Abstract
In invertebrates, peptides possessing the carboxy (C)-terminal motif -RXRFamide have been proposed as the homologs of vertebrate neuropeptide Y (NPY). Using matrix assisted laser desorption/ionization mass spectrometry, in combination with sustained off-resonance irradiation collision-induced dissociation and chemical and enzymatic reactions, we have identified the peptide pEGFYSQRYamide from the neuroendocrine pericardial organ (PO) of the crab Pugettia producta. This peptide is likely the same as that previously reported, but misidentified, as PAFYSQRYamide in several earlier reports (e.g. Li et al. [2003] J. Neurochem. 87,642–656; Fu et al. [2005] J. Comp. Neurol. 493,607–626). The -QRYamide motif contained in pEGFYSQRYamide is identical to that present in many vertebrate members of the NPY superfamily. Mass spectrometric analysis conducted on the POs of several other decapods showed that pEGFYSQRYamide is present in three other brachyurans (Cancer borealis, Cancer irroratus and Cancer productus) as well as in one species from another decapod infraorder (Lithodes maja, an anomuran). Thus, our findings show that at least some invertebrates possess NPY-like peptides in addition to those exhibiting an -RXRFamide C-terminus, and raise the question as to whether the invertebrate -QRYamides are functionally and/or evolutionarily related to the NPY superfamily.
Keywords: Pugettia producta, Cancer borealis, Cancer irroratus, Cancer productus, Lithodes maja, PAFYSQRYamide, matrix assisted laser desorption/ionizaton Fourier transform mass spectrometry (MALDI-FTMS), sustained off-resonance irradiation collision-induced dissociation (SORI-CID), pericardial organ (PO), stomatogastric nervous system (STNS)
1. Introduction
The neuropeptide Y superfamily is one of the most well studied peptide families in the animal kingdom (for reviews, see: Larhammar, 1996a; Larhammar, 1996b; Larhammar et al., 1998; Hoyle, 1998; Hoyle, 1999; Cerda-Reverter and Larhammar, 2000; Holmgren and Jensen, 2001; Conlon, 2002; Larhammar and Salaneck 2004; Conlon and Larhammar, 2005). In vertebrates, the neuropeptide Y (NPY) superfamily consists of the NPY, pancreatic polypeptide, peptide YY and polypeptide Y subfamilies. Members of each of these peptide subfamilies exhibit similar and highly conserved structures, many possessing the carboxy (C)-terminal motif -RQRYamide. Members of the NPY superfamily are widely distributed within vertebrate nervous systems; some members are also found in other tissues (e.g. pancreatic polypeptide). Physiological investigations have shown that the NPY superfamily is extremely pleiotropic, participating in the regulation of diverse physiological functions, including satiety and sexual behavior.
It has long been noted that antibodies generated against vertebrate NPY family members produce labeling in the nervous system as well as in other tissues, such as the gut, of invertebrates (e.g. Schoofs et al., 1988; Skuce et al., 1990). Using a vertebrate antibody to pancreatic polypeptide, Maule et al. (1991) isolated from the tapeworm Moniezia expansa a peptide possessing some sequence homology to members of the NPY family. The discovery of this peptide, PDKDFIVNPSDLVLDNKAALRDYLRQINEYFAIIGRPRFamide, commonly termed neuropeptide F (NPF), was followed by the isolation of similar peptides, most possessing the C-terminus -RXRFamide, from other invertebrate species (e.g. Smart et al., 1992; Leung et al., 1992; Spittaels et al., 1996; Brown et al., 1999). The sequence similarity between the NPFs and members of the vertebrate NPY superfamily, as well recent molecular comparisons of protein and gene structure (both of preprohormones and peptide receptors), has led to the hypothesis that the NPFs are invertebrate homologs of the vertebrate NPYs (for reviews, see: Hoyle, 1999; de Jong-Brink et al., 2001; Hewes and Taghert, 2001; McVeight et al., 2005). This hypothesis is further supported by physiological studies, which have shown that many functions served by NPY family members in vertebrates are likewise regulated by NPF in invertebrates (e.g. Wu et al., 2003; Le et al., 2004).
