin D-binding protein concentrations in the plasma and milk of human subjects. J Clin Endocrinol Metab. 1986;62:41–4. doi: 10.1210/jcem-62-1-41. [DOI] [PubMed] [Google Scholar]
- 13.Zinser GM, McEleney K, Welsh J. Characterization of mammary tumor cell lines from wild type and vitamin D3 receptor knockout mice. Mol Cell Endocrinol. 2003;200:67–80. doi: 10.1016/s0303-7207(02)00416-1. [DOI] [PubMed] [Google Scholar]
- 14.Esteban C, Geuskens M, Ena JM, Mishal Z, Macho A, Torres JM, Uriel J. Receptor-mediated uptake and processing of vitamin D-binding protein in human B-lymphoid cells. J Biol Chem. 1992;267:10177–83. [PubMed] [Google Scholar]
- 15.Colston KW, Welsh JE. Vitamin D and breast cancer. In: Feldman D, Pike JW, Gloriuex F, editors. Vitamin D. 2. Amsterdam: Elsevier; [Google Scholar]
- 16.Hollis BW, Roos BA, Draper HH, Lambert PW. Vitamin D and its metabolites in human milk. J Nutr. 1981;111:1240–8. doi: 10.1093/jn/111.7.1240. [DOI] [PubMed] [Google Scholar]
- 17.Lundgren S, Carling T, Hjalm G, Juhlin C, Rastad J, Pihlgren U, Rask L, Akerstrom G, Hellman P. Tissue distribution of human gp330/megalin, a putative calcium sensing protein. J Histochem Cytochem. 1997;45:383–92. doi: 10.1177/002215549704500306. [DOI] [PubMed] [Google Scholar]
- 18.Matarese V, Lodish HF. Specific uptake of retinol-binding protein by variant F9 cell lines. J Biol Chem. 1993;268:18859–65. [PubMed] [Google Scholar]
- 19.Hammes A, Andreassen TK, Spoelgen R, Raila J, Hubner N, Schulz H, Metzger J, Schweigert FJ, Luppa PB, et al. Role of endocytosis in cellular uptake of sex steroids. Cell. 2005;122:751–62. doi: 10.1016/j.cell.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 20.Raila J, Willnow TE, Schweigert FJ. Megalin-mediated reuptake of retinol in the kidneys of mice is essential for vitamin A homeostasis. J Nutr. 2005;135:2512–6. doi: 10.1093/jn/135.11.2512. [DOI] [PubMed] [Google Scholar]
- 21.Christensen EI, Moskaug JO, Vorum H, Jacobsen C, Gundersen TE, Nykjaer A, Blomhoff R, Willnow TE, Moestrup SK. Evidence for an essential role of megalin in transepithelial transport of retinol. J Am Soc Nephrol. 1999;10:685–95. doi: 10.1681/ASN.V104685. [DOI] [PubMed] [Google Scholar]
- 22.Marino M, Zheng G, Chiovato L, Pinchera A, Brown D, Andrews D, McCluskey RT. Role of megalin (gp330) in transcytosis of thyroglobulin by thyroid cells. A novel function in the control of thyroid hormone release. J Biol Chem. 2000;275:7125–37. doi: 10.1074/jbc.275.10.7125. [DOI] [PubMed] [Google Scholar]
- 23.Marino M, Zheng G, McCluskey RT. Megalin (gp330) is an endocytic receptor for thyroglobulin on cultured fisher rat thyroid cells. J Biol Chem. 1999;274:12898–904. doi: 10.1074/jbc.274.18.12898. [DOI] [PubMed] [Google Scholar]
- 24.Muller D, Nykjaer A, Willnow TE. From holoprosencephaly to osteopathology: role of multifunctional endocytic receptors in absorptive epithelia. Ann Med. 2003;35:290–9. doi: 10.1080/07853890310006488. [DOI] [PubMed] [Google Scholar]
- 25.Hermo L, Lustig M, Lefrancois S, Argraves WS, Morales CR. Expression and regulation of LRP-2/megalin in epithelial cells lining the efferent ducts and epididymis during postnatal development. Mol Reprod Dev. 1999;53:282–93. doi: 10.1002/(SICI)1098-2795(199907)53:3<282::AID-MRD4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 26.Argraves WS, Morales CR. Immunolocalization of cubilin, megalin, apolipoprotein J, and apolipoprotein A-I in the uterus and oviduct. Mol Reprod Dev. 2004;69:419–27. doi: 10.1002/mrd.20174. [DOI] [PubMed] [Google Scholar]
- 27.Erranz B, Miquel JF, Argraves WS, Barth JL, Pimentel F, Marzolo MP. Megalin and cubilin expression in gallbladder epithelium and regulation by bile acids. J Lipid Res. 2004;45:2185–98. doi: 10.1194/jlr.M400235-JLR200. [DOI] [PubMed] [Google Scholar]


