Abstract
Condensed Abstract
The timing of biochemical failure and distant metastasis after radiotherapy for low, intermediate and high-risk prostate cancer was determined. The patterns of failure suggest that the majority of early failures were due to subclinical micrometastases present at diagnosis, whereas a late wave of metastasis at 10–12 years in every risk group was consistent with tumor spread from local persistence of disease.
Background
The relationship of prostate cancer risk group stratification and the timing of biochemical failure (BF) and distant metastasis (DM) is not well defined. We sought to differentiate early failures due to subclinical micrometastasis at presentation from late failures due to local persistence.
Methods
A total of 1833 men with clinically localized prostate cancer treated with 3D-conformal radiotherapy with or without short-term androgen deprivation were retrospectively analyzed. The interval hazard rates of DM and BF, using ASTRO and Phoenix (Nadir+2) definitions, were determined for men with low, intermediate, and high risk disease.
Results
Median follow-up was 67 months. Multivariate analysis showed that increasing risk group was independently associated with higher ASTRO BF (P<.0001) and Nadir+2 BF (P<.0001). The preponderance (87%) of ASTRO BF occurred ≤4 years after RT, while Nadir+2 BF was more evenly spread over years 1–12, with 43% at >4 years. The hazard of Nadir+2 BF persisted in years 8–12 in all risk groups. The interval hazard function for DM appeared to be biphasic (early and late peaks) for intermediate and high risk patients, but no distinct early wave was evident for low risk patients.
Conclusions
ASTRO BF underestimates late BF due to backdating. Local persistence of disease is suggested by delayed Nadir+2 BF and subsequent late DM in every risk group. The paucity of early DM among those with low risk tumors supports the hypothesis that occult micrometastases contributed to the early wave.
Keywords: prostate carcinoma, radiotherapy, prognostic factors, outcome, biochemical control, neoplasm metastasis
INTRODUCTION
The most widely used risk group classification systems for men with prostate cancer are based on clinical T-stage, Gleason score, and initial pretreatment prostate specific antigen (iPSA). Low, intermediate, and high risk prostate cancers have different natural histories and responses to treatment. Chism et al1 described the outcome of men treated with radiotherapy and subdivided by a simple risk grouping based on a single factor high risk model that gained popularity after a report by D’Amico et al.2 In the current report, we sought to characterize in greater detail the differences in patterns of failure by looking at the hazard of biochemical failure (BF) and distant metastasis (DM) over discrete time intervals following RT in each risk group.
Coen et al3 demonstrated that local failure after RT is related to a late wave of DM. To our knowledge, their observation has not been substantiated in another patient population. From these data, we hypothesized that early distant metastasis are related more to unrecognized micrometastatic disease present at the time of radiotherapy and that late metastasis are related more to locally persistent disease after radiotherapy. Moreover, each wave of DM should be preceded by a wave of BF. Based on this model, the expectation is that when patients are subdivided by risk group, those at low risk should have few, if any early metastasis, while those at higher risk would have a greater incidence of early metastasis. Likewise, increasing risk should be associated with an increasing proportion of late metastasis from local persistence of disease due to intrinsic radioresistance and/or factors related to bulk of disease (e.g. hypoxia).
METHODS
Patient Selection and Treatment
The study group was comprised of 1833 patients with clinically localized (N0/Nx, M0/Mx) prostate cancer consecutively treated with definitive radiotherapy at Fox Chase Cancer Center (Philadelphia, PA) from March 1987 to October 2001. Institutional review board approval was obtained for data collection and outcome analysis of patients in this institutional database. T-category was based solely on palpation findings on digital rectal exam (no upstaging based on prostate biopsy results or radiographic imaging) using the 2002 AJCC staging guidelines.4 All patients had a PSA available prior to treatment and had serial PSA values post-treatment. Men who received neoadjuvant or adjuvant androgen deprivation (AD) of ≤6 months in duration (initiated prior to completion of RT) were included. Patients receiving >6 months neoadjuvant or adjuvant AD were excluded. Patients who received salvage AD for a rising PSA or DM after the completion of RT were included.
Patients were divided into three risk groups (low, intermediate, and high risk) according to the single-factor high risk classification scheme described by Chism et al.1 The low-risk group included patients with palpation stage T1-2, iPSA <10 ng/mL, and Gleason score of ≤6. The high risk group contained any patient with stage T3-4, iPSA ≥20 ng/mL, or Gleason score of ≥8. The intermediate risk group contained patients who did not fulfill the criteria of the low- or high-risk groups.
Prior to March 1989, 116 study patients were treated with conventional radiotherapy to small pelvic fields using fluoroscopic simulation with rectal and bladder contrast, as previously described.5 Typical pelvic field borders were the middle of the sacroiliac joints superiorly, the bottom on the ischial tuberosities inferiorly, the symphysis pubis anteriorly, the S2/S3 interspace posteriorly, and 1.5 centimeters beyond the pelvic brim laterally. These conventional pelvic fields were shaped only by corner blocks and were delivered with 2-field, 3-field, or 4-field beam arrangements. After March 1986, the remaining 1717 men were treated using a 3-D conformal technique, as previously reported.6 In general, a treatment planning computed tomography scan was performed in the treatment position, supine on a custom-made alpha cradle for immobilization.
