Abstract
Transcription factor cAMP-responsive element modulator (CREM) plays a key physiological and developmental role within the hypothalamic-pituitary-gonadal axis. The use of an alternative, intronic promoter within the CREM gene is responsible for the production of a cAMP-inducible repressor, inducible cAMP early repressor (ICER). ICER negatively autoregulates the ICER promoter, thus generating a feedback loop. We have previously documented a striking, clock-driven circadian fluctuation of CREM expression in the pineal gland. Oscillating ICER levels tightly correlate with fluctuations in the synthesis of the pineal hormone melatonin, whose production is also driven by the endogenous clock. Melatonin in turn regulates the hypothalamic-pituitary axis. The enzyme serotonin N-acetyltransferase (NAT) catalyzes the rate limiting step in melatonin synthesis. Thus, oscillations in NAT levels determine the circadian synthesis of melatonin. Here we demonstrate that NAT expression is dramatically increased in CREM-deficient mice that we have generated by homologous recombination. Characterization of the NAT promoter shows the presence of a ICER binding site. In addition, transfection studies show that ICER powerfully represses NAT transcription. Our results implicate CREM as a central regulator of output functions of the clock. Indeed, CREM acts as a key regulator of oscillatory hormonal synthesis.
A fundamental role of endocrine regulatory systems is to time physiological processes. Pulsatility, circadian, and seasonal rhythmicity characterize the action of many hormones (1, 2). Long-term physiological adaptations are mediated by changes in gene expression. Thus, the dynamic properties of transcriptional regulators constitute an essential link in the relay of hormonal information.
Rhythmic production of the hormone melatonin serves as an important day-night and seasonal endocrine signal (3, 4, 5). Clock-derived adrenergic signals are transduced into nighttime melatonin synthesis by the pineal gland (6, 7, 8). The cAMP signaling pathway activates the enzyme serotonin N-acetyltransferase (NAT) that catalyses the rate limiting step of melatonin synthesis (9, 10). Thus, NAT represents a key regulatory step for melatonin synthesis.
Transcriptional regulation by cAMP is mediated by a family of bZip transcription factors that bind to cAMP-responsive elements (CREs) (for review, see ref. 11). These factors function either as activators or repressors and their activity is tightly regulated by phosphorylation. Constitutively expressed factors such as CRE-binding protein (CREB, ref. 12) are phosphorylated by the cAMP-dependent protein kinase A (PKA) and converted into powerful transcriptional activators. This step constitutes the link between cAMP signaling and gene expression (13, 14, 15).
Among CRE regulators, the products of the CRE modulator (CREM) gene play a key role in the pituitary-hypothalamic-gonadal axis (16, 17, 18, 19, 20). CREM encodes a family of repressors and activators with characteristic tissue- and cell-specific expression. Specifically, a cAMP-inducible alternative promoter (P2), which lies near the 3′ end of the gene, directs expression of inducible cAMP early repressor (ICER) (21, 22). This small factor lacks the activation and kinase-inducible domains, and thereby functions as a dominant repressor of cAMP induced transcription (21, 22). Importantly, ICER down-regulates its own transcription via binding to CRE sites in the P2 promoter, thereby constituting a negative autoregulatory loop (21). We have shown that ICER plays an important regulatory role in several endocrine tissues (23, 24).
We have described a striking day–night rhythm of ICER mRNA expression in the rat pineal gland (22). ICER transcription is directed by the same clock-derived adrenergic signals that drive melatonin synthesis (22). Specifically, the peak of ICER mRNA occurs during the second part of the night, just preceding the decline of melatonin synthesis. The ICER peak also precedes the downregulation of NAT mRNA (25). The CREM day–night switch is developmentally regulated and matures postnatally in synchrony with rhythmic melatonin production (26, 27). We have also shown that the rate of nighttime induction of the CREM gene as well as its cAMP inducibility decrease upon adaptation to increasing night length (28, 29). Photoperiod-dependent changes in the mode of CREM induction accompany changes in the profile of melatonin synthesis (28, 29). Together, these observations support the notion that rhythmic ICER expression, and thus the CREM feedback loop, make an essential contribution to the downregulation of melatonin synthesis by repressing cAMP induced NAT transcription (25).
Here we directly address the role of ICER in shaping the rhythmic expression of NAT in the pineal gland. We demonstrate that NAT expression is deregulated in CREM-mutant mice and confirm that the NAT promoter is repressed by ICER. These findings implicate CREM as a key player in shaping oscillatory hormone synthesis.
