Abstract
Purpose
We examined the association between the number of LNs removed, the number of positive LNs and disease progression in patients undergoing pelvic lymph node dissection and radical retropubic prostatectomy for clinically localized prostate cancer.
Materials and Methods
We analyzed 5,038 consecutive patients who underwent radical retropubic prostatectomy between 1983 and 2003. Clinicopathological parameters, including the administration of neoadjuvant hormonal therapy, preoperative prostate specific antigen, specimen Gleason score, surgeon and pathological stage, were collected prospectively in our prostate cancer database. We excluded men treated with radiation or chemotherapy before surgery. BCR was defined as 2 postoperative prostate specific antigen increases greater than 0.2 ng/ml. Cox models were used to determine whether the number of nodes removed or the number of positive nodes predicted freedom from BCR after adjustment for prognostic covariates.
Results
The 4,611 eligible patients had a median of 9 LNs (IQR 5 to 13) removed. Positive nodes were found in 175 patients (3.8%). Overall the number of LNs removed did not predict freedom from BCR (HR per additional 10 nodes removed 1.02, 95% CI 0.92 to 1.13, p = 0.7). Results were similar in patients receiving and not receiving neoadjuvant hormonal therapy. Finding any LN involvement was associated with a BCR HR of 5.2 (95% CI 4.2 to 6.4, p <0.0005). However, in men without nodal involvement an increased number of nodes removed correlated significantly with freedom from BCR (p = 0.01).
Conclusions
Nodal disease increased the risk of progression. Extensive lymphadenectomy enhances the accuracy of surgical staging. However, we were unable to determine that removing more nodes improves freedom from BCR uniformly. Since the proportion of patients with prostate cancer with positive nodes is low, the value of extensive lymphadenectomy requires a multi-institutional, randomized clinical trial.
Keywords: prostate, prostatic neoplasms, lymph nodes, lymph node excision, neoplasm recurrence, local
In 2005 an estimated 232,090 men were diagnosed with prostate cancer in the United States.1 The advent of serum PSA testing has led to stage migration, whereby the majority of prostate cancers are being detected while the tumor is still contained to the prostate. Thus, the incidence of LN involvement in patients with clinically localized prostate cancer has decreased considerably.2,3 RRP remains the most common primary definitive therapy for clinically localized prostate cancer in the United States. PLND is routinely performed at RRP to provide valuable pathological information for tumor staging.4–6
LN metastases in clinically localized prostate cancer has been shown to be a poor prognostic factor for BCR and survival.3,6,7 Because of the importance of detecting nodal metastases, the question of what is the optimal number of LNs to remove during RRP has been raised. Removing more than 13 LNs has been reported to improve prostate cancer staging and increase the detection of positive nodes.5
Surgical removal of a minimum number of LNs has been associated with survival for some tumors. In carcinoma of the colon,8 breast,9 lung10 and bladder11 improved survival outcomes have generally been seen with higher LN counts regardless of nodal status. However, in prostate cancer we know of no studies that evaluate the number of LNs removed as a predictor of BCR on multivariate analysis. We investigated this question and the effect of the number of positive nodes retrieved in patients treated with RRP and PLND for clinically localized prostate cancer.
MATERIALS AND METHODS
Our prospective database documented information on 5,038 men with clinically localized prostate cancer treated with RRP and PLND between June 1983 and February 2003, as performed by all surgeons at our institution and by 1 (PTS) at Baylor College of Medicine, Houston, Texas. Patients treated with prior radiation modalities (137) or chemotherapeutic protocols (95) before surgery were excluded from this analysis. Of the 4,806 eligible patients data were missing on PSA or clinical stage in 192 and on the number of nodes removed in 3, resulting in a study group of 4,611. Clinical stage was assigned using the 1992 American Joint Committee on Cancer TNM classification. Serum PSA was measured by the Hybritech® Tandem-R® assay. Pathological specimens were serially step-sectioned at 3 to 4 mm intervals and reviewed by dedicated genitourinary pathologists. Tumor present at ink represented a positive surgical margin. Extracapsular extension was classified as negative, capsular invasion, focal extension or established extension. LN dissection included the lymphatic tissues bordered proximal by the bifurcation of the common iliac arteries, caudal by the femoral canal and the deep circumflex vessels, along the external iliac vein and limited laterally by the pelvic side wall. Lymphatics at the confluence of the internal and external iliac veins, and the obturator fossa were removed, sparing only the obturator vessels and nerve.
