Abstract
Ovariectomized rats with bilateral cannulae near the ventromedial nucleus of the hypothalamus were hormonally primed with 10 μg estradiol benzoate and 500 μg progesterone. Sexually receptive females were infused bilaterally with 200 ng of the 5-HT1A receptor agonist, 8-hydroxy-2-(di-n-propylamino) tetralin (8-OH-DPAT), or with a combination of 200 ng 8-OH-DPAT and 2000 ng of the 5-HT2 receptor agonist, (±)-2,5-dimethoxy-4-iodophenyl-2-aminopropane HCl (DOI). 8-OH-DPAT inhibited lordosis behavior and DOI reduced this inhibition. However, if females were preinfused with the PKC inhibitor, bisindolymaleimide I hydrochloride (BIM), DOI’s effect was eliminated. BIM’s attenuation of the effects of DOI was time-dependent. When BIM was infused 90 min, but not 30 min, before the 5-HT receptor agonists, BIM eliminated DOI’s protection against the lordosis-inhibiting effects of 8-OH-DPAT. A concentration of BIM as low as 10−5 nmol in a 0.5 μl infusion volume was effective and there was little evidence of dose responsivity between 10−5 and 10−1 nmol of BIM. In contrast, prior infusion with vehicle or with 10−7 nmol BIM had no impact on the female’s response to the 5-HT receptor agonists. These findings allow the suggestion that DOI’s ability to increase PKC may be responsible for attenuation of the effects of 8-OH-DPAT on lordosis behavior.
Keywords: PKC inhibitor, 5-HT1A receptors, 5-HT2 receptors, ovariectomized, sexual behavior
1.0 Introduction
Within the ventromedial nucleus of the hypothalamus (VMN), serotonin (5-HT) modulates female rat sexual behavior by acting on multiple 5-HT receptors [39]. Lordosis behavior, a supraspinal estrogen-dependent reflex of sexually receptive females [29], is inhibited by 5-HT1A receptor agonists and facilitated by 5-HT2 receptor agonists and their coactivation attenuates the lordosis-inhibitory effects of 5-HT1A receptor agonists [39]. We have proposed that this 5-HT1A/5-HT2 receptor interaction enables the female to fine-tune her behavior so that it is most adaptive to environmental conditions [42]. However, the mechanisms for this interaction are not known.
5-HT1A receptors are coupled to Gi/o/z proteins and mediate multiple intracellular responses including inhibition of adenylate cyclase, opening of a K+ channel, and inhibition of Ca2+ channels [32]. Effects of 5-HT1A receptors on ion channels probably contribute to the decrease in neuronal firing, characteristic of activation of 5-HT1A receptors [1,19,28]. An increase in firing of VMN neurons occurs when lordosis behavior is increased, while reduced firing of VMN neurons occurs under conditions where the behavior is decreased [11,30]. Kow and Pfaff [18] reported that estrogen enhanced the overall excitability of VMN neurons and speculated that this excitability contributed to the hormone’s facilitation of lordosis behavior. Therefore, 5-HT1A receptor agonists may reduce lordosis behavior via reduced firing of VMN neurons.
However, modulation of cAMP within lordosis-relevant brain areas also alters lordosis behavior. Agents which inhibit adenylate cyclase in the VMN reduce lordosis behavior while agents that increase adenylate cyclase in the VMN facilitate lordosis behavior [17]. Moreover, compounds (including estrogen) that increase cAMP not only facilitate lordosis behavior but can attenuate the lordosis-inhibiting effects of 5-HT1A receptor agonists [13,41].
5-HT2 receptors are Gq/11 coupled to phospholipase C and their activation increases diacylglycerol (DAG) and inositol triphosphate (IP3) and leads to activation of protein kinase C (PKC) and elevation of intracellular Ca2+ [23]. Since agents which increase DAG and IP3 facilitate lordosis behavior [17], PKC may be involved in the facilitatory effects of 5-HT2 receptor agonists on lordosis behavior. Relevant to the ability of 5-HT2 receptor agonists to attenuate effects of 5-HT1A receptor agonists on lordosis behavior [27,40,46] is evidence that PKC can rapidly desensitize 5-HT1A receptors in a cell culture system [31]. The 5-HT1A receptor contains multiple putative phosphorylation sites for both PKC and cAMP dependent protein kinase (PKA) [33] and both kinases can lead to desensitization of 5-HT1A receptors [12,34]. In addition, Giα proteins may be subject to phosphorylation, leading to attenuation of 5-HT1A receptor signaling [15,20]. Thus multiple opportunities exist for cross talk between 5-HT1A and 5-HT2 receptors and there is substantial evidence for their functional interaction [7,21,26].
For example, both flattened body posture and hypothermia, two behaviors elicited by 5-HT1A receptor agonists, are reduced when 5-HT2 receptor agonists are administered together with 5-HT2 receptor agonists [2,5]. Consistent with our findings that VMN infusion with DOI prevented the lordosis-inhibiting effect of 8-OH-DPAT [40], addition of the 5-HT2 receptor agonist, DOI, to tissue slices of the VMN attenuated the inhibitory effects of 5-HT1A receptor agonists on neuronal firing [19]. Moreover, 5-HT2 receptor antagonists have been reported to increase 5-HT1A receptor mediated behaviors [3]. For example, ketanserin increased the inhibitory effect of iontophoretically applied 5-HT on neurons in rat prefrontal cortex [22] and potentiated the effect of 5-HT1A receptors on vasoactive intestinal peptide (VIP)-stimulated cAMP production [43].
The mechanisms responsible for 5-HT2 receptor mediated attenuation of the effects of 5-HT1A receptors are likely to be multifaceted, but an important role for phospholipid signaling, including PKC, has emerged [8,31,33]. Products of phosopholipid hydrolysis, such as PKC, by phosphorylation of 5-HT1A receptors, may reduce the effectiveness of 5-HT1A receptors [31]. Alternatively, activation of 5-HT2 receptors may increase cAMP and thereby attenuate the inhibition of cAMP following 5-HT1A receptor activation. In A1A1 cells, 5-HT2 receptors amplify the response to cAMP stimulators such as forskolin, but such amplification is decreased in the presence of the PKC inhibitor, staurosporine [6].
In the following experiments, the potential involvement of PKC in 5-HT2 receptor-mediated attenuation of the effects of a 5-HT1A receptor agonist were examined in sexually receptive female rats. Rats were infused with bisindolymaleamide I HCl (BIM; GF109203X), a potent and selective PKC inhibitor [25,36], prior to treatment with the 5-HT receptor agonists. It was hypothesized that pretreatment with the PKC inhibitor would reduce 5-HT2 receptor attenuation of the effects of a 5-HT1A receptor agonist.
2. Materials and Methods
2.1 General Methods
2.1.1 Animals and housing conditions
Female, Fischer rats (CDF-344) were purchased from Sasco Laboratories (Wilmington, MA) or were bred in the TWU animal facility from stock obtained from Sasco Laboratories. Rats were housed three or four per cage in polycarbonate shoebox cages in a housing area maintained at 22 ºC and 55% humidity with a 12:12-h dark-light cycle (lights on at 12.00 a.m.). Food and water were available ad lib.