MATERIALS AND METHODS
Experimental Animals.
Wistar male rats (40-day-old) together with wild-type (wt) strains of mice (8- to 10-week-old 129/Sv and C3H/He) were purchased from Iffa Credo (France) and housed in accordance with institutional guidelines under light/dark 12:12 lighting conditions (night from 19:00 to 07:00). For isoproterenol treatment, animals were i.p. injected with isoproterenol dissolved in physiological saline (10 mg/kg body weight). Five rats or 10 mice were sacrificed per point by decapitation.
RNA Analysis.
RNA was extracted from dissected pineal glands as described (22) and 0.5 μg aliquots were assayed using a miniaturized version of a standard RNase protection assay (16). All denaturing, annealing, and digestion steps were performed in 0.5 ml microcentrifuge tubes in a PCR thermal cycler. The CREM probe p6N1 has been described (16). The NAT probe was derived from the 5′ end of a rat NAT cDNA clone (30). The NAT protected fragment extends 157 nucleotide upstream of a unique KpnI site (located at position +377 relative to the rat transcription start site; ref. 30). The Fra-2 probe was derived from a mouse Fra-2 cDNA clone (31). The 147-nucleotide Fra-2 protected fragment extends from a KpnI site to an upstream AvaII site located at positions +319 and +172 relative to the translation start site respectively. Equal loading and quality of RNA was determined following reverse transcription–PCR quantitation of glucose 6-phosphate dehydrogenase transcript (20).
NAT Promoter Analysis.
The entire NAT rat gene was isolated from λEMBL3-SP6/T7 genomic phage clones as a 6.5-kb HindIII fragment. A 378-bp AvaII blunt-ended fragment was subsequently subcloned into the vector pBLCAT2 to generate the clone pPromNAT-chloramphenicol acetyltransferase (CAT) (32). A double-stranded oligonucleotide (5′-CCGATGACGCCAGCCCTCAGCA-3′) was cloned into the HindIII–XbaI sites of the vector pBLCAT3 making pNAT-CRE-CAT (32). Human choriocarcinoma JEG-3 cells were transfected and assayed for CAT activity as described (16). A standard gel mobility shift assay was used to test for binding to the CRE element (16) with the following modifications. Miniextracts were prepared from pools of five rat or 10 mouse pineal glands using a modified Dignam et al. method (33). Protein concentrations of each extract was determined using a Bio-Rad protein assay kit prior to each assay.
RESULTS
NAT Oscillates in the Mouse.
To directly define the contribution of ICER to the rhythmic expression of NAT, we chose to analyze NAT expression in mice that carry a null mutation at the CREM locus (34). Many inbred mouse strains have genetic defects in melatonin synthesis (35, 36). Amongst melatonin-deficient strains are those routinely used for transgenic and homologous recombination experiments (e.g., 129/Sv) (35). Accordingly, we first tested day–night CREM and NAT expression in pineal glands from melatonin- positive (C3H/He) and -negative (129/Sv) strains. Using RNase protection analysis we observe a robust day–night rhythm of both ICER and NAT expression in both strains (Fig. 1, lanes 2–5 and 7–10). This result demonstrates that the mutation that prevents melatonin synthesis in 129/Sv mice does not affect normal NAT and CREM rhythmic expression. We then tested the adrenergic inducibility of CREM expression. We confirmed that, as previously shown in the rat (22), isoproterenol injection induces CREM expression in the mouse (Fig. 1, lanes 11–14). We next wished to determine the precise pattern of CREM and NAT expression in the mouse pineal gland. Under light/dark 12:12 conditions, the kinetics of both ICER and NAT are identical to those previously reported for the rat (Fig. 2 A, C, and D; ref. 22).
Figure 1.