Patients with clinical stage T1/T2a-b and Gleason score 2–6 typically received treatment to the prostate only. Patients with more advanced prostate cancer, T2c/T3/T4 or Gleason score 7–10, received 46–50 Gray (Gy) to the small pelvic field described above, followed by a conformal boost to the prostate and seminal vesicles. Daily fractions of 2 Gy were used. The planning target volume (PTV) for conformal radiotherapy included the prostate with or without the seminal vesicles, with a margin of 1–1.5 centimeters to the block edge. All conformal treatments utilized 10 to 18 MV photons with a 4-field or 5-field beam arrangement. The radiation dose was typically prescribed to the 95% isodose line of the beam arrangements. The mean dose to the PTV was typically between −5% and +7% of the prescribed dose, and 99% of the PTV received at least 95% of the prescribed dose. As recommended by the International Commission on Radiation Units and Measurements, radiation dose is reported here as the dose delivered to isocenter (the ICRU reference point).7 The range of radiotherapy doses was 51–82 Gy.
Follow-up
Typical follow-up consisted of a serum PSA and digital rectal exam every 3–6 months after RT for 2 years, then every 6–12 months thereafter. Biochemical failure was examined using two definitions, one encouraged by a consensus conference sponsored by ASTRO (three consecutive rises in PSA with backdating to between the nadir and the first rise)8 and the other from a joint ASTRO-RTOG conference in Phoenix.9 The Phoenix definition (rise of at least 2 ng/mL greater than the PSA nadir after RT) is a better approximation of eventual clinical failure.9-14 A primary reason for making the comparison is that the ASTRO definition incorporates backdating, which will make the estimation of the risk of BF at a given interval of time inaccurate. Using the Phoenix (Nadir+2) definition, BF is recorded at the time the event occurs. The classification of DM was determined based on radiological evidence of hematogenous spread. Time to BF and DM was measured from the end of RT or the end of neoadjuvant/adjuvant AD, whichever occurred later. Although the follow-up for a full analysis of mortality is premature, we thought it would be informative to determine if early DM translated into subsequent early deaths. Therefore, we quantified overall mortality (OM) and prostate cancer cause-specific mortality (CSM) rates.
Statistical Analysis
Estimates of BF, DM, OM, and CSM were calculated using the Kaplan-Meier product-limit method.15 Univariate comparisons of outcome were performed using the log-rank test.16 For the univariate analyses, RT dose and iPSA were treated as categorical variables, with groupings of <74, 74-<76, and ≥76 Gy and <10, 10-<20, and ≥20 ng/mL, respectively. The 74 Gy and 76 Gy RT dose cut-points were chosen because multiple studies have identified the greatest dose-response in this range.6, 17-21 Multivariate analysis (MVA) utilized stepwise Cox proportional hazards regression models. Radiotherapy dose, iPSA and age were included as continuous variables in the MVAs. Categorical covariates for MVA included T–category (T1-2 vs. T3-4), Gleason score (2–6 vs. 7–10), risk group (low vs. intermediate vs. high), neoadjuvant/adjuvant AD (yes vs. no), and RT technique (3-D conformal vs. conventional). Hazard functions were estimated using life-table methodology, with comparisons assessed using the log-rank test for overall differences and the Wilcoxon statistic for early differences. All statistical tests were two-sided; P<.05 was considered statistically significant.
RESULTS
The clinical characteristics of the 1833 patients are summarized in Table 1. As expected from the risk group stratification criteria, the high risk cohort had significantly higher T-categories, iPSAs, and Gleason scores than the low risk group (P<.0001 for all three variables). The intermediate risk group similarly had higher iPSAs and Gleason scores (P <.0001 for both). Median age differed by only one year, although the age difference was statistically significant. Fewer patients in the low risk group received a radiation dose ≥76 Gy. Median follow-up for the low and intermediate risk groups was identical at 65 months. The high risk group had longer median follow-up (79 months, P<.0001). A greater percentage of patients received neoadjuvant or adjuvant AD in the intermediate (P=.02) and the high risk (P<.0001) groups.
TABLE 1.
Patient characteristics according to risk group*
| Characteristic | Low risk (n=766) | Intermediate risk (n=677) | High risk (n=390) |
|---|---|---|---|
| Age, years | |||
| Median (range) | 68 (43–84) | 69 (45–86) | 69 (43–89) |
| T-category | |||
| T1–T2 | 766 (100%) | 677 (100%) | 252 (65%) |
| T3–T4 | 0 (0%) | 0 (0%) | 138 (35%) |
| iPSA, ng/mL | |||
| <10 | 766 (100%) | 235 (35%) | 86 (22%) |
| 10-<20 | 0 (0%) | 442 (65%) | 53 (14%) |
| ≥20 | 0 (0%) | 0 (0%) | 251 (64%) |
| Gleason score | |||
| 2–6 | 766 (100%) | 331 (49%) | 199 (51%) |
| 7 | 0 (0%) | 346 (51%) | 112 (29%) |
| 8–10 | 0 (0%) | 0 (0%) | 79 (20%) |
| RT dose, gray | |||
| <74 | 339 (44%) | 177 (26%) | 152 (39%) |
| 74-<76 | 347 (45%) | 211 (31%) | 147 (38%) |
| ≥76 | 80 (10%) | 289 (43%) | 91 (23%) |
| RT dose, gray | |||
| Mean | 74 | 77 | 75 |
| Median (range) | 76 (65–82) | 76 (67–82) | 76 (51–82) |
| Neoadjuvant/adjuvant androgen deprivation, duration 0–6 months | 41 (5%) | 57 (8%) | 96 (25%) |
| Follow-up, months | |||
| Median (range) | 65 (5–206) | 65 (4–185) | 79 (4–174) |
RT indicates radiotherapy; iPSA, initial pretreatment prostate specific antigen level.
Every patient characteristic listed had P<0.05 when comparing all three risk groups.