In our study, we used matrix assisted laser desorption/ionization Fourier transform mass spectrometry (MALDI-FTMS), in combination with sustained off-resonance irradiation collision-induced dissociation (SORI-CID) and chemical and enzymatic reactions, to identify a peptide from the neuroendocrine pericardial organ (PO) of the crab Pugettia producta. This peptide, pEGFYSQRYamide, possesses sequence homology to NPY, notably including the C-terminal motif -QRYamide. Using MALDI-FTMS, we show that this peptide is present in several other brachyuran species, and is present in at least one anomuran as well. Collectively, our results indicate that in some invertebrates, NPY-like peptides possessing C-termini identical to that of vertebrate NPY, but distinct from those of the NPF peptides, are present. These results thus raise the question as to whether these -QRYamides are also functionally and/or evolutionarily related to the NPY superfamily. Moreover, our identification of a crustacean peptide with a true NPY C-terminus raises the question as to whether previous mapping studies using antibodies to NPY family members in invertebrate tissues are detecting only the NPFs, or if they may be detecting a combination of NPFs and members of the -QRYamide family. Some of these data have appeared previously in abstract form (Messinger et al., 2006).
2. Materials and methods
2.1. Animals
Northern kelp crabs Pugettia producta (Decapoda, Brachyura) were collected by hand off dock piling and in kelp beds at Friday Harbor Laboratories (Friday Harbor, WA). Jonah crabs Cancer borealis (Decapoda, Brachyura), Atlantic rock crabs Cancer irroratus (Decapoda, Brachyura) and Northern stone crabs Lithodes maja (Decapoda, Anomura) were purchased from local seafood dealers in Brunswick, ME. Red rock crabs Cancer productus (Decapoda, Brachyura) were collected by hand or trap at multiple sites throughout the greater Puget Sound area of Washington State. Regardless of species, animals were kept in aerated natural seawater aquaria at 8–10°C until dissection.
2.2. Tissue dissection and MALDI sample preparation
Before dissection, animals were anesthetized on ice for approximately 30 minutes. Tissue samples were dissected from the animal in chilled (10 °C) physiological saline (442mM NaCl, 11mM KCl, 13mM CaCl2, 26mM MgCl2, 12mM Trizma base, 1.2 mM maleic acid, pH 7.4). POs were obtained by isolating the lateral walls of the pericardial chamber, pinning them in a wax-lined Pyrex dish, and manually dissecting the nerve roots comprising each PO from the surrounding connective and muscle tissues. Isolated POs were removed from the saline with fine forceps, rinsed sequentially in two 25 μl droplets of 0.75 M (135 mg/ml) fructose and placed on a face of the ten-facetted probe tip, minimizing co-transfers of solution. The tissue was sliced 10–20 times with a 0.1 mm needle, gathered together, covered with a 0.5 μl droplet of 2,5-dihydroxybenzoic acid matrix solution (DHB, SigmaAldrich, MO) (1.0 M DHB, 1:1 acetonitrile:2% phosphoric acid water), and allowed to co-crystallize with the matrix at room temperature (approximately 20 °C).
2.3. Tissue extraction
Individual POs were extracted, delipidated, and concentrated using the following procedure. A single PO was placed in 30 μl of extraction solvent (65% methanol, 5% acetic acid, and 30% water) and homogenized manually using Vannas-type spring scissors. The mixture was centrifuged for five minutes to form a pellet of any remaining tissue, the supernatant was transferred to a new microcentrifuge tube, and the remaining pellet was washed with 5 μl of nanopure water. Nanopure water (20 μl) was added to the supernatant followed by 25 μl of chloroform to form an organic and aqueous layer. The aqueous layer was removed and dried using a SpeedVac. Dried extracts were either dissolved in 50:50 acetonitrile:water for MALDI-FTMS analysis or were subjected to chemical derivatization or enzymatic digestion prior to analysis by MALDI-FTMS. For MALDI-FTMS, samples were prepared by mixing reconstituted extract 1:1 with 1.0 M DHB matrix (prepared as described earlier) on a face of a ten-facetted MALDI probe tip.
2.4. Chemical and enzymatic reactions
Acetylation was achieved by adding acetic anhydride (5 μl; Alltech, Deerfield, IL) and nanopure water (2.5 μl water) to a dried extract from a single PO for one hour. Enzymatic digestion used pyroglutamate aminopeptidase (10 mU, Sigma Aldrich, St. Louis, MO) reconstituted in 50 μl of buffer solution (50 mM sodium phosphate, pH 7.0, 10 mM DDT, 1 mM EDTA). Enzyme solution (5 μl) was added to a dried extract from a single PO and reacted overnight at 37° C. Samples were prepared for MALDI-FTMS as described earlier.