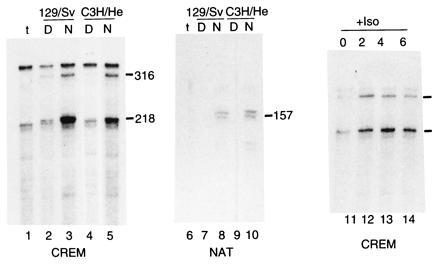
CREM and NAT rhythmic expression is conserved in melatonin-deficient and normal mouse strains. RNase protection assays of NAT and CREM mRNA expression in a melatonin deficient (129/Sv) and normal (C3H/He) mice (37). Mice were sacrificed at 12:00 (D) or 24:00 (N) as indicated (lanes 2–5 and 7–10). Also, 129/Sv mice were injected with isoproterenol (+Iso) and sacrificed 2, 4, or 6 hr later. Together with a noninjected control (0), these mice were assayed for CREM expression (lanes 11–14). t represents tRNA controls for both probes (lanes 1 and 6). For the CREM probe (p6N1 in ref. 16) the 316- and 218-nucleotide bands correspond to CREM transcripts incorporating DBDI and DBDII, respectively (16). For NAT, the mouse protected fragment is 157 nucleotides as in the rat. In both 129/Sv and C3H/He mice, rhythmic day–night expression of both NAT and CREM is evident. Furthermore CREM expression is induced by isoproterenol injection as reported for the rat (22).
Figure 2.
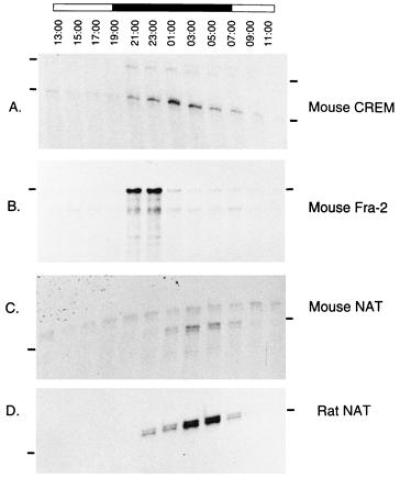
Day–night expression profiles of CREM, NAT and Fra-2 in the mouse and rat are identical. (A–C) Pineal RNA was extracted from mice sacrificed at the times indicated above the lanes and assayed for CREM, Fra-2, and NAT expression. (D) Rat RNA was extracted at the same time points and assayed for NAT mRNA. Both mice and rats were housed for 2 weeks prior to analysis in light/dark 12:12 lighting conditions (night from 19:00 to 07:00) as indicated by the black and white bar above the panels.
Another gene whose expression has been shown to oscillate in the pineal gland is the fos-related Fra-2 (31). Indeed, in the rat Fra-2 mRNA expression also appears to be directed by adrenergic signals and to be elevated during the first part of the night (37). Furthermore, Fra-2 has been implicated as a negative regulator of NAT expression (37). In the mouse we observe the same kinetics of Fra-2 expression as in the rat (Fig. 2B; ref. 37). Thus, the patterns of CREM, NAT, and Fra-2 expression indicate that rat and mouse pineal glands can be considered equivalent in terms of adrenergically regulated gene expression.
Aberrant NAT Rhythm in CREM-Deficient Mice.
We previously generated mice that are homozygous for a null mutation in the CREM locus (34). This mutation truncates the C-terminal DNA binding domain and thereby inactivates all CREM isoforms, including ICER. To define the role of ICER in the regulation of NAT, we tested NAT levels in CREM mutant mice sacrificed at selected time points throughout the night (Fig. 3A). With the exception of the 19:00 point where mutant and wt control animals display an equivalent low basal level of expression, at all other time points, the mutant animals have significantly higher levels of NAT mRNA. Consequently, the overall kinetics of NAT expression are modified in the mutants (Fig. 3C). Specifically, the NAT induction is detected earlier, arrives at a higher peak of expression and then persists longer than in wt siblings. In contrast, the kinetics and magnitude of Fra-2 expression is equivalent in wt and mutant animals (Fig. 3B). These results clearly implicate ICER as a negative regulator of NAT expression. Importantly, normal Fra-2 expression in the mutant animals demonstrates that ICER negative regulation does not extend to all adrenergically regulated genes. Furthermore the Fra-2 result confirms that clock-derived adrenergic signals are not grossly altered in the CREM mutant animals.
Figure 3.

Rhythmic NAT expression in CREM mutant mice is deregulated. Pineal RNA from wt (+/+) and CREM mutant (−/−) littermates that were sacrificed in parallel at the times indicated throughout the night, were analyzed for NAT (A) and Fra-2 (B) expression by RNase protection assay. The NAT transcript begins to increase at 23:00 in the mutant mice while a similar increase is detected later in the wt animals between 24:00 and 01:00. The peak of expression at 03:00 is considerably stronger in the mutant than in the wt animals. Furthermore, the NAT transcript remains higher in the mutant than the wt mice at 09:00. In contrast, Fra-2 expression has the same profile in both sets of mice as shown in Fig. 2B. (C). The times of sacrifice relative to the 12-hr night are shown schematically. Above is represented the kinetics of nighttime NAT mRNA expression in the wt (+/+; black shaded curve) and CREM mutant mice (−/−; grey shaded curve). Lack of CREM significantly perturbs the normal profile of NAT expression.