Biochemical failure using the ASTRO definition was documented in 447 (24%) of the 1833 study patients. The majority of these failures were registered early due to backdating, with 390/447 (87%) in years 0–4 post-RT. The 5-year actuarial ASTRO BF rates increased from 16% to 54% with higher risk group stratification and the difference in ASTRO BF among risk groups was statistically significant on univariate (P<.0001, Table 2) and multivariate analysis (P<.0001, Table 4). Univariate analyses revealed that higher T-category, Gleason score and iPSA, and use of neoadjuvant/adjuvant AD were significantly associated with a higher ASTRO BF rates (P<.0001, Table 3). Conversely, higher RT doses were significantly related to lower ASTRO BF rates (P<.0001). Multivariate analysis (MVA) for all patients confirmed that higher Gleason score, iPSA, and risk group, along with lower RT dose, were independently associated with increased ASTRO BF (Table 4). An MVA was also performed for each risk group individually (Table 4).
TABLE 2.
Biochemical failure, distant metastasis, salvage androgen deprivation, and mortality by risk group
| Event type | Low risk (n=766) | Intermediate risk (n=677) | High risk (n=390) |
|---|---|---|---|
| ASTRO biochemical failurea | |||
| Crude rate (no.) | 14% (104) | 24% (161) | 47% (182) |
| 5-year actuarial | 16% | 28% | 54% |
| 10-year actuarial | 20% | 36% | 61% |
| Nadir+2 biochemical failureb | |||
| Crude rate (no.) | 11% (85) | 21% (140) | 44% (171) |
| 5-year actuarial | 9% | 18% | 43% |
| 10-year actuarial | 25% | 48% | 67% |
| Distant metastasis | |||
| Crude rate (no.) | 2% (12) | 5% (32) | 11% (43) |
| 5-year actuarial | 1% | 4% | 8% |
| 10-year actuarial | 2% | 9% | 16% |
| Salvage androgen deprivation | |||
| Crude rate (no.) | 5% (36) 1% | 10% (70) | 27% (104) |
| Initiated after DM diagnosed | (4) | 3% (21) | 6% (22) |
| Initiated for rising PSA develop subsequent DM | 4% (32) | 7% (49) | 21% (82) |
| 1% (6) | 1% (8) | 5% (19) | |
| Overall mortality, | |||
| Crude rate (no.) | 15% (118) | 20% (137) | 38% (149) |
| 5-year actuarial | 8% | 11% | 17% |
| 10-year actuarial | 30% | 33% | 50% |
| Cause specific mortality | |||
| Crude rate (no.) | 1% (6) | 2% (12) | 7% (29) |
| 5-year actuarial | 1% | 1% | 4% |
| 10-year actuarial | 1% | 3% | 11% |
ASTRO indicates American Society of Therapeutic Radiology and Oncology; BF, biochemical failure; DM, distant metastasis; PSA, prostate-specific antigen.
P<.0001 for differences among risk groups in every category above.
ASTRO definition of biochemical failure: 3 consecutive rises in PSA, with backdating to midpoint between nadir and the first rise.8
TABLE 4.
Multivariate analyses of potential predictors of biochemical failure and distant metastasis*
| Biochemical failure
|
Distant metastasis
|
|||||
|---|---|---|---|---|---|---|
| ASTROa |
Nadir+2b |
|||||
| Covariate† | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P |
| All patients | ||||||
| Risk group | 1.80 (1.55–2.08) | <.0001 | 1.71 (1.46–2.00) | <.0001 | ||
| T-stage | 2.16 (1.30–3.61) | .003 | ||||
| Gleason score | 1.38 (1.12–1.71) | .002 | 1.61 (1.29–2.01) | <.0001 | 3.39 (2.18–5.26) | <.0001 |
| iPSA | 1.01 (1.01–1.02) | <.0001 | 1.01 (1.01–1.02) | <.0001 | 1.02 (1.01–1.02) | <.0001 |
| Conformal RT | 0.68 (0.48–0.94) | .02 | ||||
| RT dose | 0.91 (0.89–0.93) | <.0001 | 0.95 (0.92–0.97) | <.0001 | 0.94 (0.89–0.99) | .025 |
|
| ||||||
| Low risk | ||||||
| Age | 0.96 (0.93–1.00) | .03 | No covariate met the P<.05 requirement for DM in the low risk group | |||
| iPSA | 1.12 (1.01–1.23) | .03 | 1.23 (1.10–1.38) | .0004 | ||
| RT dose | 0.93 (0.87–0.99) | .03 | ||||
|
| ||||||
| Intermediate risk | ||||||
| Gleason score | 5.08 (2.24–11.52) | <.0001 | ||||
| iPSA | 1.10 (1.02–1.20) | .018 | ||||
| RT dose | 0.91 (0.88–0.94) | <.0001 | 0.95 (0.91–0.98) | .008 | 0.91 (0.83–0.996) | .041 |
|
| ||||||
| High risk | ||||||
| T-stage | 2.02 (1.09–3.75) | .025 | ||||
| Gleason score | 1.73 (1.27–2.35) | .0005 | 2.07 (1.12–3.86) | .021 | ||
| iPSA | 1.01 (1.01–1.02) | <.0001 | 1.01 (1.01–1.02) | <.0001 | 1.01 (1.00–1.02) | .003 |
| Conformal RT | 0.58 (0.38–0.88) | .01 | ||||
| RT dose | 0.92 (0.89–0.95) | <.0001 | 0.95 (0.92–0.99) | .007 | ||
ASTRO indicates American Society of Therapeutic Radiology and Oncology; HR, hazard ratio; CI, confidence interval; iPSA, initial pretreatment prostate-specific antigen level; RT, radiotherapy.
Only variables with P<.05 are listed.
Four MVAs are shown: all patients, low, intermediate and high risk patients.
ASTRO definition of biochemical failure: 3 consecutive rises in PSA, with BF backdated to midpoint between nadir and the first rise.8
TABLE 3.