Our followup protocol consisted of clinical visits or telephone calls. Consented patient surveys or questionnaires, imaging and laboratory information were collected prospectively and uploaded directly into our prostate cancer database by specialized research assistants. In general during postoperative year 1 patients underwent serum PSA measurement and digital rectal examination every 3 months. When negative, these tests were done semiannually during year 2 and annually thereafter. BCR was defined as serum PSA greater than 0.2 ng/ml, followed by any increase above this level or initiation of secondary therapies due to increasing PSA. Adjuvant hormonal therapy was administered in 2 patients 4 and 11 months after surgery, respectively. Upon the discontinuation of hormonal suppression each patient experienced increased PSA and was classified as having BCR. They were restarted on hormonal deprivation.
Linear regression was used to determine associations between clinical variables and the number of nodes removed. To determine associations between the number of positive and removed nodes, and freedom from BCR multivariate Cox proportional hazards models were developed. Clinicopathological variables analyzed in the models were patient age, pretreatment serum PSA, clinical stage, biopsy Gleason sum, RRP specimen Gleason sum, the total and positive number of LNs removed, and NHT or no NHT. However, variability in technique among surgeons was expected and surgeon was incorporated into the analysis to control for its effect. The clinicopathological variables described were used as covariates because they are well known to be predictive of BCR. Date of surgery was also included to control for stage migration and decreased tumor burden. Pretreatment PSA was entered into the model as restricted cubic splines with knots at the tertiles. No variable selection procedures were used and no interaction terms were added to the model. Data were analyzed using Stata® 8.
RESULTS
Patients
Table 1 lists patient clinicopathological and demographic features. The median number of LNs yielded was 9 (IQR 5 to 13). Figure 1 shows the distribution of the number of nodes resected. Positive nodes were found in 175 patients (3.8%), including 1 to 4 or more positive nodes in 116 (66%), 39 (22%), 7 (4%) and 13 (7%), respectively. The highest number of positive nodes was 9. There was no difference in the rate of nodal disease in men treated with NHT vs those treated with radical prostatectomy alone (3.6% and 3.8%, respectively, p = 0.8). Table 2 shows an overview of the number of nodes by node status.
Table 1.
Study sample characteristics
| No. surgery yr (%): | |
| 1983–1989 | 238 (5) |
| 1990–1994 | 1,156 (25) |
| 1995–1999 | 1,680 (36) |
| 2000–2003 | 1,537 (33) |
| Median age (IQR) | 61 (56–66) |
| No. Gleason grade (%): | |
| 6 or Less | 3,121 (68) |
| 7 | 1,224 (27) |
| 8–10 | 266 (6) |
| Median ng/ml pretreatment PSA (IQR) | 7.3 (5.1–11.9) |
| No. extracapsular extension (%): | |
| Focal | 437 (9) |
| Established | 821 (18) |
| No. seminal vesicle invasion (%) | 400 (9) |
| No. pos surgical margins (%) | 1,011 (22) |
| No. clinical T stage (%): | |
| T1a,b | 112 (2) |
| T1c | 1,859 (40) |
| T2, T2a | 1,026 (22) |
| T2b | 501 (11) |
| T2c | 960 (21) |
| T3a–c | 153 (3) |
| No. neoadjuvant hormonal therapy (%) | 673 (15) |
| No. adjuvant radiotherapy (%) | 50 (1) |
Fig. 1.
