Abstract
A recent study found high levels of HIV-1 bound to erythrocytes in HIV-1-infected patients with long-term undetectable plasma HIV-1 viral load (VL), potentially representing a novel and important reservoir of HIV-1 infection. To attempt to confirm this finding, we purified erythrocytes from 13 HIV-1-infected patients with long-term undetectable plasma VL and depleted contaminating CD3+/CD4+ lymphocytes using magnetic beads. HIV-1 VL of the purified erythrocyte fraction was < 20 copies/mL in 11 of 13 patients and 42 and 52 copies/mL in 2 patients. Contrary to the prior report, therefore, erythrocytes do not represent a novel reservoir of HIV-1 infection in these patients.
Keywords: HIV-1 reservoir, viral load, erythrocytes, circulating immune complex, complement receptor
HIV-1 infection cannot be eradicated from patients despite long-term suppression of viremia with antiretroviral therapy (ART) due, in large part, to persistent reservoirs of infection. Persistent reservoirs that have been described include kidney [1], central nervous system [1,2], the female and male genital tracts [3], lymphoid organs including the gut [4], and most prominently, resting CD4 lymphocytes [4]. HIV-1 has been demonstrated within circulating immune complexes of patients who were not receiving antiretroviral therapy (ART) [5, 6], and subsequent studies have shown that these HIV-1-containing immune complexes can bind to erythrocytes in vitro, presumably to CR1, the abundant erythrocyte complement receptors [7, 8]. In a more recent report, Hess et al. [9] fractionated whole blood from patients receiving ART and found significantly higher levels of HIV-1 RNA in the erythrocyte fraction than in the plasma fraction of the same individuals; the differences were especially marked in patients who had plasma viral loads (VL) < 20 copies/mL. These findings, especially in those patients who had undetectable plasma viremia, would represent a novel reservoir of HIV-1 infection and suggest that there is a much larger burden of circulating infectious virus than current models of HIV-1 pathogenesis predict. We therefore attempted to confirm these findings, but we used a more efficient method of lymphocyte depletion to obtain erythrocytes of higher purity.
METHODS
Patients
Patients were recruited from the outpatient HIV clinic at The Mount Sinai Medical Center. Patients were enrolled under a protocol approved by the Mount Sinai School of Medicine IRB and written informed consent was obtained from all patients prior to enrollment. Experimentation guidelines of the US Department of Health and Human Services and the Mount Sinai School of Medicine IRB were followed in the conduct of this research.
Laboratory Procedures
Erythrocyte purification
Whole blood was drawn into EDTA-anticoagulated tubes, immediately placed on ice, and separated by centrifugation at 600 x g for 10 minutes at 4°C. The platelet-rich plasma and buffy coat were aspirated and discarded and the pelleted erythrocyte fraction was washed 4 times in phosphate-buffered saline (PBS) on ice. Washed erythrocytes were depleted of CD3+ lymphocytes using magnetic beads coated with a monoclonal antibody to CD3 (Dynabeads CD3, Dynal). Erythrocytes were also purified using the protocol of Hess et al. [9] for comparison purposes. Briefly, the pelleted erythrocyte fraction was prepared as above, brought to 1% dextran final, and allowed to sediment on ice at 1 x g for approximately one hour until maximal separation occurred. The supernatant and interface layer were aspirated and discarded, and the erythrocyte fraction was washed 4 times in PBS on ice.
HIV-1 VL assay
For measurement of erythrocyte-associated HIV-1 VL, an aliquot of purified erythrocytes was lysed with sterile water and HIV-1 VL quantitation was performed by The Mount Sinai Medical Center clinical laboratory on 1 mL using the Roche Amplicor kit and the ultrasensitive methodology (Roche Amplicor HIV-1 Monitor Test v.1.5, sensitivity 20 copies/mL).
Assay for residual CD3+/CD4+ lymphocytes
Purified erythrocytes were assayed for residual contaminating CD3+/CD4+ lymphocytes by FACS analysis using a quantitative internal bead standard (TriTEST CD3/CD4/CD45 staining reagent; TruCOUNT tubes; MultiSET software; FACSCalibur flow cytometer, all Becton Dickinson). Briefly, 50 μL of the purified erythrocyte fraction was added to a TruCOUNT tube that contained the TriTEST CD3-FITC/CD4-PE/CD45-PerCP staining reagent and a fixed, specified number of beads. The tubes were incubated for 20 minutes at room temperature, 0.45 mL of FACS lysing solution was added, and the material assayed on the FACSCalibur flow cytometer. The CD45+ and CD4+ cell populations and bead the population were gated automatically by the MultiSET software, but the gates were adjusted manually for added precision, especially in the samples post-CD4-depletion. The absolute counts of CD3+/CD4+ lymphocytes were calculated automatically by the MultiSET software. Counts were validated by manual calculation using the formula: (number of events in gated region containing CD3+/CD4+ lymphocytes ÷ number of events in gated region containing beads) × (number of beads per tube ÷ 50 μL) = absolute count of CD3+/CD4+ lymphocytes.