Univariate analyses of potential predictors of biochemical failure and distant metastasis
| Biochemical failure
|
Distant metastasis
|
||||||
|---|---|---|---|---|---|---|---|
| ASTROa |
Nadir+2b |
||||||
| Covariate | No. of men | (5-year) | P | (5-Year) | P | (5-Year) | P |
| Age (years) | |||||||
| ≤ 68 | 894 | 29% | 20% | 4% | |||
| >68 | 939 | 29% | .9501 | 20% | .8251 | 3% | .2034 |
| T-category | |||||||
| T1–T2 | 1695 | 26% | 18% | 3% | |||
| T3–T4 | 138 | 58% | <.0001 | 45% | <.0001 | 12% | <.0001 |
| Gleason score | |||||||
| 2–6 | 1296 | 25% | 16% | 2% | |||
| 7 | 458 | 39% | 28% | 7% | |||
| 8–10 | 79 | 41% | <.0001 | 44% | <.0001 | 11% | <.0001 |
| iPSA (ng/mL) | |||||||
| <10 | 1087 | 25% | 13% | 2% | |||
| 10-<20 | 495 | 29% | 20% | 4% | |||
| ≥20 | 251 | 61% | <.0001 | 48% | <.0001 | 8% | <.0001 |
| Neoadjuvant/adjuvant AD | |||||||
| No | 1639 | 27% | 20% | 4% | |||
| Yes | 194 | 43% | <.0001 | 23% | <.0001 | 4% | .5193 |
| RT dose (Gy) | |||||||
| <74 | 668 | 37% | 24% | 5% | |||
| 74-<76 | 705 | 26% | 20% | 3% | |||
| ≥76 | 460 | 20% | <.0001 | 15% | .0523 | 3% | .2917 |
ASTRO indicates American Society of Therapeutic Radiology and Oncology; iPSA, initial pretreatment prostate-specific antigen level; AD, androgen deprivation; RT, radiotherapy; Gy, Gray.
ASTRO definition of biochemical failure: 3 consecutive rises in PSA, with BF backdated to midpoint between nadir and the first rise.8
Biochemical failure using the Nadir+2 definition was documented in 396 (22%) of the 1833 study patients. These failures were recorded at call (time of event) following treatment, with 170/396 (43%) occurring >4 year after RT. The 5-year actuarial Nadir+2 BF rates increased from 9% to 43% with higher risk group stratification. The difference in Nadir+2 BF rates among the risk groups was statistically significant on univariate (P<.0001, Table 2) and multivariate analyses (P<.0001, Table 4). As with ASTRO BF, univariate analyses revealed that higher T-category, Gleason score, and iPSA, and use of neoadjuvant/adjuvant AD were significantly associated with higher Nadir+2 BF rates (P<.0001, Table 3). Multivariate analyses confirmed that higher iPSA, higher Gleason score, lower RT dose, and some other covariates, were independently associated with increased Nadir+2 BF (P<.0001; Table 4).
Using the ASTRO and Nadir+2 definitions, the hazard rates of BF for each 2-year time interval of follow-up after treatment are shown in Figure 1. Due to backdating, ASTRO BF was documented at earlier times than Nadir+2 BF. ASTRO BFs are nearly absent in years 8–12. The most revealing findings are derived from the examination of interval hazard rates using the Nadir+2 definition. The hazard of Nadir+2 BF persisted in years 8–12 in all risk groups. For low risk patients there was a peak incidence at 6–8 years post-treatment. For intermediate risk patients, Nadir+2 BF continued to gradually increase throughout the period of the study. For high risk patients, there was an early peak at 4–6 years, followed by a decline. The decline in Nadir+2 BF in the 10–12 year range was undoubtedly affected by the lack of patients at risk; but prior to that the decline is probably real. The difference in the BF hazard function between the low, intermediate, and high risk groups was statistically significant using the log-rank test for overall differences (ASTRO BF, P<.0001; Nadir+2 BF, P<.0001) and the Wilcoxon statistic for early differences (ASTRO BF, P<.0001; Nadir+2 BF, P<.0001).
FIGURE 1.
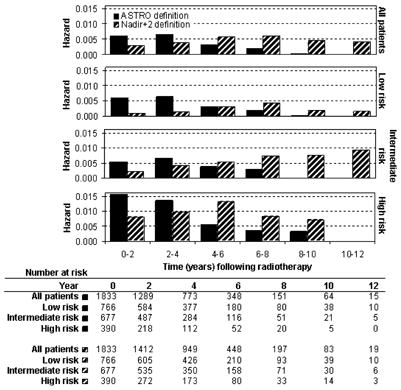
Interval hazard rates for biochemical failure during 2-year time intervals after radiotherapy, according to the ASTRO definition (3 consecutive rises in PSA, backdated)8 and the Nadir+2 definition (PSA ≥ nadir + 2 ng/mL).9-14
Distant metastasis was documented in 87 (5%) of the 1833 study patients. The 5-year actuarial DM rates increased from 1% to 8% with increasing risk group stratification (P<.0001, Table 2). The 10-year actuarial DM rates were higher and were consistent with this pattern. Univariate analyses revealed that higher T-category, Gleason score, and iPSA were significantly associated with higher DM (P<.0001 for each, Table 3). RT dose as a categorical variable did not reach significance (P=.2917). On MVA, the significant associations of T-category, Gleason score, and iPSA with DM were confirmed (Table 4). Moreover, increasing RT dose as a continuous variable was significantly associated with reduced DM (P=.025); the effect of dose was most apparent in the intermediate risk patients. The risk of DM was decreased by 6% with each additional Gy of radiation administered, within the dose range of the study (51–82 Gy).