2.1.2 Surgical procedures
When at least 60 days of age and 140–170 g body weight, rats were anesthetized with AErrane® (Isoflurane, Pitman-Moore, Mundelein, IL) and were implanted with 22-gauge stainless steel guide cannulae (Plastics One, Roanoke, VA) directed toward the ventromedial nucleus of the hypothalamus (VMN) (atlas coordinates from König and Klippel [16], anterior + 4.38, DV −7.8, ML ± 0.4) as previously described [40]. Bilateral ovariectomy was performed immediately after implant surgery [37] and the rats were allowed to recover for at least 2 weeks. Dental acrylic was purchased from Reliance Dental Mfg Co (Worth, IL) and suture materials were obtained from Henry Schein (Melville, NY). All procedures were carried out according to Public Health Service Policy Guide for the Care and Use of Laboratory Animals and were approved by the IACUC at Texas Woman’s University.
2.1.3 Hormonal priming
Hormonal priming began 2–3 weeks after ovariectomy and all hormone injections were administered between 9 and 10 a.m. Rats were injected with 10 μg estradiol benzoate (EB; Sigma Chemical Co., St. Louis, MO) followed 48 h later with 500 μg progesterone (P, Sigma Chemical Co.). Hormones were dissolved in sesame seed oil (Fischer Scientific, Houston, TX) and injections were given subcutaneously (SC) in a volume of 0.1 ml per rat.
2.1.4 Drug infusions
The 5-HT1A receptor agonist, 8-hydroxy-2-(di-n-propylamino) tetralin (8-OH-DPAT) and the 5-HT2A/2C receptor agonist, (±)-2,5-dimethoxy-4-iodophenyl-2-aminopropane HCl (DOI) were obtained from Sigma Chemical Co. (St. Louis, MO) and were dissolved in 0.9% saline. Bisindolymaleimide I HCl (BIM; GF 109203X; Sigma Chemical Co.) was dissolved in distilled/deionized water. Receptive females had their dummy cannulae replaced with 28 gauge stainless steel internal cannulae, attached by tubing (i.d.= 0.58 mm; o.d. = 0.96 mm) to a BAS (CMA/100) microinjector. All infusions were delivered at a rate of 0.24–0.26 μl/min to a final infusion volume of 0.5 μl per bilateral site.
2.1.5 Behavioral testing
In all experiments, behavioral testing took place during the dark phase of the light-dark cycle. Red lighting was used to facilitate visibility. Four to six h after P, females were screened for sexual receptivity by placing the female in the home cage of a sexually experienced male and observing the behavior for 10 mounts. After this pretest, the female was infused with the appropriate 5-HT receptor agonist(s). A single female was used only once for a single experimental condition. When effects of BIM were examined, rats were preinfused with BIM or distilled/deionized water after the pretest but 30 or 90 min prior to infusion with the 5-HT receptor agonist(s). Sexual behavior after infusion with 5-HT receptor compounds was monitored for 30 consecutive min as previously described [37]. Sexual receptivity was quantified as the lordosis to mount (L/M) ratio (e.g. number of lordosis responses by the female divided by the number of mounts by the male). When a female’s L/M ratio fell below 0.7 for two consecutive intervals after drug infusion, the female’s behavior was considered to have been inhibited by the drug. Lordosis quality was recorded on a scale of 1 to 4 as previously described [44]. The absence of a lordosis response was given a score of 0.0. Minimal arching of the back was given a score of 1.0. An intermediate reflex was scored as 2.0; a normal reflex was scored as 3.0, and an exaggerated reflex was scored as 4.0.
2.1.6 Histological procedures
After behavioral testing, rats were anesthetized with AErrane® and perfused with phosphate-buffered saline followed by 10% buffered formalin. Brain tissue was placed in 10% buffered formalin for at least 24 h before sectioning. Coronal sections (100 μm) were stained with cresyl violet and examined for cannulae placement according to the atlas of König and Klippel [16]. Rats with cannulae placements outside the vicinity of the VMN or its most anterior extension were excluded from the data analysis. All rats with cannulae in the third ventricle were excluded.
2.1.7 Statistical methods
Data were grouped into the pretest interval and five consecutive 5 min intervals after treatment. The data were analyzed by repeated measures ANOVA as described in the specific procedures. Only animals with cannulae locations near the VMN were included in the ANOVA. Time dependent differences, within treatment, were compared to the pretest interval with Dunnett’s test; differences between groups within a time interval were compared by Tukey’s test. The statistical reference was Zar [48] and an α level of 0.05 was required to reject the null hypothesis.
2.2 Specific Methods
2.2.1 Effects of DOI on 8-OH-DPAT-mediated inhibition of lordosis behavior
After the pretest for sexual behavior, ovariectomized hormonally primed, sexually receptive females were infused bilaterally with 200 ng 8-OH-DPAT (N = 13) or 200 ng 8-OH-DPAT plus 2000 ng DOI (N = 10). Data were grouped into the pretest interval and six consecutive 5 min intervals after treatment and were analyzed by repeated measures ANOVA with time after infusion as the repeated measure and type of infusion as the independent factor.
2.2.2 Effects of preinfusion with BIM 30 or 90 min before infusion with 5-HT receptor agonists
After the pretest, receptive rats were infused with 10−1 nmol BIM or deionized water. Thirty or ninety min later, rats were infused bilaterally with either 200 ng 8-OH-DPAT or 200 ng 8-OH-DPAT plus 2000 ng DOI. N’s for the 30 min preinfusion conditions were as follows: rats preinfused with distilled/deionized water and infused with 8-OH-DPAT or 8-OH-DPAT plus DOI were 6 and 4, respectively; rats preinfused with BIM and infused with 8-OH-DPAT or 8-OH-DPAT plus DOI were 6 and 7, respectively. N’s for the 90 min conditions were: for rats preinfused with distilled/deionized water and infused with 8-OH-DPAT or 8-OH-DPAT plus DOI, 4 and 9, respectively; for rats preinfused with BIM and infused with 8-OH-DPAT or 8-OH-DPAT plus DOI, 5 and 13, respectively.
Sexual behavior was monitored for 30 consecutive min after infusion with the 5-HT receptor active drugs. Data were grouped into the pretest interval and six consecutive 5 min intervals after treatment and were analyzed by repeated measures ANOVA with time after 5-HT receptor agonists as the repeated measure and type of agonist and preinfusion condition as the independent factors.
2.2.3 Dose dependent effects of BIM
After the pretest, receptive rats were infused bilaterally with varying concentrations of BIM (1 × 10−1 to 10−7 nmol) or deionized water. Ninety min later, rats were infused bilaterally with 200 ng 8-OH-DPAT plus 2000 ng DOI. Sexual behavior was monitored for 30 consecutive min after infusion with the 5-HT receptor active drugs. The number of subjects per treatment were as follows: distilled/deionized water, N = 4; 10−7 nmol BIM, N = 6; 10−5 nmol BIM, N = 3; 10−3 nmol BIM, N = 3; 2.5 × 10−2 nmol BIM, N = 3; 5 × 10−2 nmol BIM, N = 7; and 10−1 nmol BIM, N = 7. Data were grouped into the pretest interval and six consecutive 5 min intervals after treatment and were analyzed by repeated measures ANOVA with time after 5-HT receptor agonists as the repeated measure and preinfusion condition as the independent factor.