RESULTS
Thirteen consecutive HIV-1-infected patients who met the inclusion criterion of long-term aviremia were enrolled. Eleven patients were receiving ART and 2 “long-term non-progressors” were not receiving ART. The duration of aviremia and the CD4+ count at the time of the study for each patient are shown in Table 1. Median time of aviremia (< 50 copies/mL) was 48 months; median CD4 count was 513 cells/μL. Dextran sedimentation of the erythrocyte fraction using the protocol of Hess et al. [9] (N = 12) resulted in significant lymphocyte contamination of the erythrocyte fraction in 70% of specimens (median residual lymphocytes, 200/μL; median depletion, 83%). Magnetic bead depletion of CD3+/CD4+ lymphocytes, however, was consistently extremely efficient. Quantitative FACS analysis of these specimens using TruCOUNT beads, a highly reproducible and accurate technique validated in patients with HIV infection [10], demonstrated a median of 4 contaminating CD3+/CD4+ lymphocytes per 1 μL of blood (Table 1; see Figure 1 for representative FACS plots). HIV-1 VL in these CD3+/CD4+ lymphocyte-depleted erythrocyte fractions were indistinguishable from background (< 20 copies/mL blood) in 11 of 13 patients; only 2 patients’ specimens had values over background (42 and 52 copies/mL blood) (Table 1), well within the false-positive measurement range of this PCR-based HIV-1 VL method. The internal quantitation standards (QS) of all samples assayed were amplified within the normal efficiency range (data not shown). In summary, we have found no evidence that there is a significant quantity of HIV-1 bound to erythrocytes in patients with long-term aviremia. We therefore conclude that erythrocytes do not represent a reservoir of HIV-1 infection in these patients.
Table 1.
Patient Characteristics and Erythrocyte HIV-1 VL after CD4-depletion
| Patient | Duration plasma HIV-1 VL < 50/mL (months) | CD4 count (cells/μL) | CD4 count post depletion (cells/μL) | CD4 depletion (%) | Erythrocyte HIV-1 VL (copies/mL blood) |
|---|---|---|---|---|---|
| 407D | 24 | 579 | 2 | 99.7 | < 20 |
| 412C | 54 | 474 | 1 | 99.8 | < 20 |
| 415Ca | 45 | 923 | 3 | 99.7 | 42 |
| 423C | 59 | 905 | 9 | 99.0 | < 20 |
| 430Ca | 54 | 1280 | 6 | 99.5 | < 20 |
| 435C | 16 | 413 | 8 | 98.1 | < 20 |
| 437B | 13 | 568 | <1 | 99.9 | < 20 |
| 438B | 3 | 54 | 4 | 92.6 | 52 |
| 439B | 52 | 443 | <1 | 99.8 | < 20 |
| 443A | 53 | 1645 | 40 | 97.6 | < 20 |
| 444A | 44 | 232 | <1 | 99.7 | < 20 |
| 445A | 59 | 513 | 4 | 99.2 | < 20 |
| 446A | 16 | 382 | 5 | 98.7 | < 20 |
long-term non-progressor
Figure 1. FACS analysis of erythrocyte fraction of patient 437B.