The interval hazard rates for DM are shown in Figure 2. The distribution of DM over time was biphasic in the intermediate and high risk groups, with an early wave peaking at 0–4 years and a late wave at 8–12 years. The waves in the high risk group had greater magnitude and began about 2 years earlier than in the intermediate risk group. In the low risk group, the pattern of DM was not biphasic, due to the absence of a distinct early wave; the risk of DM remained low after RT until a late wave of DM was seen in years 10–12. The difference in the DM hazard function between the risk groups was statistically significant using the log-rank test for overall differences (P<.0001) and the Wilcoxon statistic for early differences (P<.0001).
FIGURE 2.
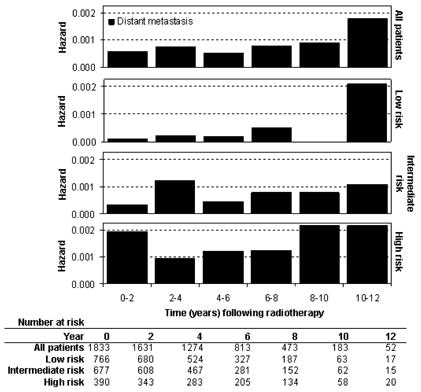
Interval hazard rates for distant metastasis during 2-year time intervals after radiotherapy.
One potentially confounding factor in an analysis of the timing of failure, particularly DM, is the use of androgen deprivation therapy. In this study, 194 patients (11%) received AD in the neoadjuvant or adjuvant setting for ≤6 months duration. A significantly higher percentage of men in the intermediate and high risk groups received neoadjuvant/adjuvant AD (Table 1). When patients who received neoadjuvant/adjuvant AD were excluded from the DM hazard analysis (Figure 3), the timing of DM was largely unchanged compared to the entire study group, with the exception of the late wave of DM peaking at a slightly earlier time during the 8–12 year period among high risk patients.
FIGURE 3.
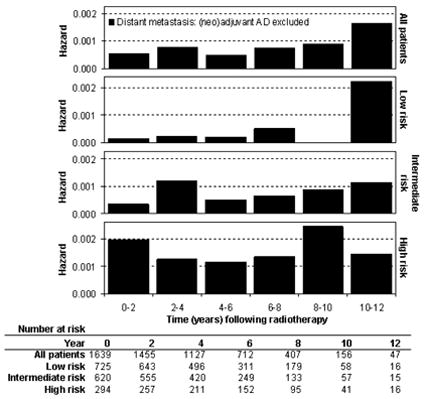
Interval hazard rates for distant metastasis during 2-year time intervals after radiotherapy alone, excluding patients who received neoadjuvant/adjuvant androgen deprivation (AD).
Salvage AD initiated after RT is another confounding factor. Androgen deprivation given to patients for a rising PSA could postpone or eliminate the subsequent appearance of DM, thus altering the DM interval hazard function. Salvage AD was administered to 210 (11%) of the 1833 study patients. If the salvage AD was initiated after a diagnosis of DM, the DM hazard function would not be affected. However, 163 (9%) of the study patients had hormone therapy initiated for a rising PSA, at a time when no DM had been identified. Half of these patients (n=82) were in the high risk group. Table 2 shows that with increasing patient risk, there is an increase in the use of salvage AD before the diagnosis of DM (4%, 7% to 21% for low, intermediate to high risk). Thus, the greatest potential effect on the shape of the interval hazard curves would be in the high risk group; yet, this was the group showing the earliest onset of DM in the early and late waves.
Regarding mortality, a total of 404 deaths occurred, including 47 prostate cancer cause-specific deaths (Table 2). Multivariate analysis demonstrated that higher age, Gleason score, and risk group, along with lower RT dose, were significant predictors of increased OM (P<.01 for each). For CSM, higher iPSA, Gleason score, and T-stage were significantly associated with increased cause-specific death (P≤.01 for each). The interval hazard rates for OM and CSM are shown in Figure 4. The preponderance of deaths was from intercurrent disease, with the hazard of OM increasing steadily from time 0 out to 12 years in every risk group. A small wave of CSM is seen in the 10–12 year time interval in the intermediate and high risk groups, which was less apparent in the low risk patients. These data suggest that the early wave of DM does not translate into mortality from prostate cancer until 5–8 years later.
FIGURE 4.
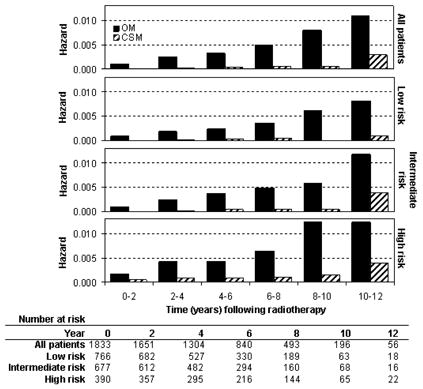
Interval hazard rates for overall mortality (OM) and cause specific mortality (CSM) during 2-year time intervals after radiotherapy.
DISCUSSION
Radiotherapy is an effective local treatment for prostate cancer, as is radical prostatectomy.22 However, a rising PSA occurs too frequently, in an average of 30–40% at 10 years depending on patient features. Many of these failures are beyond 5 years. Amling et al23 have described a persistent yearly risk of biochemical failure of approximately 5% at 5–10 years after radical prostatectomy. Likewise, there is a continued risk of biochemical failure after radiotherapy beyond 5 years.24 This pattern of late biochemical failures is suggestive of local tumor persistence.
Local persistence of tumor cells after radical prostatectomy25-27 and radiotherapy28-30 has been documented in studies of selected patients. Because routine biopsies of the prostate bed/prostate are not performed in the PSA era, the full extent of the problem, as well as the relationship of local persistence to distant metastasis is not well-described. The results described here support the concept that local persistence of disease after radiotherapy results in a late wave of metastasis, whereas early DM are primarily due to progression of subclinical metastases already present at diagnosis.