3.0 Results
3.1 Effects of DOI on 8-OH-DPAT-mediated inhibition of lordosis behavior
Every rat had cannulae located in the vicinity of the VMN. As previously reported [27], infusion of the 5-HT1A receptor agonist, 8-OH-DPAT, into the vicinity of the VMN inhibited lordosis behavior and this inhibition was attenuated by coinfusion with the 5-HT2 receptor agonist, DOI (Figure 1). There was a significant effect of drug (F1,21 = 5.27, P ≤ 0.05), time after infusion (F6,126 = 9.95, P ≤ 0.0001), and the time by drug interaction (F6,126 = 2.62, P ≤ 0.05). Relative to the pretest, both groups of rats showed a decline in the L/M ratio after infusion. However, L/M ratios of rats coinfused with 8-OH-DPAT and DOI were significantly higher than those of rats infused only with 8-OH-DPAT at all time intervals except the first 5 min interval after infusion (Tukey’s, all q126,2 ≥ 2.77, P ≤ 0.05). Eleven out of 13 rats infused with 8-OH-DPAT showed a decline in lordosis behavior, while only 6/10 of the rats coinfused with 8-OH-DPAT plus DOI showed such a decline. Moreover, for 2 rats infused with 8-OH-DPAT plus DOI, the decline did not occur until 20 min after infusion while, for the majority of 8-OH-DPAT rats, inhibition occurred within 10–15 min of the infusion.
Figure 1. Effect of DOI on the lordosis-inhibiting effects of 8-OH-DPAT.
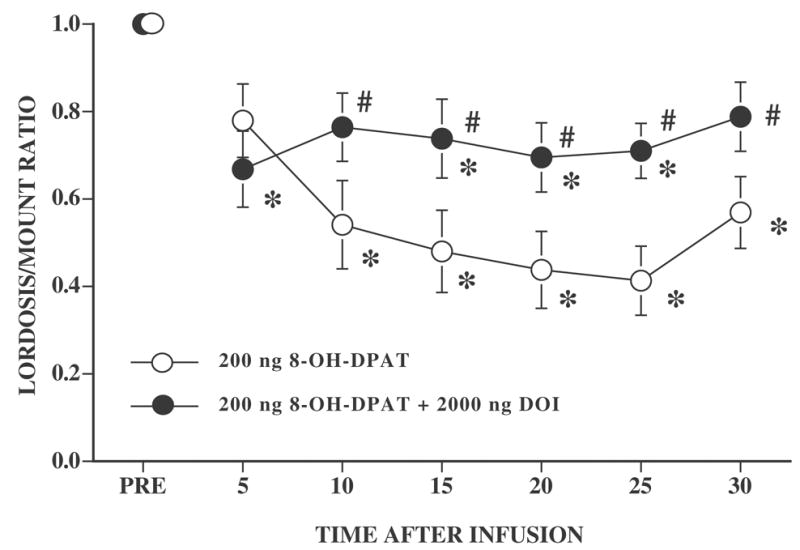
Ovariectomized rats, with bilateral cannulae near the VMN, were hormonally primed with 10 μg EB and 500 μg P. Four to six h after the P injection, rats were tested for sexual receptivity (PRE). After the pretest for sexual behavior, females were infused with 200 ng 8-OH-DPAT or were coinfused with 200 ng 8-OH-DPAT and 2000 ng DOI. The numbers of subjects for 8-OH-DPAT and 8-OH-DPAT plus DOI rats were 13 and 10, respectively. Data are the mean ± S.E. L/M ratios before infusion (PRE) and for six consecutive 5 min intervals after infusion. * indicates a significant difference from the pretest within each 5 min test interval. # indicates a significant difference between rats infused with 8-OH-DPAT and rats coinfused with 8-OH-DPAT and DOI within each 5 min time interval.
Four of the rats treated with 8-OH-DPAT had L/M ratios of zero after infusion so that their lordosis quality could not be assessed. For the remaining rats, there was a small decline in overall lordosis quality during testing (F6,102 = 4.61, P ≤ 0.05), but neither the main effect of drug nor the time by drug interaction were significant (P > 0.05; data not shown). Number of mounts received by the females did not differ between the two infusion conditions (P > 0.05). For rats infused with 8-OH-DPAT or 8-OH-DPAT and DOI, the mean ± S.E. number of mounts per 5 min interval, respectively, were 8.36 ± 0.45 and 7.85 ± 0.48.
3.2 Effects of preinfusion with BIM 30 or 90 min before infusion with 5-HT receptor agonists
3.2.1 Bim 90 min preinfusion
The effects of the PKC inhibitor, BIM, on DOI’s ability to attenuate the effects of 8-OH-DPAT are shown in Figures 2 and 3. Representative cannulae locations are shown in Figure 4. 8-OH-DPAT significantly inhibited lordosis behavior and DOI attenuated this decline. However, pretreatment with BIM 90 min (Figure 2), but not 30 min (Figure 3), before infusion with 8-OH-DPAT and DOI abolished the effects of DOI. After the 90 min preinfusion, there was a significant main effect of type of pretreatment (water versus BIM) (F1,27 = 18.65, P ≤ 0.0002), type of second infusion (8-OH-DPAT versus 8-OH-DPAT plus DOI) (F1,27 = 6.27, P≤ .02), and their interaction (F1,27 = 4.25, P ≤ 0.05). Overall, L/M ratios declined with time (F6,162 = 1881, P ≤ 0.0001) and interacted significantly with type of pretreatment (F6,162 = 3.53, P ≤ 0.003) as well as with the second infusion (F6,162 = 3.37, P ≤ 0.004). The three-way interaction was not significant.
Figure 2. Effect of preinfusion with 10−1 nmol BIM 90 min before 8-OH-DPAT infusion or 8-OH-DPAT and DOI coinfusion.
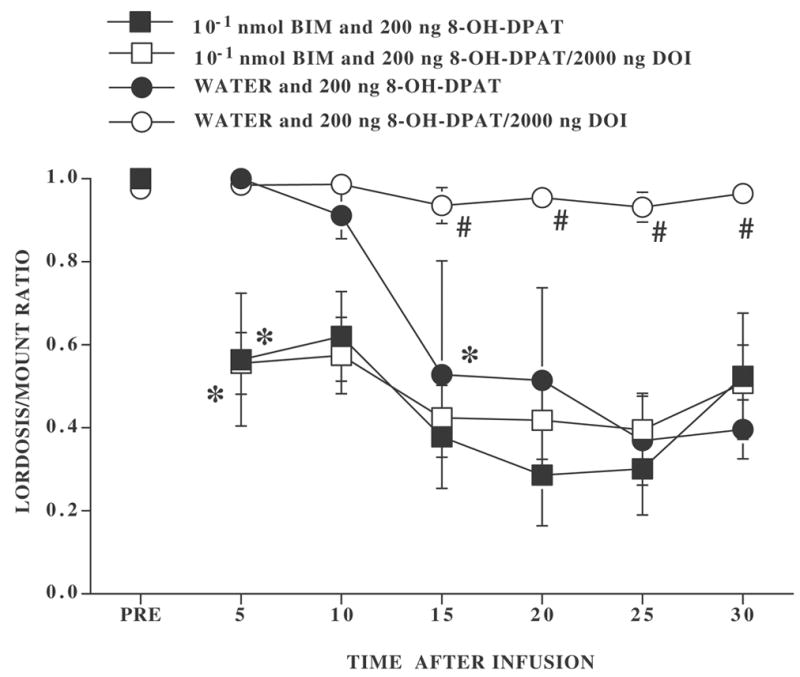
Ovariectomized rats, with bilateral cannulae, were hormonally primed with 10 μg EB and 500 μg P. Four to six h after the P injection, rats were tested for sexual receptivity during a pretest (PRE). After the pretest for sexual behavior, rats were infused with either distilled/deionized water or BIM. Ninety min later they were then infused with either 200 ng 8-OH-DPAT or 200 ng 8-OH-DPAT plus 2000 ng DOI. Ns for rats preinfused with distilled/deionized water and infused with 8-OH-DPAT or 8-OH-DPAT plus DOI were 4 and 9, respectively. Ns for rats preinfused with BIM and infused with 8-OH-DPAT or 8-OH-DPAT plus DOI were 5 and 13, respectively. Data are the mean ± S.E. L/M ratios before infusion (PRE) and for six consecutive 5 min intervals after infusion. # indicates a significant difference between rats infused with 8-OH-DPAT or 8-OH-DPAT plus DOI within preinfusion condition. * indicates the first time interval within treatment conditions at which there was a significant difference from the pretest.