2.5. Instrumentation
All data were collected using a HiResMALDI-FTMS (IonSpec, Lake Forest, CA) with a 4.7 Tesla actively shielded superconducting magnet (Cryomagnetics, Oak Ridge, TN). Seven pulsed nitrogen laser shots (337 nm) were accumulated in the FTMS cell prior to detection. Spectra were internally calibrated with poly(propylene glycol) calibrants 725 and 2,000 (SigmaAldrich, MO). For SORI-CID experiments, argon was used as the collision gas, the frequency offset was set equal to −1.8% of the reduced cyclotron frequency, and the voltage amplitude was in the range of 6–8.5 Vbp. A delay of 5–10 seconds preceded ion detection, which occurred with analyzer pressures of 1–2 × 10−10 Torr. Transients from direct tissue spectra were apodized using a Blackman function and zero-filled prior to fast Fourier transformation; SORI-CID spectra were processed without apodization and calibrated with a one-point calibration using the [M+H]+ or [MH−H2O]+ ion.
3. Results and discussion
3.1. Identification of pEGFYSQRYamide from the pericardial organs of Pugettia producta
As part of a study where we used MALDI-FTMS to characterize peptides in tissue samples from the kelp crab, P. producta (Dickinson et al., 2004), we consistently detected an abundant peak at m/z 1030.47 in the PO (see Fig. 1a). Lower abundance peaks at this mass were also found in the commissural ganglia (part of the stomatogastric nervous system), but not in the neuroendocrine sinus gland (data not shown). To further characterize the m/z 1030.47 peak, we used SORI-CID to generate an MS/MS spectrum to determine the amino acid sequence (see Fig. 2a). The fragment ion masses provided evidence for the C-terminal sequence -FYSQRYamide, with all y-type ions showing mass measurement errors that were less than 1.5 ppm. We also noted that the SORI-CID mass spectrum showed fragment ion masses and intensity patterns that were very similar to those observed for a peptide at m/z 1030 that was previously detected in the POs of C. borealis (Li et al., 2003; Kutz et al., 2004) and C. productus (Fu et al., 2005) and assigned the sequence PAFYSQRYamide.
Figure 1.
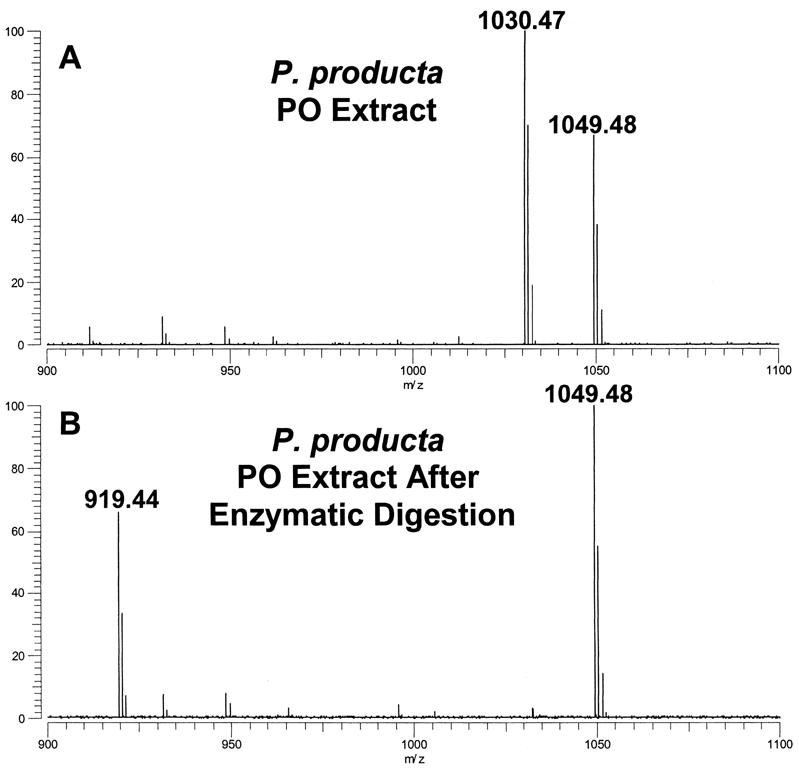
MALDI-FT mass spectra of (A) an extract of a single pericardial organ (PO) from Pugettia producta and (B) an extract of a single P. producta PO taken after enzymatic digestion with pyroglutamate aminopeptidase. Following digestion, the peptide at m/z 1030.47 is converted to m/z 919.44, a mass change of 111.03 resulting from the loss of a pyroglutamate residue. Both spectra were measured using DHB as the MALDI matrix.