We observed that increasing risk group stratification was significantly associated with increased DM (P<.0001, Table 2). Our data show for the first time that the DM interval hazard function for men with intermediate and high risk prostate cancer is biphasic (Figure 2). The initial wave peaked at 0–4 years and the second wave at 8–12 years following completion of RT. In the high risk group, the early wave was higher in magnitude and began earlier, suggesting that occult micrometastases are more common at diagnosis and progress more quickly in men with high risk disease. A low incidence of occult DM among low risk patients likely explains the paucity of early DM in this group. There was, however, a significant late wave of DM among low risk patients, suggesting that local persistence of disease with hematogenous spread of tumor cells remains a problem even in these more indolent cancers. Many of these patients received lower RT doses, which could predispose to incomplete tumor eradication.
An alternative explanation for the late wave of DM among low risk patients is that occult micrometastatic disease at diagnosis grows so slowly that clinical manifestation of DM takes 10 years or more to become apparent. This mechanism is not likely to be dominant, considering the following results. First, Coen et al3 showed that late DM are more pronounced in prostate cancer patients with local failure after RT. More importantly, those who remained controlled locally had a decreasing risk of DM over time, with no late wave. Second, if local persistence is the primary contributor to the late wave, then RT dose escalation should reduce late DM. Although the randomized dose escalation trials have not demonstrated a significant effect on DM, these trials did not have sufficient follow-up past 8 years, when the late wave occurs.18, 31, 32 The last report of the M.D. Anderson trial18 showed a borderline trend for DM reduction (P=.056; median follow-up 5 years) and a more recent preliminary analysis with longer follow-up (median follow-up 8.7 years) more conclusively demonstrates that higher RT dose reduces DM.33 They observed a significantly lower DM rate (4%) after 78 Gy, compared to after 70 Gy (17%). These results indicate that the local control benefit of RT dose escalation translates into a reduction in DM with sufficient follow-up. Lastly, local persistence as a mechanism for late BF and DM after RT is suggested by the success of local salvage therapy, including prostatectomy,34 brachytherapy,35 and cryosurgery.36 These treatments are most effective in men who develop a rising PSA after RT for low risk disease.
Our results are also concordant with the findings of observational studies. Albertsen et al37 have documented how the natural history of prostate cancer is influenced by Gleason score, with lower Gleason scores of 2–6 demonstrating indolent progression with cause-specific mortality rates of 7–27% over 20-years. The isolated late wave of DM observed in low risk patients in the current study suggests that tumor cells were left behind after RT, and the potential progression to DM takes many years, reflecting the natural history of the disease.
Figure 1 illustrates the problem of backdating of BF using the ASTRO definition. Under these conditions, late biochemical failures are nearly absent, suggesting that almost all of the failures occurred early. This artifact of the ASTRO BF definition has previously been interpreted as suggesting prostate cancer cure.38 In contrast, the interval hazard function for BF using the Nadir+2 definition is spread more evenly over the study period because failure is specified at the call date. The Nadir+2 definition is more appropriate for examining the timing of BF.
In contrast to the results in low risk patients, each wave of DM in intermediate-to-high risk patients was not immediately preceded by a wave of BF. The Nadir+2 BF hazard function was not biphasic. Among intermediate risk tumors, the hazard of Nadir+2 BF continued to rise over time, suggesting that local persistence of disease continues to declare itself even 10 years after RT. This late rise in the Nadir+2 BF suggests that RT may be postponing, rather than truly eliminating, the risk of late DM beyond the follow-up period in some patients. In high risk patients, Nadir+2 BF was relatively high from the outset, peaked at 4–6 years and then appeared to decline. These data suggest that locally persistent high risk disease declares itself earlier than for intermediate risk disease. This shorter latency and higher propensity for BF and DM reflect the more aggressive biology of these tumors.
Several potential confounding factors exist due to the retrospective design of this study. It is well established that androgen deprivation therapy delays the time to DM. Neoadjuvant/adjuvant AD of <6 months duration was administered in 5%, 8%, and 25% of the low, intermediate and high risk groups, respectively. This introduces a bias toward delayed failure in the high risk group, which we attempted to lessen by measuring the time to BF and DM from the end of AD in these patients. Despite this bias, failures generally occurred at earlier time intervals in the high risk group. When patients who received neoadjuvant/adjuvant AD were excluded from the DM hazard analysis, the late wave of DM in the high risk group peaked earlier in the 8–12 year time interval (Figure 3). Otherwise there were no notable differences in the timing of DM in any risk group.
Salvage AD is another confounding factor. If AD is initiated after a diagnosis of DM has been established, then our analysis would not be affected. However, 163 (9%) of the study patients received salvage AD for a rising PSA, at a time when they had no evidence of DM. A higher percentage of patients in the high risk group received this treatment (21% versus 4–7% in the lower risk groups, Table 2); but nevertheless had higher DM rates over the follow-up period. Although, there was a slight follow-up bias favoring the lower risk groups (median follow-up 65 months vs 79 months in the high risk group), DMs were generally seen earlier in the higher risk group.
In conclusion, analysis of the interval hazard function for BF and DM in discrete 2-year time intervals following RT provides unique insight into the mechanisms of progression to BF and DM in low, intermediate and high risk prostate cancers. The data indicate that intermediate and high risk patients have a greater risk of occult micrometastasis, manifested by an early wave of DM, as compared to low risk patients (absence of an early wave). Early DM appear to translate into increased CSM after a lag time of about 5–8 years. The late DM data, combined with the timing of Nadir+2 BF, suggest that local disease in the prostate remains a problem in some patients after RT, even among low risk patients. RT dose escalation would be expected to reduce local persistence and consequently late DM in all risk groups, but follow-up of greater than 10 years would be required to fully appreciate this benefit.