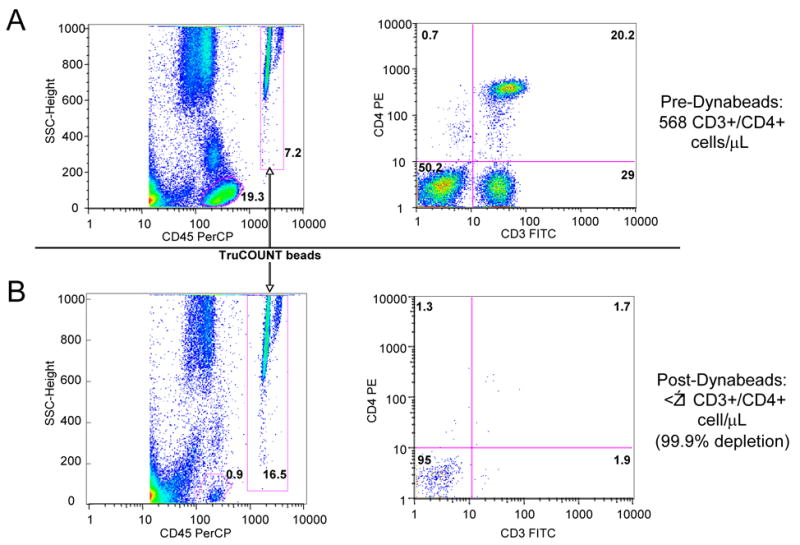
Row A shows FACS profile of erythrocytes before CD3+/CD4+ bead depletion. Row B shows FACS profile of erythrocytes after CD3+/CD4+ bead depletion. Left hand panels (above and below) show the location of the CD45+ cell gate (circled) and TruCOUNT beads gate (in box). Right hand panels (above and below) show CD4+ cells versus CD3+ cells (without beads). Calculated values to the right of the panels are the quantitative measurements based on absolute bead counts, not based on relative depletion, as described in the Methods section.
DISCUSSION
The implications of the presence of a pool of infectious HIV-1 particles associated with erythrocytes are significant. Erythrocytes, through surface complement receptors, bind complement- and antibody-opsinized pathogens [11], facilitating their removal from the blood stream by delivery to the splenic macrophages where the immune complexes are phagocytosed. HIV-1 has been demonstrated within circulating immune complexes (CIC) of patients who were not receiving antiretroviral therapy (ART) [5, 6]. Subsequent studies have shown that these HIV-1-containing immune complexes can bind to erythrocytes in vitro, presumably via their abundant surface complement receptors [7, 8], and it has been shown that levels of the complement receptor CR1 on erythrocytes from HIV-1-infected patients are significantly depressed compared to healthy controls [12], consistent with removal of the complex of CR1-CIC in the spleen. HIV-1, however, is only partially inactivated by complement or antibody binding [13], and could therefore be delivered as an infectious particle directly to an organ highly enriched with susceptible target cells for HIV-1 infection. It was therefore crucial to confirm the findings of Hess et al. [9] that erythrocytes purified from HIV-1-infected patients with undetectable plasma VL contained high levels of HIV-1 RNA, including infectious particles. What we found instead is that there was essentially no PCR-amplifiable HIV-1 RNA associated with circulating erythrocytes in 13 consecutive patients who had undetectable plasma viremia when we carefully quantitatively depleted lymphocytes from the erythrocyte fraction. Proviral HIV-1 DNA, which is present in CD3+/CD4+ lymphocytes of all HIV-1-infected patients regardless of duration of plasma HIV-1 VL suppression [4], is measured with at least equal efficiency as the HIV-1 RNA in viral particles in the Amplicor HIV-1 VL assay. Therefore we were careful to minimize contamination of the purified erythrocytes by CD3+/CD4+ lymphocytes to avoid obtaining spuriously high HIV-1 VL measurements. We also used the dextran sedimentation technique as described by Hess et al. but in our hands there were always significant numbers of residual contaminating lymphocytes. While this might be considered a negative finding in relationship to the report of Hess et al. [9], we have identified no step in our simple methodology that resulted in falsely negative VL measurements. For instance, hemoglobin has been reported to inhibit PCR reactions [14], but the QS of the Amplicor HIV-1 VL assay was uniformly amplified to expected levels in all cases, thereby excluding the possibility of spuriously negative results due to inhibition of the assay. In summary, while we cannot fully explain the findings of Hess et al. [9] of HIV-1 associated with erythrocytes in HIV-1-infected patients in vivo, the dextran gradient sedimentation purification of erythrocytes did not result in an adequate depletion of CD3+/CD4+ lymphocytes in our hands. We therefore believe that this report of HIV-1 associated with erythrocytes of HIV-1-infected patients in vivo [9] may have been due to undetected contamination of the erythrocytes by CD3+/CD4+ cells containing HIV-1 proviral DNA. In conclusion, we found that HIV-1 is not associated with erythrocytes in HIV-1-infected patients with long-term undetectable HIV-1 VL in plasma and therefore erythrocytes do not represent a novel reservoir of HIV-1 infection in these patients.