Figure 3. Effect of preinfusion with 10−1 nmol BIM 30 min before 8-OH-DPAT infusion or 8-OH-DPAT and DOI coinfusion.
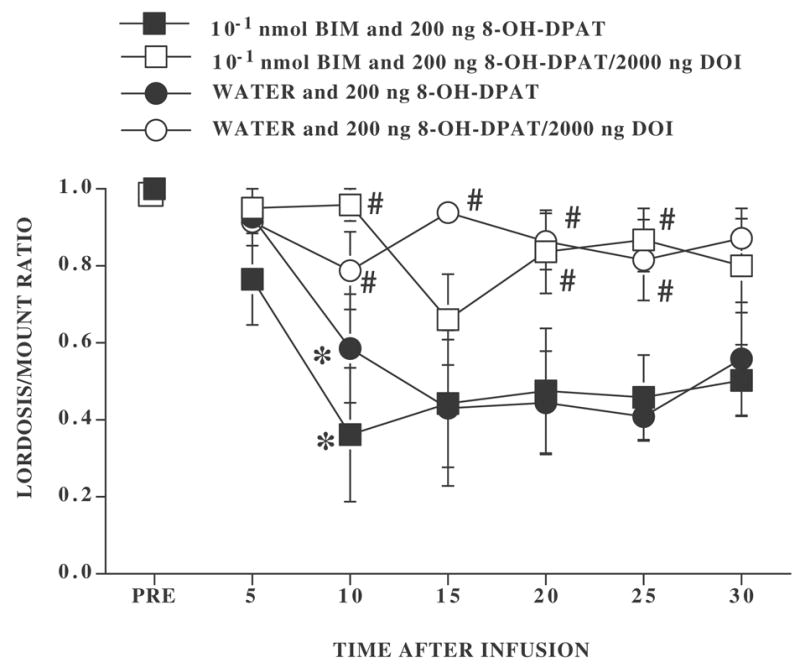
Ovariectomized rats, with bilateral cannulae, were hormonally primed with 10 μg EB and 500 μg P. Four to six h after the P injection, rats were tested for sexual receptivity (PRE). After the pretest for sexual behavior, rats were infused with either distilled/deionized water or BIM. Thirty min later they were infused with either 200 ng 8-OH-DPAT or 200 ng 8-OH-DPAT plus 2000 ng DOI. The numbers of subjects for rats preinfused with distilled/deionized water and infused with 8-OH-DPAT or 8-OH-DPAT plus DOI were 6 and 4, respectively. The numbers of subjects for rats preinfused with BIM and infused with 8-OH-DPAT or 8-OH-DPAT plus DOI were 6 and 7, respectively. Data are the mean ± S.E. L/M ratios before infusion (PRE) and for six consecutive 5 min intervals after infusion. # indicates a significant difference between rats infused with 8-OH-DPAT or 8-OH-DPAT plus DOI within preinfusion condition. * indicates the first time interval within treatment conditions at which there was a significant difference from the pretest.
Figure 4. Location of cannulae from rats preinfused with 10−1 nmol BIM 90 min before 8-OH-DPAT or 8-OH-DPAT plus DOI.
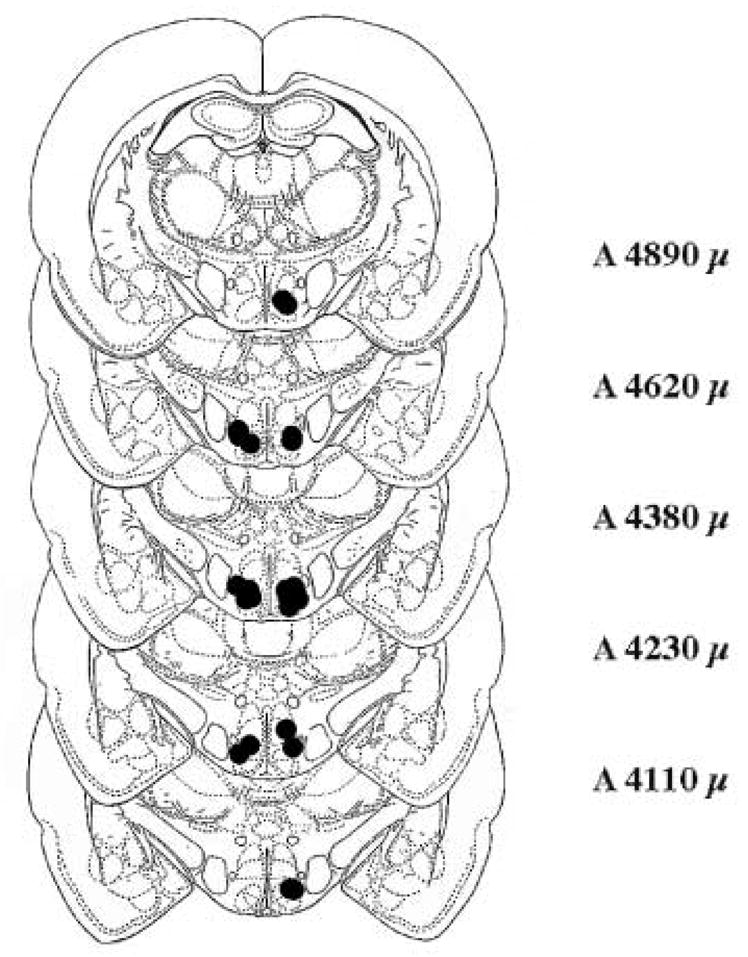
The figure represents coronal sections taken from König and Klippel [16]at the level of the VMN from A 4110 μ to A 4890 μ. Dots on the left side of the sections indicate locations of bilateral cannulae from rat infused with 200 ng 8-OH-DPAT. Dots on the right represent locations of bilateral cannulae from rats infused with 200 ng 8-OH-DPAT and 2000 ng DOI.