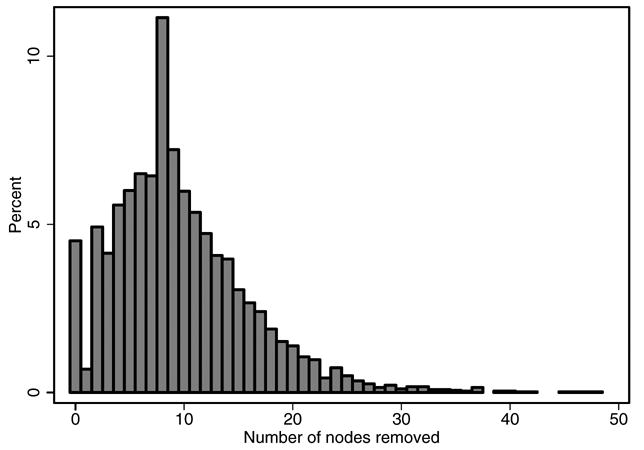
Distribution of LNs removed
Table 2.
LN findings and distribution
| LN Status | No. Pts (%) | Median No. LNs Removed/Pt (IQR) |
|---|---|---|
| Neg | 4,436 (96) | 8 (5–13) |
| Pos: | 175 (4) | 12 (8–17) |
| 1 + LN | 116 (66) | 12 (8–16) |
| 2 or Greater + LN | 59 (37) | 14 (9–19) |
Removing a greater number of LNs was associated with an increased likelihood of finding a positive node (p <0.0005, fig. 2). In a multivariate linear model the number of nodes removed was associated with case surgeon (p <0.0005), surgery date (p = 0.012) and neoadjuvant therapy (p = 0.001) when adjusting for patient age (p = 0.042), biopsy Gleason sum (p = 0.058), pretreatment serum PSA (p <0.0005) and clinical stage (p = 0.8). Patients receiving NHT had 0.9 fewer nodes removed (95% CI 0.4 to 1.5). Recent year of surgery was also associated with more limited node dissection, although the effect was small at 0.08 fewer nodes removed yearly (95% CI 0.02 to 0.14). Accordingly the mean number of nodes removed in the first half of the cohort (1983 to early 1997) was only slightly higher than the mean in the second half (10 and 9.3, respectively). There was noticeable variability among the surgeons who performed all procedures. In the first half of the cohort the median number of nodes removed was 6 to 10 by 2, 1, 2, 2 and 1 surgeons, respectively. In the later half of the cohort the number of surgeon who removed a median of 6 to 10 nodes was 2, 4, 4, 1 and 1, respectively.
Fig. 2.
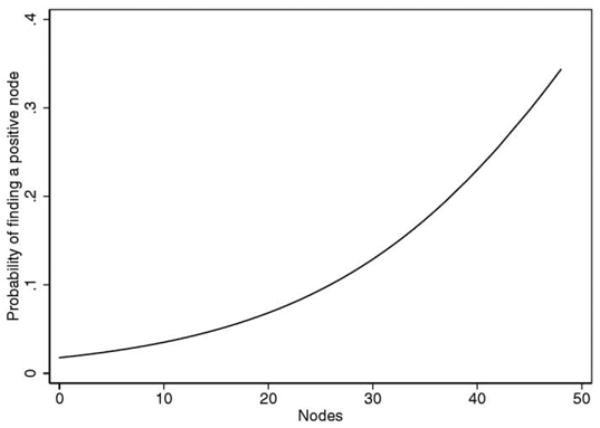
Predicted probability of finding positive LN by number of nodes removed.
Disease progression (BCR)
There were 807 (18%) patients with BCR. Median followup in men free of BCR was 53 months. Five and 10-year freedom from BCR probability in the entire cohort was 81% and 75%.
An increasing number of LNs removed predicted a slightly higher risk of BCR on univariate analysis (HR per additional 10 nodes 1.11, 95% CI 1.00 to 1.23, p = 0.055). This association disappeared after adjustment for covariates (HR per additional 10 nodes removed 1.02, 95% CI 0.92 to 1.13, p = 0.7). Results were similar in patients receiving or not receiving NHT. Similarly there was some evidence that the number of LNs removed predicted freedom from BCR in patients with node negative compared to node positive disease (HR 0.91, p = 0.01 vs HR 1.08, p = 0.6). The interaction tested between nodes removed and nodal status was not significant (p = 0.2).