Acknowledgments
This research was supported in part by grants CA-006927 and CA101984-01 from the National Cancer Institute, and a grant from Varian Medical Systems, Palo Alto, CA. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or Varian Medical Systems. The authors thank Dr. Gerald Hanks for his leadership in the establishment of the Fox Chase Cancer Center database for the treatment of prostate cancer reported herein and Ruth Peter for her dedication to its maintenance.
References
- 1.Chism DB, Hanlon AL, Horwitz EM, Feigenberg SJ, Pollack A. A comparison of the single and double factor high-risk models for risk assignment of prostate cancer treated with 3D conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2004 Jun 1;59:380–385. doi: 10.1016/j.ijrobp.2003.10.059. [DOI] [PubMed] [Google Scholar]
- 2.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. Jama. 1998 Sep 16;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 3.Coen JJ, Zietman AL, Thakral H, Shipley WU. Radical radiation for localized prostate cancer: local persistence of disease results in a late wave of metastases. J Clin Oncol. 2002 Aug 1;20:3199–3205. doi: 10.1200/JCO.2002.01.086. [DOI] [PubMed] [Google Scholar]
- 4.American Joint Committee on Cancer. Prostate. In: Greene FL, Page DL, Fleming ID, et al., editors. AJCC Cancer Staging Manual. New York: Springer; 2002. pp. 337–345. [Google Scholar]
- 5.Hanks GE. The Prostate. St. Louis: C.V. Mosby; 1989. [Google Scholar]
- 6.Horwitz EM, Hanlon AL, Pinover WH, Anderson PR, Hanks GE. Defining the optimal radiation dose with three-dimensional conformal radiation therapy for patients with nonmetastatic prostate carcinoma by using recursive partitioning techniques. Cancer. 2001 Sep 1;92:1281–1287. doi: 10.1002/1097-0142(20010901)92:5<1281::aid-cncr1449>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 7.Monti AF, Ostinelli A, Frigerio M, et al. An ICRU 50 radiotherapy treatment chart. Radiother Oncol May. 1995;35:145–150. doi: 10.1016/0167-8140(95)01541-n. [DOI] [PubMed] [Google Scholar]
- 8.Cox J, Grignon D, Kaplan R, Parsons J, Schellhammer P. Consensus statement: guidelines for PSA following radiation therapy. Int J Radiat Oncol Biol Phys. 1997;37:1035–1041. [PubMed] [Google Scholar]
- 9.Roach M, 3rd, Hanks G, Thames H, Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006 Jul 15;65:965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 10.Horwitz EM, Thames HD, Kuban DA, et al. Definitions of biochemical failure that best predict clinical failure in patients with prostate cancer treated with external beam radiation alone: a multi-institutional pooled analysis. J Urol Mar. 2005;173:797–802. doi: 10.1097/01.ju.0000152556.53602.64. [DOI] [PubMed] [Google Scholar]
- 11.Kestin LL, Vicini FA, Martinez AA. Practical application of biochemical failure definitions: what to do and when to do it. Int J Radiat Oncol Biol Phys. 2002 Jun 1;53:304–315. doi: 10.1016/s0360-3016(02)02707-4. [DOI] [PubMed] [Google Scholar]
- 12.Pickles T, Kim-Sing C, Morris WJ, Tyldesley S, Paltiel C. Evaluation of the Houston biochemical relapse definition in men treated with prolonged neoadjuvant and adjuvant androgen ablation and assessment of follow-up lead-time bias. Int J Radiat Oncol Biol Phys. 2003 Sep 1;57:11–18. doi: 10.1016/s0360-3016(03)00439-5. [DOI] [PubMed] [Google Scholar]
- 13.Vicini FA, Kestin LL, Martinez AA. The correlation of serial prostate specific antigen measurements with clinical outcome after external beam radiation therapy of patients for prostate carcinoma. Cancer. 2000 May 15;88:2305–2318. doi: 10.1002/(sici)1097-0142(20000515)88:10<2305::aid-cncr15>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 14.Thames H, Kuban D, Levy L, et al. Comparison of alternative biochemical failure definitions based on clinical outcome in 4839 prostate cancer patients treated by external beam radiotherapy between 1986 and 1995. Int J Radiat Oncol Biol Phys. 2003 Nov 15;57:929–943. doi: 10.1016/s0360-3016(03)00631-x. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statistical Assoc. 1958;53:447–457. [Google Scholar]
- 16.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep Mar. 1966;50:163–170. [PubMed] [Google Scholar]
- 17.Hanks GE, Hanlon AL, Epstein B, Horwitz EM. Dose response in prostate cancer with 8–12 years’ follow-up. Int J Radiat Oncol Biol Phys. 2002 Oct 1;54:427–435. doi: 10.1016/s0360-3016(02)02954-1. [DOI] [PubMed] [Google Scholar]
- 18.Pollack A, Zagars GK, Starkschall G, et al. Prostate cancer radiation dose response: results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys. 2002 Aug 1;53:1097–1105. doi: 10.1016/s0360-3016(02)02829-8. [DOI] [PubMed] [Google Scholar]
- 19.Zelefsky MJ, Fuks Z, Hunt M, et al. High dose radiation delivered by intensity modulated conformal radiotherapy improves the outcome of localized prostate cancer. J Urol Sep. 2001;166:876–881. [PubMed] [Google Scholar]