Whether preinfused with water or BIM 90 min earlier, every rat infused with 8-OH-DPAT showed a reduction in the L/M ratio for at least 2 consecutive 5 min intervals. Similarly 12 of 13 of the rats preinfused with BIM and then infused with 8-OH-DPAT plus DOI exhibited such a decline in lordosis behavior. In contrast, none of the rats that were preinfused with water and then infused with 8-OH-DPAT and DOI showed a reduction in the lordosis response (Figure 2).
Rats preinfused with distilled/deionized water and 8-OH-DPAT plus DOI had significantly higher L/M ratios than their 8-OH-DPAT counterparts at 15 min and this lasted throughout the testing period (Tukey’s q162,4 ≥ 3.63, P ≤ 0.05). In contrast, in rats preinfused 90 min earlier with BIM, at no time interval did L/M ratios of rats infused with 8-OH-DPAT and 8-OH-DPAT plus DOI differ (Tukey’s q162,4 < 3.63, P > 0.05). In rats preinfused with BIM, 8-OH-DPAT and 8-OH-DPAT plus DOI treated rats had significantly lower L/M ratios than the pretest at every test interval (Dunnett’s q162,7 ≥ 2.57, P ≤ 0.05).
Eleven rats (6 preinfused with BIM and 5 preinfused with water) had cannulae either in the third ventricle or located in the anterior hypothalamus and were excluded from the analysis. For rats preinfused with water and then infused with 8-OH-DPAT, all showed a decrease in lordosis behavior while none of the rats infused with both agonists showed such a decline. For rats preinfused with BIM, all 6 rats showed a decline in lordosis behavior.
3.2.2 BIM 30 min preinfusion
In contrast to the effects of BIM 90 min before treatment, when the BIM infusion preceded the second infusion by 30 min (Figure 3), there was not a significant effect of the pretreatment (P > 0.05). Independent of the type of pretreatment, 8-OH-DPAT significantly reduced L/M ratios leading to a significant main effect for the second infusion (F1,19 = 16.00, P ≤ 0.0008). There was also a significant effect of time after the second infusion (F6,114 = 10.12, P ≤ 0.0001) and a significant interaction between time and the type of second infusion (F6,114 = 3.22, P ≤ 0.006). None of the interactions with pretreatment were significant (all P > 0.05).
Whether pretreatment 30 min earlier was with water or with BIM, every rat infused with 8-OH-DPAT showed a decline in the L/M ratio during the testing period. After both preinfusion conditions, rats infused with 8-OH-DPAT differed significantly from their pretest by 10 min and at every interval thereafter. In contrast, only 2 rats (1 preinfused with water and 1 preinfused with BIM) infused with 8-OH-DPAT and DOI showed such a decline. Rats preinfused with distilled/deionized water and 8-OH-DPAT plus DOI had significantly higher L/M ratios than their 8-OH-DPAT counterparts at 10, 15, 20, and 25 min time intervals after infusion. DOI was also effective in decreasing effects of 8-OH-DPAT in rats preinfused 30 min earlier with BIM at the 10, 20, and 25 min time intervals (Tukey’s q114,4 = 3.68, P ≤ 0.05).
Unlike L/M ratios, treatments had only minor effects on lordosis quality (data not shown) and none of the treatment conditions significantly influenced the male’s tendency to mount the female. Average mounts per interval ranged from a low of 6.22 ± 0.39 for rats infused with water 90 min before 8-OH-DPAT and DOI to a high of 9.65 ± 0.74 for rats preinfused with BIM 30 min before 8-OH-DPAT.
Five rats had one or both cannulae located outside the VMN and were excluded from the analysis. Three rats had been preinfused with water. One of these rats, later infused with 8-OH-DPAT, had one cannulae in the third ventricle and showed a decline in lordosis behavior. Two rats had one cannula in the third ventricle and one dorsal to the VMN and had been infused with both 8-OH-DPAT and DOI. Neither rat showed a decline in L/M ratios. Two of the 5 rats had been infused with BIM (one later infused with 8-OH-DPAT and one with both agonists) and had one cannula in the third ventricle. The rat infused with 8-OH-DPAT showed a decrease in lordosis behavior while the rat infused with both agonists showed no such decline.
3.3 Dose-dependent effects of BIM
When varying concentrations of BIM were evaluated for their ability to attenuate the effects of DOI (Figure 5), there was an overall effect of concentration (F6,26 = 8.31, P ≤ 0.0001) and a significant concentration by time interval interaction (F36,156 = 1.67, P ≤ 0.02). Every concentration of BIM greater than or equal to 1 × 10−5 nmol reduced the ability of DOI to attenuate the effects of 8-OH-DPAT while a concentration of 10−7 nmol BIM was ineffective. Consequently, there was limited evidence for dose-responsivity over the concentration range of 10−5 to 10−1 nmol BIM. In rats preinfused with 10−5 nmol BIM and then infused with 8-OH-DPAT plus DOI, L/M ratios were significantly different from the pretest at 15, 20, and 25 min time after infusion. Rats preinfused with 10−3 nmol BIM were significantly different from the pretest at 10 min and at every interval thereafter. Rats preinfused with 2.5 × 10−2, 5 × 10−2, and 10−1 nmol BIM were significantly different from the pretest at every interval after infusion (Dunnett’s all q156,7 ≥ 2.57, P ≤ 0.05). Rats preinfused with either water or 10−7 nmol BIM were significantly different from those treated with all other doses throughout the testing period (Tukey’s q156,7 ≥ 4.17, P ≤ 0.05).
Figure 5. Effect of varying concentrations of the PKC inhibitor, BIM, on DOI mediated attenuation of 8-OH-DPAT’s effect on lordosis behavior.
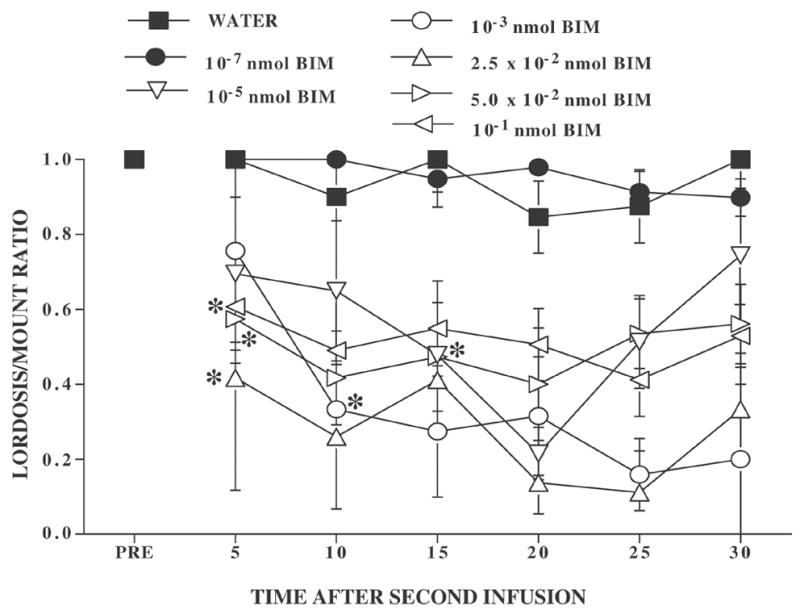
Ovariectomized rats were hormonally primed with 10 μg EB followed 48 h later with P. Four-six h later, rats were pretested for sexual behavior (PRE). They were then infused with one of several concentrations of BIM or the water vehicle. Ninety min later, rats were infused with 200 ng 8-OH-DPAT plus 2000 ng DOI. Data are the mean ± S.E. L/M ratios for the pretest (PRE) and 6 consecutive 5 min intervals after the second infusion. Ns per treatment were as follows: distilled/deionized water, N = 4; 10−7 nmol BIM, N = 6; 10−5 nmol BIM, N = 3; 10−3 nmol BIM, N = 3; 2.5 × 10−2 nmol BIM, N = 3; 5 × 10−2 nmol BIM, N = 7; and 10−1 nmol BIM, N = 7. For ease of viewing, S.E. error bars have been omitted. The largest S.E. was 0.33 in the 1 × 10−3 nmol BIM group at 10 min and the overall MS was 0.048. * indicates the first interval at which a group differed significantly from the pretest, within treatment condition.