Figure 2.
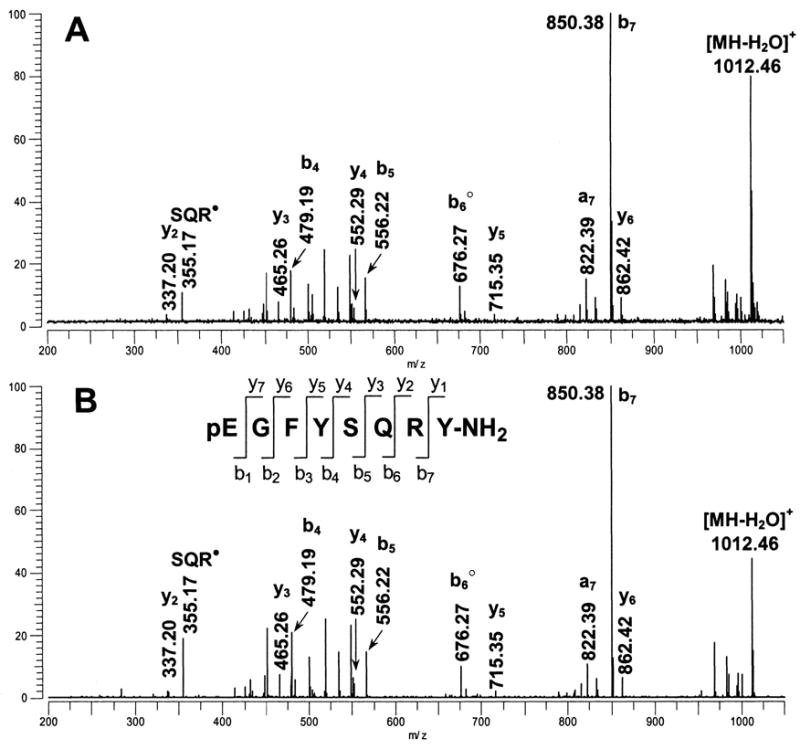
SORI-CID MALDI-FTMS spectra of m/z 1030.47. (A) SORI-CID of the m/z 1030.47 peak detected in the direct tissue MALDI-FT mass spectrum of a pericardial organ from Pugettia producta. The m/z 1030.47 peak was dissociated following ion isolation using argon as the collision gas with an excitation amplitude of 6.5 Vbp, n=5; (B) SORI-CID of the m/z 1030.47, [M+H]+ peak, from a synthetic standard of pEGFYSQRYamide. The m/z 1030.47 peak was dissociated following ion isolation and argon was used as the collision gas with an excitation amplitude of 6.5 Vbp, n=1. The y-, b-, and a-type fragment ions are identified using the nomenclature established by Roepstorff (1984). Ions that have lost NH3 are shown with a filled circle, ions that have lost H2O are shown with an open circle.
Initially, we assumed that the m/z 1030.47 peak we detected in P. producta was the previously identified PAFYSQRYamide; however, when we turned our attention to carefully calibrated mass measurements, we found that PAFYSQRYamide could not be the correct peptide sequence. When we measured the exact mass for the m/z 1030.47 peak from P. producta using internal calibration with PPG as the calibrant (Table 1), a mass measurement error of −36 ppm was obtained when compared with the calculated mass for the PAFYSQRYamide peptide sequence. The magnitude of this error provided definitive evidence that the peptide present in P. producta was not PAFYSQRYamide. Calibration of the SORI-CID spectrum of the m/z 1030.47 peak provided insights regarding the sequence assignment. As noted above, mass measurement errors for y-type ions detected in the spectrum, ions that retain the C-terminal sequence of the peptide, were less than 1.5 ppm and were consistent with the -FYSQRYamide portion of the peptide sequence; however, all b-type ions detected in the spectrum, ions that retain the N-terminal sequence, showed unacceptably high mass measurement errors (−36 to −80 ppm) with respect to the PAFYSQRYamide sequence. This result indicated that the first two amino acids of the sequence, proline and alanine, were incorrectly assigned. Unfortunately, the SORI-CID spectrum did not show the b- or y-type ions (b2 or y7) that were needed to elucidate this part of the N-terminal peptide sequence.
Table 1.