In contrast, finding any number of positive LNs was associated with BCR on univariate analysis (HR 9.2, 95% CI 7.6 to 11.2, p <0.0005, fig. 3). On multivariate regression adjusting for covariates finding any LN involvement was associated with an HR of 5.2 (95% CI 4.2 to 6.4, p <0.0005). Table 3 shows the HR associated with each number of nodes after covariate adjustment.
Fig. 3.
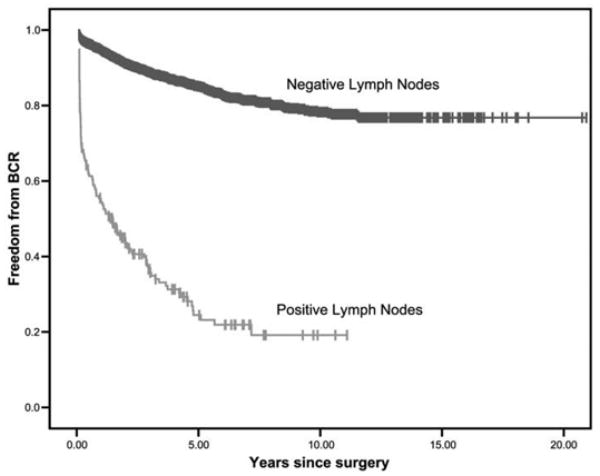
Kaplan-Meier plot of BCR-free probability by node negative or node positive status.
Table 3.
Multivariate analysis of BCR HR according to number of positive LNs identified
| No. Pos LNs | No. Pts (%) | HR | 95% CI |
|---|---|---|---|
| 0 | 4,436 (96.2) | Referent | – |
| 1 | 116 (2.5) | 4.3 | 3.4–5.5 |
| 2 | 39 (0.9) | 8.5 | 5.7–12.6 |
| 3 | 7 (0.2) | 7.7 | 3.5–17.0 |
| 4+ | 13 (0.3) | 11.4 | 6.1–21.2 |
There was evidence that the prognostic importance of positive nodes is increasing with time. Due to different followups between years we examined 2-year BCR-free survival in a logistic model, adjusting for predicted hazard. The OR for positive nodes in the first half of the cohort, treated up to early 1997, was 7.2, while in the second half the OR was 14.1. The interaction term between time and positive nodes had borderline significance (p = 0.06).
DISCUSSION
While pelvic lymphadenectomy has been useful for tumor staging, to our knowledge its therapeutic benefit for prostate cancer has yet to be defined. With the advent of PSA screening and the resulting stage migration of prostate cancers the incidence of LN metastases has decreased considerably. In our series the rate was about 4% in a cohort of 4,611 patients. Other contemporary series have shown rates between 1% and 12%.2,3,6,7 Some investigators recommend omitting PLND since the incidence of positive nodes is low and there is some morbidity risk.12–15
Several groups have investigated the relationship between the number of LNs removed and the incidence of positive nodes.4,5,15 Bader et al reported on 367 consecutive men treated with RRP and extended LN dissection during a decade.4 The median number of nodes removed was 21 and the overall nodal metastasis rate was 25%. Survival was inversely related to the number of positive nodes. Barth et al analyzed 238 patients treated with RRP and PLND for clinically localized prostate cancer.5 They found that removing greater than 13 LNs was associated with a 2-fold increase in the detection of positive nodes. They concluded that at least 13 nodes should be removed for adequate prostate cancer staging. Similarly Heidenreich et al found evidence of LN metastasis in 26% of patients with extended PLND, including dissection of the external iliac, internal iliac, obturator and presacral regions, compared to 12% of those with dissection incorporating only the external iliac and obturator regions.15 However, the effect on freedom from BCR and survival was not clear. Weingartner et al suggested that at least 20 LNs should be removed for adequate staging but they did not examine the relationship between the number of nodes removed and the detection of positive nodes.16 Our data demonstrate an association between increasing numbers of LNs removed and the likelihood of finding a positive node. These findings suggest that removing a greater number of LNs is related to the surgeon, possibly due to the extent of dissection. However, other unidentified factors that escape this analysis might influence the likelihood of positive nodal disease, such as pathological processing. Submitting individual regional packets has resulted in increased nodal counts in patients with prostate and bladder cancer.4,17
In our series a positive impact due to an increased total number of nodes on BCR-free probabilities was only seen in patients with a negative nodal pathological examination, not in the entire group. This unexpected observation was intriguing. We could speculate that this might have been due to cellular disease that escaped identification, to such an unrecognizably low disease burden that it was treated effectively with surgery present in 1 or more nodes or to bias because of the retrospective nature of our study. In studies of series of patients from the same institution single surgeon technique analysis suggested that the number of nodes was not associated with the BCR-free interval.18 However, when comparing extensive vs limited lymphadenectomy, despite subtle differences in the mean number of nodal counts the extent of dissection may prove beneficial if overall positive nodal density is less than 15%.2 The question of whether the most benefit for extensive dissection on cancer control is in patients with a lower nodal burden deserves further studies.