L/M ratios of 6 rats (3 water 8-OH-DPAT and 3 BIM 8-OH-DPAT) fell to zero during the test so that their lordosis quality could not be assessed. For the remaining rats, there was not a significant effect of pretreatment on lordosis quality (P > .05, data not shown). Nor did the number of mounts received by the female differ significantly. The mean ± S.E. mounts per interval ranged from a low of 4.63 ± .58 to a high of 7.79 ± .40.
Seven rats had cannulae outside the VMN and were excluded from the analysis. Three had been preinfused with water (2 had cannulae dorsal to the VMN; one had cannulae posterior to the VMN) and showed no decline in lordosis behavior. One rat preinfused with 10−7 nmol BIM and two rats preinfused with 10−3 nmol BIM had cannulae dorsal to the VMN. None showed a decline in lordosis behavior. One rat, preinfused with 10−1 nmol BIM had cannulae located posterior to the VMN and did show a decrease in lordosis behavior.
4. Discussion
Although in several behavioral studies, 5-HT1A/5-HT2 receptor interaction has been observed, this is the first behavioral study in which a potential mechanism for such interaction has been examined. In the present studies, a PKC inhibitor blocked an effect of a 5-HT2 receptor agonist’s attenuation of the effects of a 5-HT1A receptor agonist in the hypothalamus of female rats. These findings are consistent with studies in cell culture where PKC was shown to attenuate effects of 5-HT1A receptors [31].
Consistent with prior studies, DOI prevented the lordosis-inhibiting effects of 8-OH-DPAT infusion into the VMN [27], but the effect of DOI was blocked by prior infusion with the PKC inhibitor, BIM. These findings reinforce prior observations for an antagonistic interaction between 5-HT2 and 5-HT1A receptors [7]. 5-HT2 receptors are Gq/11 coupled receptors that, among other effector mechanisms, activate phospholipase C leading to IP3 and DAG [23]. Increased IP3 leads to an increase in intracellular calcium while DAG activates PKC. In a variety of cell culture systems, PKC has been shown to lead to desensitization of 5-HT1A receptors [32,33] and it has been hypothesized that a PKC-mediated mechanism might account for DOI’s ability to attenuate the effects of a 5-HT1A receptor agonist on lordosis behavior [39]. The current findings are consistent with this hypothesis.
At the highest concentration investigated (10−1 nmol/0.5 μl infusion), the PKC inhibitor, BIM, completely abolished DOI’s attenuation of the effects of 8-OH-DPAT. BIM inhibits most isoforms of PKC with an IC50 in the nmol range but α and β isoforms show the greatest sensitivity [25,36]. In the current study, concentrations above 10−5 nmol/0.5 μl infusion successfully attenuated the effects of DOI while lower concentrations did not. Thus, the effective concentration of BIM was between 10−7 and 10−5 nmol (delivered in 0.5 μl). Although it is difficult to translate the amount of drug delivered intracranially to concentrations of drugs delivered in vitro, the range of concentrations effective in the current experiment are well within the range of concentrations where selective inhibition of PKC might be expected to occur [25,36]. However, since only a single PKC inhibitor concentration was examined, the possibility of alternative explanations cannot be excluded.
Effective concentrations of BIM in the current experiment were comparable to or below concentrations reported to be effective in other in vivo neural models [4,24,38,47]. In the current experiment, BIM was effective when administered 90 min, but not 30 min, before the 5-HT receptor agonists. Although comparable time comparisons have not been examined in prior studies, in most in vivo work, at least 30 min have been allowed to elapse before the effects of the PKC inhibitor have been assessed [4,24,38,47]. Thus the current time-dependency is consistent with prior findings and is likely to reflect the time required for the inhibitor to effectively block DOI’s ability to increase PKC. Therefore, these findings lend support to the hypothesis that DOI’s attenuation of the effects of the 5-HT1A receptor involves activation of PKC. However, these conclusions assume specificity of PKC inhibition by BIM.
For example, at 500 fold higher concentrations than effective against PKC, BIM can inhibit PKA [36]. Moreover, activation of 5-HT2 receptors can increase cAMP formation via either a PKC pathway or a calcium/calmodulin pathway [6]. Therefore, further studies are required to determine the contribution of other signaling mechanisms to DOI’s attenuation of the effects of 8-OH-DPAT. Gonzales-Flores et al. [10] recently reported that kinase A or kinase C blockers reduced the facilitatory effects of progesterone or its metabolite, 5α-pregnanedione, respectively, on lordosis facilitation. Therefore, both PKA and PKC could potentially participate in the interaction between 5-HT1A and 5-HT2 receptors on lordosis behavior.
Alternatively, given the potential involvement of PKC in progesterone’s facilitation of lordosis [10], it is possible that BIM interfered with progesterone’s facilitative action and, thereby, increased the female’s vulnerability to inhibition by 8-OH-DPAT. Since the dose of 8-OH-DPAT used in the current study was intended to inhibit lordosis behavior of a majority of the receptive females, we cannot rule out such a possibility. However, since BIM was not infused until 4–6 h after progesterone priming and after females were sexually receptive, this explanation is unlikely. Nevertheless, lower doses of 8-OH-DPAT would have to be examined to rule out this possibility.
Although the current findings are consistent with the suggestion that DOI enhances PKC and thereby reduces the effectiveness of 5-HT1A receptors, it is also possible that 5-HT1A and 5-HT2 receptor agonists influence lordosis behavior by completely independent mechanisms and that their apparent interaction reflects an additive effect of their separate actions. For example, 8-OH-DPAT reduces and DOI increases neuronal firing [1,18,35]; or DOI may influence the functioning of other neuronal systems which then impinge on the function of 5-HT1A receptors. Alternatively, DOI, by activating PKC, may alter the functioning of the serotonin transporter [14] and thereby influence extracellular concentrations of serotonin.
Finally, DOI is an effective agonist for all members of the 5-HT2 receptor family [23]. Within the VMN, we have suggested that DOI’s facilitation of lordosis behavior involves 5-HT2C rather than 5-HT2A receptors [45], although other investigators have emphasized the 5-HT2A rather than the 5-HT2C [9]. While it may be reasonable to assume involvement of 5-HT2C receptors in the current study, the receptor subtype responsible for DOI’s attenuation of the effects of 5-HT1A receptors has not yet been established.