Exact mass measurements for putative pEGFYSQRYamide detected in pericardial organs using MALDI-FTMS
| Species | Expected mass, [M+H]+ | Measured massa, [M+H]+ | Error(ppm) |
|---|---|---|---|
| P. producta | 1030.4741 | 1030.4736 | −0.5 |
| C. borealis | 1030.4741 | 1030.4744 | 0.3 |
| C. productus | 1030.4741 | 1030.4726 | −1.4 |
| C. irroratus | 1030.4741 | 1030.4763 | 2.1 |
| L. maja | 1030.4741 | 1030.4743 | 0.2 |
Mass measured using internal calibration
For insights regarding the N-terminal sequence, we made use of our exact mass measurements and calculated the mass of the undetermined portion of the sequence, 168.053 Da, and found that this value could be accounted for by the combination of a pyroglutamate and glycine residue. Because this sequence identity was not provided by the SORI-CID experiments, we resorted to chemical and enzymatic reactions to test this hypothesis. First, using an extract of a single PO from P. producta, we carried out an acetylation reaction to determine if the N-terminus of the peptide was blocked. While other peptides in the sample were completely acetylated, the m/z 1030.47 peptide was not derivatized, providing evidence for a blocked N-terminus (data not shown). Next, we treated a second extract from a single PO from P. producta with the enzyme pyroglutamate aminopeptidase and found that the m/z 1030.47 peptide was replaced by a peak at m/z 919.44 (Fig. 1b), which exact mass measurements showed to be lower in mass by 111.030 Da, corresponding to a pyroglutamate residue (111.032 Da). These experiments provided convincing evidence for pyroglutamate and glycine residues at the N-terminus. As a final confirmation of the corrected sequence, we had the peptide pEGFYSQRYamide synthesized. SORI-CID spectra for the peptide standard showed excellent agreement with that obtained for the tissue-derived peptide (see Fig. 2b). Thus, we conclude the structure of the P. producta peptide at m/z 1030.47 to be pEGFYSQRYamide.
3.2. Detection of pEGFYSQRYamide in the pericardial organs of other decapod species
In subsequent work, expanded to include the analysis of tissues from other decapod crustaceans, an m/z 1030.47 peptide was also observed as an abundant peak in the MALDI-FT mass spectra of POs from the brachyuran species C. borealis, C. irroratus, and C. productus (Fig. 3). As stated earlier, in both C. borealis and C. productus, the structure of this peptide had previously been assigned as PAFYSQRYamide (Li et al., 2003; Kutz et al., 2004; Fu et al., 2005). For all three Cancer species, our exact mass measurements (Table 1) and SORI-CID spectra (data not shown) disputed this assignment, instead supporting pEGFYSQRYamide as the origin of the m/z 1030.47 peak. Thus, we conclude that PAFYSQRYamide, initially reported in the POs of C. borealis and C. productus, is actually pEGFYSQRYamide, and that this peptide may well be broadly conserved in brachyuran species.
Figure 3.
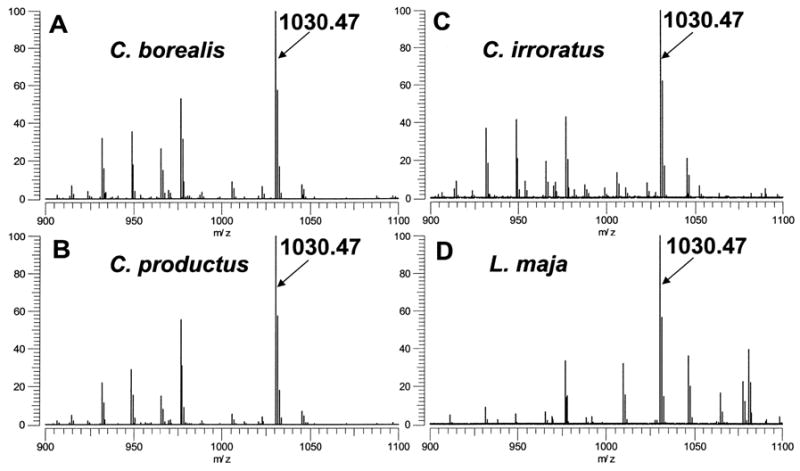
Direct MALDI-FTMS spectra of freshly dissected pericardial organs from (A) Cancer borealis, (B) Cancer productus, (C) Cancer irroratus, and (D) Lithodes maja. All spectra were measured using DHB as the matrix and conditions optimized for accumulation of m/z 1500. Labeled peaks correspond to the [M+H]+ ion for pEGFYSQFRYamide at m/z 1030.47.