Acknowledgments
2) This work was supported by National Institutes of Health K23 AI50449 to D.S.F. and MO1-RR-00071 to the Mount Sinai School of Medicine
Footnotes
None of the authors have a commercial or other association that might pose a conflict of interest.
Presented in part at the 1st International Workshop on HIV Persistence During Therapy; St. Martin; 12 December 2003
References
- 1.Fierer DS, Klotman ME. Kidney and central nervous system as reservoirs of HIV infection. Curr Opin HIV AIDS. 2006;1:115–20. doi: 10.1097/01.COH.0000209581.88166.89. [DOI] [PubMed] [Google Scholar]
- 2.Kramer-Hammerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res. 2005;111:192–213. doi: 10.1016/j.virusres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Galvin SR, Cohen MS. Genital tract reservoirs. Curr Opin HIV AIDS. 2006;1:162–6. doi: 10.1097/01.COH.0000199799.06454.10. [DOI] [PubMed] [Google Scholar]
- 4.Stebbing J, Gazzard B, Douek DC. Where does HIV live? N Engl J Med. 2004;350:1872–80. doi: 10.1056/NEJMra032395. [DOI] [PubMed] [Google Scholar]
- 5.Morrow WJ, Wharton M, Stricker RB, Levy JA. Circulating immune complexes in patients with acquired immune deficiency syndrome contain the AIDS-associated retrovirus. Clin Immunol Immunopathol. 1986;40:515–24. doi: 10.1016/0090-1229(86)90196-0. [DOI] [PubMed] [Google Scholar]
- 6.McHugh TM, Stites DP, Busch MP, Krowka JF, Stricker RB, Hollander H. Relation of circulating levels of human immunodeficiency virus (HIV) antigen, antibody to p24, and HIV-containing immune complexes in HIV-infected patients. J Infect Dis. 1988;158:1088–91. doi: 10.1093/infdis/158.5.1088. [DOI] [PubMed] [Google Scholar]
- 7.Montefiori DC, Graham BS, Zhou JY, Zhou JT, Ahearn JM. Binding of human immunodeficiency virus type 1 to the C3b/C4b receptor CR1 (CD35) and red blood cells in the presence of envelope-specific antibodies and complement. J Infect Dis. 1994;170:429–32. doi: 10.1093/infdis/170.2.429. [DOI] [PubMed] [Google Scholar]
- 8.Olinger GG, Saifuddin M, Spear GT. CD4-Negative cells bind human immunodeficiency virus type 1 and efficiently transfer virus to T cells. J Virol. 2000;74:8550–7. doi: 10.1128/jvi.74.18.8550-8557.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hess C, Klimkait T, Schlapbach L, et al. Association of a pool of HIV-1 with erythrocytes in vivo: a cohort study. Lancet. 2002;359:2230–4. doi: 10.1016/S0140-6736(02)09291-7. [DOI] [PubMed] [Google Scholar]
- 10.Schnizlein-Bick CT, Spritzler J, Wilkening CL, Nicholson JK, O’Gorman MR. Evaluation of TruCount absolute-count tubes for determining CD4 and CD8 cell numbers in human immunodeficiency virus-positive adults. Site Investigators and The NIAID DAIDS New Technologies Evaluation Group. Clin Diagn Lab Immunol. 2000;7:336–43. doi: 10.1128/cdli.7.3.336-343.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson RA. The immune adherence phenomenon: an immunologically specific reaction between microorganisms and erythrocytes leading to enhanced phagocytosis. Science. 1953;118:733–737. doi: 10.1126/science.118.3077.733. [DOI] [PubMed] [Google Scholar]
- 12.Tausk FA, McCutchan A, Spechko P, Schreiber RD, Gigli I. Altered erythrocyte C3b receptor expression, immune complexes, and complement activation in homosexual men in varying risk groups for acquired immune deficiency syndrome. J Clin Invest. 1986;78:977–82. doi: 10.1172/JCI112688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stoiber H, Speth C, Dierich MP. Role of complement in the control of HIV dynamics and pathogenesis. Vaccine. 2003;21 (Suppl 2):S77–82. doi: 10.1016/s0264-410x(03)00203-2. [DOI] [PubMed] [Google Scholar]
- 14.Akane A, Matsubara K, Nakamura H, et al. Identification of the heme compound copurified with deoxyribonucleic acid (DNA) from bloodstains, a major inhibitor of polymerase chain reaction (PCR) amplification. J Forensic Sci. 1994;39:362–72. [PubMed] [Google Scholar]