In summary, coinfusion of DOI with 8-OH-DPAT attenuated the lordosis-inhibiting effects of 8-OH-DPAT on female rat lordosis behavior. Preinfusion of a PKC inhibitor 90, but not 30 min, before infusion with the 5-HT receptor agonists, eliminated the effect of DOI but had no influence on the response to 8-OH-DPAT. Therefore, these findings emphasize a potentially important role for PKC in the 5-HT2/5-HT1A receptor interaction of female rat lordosis behavior.
Acknowledgments
The authors express appreciation to Mr. Dan Wall and Ms. Karolina Blaha-Black for animal care and to Ms. Jutatip Guptarak and Ms. Cindy Hiegel for technical assistance. The research was supported by NIH HD28419 and GM 55380 and a TWU REP award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andrade R, Nicoll RA. Pharmacologically distinct actions of serotonin on single pyramidal neurones of the rat hippocampus recorded in vitro. J Physiol. 1987;394:99–124. doi: 10.1113/jphysiol.1987.sp016862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnt J, Hyttel J. Facilitation of 8-OHDPAT-induced forepaw treading of rats by the 5-HT2 agonist DOI. Eur J Pharmacol. 1989;161:45–51. doi: 10.1016/0014-2999(89)90178-7. [DOI] [PubMed] [Google Scholar]
- 3.Backus LI, Sharp T, Grahame-Smith DG. Behavioural evidence for a functional interaction between central 5-HT2 and 5-HT1A receptors. Br J Pharmacol. 1990;100:793–9. doi: 10.1111/j.1476-5381.1990.tb14094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balaban CD, Freilino M, Romero GG. Protein kinase C inhibition blocks the early appearance of vestibular compensation. Brain Res. 1999;845:97–101. doi: 10.1016/s0006-8993(99)01958-7. [DOI] [PubMed] [Google Scholar]
- 5.Berendsen HH, Broekkamp CL. Behavioural evidence for functional interactions between 5-HT-receptor subtypes in rats and mice. Br J Pharmacol. 1990;101:667–73. doi: 10.1111/j.1476-5381.1990.tb14138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berg KA, Clarke WP, Chen Y, Ebersole BJ, McKay RD, Maayani S. 5-Hydroxytryptamine type 2A receptors regulate cyclic AMP accumulation in a neuronal cell line by protein kinase C-dependent and calcium/calmodulin-dependent mechanisms. Mol Pharmacol. 1994;45:826–36. [PubMed] [Google Scholar]
- 7.Darmani NA, Martin BR, Pandey U, Glennon RA. Do functional relationships exist between 5-HT1A and 5-HT2 receptors? Pharmacol Biochem Behav. 1990;36:901–6. doi: 10.1016/0091-3057(90)90098-3. [DOI] [PubMed] [Google Scholar]
- 8.Evans KL, Cropper JD, Berg KA, Clarke WP. Mechanisms of regulation of agonist efficacy at the 5-HT(1A) receptor by phospholipid-derived signaling components. J Pharmacol Exp Ther. 2001;297:1025–35. [PubMed] [Google Scholar]
- 9.Gonzalez MI, Greengrass P, Russell M, Wilson CA. Comparison of serotonin receptor numbers and activity in specific hypothalamic areas of sexually active and inactive female rats. Neuroendocrinology. 1997;66:384–92. doi: 10.1159/000127277. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Flores O, Ramirez-Orduna JM, Javier Lima-Hernandez F, Garcia-Juarez M, Beyer C. Differential effect of kinase A and C blockers on lordosis facilitation by progesterone and its metabolites in ovariectomized estrogen-primed rats. Horm Behav. 2006;49:398–404. doi: 10.1016/j.yhbeh.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Harlan RE, Shivers BD, Kow LM, Pfaff DW. Estrogenic maintenance of lordotic responsiveness: requirement for hypothalamic action potentials. Brain Res. 1983;268:67–78. doi: 10.1016/0006-8993(83)90390-6. [DOI] [PubMed] [Google Scholar]
- 12.Harrington MA, Shaw K, Zhong P, Ciaranello RD. Agonist-induced desensitization and loss of high-affinity binding sites of stably expressed human 5-HT1A receptors. J Pharmacol Exp Ther. 1994;268:1098–106. [PubMed] [Google Scholar]
- 13.Jackson A, Uphouse L. Dose-dependent effects of estradiol benzoate on 5-HT1A receptor agonist action. Brain Res. 1998;796:299–302. doi: 10.1016/s0006-8993(98)00238-8. [DOI] [PubMed] [Google Scholar]
- 14.Jayanthi LD, Samuvel DJ, Blakely RD, Ramamoorthy S. Evidence for biphasic effects of protein kinase C on serotonin transporter function, endocytosis, and phosphorylation. Mol Pharmacol. 2005;67:2077–87. doi: 10.1124/mol.104.009555. [DOI] [PubMed] [Google Scholar]
- 15.Katada T, Gilman AG, Watanabe Y, Bauer S, Jakobs KH. Protein kinase C phosphorylates the inhibitory guanine-nucleotide-binding regulatory component and apparently suppresses its function in hormonal inhibition of adenylate cyclase. Eur J Biochem. 1985;151:431–7. doi: 10.1111/j.1432-1033.1985.tb09120.x. [DOI] [PubMed] [Google Scholar]
- 16.König J, Klippel R. A Stereotaxic Atlas of the Forebrain and Lower Parts of the Brain Stem. Williams and Wilkins; Baltimore: 1963. The Rat Brain. [Google Scholar]
- 17.Kow LM, Mobbs CV, Pfaff DW. Roles of second-messenger systems and neuronal activity in the regulation of lordosis by neurotransmitters, neuropeptides, and estrogen: a review. Neurosci Biobehav Rev. 1994;18:251–68. doi: 10.1016/0149-7634(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 18.Kow LM, Pfaff DW. Estrogen effects on neuronal responsiveness to electrical and neurotransmitter stimulation: an in vitro study on the ventromedial nucleus of the hypothalamus. Brain Res. 1985;347:1–10. doi: 10.1016/0006-8993(85)90883-2. [DOI] [PubMed] [Google Scholar]
- 19.Kow LM, Tsai YF, Wang L, Pfaff DW. Electrophysiological analyses of serotonergic actions on neurons in hypothalamic ventromedial nucleus in vitro: receptor subtypes involved and implications for regulation of feeding and lordosis behaviors. Chin J Physiol. 1992;35:105–21. [PubMed] [Google Scholar]
- 20.Kozasa T, Gilman AG. Protein kinase C phosphorylates G12 alpha and inhibits its interaction with G beta gamma. J Biol Chem. 1996;271:12562–7. doi: 10.1074/jbc.271.21.12562. [DOI] [PubMed] [Google Scholar]
- 21.Krebs-Thomson K, Geyer MA. Evidence for a functional interaction between 5-HT1A and 5-HT2 receptors in rats. Psychopharmacology (Berl) 1998;140:69–74. doi: 10.1007/s002130050740. [DOI] [PubMed] [Google Scholar]
- 22.Lakoski JM, Aghajanian GK. Effects of ketanserin on neuronal responses to serotonin in the prefrontal cortex, lateral geniculate and dorsal raphe nucleus. Neuropharmacology. 1985;24:265–73. doi: 10.1016/0028-3908(85)90130-3. [DOI] [PubMed] [Google Scholar]