- 20.Pollack A, Smith LG, von Eschenbach AC. External beam radiotherapy dose response characteristics of 1127 men with prostate cancer treated in the PSA era. Int J Radiat Oncol Biol Phys. 2000 Sep 1;48:507–512. doi: 10.1016/s0360-3016(00)00620-9. [DOI] [PubMed] [Google Scholar]
- 21.Pollack A, Hanlon AL, Horwitz EM, Feigenberg SJ, Uzzo RG, Hanks GE. Prostate cancer radiotherapy dose response: an update of the fox chase experience. J Urol Mar. 2004;171:1132–1136. doi: 10.1097/01.ju.0000111844.95024.74. [DOI] [PubMed] [Google Scholar]
- 22.Kupelian PA, Elshaikh M, Reddy CA, Zippe C, Klein EA. Comparison of the efficacy of local therapies for localized prostate cancer in the prostate-specific antigen era: a large single-institution experience with radical prostatectomy and external-beam radiotherapy. J Clin Oncol. 2002 Aug 15;20:3376–3385. doi: 10.1200/JCO.2002.01.150. [DOI] [PubMed] [Google Scholar]
- 23.Amling CL, Blute ML, Bergstralh EJ, Seay TM, Slezak J, Zincke H. Long-term hazard of progression after radical prostatectomy for clinically localized prostate cancer: continued risk of biochemical failure after 5 years. J Urol Jul. 2000;164:101–105. [PubMed] [Google Scholar]
- 24.Rosser CJ, Levy LB, Kuban DA, et al. Hazard rates of disease progression after external beam radiotherapy for clinically localized carcinoma of the prostate. J Urol Jun. 2003;169:2160–2165. doi: 10.1097/01.ju.0000058212.00170.a0. [DOI] [PubMed] [Google Scholar]
- 25.Naya Y, Okihara K, Evans RB, Babaian RJ. Efficacy of prostatic fossa biopsy in detecting local recurrence after radical prostatectomy. Urology Aug. 2005;66:350–355. doi: 10.1016/j.urology.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 26.Leventis AK, Shariat SF, Slawin KM. Local recurrence after radical prostatectomy: correlation of US features with prostatic fossa biopsy findings. Radiology May. 2001;219:432–439. doi: 10.1148/radiology.219.2.r01ma20432. [DOI] [PubMed] [Google Scholar]
- 27.Saleem MD, Sanders H, Abu El Naser M, El-Galley R. Factors predicting cancer detection in biopsy of the prostatic fossa after radical prostatectomy. Urology Feb. 1998;51:283–286. doi: 10.1016/s0090-4295(97)00509-8. [DOI] [PubMed] [Google Scholar]
- 28.Levegrun S, Jackson A, Zelefsky MJ, et al. Risk group dependence of dose-response for biopsy outcome after three-dimensional conformal radiation therapy of prostate cancer. Radiother Oncol Apr. 2002;63:11–26. doi: 10.1016/s0167-8140(02)00062-2. [DOI] [PubMed] [Google Scholar]
- 29.Pollack A, Zagars GK, Antolak JA, Kuban DA, Rosen II. Prostate biopsy status and PSA nadir level as early surrogates for treatment failure: analysis of a prostate cancer randomized radiation dose escalation trial. Int J Radiat Oncol Biol Phys. 2002 Nov 1;54:677–685. doi: 10.1016/s0360-3016(02)02977-2. [DOI] [PubMed] [Google Scholar]
- 30.Dugan TC, Shipley WU, Young RH, et al. Biopsy after external beam radiation therapy for adenocarcinoma of the prostate: correlation with original histological grade and current prostate specific antigen levels. J Urol Nov. 1991;146:1313–1316. doi: 10.1016/s0022-5347(17)38077-1. [DOI] [PubMed] [Google Scholar]
- 31.Peeters ST, Heemsbergen WD, Koper PC, et al. Dose-response in radiotherapy for localized prostate cancer: results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol. 2006 May 1;24:1990–1996. doi: 10.1200/JCO.2005.05.2530. [DOI] [PubMed] [Google Scholar]
- 32.Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. Jama. 2005 Sep 14;294:1233–1239. doi: 10.1001/jama.294.10.1233. [DOI] [PubMed] [Google Scholar]
- 33.Kuban D, Tucker S, Dong L, et al. Long-term results of a randomized dose escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2006;66:S8–S9. doi: 10.1016/j.ijrobp.2007.06.054. (abstr) [DOI] [PubMed] [Google Scholar]
- 34.Ward JF, Sebo TJ, Blute ML, Zincke H. Salvage surgery for radiorecurrent prostate cancer: contemporary outcomes. J Urol Apr. 2005;173:1156–1160. doi: 10.1097/01.ju.0000155534.54711.60. [DOI] [PubMed] [Google Scholar]
- 35.Grado GL, Collins JM, Kriegshauser JS, et al. Salvage brachytherapy for localized prostate cancer after radiotherapy failure. Urology Jan. 1999;53:2–10. doi: 10.1016/s0090-4295(98)00492-0. [DOI] [PubMed] [Google Scholar]
- 36.Bahn DK, Lee F, Silverman P, et al. Salvage cryosurgery for recurrent prostate cancer after radiation therapy: a seven-year follow-up. Clin Prostate Cancer Sep. 2003;2:111–114. doi: 10.3816/cgc.2003.n.018. [DOI] [PubMed] [Google Scholar]
- 37.Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. Jama. 2005 May 4;293:2095–2101. doi: 10.1001/jama.293.17.2095. [DOI] [PubMed] [Google Scholar]
- 38.Hanlon AL, Hanks GE. Failure patterns and hazard rates for failure suggest the cure of prostate cancer by external beam radiation. Urology May. 2000;55:725–729. doi: 10.1016/s0090-4295(99)00605-6. [DOI] [PubMed] [Google Scholar]