ICER Directly Regulates NAT Expression.
To uncover the molecular mechanisms whereby ICER downregulates NAT expression we characterized the promoter regulatory sequences of the NAT gene. We screened a rat phage genomic library with a 200-bp oligonucleotide probe derived from the 5′ end a full-length NAT clone (30). We isolated two overlapping phage clones that encompass the entire NAT gene and extend 10-kb upstream of the cDNA start point. After subcloning the coding and 5′ flanking region, sequence comparison with the original NAT cDNA clones revealed that the gene is divided into four exons. The first intron is the largest (≈2 kb) and is sited within the 5′ untranslated region, while the remaining two introns are significantly smaller and positioned within the coding region. We demonstrated that the 5′ boundary of the cDNA clones corresponds to the most 5′ transcription start site by both primer extension and RNase protection analysis using nighttime rat pineal RNA (data available upon request). Sequence analysis up to 154-bp upstream of the transcription start site is reported in Fig. 4A. The sequence reveals a region with a base composition of 43% AT, 57% GC, and the absence of any canonical CAAT or TATA boxes. However, a CRE element (TGACGCCA) different from the consensus (11) by only one mismatch is centered at position −108.
Figure 4.
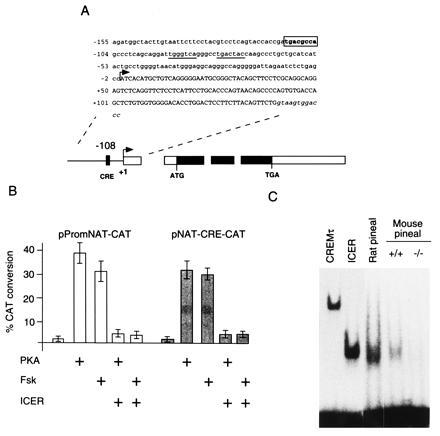
The NAT promoter is directly regulated by ICER. (A) Schematic representation of the exon-intron organization of the rat NAT gene. Black bars denote the coding region while white bars show transcribed, noncoding sequences. An arrowhead indicates the position of the most 5′ transcription start site. Three introns interrupt the cDNA at positions +141, +359, and +514 relative to the transcription start site. A hatched box represents the CRE-like element. Above is shown the sequence of the first exon and the 5′ flanking region. Lower case and upper case letters denote nontranscribed and transcribed sequences, respectively. Italic, lowercase letters show the sequence at the 5′ boundary of the first intron. Numbers in the left-hand margin indicate the position of the adjacent base relative to the transcription start site. A CRE site in the promoter region is boxed and shown in boldface. Two AP-1-like sites are underlined (between positions −70 and −90). The sequence of the 5′ AP-1-like site (TGGGTCA), however, has been previously reported not to constitute a functional AP-1 site (38). (B) Results of transient transfection experiments using plasmids with an NAT promoter fragment cloned upstream of the CAT reporter gene (pPromNAT-CAT) and the NAT CRE element (TGACGCCA) inserted 5′ of a thymidine kinase promoter-CAT heterologous gene (pNAT-CRE-CAT). Transfected cells were either treated with forskolin (Fsk) or cotransfected with an expression vector for the catalytic subunit of PKA, in the presence or absence of a cotransfected expression vector for ICERIIγ (20, 21). CAT assay results were quantified by counting radioactivity of chromatographic plates and expressed as a fold induction. Both the NAT promoter and CRE element direct cAMP-inducible transcription that is strongly repressed by ICER. (C) Gel mobility shift assay of binding to the NAT CRE element. Bacterially generated CREMτ and ICERII protein (CREMτ and ICER, respectively) and nuclear extracts prepared from the pineal glands of rat and wt (+/+) and CREM-mutant (−/−) mice were assayed for binding. Both rat and wt mice show a high mobility binding complex that comigrates with that generated by bacterial ICER protein. This complex is absent in the CREM-mutant extracts.