In addition to our detection of a peptide at m/z 1030.47 in brachyuran crabs, we also found the same peak present in one anomuran decapod, specifically L. maja (Fig. 3). As was the case for P. producta and the three Cancer species, exact mass measurements (Table 1) and SORI-CID spectra (data not shown) indicated that this peptide too was pEGFYSQRYamide. Thus, the peptide pEGFYSQRYamide is not limited to members of the infraorder Brachyura, but may in fact be a broadly conserved decapod hormone.
Whether or not other isoforms of -QRYamide exist is an open question. Clearly other peptides ending in -RYamide have been identified in crustaceans (e.g. FYANRYamide [Li et al., 2003] and SSRFVGGSRYamide [Fu et al., 2005] and mollusks (e.g. pQPPLPRYamide [Ohtani et al., 1997]) and it possible that these peptides and pEGFYSQRYamide may form an extended invertebrate peptide family. Moreover, preliminary data suggests that a different N-terminally blocked -QRYamide isoform is present in the American lobster Homarus americanus (E.A. Stemmler, unpublished). As additional studies are conducted it will be interesting to see the extent to which peptides similar to pEGFYSQRYamide are distributed in invertebrate species.
3.3. Is pEGFYSQRYamide evolutionarily and/or functionally related to the NPY superfamily?
As stated in the Introduction of this study, many members of the vertebrate NPY superfamily exhibit the C-terminal motif -RQRYamide. In invertebrates, a group of peptides collectively termed the NPFs, and exhibiting -RXRFamide C-termini, are thought to be evolutionary and functional homologs of the NPYs. Our identification of pEGFYSQRYamide is notable as its C-terminus, -SQRYamide, is nearly identical to that of a number of vertebrate NPY family members. Moreover the serine for arginine substitution at position 4 from the C-terminus can be accounted for by a single nucleotide substitution in the codons for each residue (i.e. AGU or AGC for serine versus AGA or AGG for arginine).
While the structural homology between pEGFYSQRYamide of crustaceans and the vertebrate NPYs is intriguing, much work will be needed to determine what, if any, ancestral relationship exists between these peptides. Clearly, identification of the gene encoding prepro-pEGFYSQRYamide would be helpful in that it may reveal similarities to the vertebrate NPY prepro-hormones. This is especially important given the much smaller size of the crustacean peptide relative to the vertebrate NPYs. Likewise, identification and characterization of the receptor or receptors targeted by pEGFYSQRYamide would also be helpful in ascribing a common ancestry for this peptide and the NPYs since this would permit both structural and biochemical comparisons with the receptor(s) targeted by the NPY family members. Finally, physiological investigations of the role(s) played by pEGFYSQRYamide in crustacean are needed to determine whether functional conservations between this peptide and the vertebrate NPYs exist.
While it is currently too early to assess what if any evolutionary and/or functional link pEGFYSQRYamide has to the NPY superfamily, the presence of an invertebrate peptide possessing a -QRYamide N-terminal motif does show that NPY-like peptides, in addition to those exhibiting NPF N-termini, exist in invertebrates. Minimally, this finding raises the question as to whether the immunohistochemistry done previously in invertebrates using antibodies to NPY family members is detecting only the NPFs. For those polyclonals generated against vertebrate peptides containing intact N-termini, it is clearly possible, and even likely, that peptides such as pEGFYSQRYamide would also be detected.
As additional studies are done, it will be interesting to see how extensive the conservation of -QRYamide peptides is in invertebrates, and if these peptides are present in taxa other than the decapods. With the identification of family members, such as pEGFYSQRYamide, physiological investigations of the roles played by -QRYamides become possible. These studies, in combination with molecular analyses, should allow for future assessment of what if any evolutionary and/or functional homology exists between the invertebrate -QRYamides, the NPFs and/or vertebrate members of NPY superfamily.