- 23.Leysen JE. 5-HT2 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3:11–26. doi: 10.2174/1568007043482598. [DOI] [PubMed] [Google Scholar]
- 24.Liu SJ, Zhang AH, Li HL, Wang Q, Deng HM, Netzer WJ, Xu H, Wang JZ. Overactivation of glycogen synthase kinase-3 by inhibition of phosphoinositol-3 kinase and protein kinase C leads to hyperphosphorylation of tau and impairment of spatial memory. J Neurochem. 2003;87:1333–44. doi: 10.1046/j.1471-4159.2003.02070.x. [DOI] [PubMed] [Google Scholar]
- 25.Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, Marme D, Schachtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J Biol Chem. 1993;268:9194–7. [PubMed] [Google Scholar]
- 26.Maswood N, Uphouse L. Modulation of the behavioral effects of 8-OH-DPAT by estrogen and DOI. Pharmacol Biochem Behav. 1997;58:859–66. doi: 10.1016/s0091-3057(97)00048-8. [DOI] [PubMed] [Google Scholar]
- 27.Maswood S, Andrade M, Caldarola-Pastuszka M, Uphouse L. Protective actions of the 5-HT2A/2C receptor agonist, DOI, on 5-HT1A receptor-mediated inhibition of lordosis behavior. Neuropharmacology. 1996;35:497–501. doi: 10.1016/0028-3908(95)00195-6. [DOI] [PubMed] [Google Scholar]
- 28.Newberry NR. 5-HT1A receptors activate a potassium conductance in rat ventromedial hypothalamic neurones. Eur J Pharmacol. 1992;210:209–12. doi: 10.1016/0014-2999(92)90673-r. [DOI] [PubMed] [Google Scholar]
- 29.Pfaff DW, Modianos D. Neural mechanisms of female reproductive behavior. In: Adler D, Pfaff D, Goy RW, editors. Handbook of behavioral neurobiology. Plenum Press; New York: 1985. pp. 423–493. [Google Scholar]
- 30.Pfaff DW, Sakuma Y. Facilitation of the lordosis reflex of female rats from the ventromedial nucleus of the hypothalamus. J Physiol. 1979;288:189–202. [PMC free article] [PubMed] [Google Scholar]
- 31.Raymond JR. Protein kinase C induces phosphorylation and desensitization of the human 5-HT1A receptor. J Biol Chem. 1991;266:14747–53. [PubMed] [Google Scholar]
- 32.Raymond JR, Mukhin YV, Gelasco A, Turner J, Collinsworth G, Gettys TW, Grewal JS, Garnovskaya MN. Multiplicity of mechanisms of serotonin receptor signal transduction. Pharmacol Ther. 2001;92:179–212. doi: 10.1016/s0163-7258(01)00169-3. [DOI] [PubMed] [Google Scholar]
- 33.Raymond JR, Mukhin YV, Gettys TW, Garnovskaya MN. The recombinant 5-HT1A receptor: G protein coupling and signalling pathways. Br J Pharmacol. 1999;127:1751–64. doi: 10.1038/sj.bjp.0702723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raymond JR, Olsen CL. Protein kinase A induces phosphorylation of the human 5-HT1A receptor and augments its desensitization by protein kinase C in CHO-K1 cells. Biochemistry. 1994;33:11264–9. doi: 10.1021/bi00203a023. [DOI] [PubMed] [Google Scholar]
- 35.Stanford IM, Kantaria MA, Chahal HS, Loucif KC, Wilson CL. 5-Hydroxytryptamine induced excitation and inhibition in the subthalamic nucleus: action at 5-HT(2C), 5-HT(4) and 5-HT(1A) receptors. Neuropharmacology. 2005;49:1228–34. doi: 10.1016/j.neuropharm.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 36.Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, et al. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991;266:15771–81. [PubMed] [Google Scholar]
- 37.Truitt W, Harrison L, Guptarak J, White S, Hiegel C, Uphouse L. Progesterone attenuates the effect of the 5-HT1A receptor agonist, 8-OH-DPAT, and of mild restraint on lordosis behavior. Brain Res. 2003;974:202–11. doi: 10.1016/s0006-8993(03)02581-2. [DOI] [PubMed] [Google Scholar]
- 38.Tsushima H, Mori M, Fujiwara N, Moriyama A. Pharmacological characteristics of bombesin receptor mediating hypothermia in the central nervous system of rats. Brain Res. 2003;969:88–94. doi: 10.1016/s0006-8993(03)02281-9. [DOI] [PubMed] [Google Scholar]
- 39.Uphouse L. Female gonadal hormones, serotonin, and sexual receptivity. Brain Res Rev. 2000;33:242–57. doi: 10.1016/s0165-0173(00)00032-1. [DOI] [PubMed] [Google Scholar]
- 40.Uphouse L, Andrade M, Caldarola-Pastuszka M, Maswood S. Hypothalamic infusion of the 5-HT2/1C agonist, DOI, prevents the inhibitory actions of the 5-HT1A agonist, 8-OH-DPAT, on lordosis behavior. Pharmacol Biochem Behav. 1994;47:467–70. doi: 10.1016/0091-3057(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 41.Uphouse L, Maswood S, Jackson A. Factors elevating cAMP attenuate the effects of 8-OH-DPAT on lordosis behavior. Pharmacol Biochem Behav. 2000;66:383–8. doi: 10.1016/s0091-3057(00)00179-9. [DOI] [PubMed] [Google Scholar]
- 42.Uphouse L, White S, Harrison L, Hiegel C, Majumdar D, Guptarak J, Truitt WA. Restraint accentuates the effects of 5-HT2 receptor antagonists and a 5-HT1A receptor agonist on lordosis behavior. Pharmacol Biochem Behav. 2003;76:63–73. doi: 10.1016/s0091-3057(03)00194-1. [DOI] [PubMed] [Google Scholar]
- 43.Weiss S, Sebben M, Kemp DE, Bockaert J. Serotonin 5-HT1 receptors mediate inhibition of cyclic AMP production in neurons. Eur J Pharmacol. 1986;120:227–30. doi: 10.1016/0014-2999(86)90544-3. [DOI] [PubMed] [Google Scholar]
- 44.White S, Uphouse L. Estrogen and progesterone dose-dependently reduce disruptive effects of restraint on lordosis behavior. Horm Behav. 2004;45:201–8. doi: 10.1016/j.yhbeh.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 45.Wolf A, Caldarola-Pastuszka M, DeLashaw M, Uphouse L. 5-HT2C receptor involvement in female rat lordosis behavior. Brain Res. 1999;825:146–51. doi: 10.1016/s0006-8993(99)01159-2. [DOI] [PubMed] [Google Scholar]
- 46.Wolf A, Jackson A, Price T, Trevino A, Caldarola-Pastuszka M, Uphouse L. Attenuation of the lordosis-inhibiting effects of 8-OH-DPAT by TFMPP and quipazine. Brain Res. 1998;804:206–11. doi: 10.1016/s0006-8993(98)00625-8. [DOI] [PubMed] [Google Scholar]
- 47.Xi MC, Chase MH. Neuronal mechanisms of active (rapid eye movement) sleep induced by microinjections of hypocretin into the nucleus pontis oralis of the cat. Neuroscience. 2006 March 11; doi: 10.1016/j.neuroscience.2006.01.032. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 48.Zar J. Biostatistical Analysis. 4. Prentice-Hall; Englewood Cliffs, NJ: 1999. [Google Scholar]