We initially tested whether the NAT promoter region is sufficient to direct cAMP inducible transcription and whether it can be downregulated by ICER. We cloned a 378-bp fragment of the NAT promoter including the first exon into a CAT reporter plasmid (pPromNAT-CAT) and performed transient transfection assays. Cotransfection with an expression vector for the PKA catalytic subunit or treatment of transfected cells with forskolin lead to a strong reporter activation (Fig. 4B). Cotransfection with substoichiometric concentrations of an ICER expression vector abolished PKA or forskolin-mediated induction. We next tested whether the CRE alone could confer cAMP inducibility and repression by ICER on a heterologous promoter. We therefore cloned the NAT element upstream of the thymidine kinase minimal promoter (pNAT-CRE-CAT) and performed an equivalent set of transfection assays. The results confirm that this element alone is sufficient to direct cAMP inducibility and repression by ICER (Fig. 4B).
ICER represses cAMP-induced transcription by binding directly to CRE elements as nonactivating homodimers or in heterodimeric complexes with other CRE binding factors (21). We thus wished to determine whether ICER is able to bind to the NAT CRE element. By gel mobility shift assay we confirmed that bacterially-generated ICER as well as the CREMτ activator protein are able to bind to the CRE in the NAT promoter (Fig. 4C). To test for binding in vivo, we next assayed nuclear extracts prepared from mouse and rat pineal glands. Interestingly, the NAT element binds predominantly a high mobility complex in both rat and mouse extracts that comigrates with the ICER bacterial protein complex. A slower mobility binding complex corresponding to activator proteins is also visible on longer exposures (not shown). To determine whether the pineal high mobility complex indeed contains ICER protein, we prepared nuclear extracts from the pineal glands of our CREM mutant mice. In these mutant mouse extracts, the complex is completely absent, demonstrating that endogenous ICER protein does indeed bind to the CRE element (Fig. 4C).
DISCUSSION
In this study we link rhythmic expression of a transcription factor in the pineal gland with shaping the profile of melatonin synthesis. Specifically, the timing and magnitude of nighttime induction of the melatonin synthetic hormone NAT are determined by the repressor ICER. CREM has already been shown to play diverse physiological roles in the neuroendocrine system (22, 23, 24). During spermatogenesis, the CREMτ activator is expressed at high levels in maturing spermatids and has been implicated in the regulation of several haploid-specific genes (19, 34). The notion that CREMτ functions as a master controller of haploid gene expression is supported by the complete block of spermiogenesis in mice that carry a null mutation in the CREM gene (34, 39). Furthermore, ICER has been implicated in the down-regulation of specific hormone receptor mRNAs in the process of long-term desensitization (23, 24). This report directly implicates CREM regulation in the timing of hormone production.
Here we identify a key transcriptional regulator of the NAT gene. The cloning of the NAT cDNA has facilitated the studies of the regulation of melatonin synthesis (30, 40). The remarkable species-to-species variation in the magnitude of the day–night NAT mRNA rhythm implies an inherent flexibility in its regulation. In this regard, posttranscriptional modifications also play a key role in the regulation of this enzyme (9). It will be of great interest to assess the relative contribution of ICER and other transcriptional regulators to melatonin rhythmicity in different species. This includes the avian and reptilian pineal glands where pinealocytes also possess photoreceptor function and an endogenous oscillator (41, 42, 43). An understanding of the conservation of NAT transcriptional regulation during evolution therefore may also shed light on the nature of basic clock regulatory mechanisms.
Interestingly, the expression of Fra-2 that is also directed by rhythmic adrenergic signals, is not affected in the CREM-mutant mice. Furthermore, there are clear differences in the timing of nighttime induction between the CREM, NAT, and Fra-2 genes. These findings indicate that negative regulation by ICER in the pineal gland does not extend equally to all cAMP regulated genes. Differential binding affinities of activators and repressors to various CRE elements may explain this observation. Recent results from our laboratory support this notion (unpublished work).
The normal rhythm of Fra-2 expression in the CREM mutant mice may participate in the downregulation of NAT mRNA. However, a direct assessment of the contribution of Fra-2 to NAT regulation in vivo must await the analysis of Fra-2 mutant mice. The detection of rhythmic NAT, CREM, and Fra-2 expression in the mouse should facilitate studies with transgenic and mutant mice strains. Many inbred laboratory mouse strains are melatonin deficient (35). By biochemical and genetic analyses, defects in NAT or NAT regulators and hydroxyindole-O-methyltransferase have been implicated in this deficiency (36). However our results clearly demonstrate that at least in the 129/Sv strain, the expression profile of NAT mRNA is normal.