Acknowledgments
John Weller, Daniel Messinger and Yun-Wei Hsu are thanked for their assistance in collecting some of the animals used in this study. Financial support for this work was provided by National Science Foundation grants MRI-0116416 (E.A.S.) and IBN-0111040 (P.S.D.), the Howard Hughes Medical Institute, NIH Grant Number P20 RR-016463 from the INBRE Program of the National Center for Research Resources and a Mount Desert Island Biological Laboratory New Investigator Award (Salisbury Cove Research Fund provided by the Thomas H. Maren Foundation; to A.E.C).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brown MR, Crim JW, Arata RC, Cai HN, Chun C, Shen P. Identification of a Drosophila brain-gut peptide related to the neuropeptide Y family. Peptides. 1999;20:1035–1042. doi: 10.1016/s0196-9781(99)00097-2. [DOI] [PubMed] [Google Scholar]
- Cerda-Reverter JM, Larhammar D. Neuropeptide Y family of peptides: structure, anatomical expression, function, and molecular evolution. Biochem Cell Biol. 2000;78:371–392. [PubMed] [Google Scholar]
- Conlon JM. The origin and evolution of peptide YY (PYY) and pancreatic polypeptide (PP. Peptides. 2002;23:269–278. doi: 10.1016/s0196-9781(01)00608-8. [DOI] [PubMed] [Google Scholar]
- Conlon JM, Larhammar D. The evolution of neuroendocrine peptides. Gen Comp Endocrinol. 2005;142:53–59. doi: 10.1016/j.ygcen.2004.11.016. [DOI] [PubMed] [Google Scholar]
- de Jong-Brink M, ter Maat A, Tensen CP. NPY in invertebrates: molecular answers to altered functions during evolution. Peptides. 2001;22:309–315. doi: 10.1016/s0196-9781(01)00332-1. [DOI] [PubMed] [Google Scholar]
- Dickinson PS, Hsu YA, Labenia J, Latham R, Lin M, Messinger DI, Ngo CT, Graubard K, Christie AE. Program No 657.11. 2004 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2004. The pyloric rhythm of the kelp crab contains but is insensitive to peptides that modulate this rhythm in other crustaceans. 2004 Online. [Google Scholar]
- Fu Q, Kutz KK, Schmidt JJ, Hsu YW, Messinger DI, Cain SD, de la Iglesia HO, Christie AE, Li L. Hormone complement of the Cancer productus sinus gland and pericardial organ: an anatomical and mass spectrometric investigation. J Comp Neurol. 2005;493:607–626. doi: 10.1002/cne.20773. [DOI] [PubMed] [Google Scholar]
- Hewes RS, Taghert PH. Neuropeptides and neuropeptide receptors in the Drosophila melanogaster genome. Genome Res. 2001;11:1126–1142. doi: 10.1101/gr.169901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren S, Jensen J. Evolution of vertebrate neuropeptides. Brain Res Bull. 2001;55:723–735. doi: 10.1016/s0361-9230(01)00556-1. [DOI] [PubMed] [Google Scholar]
- Hoyle CH. Neuropeptide families: evolutionary perspectives. Regul Pept. 1998;73:1–33. doi: 10.1016/s0167-0115(97)01073-2. [DOI] [PubMed] [Google Scholar]
- Hoyle CH. Neuropeptide families and their receptors: evolutionary perspectives. Brain Res. 1999;848:1–25. doi: 10.1016/s0006-8993(99)01975-7. [DOI] [PubMed] [Google Scholar]
- Kutz KK, Schmidt JJ, Li L. In situ tissue analysis of neuropeptides by MALDI FTMS in-cell accumulation. Anal Chem. 2004;76:5630–5640. doi: 10.1021/ac049255b. [DOI] [PubMed] [Google Scholar]
- Larhammar D. Evolution of neuropeptide Y, peptide YY and pancreatic polypeptide. Regul Pept. 1996a;62:1–11. doi: 10.1016/0167-0115(95)00169-7. [DOI] [PubMed] [Google Scholar]
- Larhammar D. Structural diversity of receptors for neuropeptide Y, peptide YY and pancreatic polypeptide. Regul Pept. 1996b;65:165–174. doi: 10.1016/0167-0115(96)00110-3. [DOI] [PubMed] [Google Scholar]
- Larhammar D, Salaneck E. Molecular evolution of NPY receptor subtypes. Neuropeptides. 2004;38:141–151. doi: 10.1016/j.npep.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Larhammar D, Söderberg C, Lundell I. Evolution of the neuropeptide Y family and its receptors. Ann N Y Acad Sci. 1998;839:35–40. doi: 10.1111/j.1749-6632.1998.tb10729.x. [DOI] [PubMed] [Google Scholar]