The premature rise of NAT expression in the mutant mice is consistent with photoperiod-dependent changes in the mode of cAMP-regulated gene expression (29). Decreasing basal levels of the ICER protein together with increased phosphorylation of the CREB activator correlate with a faster induction of gene expression. Unlike the case of the CREM mutant mice however, exposure to various photoperiod regimes does not alter the peak levels of expression. This might reflect repression by de novo synthesized ICER that is lacking in the mutant mice. Together, these findings support the following scenario for ICER function in the pineal gland (Fig. 5). Rhythmic adrenergic stimulation drives a cycle of CREB phosphorylation and dephosphorylation mediated by the balance in the activities of PKA and specific phosphatases. Consequent nighttime activation of the P2 promoter, together with the CREM feedback loop, sets a basal level of ICER protein. By binding directly to the CRE element in the NAT promoter, ICER thereby modulates the rate and magnitude of rhythmic melatonin induction in response to adrenergic signals (Fig. 5). It is interesting to note how autoregulatory loops are a common theme in the molecular regulation of circadian rhythms (44, 45, 46). Our work now links this feature to the oscillatory regulation of hormonal synthesis.
Figure 5.
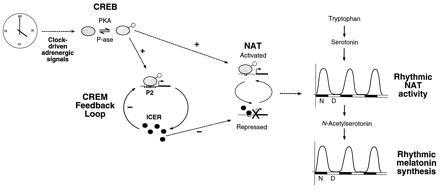
The role of the CREM feedback loop in transducing a rhythmic clock-directed signal into rhythmic hormone synthesis. Schematic representation of the regulatory pathway responsible for generating rhythmic melatonin synthesis. Nighttime adrenergic signals originating from the clock, activate PKA and thus phosphorylate CREB. During the day, dephosphorylation is achieved by phosphatase action. Thus clock-directed signals determine the equilibrium position. Phosphorylated CREB activates the P2 promoter of the CREM gene and thus induces the expression of ICER. ICER down regulates its own expression constituting the CREM feedback loop. The balance between the proportion of phosphorylated CREB (positive effect) and ICER protein levels (negative effect) determines the transcriptional activity of the NAT promoter. Thus the promoter cycles between activated and repressed states as a function of time. In this way, NAT mRNA oscillates between high nighttime and low basal daytime levels and determines the characteristic day–night oscillation of NAT activity (alternating black and white bars below the activity curve denote night and day, respectively). The conversion of serotonin to N-acetylserotonin catalyzed by NAT importantly constitutes the rate limiting step of melatonin synthesis. Therefore this oscillating transcriptional control mechanism ensures rhythmic melatonin synthesis.
The implications of our findings extend beyond melatonin synthesis in the pineal gland to other endocrine systems. The ability of a transcription factor to modulate the kinetics of hormone synthesis represents an important concept. Long-term changes in gene expression in response to hormonal signals in turn modulate the subsequent pattern of hormone production. In this way, CREM can be regarded as part of a relay of information enabling temporal adaptations of endocrine function in response to a changing environment.
Acknowledgments
We thank D. C. Klein, E. Borrelli, E. Lalli, M. Lamas, L. Monaco, F. Nantel, J. H. Stehle, E. Zazopoulos, Z. Wang, K. Tamai, P. Garcia-Villalba, and L. Richie for discussions and support; M. Yaniv for the Fra-2 cDNA; M. M. Wang for constructing the nighttime pineal cDNA library. The technical assistance of E. Heitz, G. Duval, and B. Boulay was appreciated. This study was funded by the U.S. Public Health Service Grant DA/00266 and a Research Scientist Award DA/00074 to S.H.S., and grants from Centre National de la Recherche Scientifique, Institut National de la Santé et de la Recherche Médicale, Centre Hospitalier Universitaire Régional, Fondation de la Recherche Médicale, Rhône-Poulenc Rorer (Bioavenir), and Association pour Recherche sur le Cancer to P.S.-C.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. §1734 solely to indicate this fact.
Abbreviations: CRE, cAMP-responsive element; CREM, CRE modulator; CREB, CRE-binding protein; ICER, inducible cAMP early repressor; NAT, serotonin N-acetyltransferase; PKA, protein kinase A; CAT, chloramphenicol acetyltransferase; wt, wild-type.
References
- 1.Felig P, Baxter J D, Broadus A E, Frohman L A. Endocrinology and Metabolism. New York: McGraw–Hill; 1987. [Google Scholar]
- 2.Krieger D T. Endocrine Rhythms. New York: Raven; 1979. [Google Scholar]
- 3.Karsch F J, Woodfill J I, Malpaux B, Robinson J I, Wayne N L. In: Suprachiasmatic Nucleus: The Mind’s Clock. Klein D C, Moore R Y, Reppert S M, editors. New York: Oxford Univ. Press; 1991. pp. 217–232. [Google Scholar]
- 4.Arendt J. Melatonin and the Mammalian Pineal Gland. London: Chapman & Hall; 1995. pp. 201–285. [Google Scholar]
- 5.Dollins A B, Zhdanova I V, Wurtman R J, Lynch H J, Deng M H. Proc Natl Acad Sci USA. 1994;91:1824–1828. doi: 10.1073/pnas.91.5.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamarkin L, Baird C J, Almeida O F X. Science. 1985;227:714–720. doi: 10.1126/science.3881822. [DOI] [PubMed] [Google Scholar]
- 7.Reiter R J. Trends Endocrinol Metab. 1991;1:13–19. doi: 10.1016/1043-2760(91)90055-r. [DOI] [PubMed] [Google Scholar]
- 8.Klein D C. In: Photoperiodism, Melatonin, and the Pineal Gland. Evered D, Clark S, editors. London: Pitman; 1985. pp. 38–56. [Google Scholar]
- 9.Axelrod J. Science. 1974;184:1341–1348. doi: 10.1126/science.184.4144.1341. [DOI] [PubMed] [Google Scholar]
- 10.Zatz M. In: Handbook of Experimental Pharmacology. Kebabian J W, Nathanson J A, editors. Berlin: Springer; 1982. pp. 691–710. [Google Scholar]
- 11.Sassone-Corsi P. Annu Rev Cell Dev Biol. 1995;11:355–377. doi: 10.1146/annurev.cb.11.110195.002035. [DOI] [PubMed] [Google Scholar]
- 12.Hoeffler J P, Meyer T, Yun Y, Jameson J L, Habener J F. Science. 1988;242:1430–1433. doi: 10.1126/science.2974179. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez G, Montminy M R. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- 14.de Groot R P, den Hertog J, Vandenheede J R, Goris J, Sassone-Corsi P. EMBO J. 1993;12:3903–3911. doi: 10.1002/j.1460-2075.1993.tb06068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rehfuss R P, Walton K M, Loriaux M M, Goodman R H. J Biol Chem. 1991;266:18431–18434. [PubMed] [Google Scholar]
- 16.Foulkes N S, Borrelli E, Sassone-Corsi P. Cell. 1991;64:739–749. doi: 10.1016/0092-8674(91)90503-q. [DOI] [PubMed] [Google Scholar]
- 17.Foulkes N S, Mellström B, Benusiglio E, Sassone-Corsi P. Nature (London) 1992;355:80–84. doi: 10.1038/355080a0. [DOI] [PubMed] [Google Scholar]
- 18.Mellström B, Naranjo J R, Foulkes N S, Lafarga M, Sassone-Corsi P. Neuron. 1993;10:655–665. doi: 10.1016/0896-6273(93)90167-p. [DOI] [PubMed] [Google Scholar]
- 19.Delmas V, van der Hoorn F, Mellström B, Jégou B, Sassone-Corsi P. Mol Endocrinol. 1993;7:1502–1514. doi: 10.1210/mend.7.11.8114765. [DOI] [PubMed] [Google Scholar]
- 20.Foulkes N S, Schlotter F, Pévet P, Sassone-Corsi P. Nature (London) 1993;362:264–267. doi: 10.1038/362264a0. [DOI] [PubMed] [Google Scholar]
- 21.Molina C A, Foulkes N S, Lalli E, Sassone-Corsi P. Cell. 1993;75:875–886. doi: 10.1016/0092-8674(93)90532-u. [DOI] [PubMed] [Google Scholar]
- 22.Stehle J H, Foulkes N S, Molina C A, Simonneaux V, Pévet P, Sassone-Corsi P. Nature (London) 1993;365:314–320. doi: 10.1038/365314a0. [DOI] [PubMed] [Google Scholar]