- Lee KS, You KH, Choo JK, Han YM, Yu K. Drosophila short neuropeptide F regulates food intake and body size. J Biol Chem. 2004;279:50781–50789. doi: 10.1074/jbc.M407842200. [DOI] [PubMed] [Google Scholar]
- Leung PS, Shaw C, Maule AG, Thim L, Johnston CF, Irvine GB. The primary structure of neuropeptide F (NPF) from the garden snail, Helix aspersa. Regul Pept. 1992;41:71–81. doi: 10.1016/0167-0115(92)90515-v. [DOI] [PubMed] [Google Scholar]
- Li L, Kelley WP, Billimoria CP, Christie AE, Pulver SR, Sweedler JV, Marder E. Mass spectrometric investigation of the neuropeptide complement and release in the pericardial organs of the crab, Cancer borealis. J Neurochem. 2003;87:642–656. doi: 10.1046/j.1471-4159.2003.02031.x. [DOI] [PubMed] [Google Scholar]
- Maule AG, Shaw C, Halton DW, Thim L, Johnston CF, Fairweather I, Buchanan KD. Neuropeptide F: a novel parasitic flatworm regulatory peptide from Moniezia expansa (Cestoda: Cyclophillidea) Parasitology. 1991;102:309–316. [Google Scholar]
- McVeigh P, Kimber MJ, Novozhilova E, Day TA. Neuropeptide signalling systems in flatworms. Parasitology. 2005;131:S41–55. doi: 10.1017/S0031182005008851. [DOI] [PubMed] [Google Scholar]
- Messinger DI, Bruns EA, Goiney CC, Easton CR, Hsu YA, Day-Bazhaw NM, Savage EE, Stemmler EA, Dickinson PS, Christie AE. Program No. 129.8. 2006 Neuroscience Meeting Planner. Atlanta, GA: Society for Neuroscience; 2006. Identification and distribution of pEGFYSQRYamide: a crab neuropeptide Y (NPY)-like peptide. 2006 Online. [Google Scholar]
- Ohtani M, Maneoka Y, Matsushima O, Takao T, Shimonishi Y, White AR, Pedder S, Sharma R, Lennon MA, Katugampola S, Walker RJ. Isolation of bioactive compounds from Helix aspersa nerve tissue and the effects of pQPPLPRYamide on heart, esophagus and central neurons of H. aspersa and rectum of Anodonta woodiana. Gen Pharmac. 1997;29:103–111. doi: 10.1016/s0306-3623(96)00532-0. [DOI] [PubMed] [Google Scholar]
- Roepstorff P. Proposal for a common nomenclature for sequence ions in mass spectra of peptides. Biomed Mass Spectrom. 1984;11:601. doi: 10.1002/bms.1200111109. [DOI] [PubMed] [Google Scholar]
- Schoofs L, Danger JM, Jegou S, Pelletier G, Huybrechts R, Vaudry H, De Loof A. NPY-like peptides occur in the nervous system and midgut of the migratory locust, Locusta migratoria and in the brain of the grey fleshfly, Sarcophaga bullata. Peptides. 1988;9:1027–1036. [Google Scholar]
- Skuce PJ, Johnston CF, Fairweather I, Halton DW, Shaw C, Buchanan KD. Immunoreactivity to the pancreatic polypeptide family in the nervous system of the adult human blood fluke, Schistosoma mansoni. Cell Tissue Res. 1990;261:573–581. doi: 10.1007/BF00313537. [DOI] [PubMed] [Google Scholar]
- Smart D, Shaw C, Johnston C, Thim L, Halton D, Buchanan K. Peptide tyrosine phenylalanine: a novel neuropeptide F-related nonapeptide from the brain of the squid, Loligo vulgaris. Biochem Biophys Res Commun. 1992;186:1616–1623. doi: 10.1016/s0006-291x(05)81593-1. [DOI] [PubMed] [Google Scholar]
- Spittaels K, Verhaert P, Shaw C, Johnston RN, Devreese B, Van Beeumen J, De Loof A. Insect neuropeptide F (NPF)-related peptides: isolation from Colorado potato beetle (Leptinotarsa decemlineata) brain. Insect Biochem Mol Biol. 1996;26:375–382. doi: 10.1016/0965-1748(95)00104-2. [DOI] [PubMed] [Google Scholar]
- Wu Q, Wen T, Lee G, Park JH, Cai HN, Shen P. Developmental control of foraging and social behavior by the Drosophila neuropeptide Y-like system. Neuron. 2003;39:147–161. doi: 10.1016/s0896-6273(03)00396-9. [DOI] [PubMed] [Google Scholar]