- 23.Lalli E, Sassone-Corsi P. Proc Natl Acad Sci USA. 1995;92:9633–9637. doi: 10.1073/pnas.92.21.9633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monaco L, Foulkes N S, Sassone-Corsi P. Proc Natl Acad Sci USA. 1995;92:10673–10677. doi: 10.1073/pnas.92.23.10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi J. Curr Biol. 1994;4:165–168. doi: 10.1016/s0960-9822(94)00040-0. [DOI] [PubMed] [Google Scholar]
- 26.Stehle J H, Foulkes N S, Pévet P, Sassone-Corsi P. Mol Endocrinol. 1995;9:706–716. doi: 10.1210/mend.9.6.8592516. [DOI] [PubMed] [Google Scholar]
- 27.Klein D C, Namboodiri M A A, Auerbach D A. Life Sci. 1981;28:1975–1986. doi: 10.1016/0024-3205(81)90644-5. [DOI] [PubMed] [Google Scholar]
- 28.Illnerova H, Vanecek J. J Pineal Res. 1985;2:67–78. doi: 10.1111/j.1600-079x.1985.tb00628.x. [DOI] [PubMed] [Google Scholar]
- 29.Foulkes N S, Duval G, Sassone-Corsi P. Nature (London) 1996;381:83–85. doi: 10.1038/381083a0. [DOI] [PubMed] [Google Scholar]
- 30.Borjigin J, Wang M M, Snyder S H. Nature (London) 1995;378:783–785. doi: 10.1038/378783a0. [DOI] [PubMed] [Google Scholar]
- 31.Foletta V C, Sonobe M H, Suzuki T, Endo T, Iba H, Cohen D R. Oncogene. 1994;9:3305–3311. [PubMed] [Google Scholar]
- 32.Luckow B, Schütz G. Nucleic Acids Res. 1987;13:5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andrews N C, Faller D V. Nucleic Acids Res. 1991;19:2499. doi: 10.1093/nar/19.9.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nantel F, Monaco L, Foulkes N S, Masquilier D, LeMeur M, Henricksén K, Dierich A, Parvinen M, Sassone-Corsi P. Nature (London) 1996;380:159–162. doi: 10.1038/380159a0. [DOI] [PubMed] [Google Scholar]
- 35.Goto M, Oshima I, Tomita T, Ebihara S. J Pineal Res. 1989;7:195–204. doi: 10.1111/j.1600-079x.1989.tb00667.x. [DOI] [PubMed] [Google Scholar]
- 36.Ebihara S, Marks T, Hudson D J, Menaker M. Science. 1986;231:491–493. doi: 10.1126/science.3941912. [DOI] [PubMed] [Google Scholar]
- 37.Baler R, Klein D C. J Biol Chem. 1995;270:27319–27325. doi: 10.1074/jbc.270.45.27319. [DOI] [PubMed] [Google Scholar]
- 38.Risse G, Jooss K, Neuberg M, Brüller H-J, Müller R. EMBO J. 1989;8:3825–3822. doi: 10.1002/j.1460-2075.1989.tb08560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blendy J A, Kaestner K H, Weinbauer G F, Nieschlag E, Schütz G. Nature (London) 1996;380:162–165. doi: 10.1038/380162a0. [DOI] [PubMed] [Google Scholar]
- 40.Coon S L, Roseboom P H, Baler R, Weller J L, Namboodiri M A A, Koonin E V, Klein D C. Science. 1995;270:1681–1683. doi: 10.1126/science.270.5242.1681. [DOI] [PubMed] [Google Scholar]
- 41.Deguchi T. Nature (London) 1979;282:94–96. doi: 10.1038/282094a0. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi J S, Hamm H, Menaker M. Proc Natl Acad Sci USA. 1980;77:2319–2322. doi: 10.1073/pnas.77.4.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Menaker M, Wisner S. Proc Natl Acad Sci USA. 1983;80:6119–6121. doi: 10.1073/pnas.80.19.6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aronson B D, Johnson K A, Loros J J, Dunlap J C. Science. 1994;263:1578–1584. doi: 10.1126/science.8128244. [DOI] [PubMed] [Google Scholar]
- 45.Edery I, Rutila J E, Rosbash M. Science. 1994;263:237–240. doi: 10.1126/science.8284676. [DOI] [PubMed] [Google Scholar]
- 46.Sassone-Corsi P. Cell. 1994;78:361–364. doi: 10.1016/0092-8674(94)90415-4. [DOI] [PubMed] [Google Scholar]