In our analysis the number of positive nodes had a deleterious effect on BCR-free probabilities by at least doubling the HR in patients with greater than 1 positive node. Other series presenting cancer survival data have shown a similar effect in patients with 2 positive nodes and those with 3 or more were at greater risk for cancer specific death compared to patients without nodal metastasis.4,6,7 Pelvic node dissection at RRP provides long-term cancer control, as measured by undetectable PSA and a lack of further therapy in 15% of men with nodal disease after 10 years.3,18 In the prePSA era the administration of adjuvant androgen deprivation therapy shortly after surgery was hypothesized to improve outcomes in men with node positive prostate cancer.19 This observation was later validated by a randomized clinical trial.20 The best course of action in men with positive nodes discovered after pathological examination of the specimen in the PSA era is debatable. We favor PSA testing 6 to 8 weeks after surgery and consider treatment only after serum PSA begins to increase. Perhaps a single nodal metastasis is where PLND may exert its maximal and possible curative benefit but this hypothesis can only be answered by clinical trials.
This study has several limitations. In addition to its retrospective nature and the inability to adjust for comorbid illnesses, the analysis used biochemical progression as a measure of disease progression, which as an intermediate end point is of unknown significance to survival. Furthermore, local progression, distant progression and disease specific survival could not be studied with sufficient power because this series had few patients who attained these end points. Variation in the pathological evaluation of LNs may represent a major potential confounding factor. However, our study provides novel observations about the contemporary impact of positive nodes on BCR rates despite the stage migration of prostate cancer and decrease in the incidence of nodal positivity. We present evidence that the prognostic importance of positive nodes is increasing with time, as shown by the duplication observed in the predicted hazard in men who underwent RRP after 1997. Nevertheless, definitive answers depend on prospective evaluation of these issues using standardized dissection templates, node packaging methods and pathological evaluation of randomized trials. The low proportion of patients with prostate cancer with positive nodes demands a multi-institutional approximation in the design of such clinical protocols to achieve statistical power.
Acknowledgments
Supported by a National Cancer Institute SPORE grant and by a gift from the Leon Lowenstein Foundation.
Abbreviations and Acronyms
- BCR
biochemical recurrence
- LN
lymph node
- NHT
neoadjuvant hormonal therapy
- PLND
pelvic lymphadenectomy
- PSA
prostate specific antigen
- RRP
radical retropubic prostatectomy
References
- 1.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Allaf ME, Palapattu GS, Trock BJ, Carter HB, Walsh PC. Anatomical extent of lymph node dissection: impact on men with clinically localized prostate cancer. J Urol. 2004;172:1840. doi: 10.1097/01.ju.0000140912.45821.1d. [DOI] [PubMed] [Google Scholar]
- 3.Bianco FJ, Jr, Scardino PT, Eastham JA. Radical prostatectomy: long-term cancer control and recovery of sexual and urinary function (“trifecta”) Urology. 2005;66:83. doi: 10.1016/j.urology.2005.06.116. [DOI] [PubMed] [Google Scholar]
- 4.Bader P, Burkhard FC, Markwalder R, Studer UE. Disease progression and survival of patients with positive lymph nodes after radical prostatectomy. Is there a chance of cure? J Urol. 2003;169:849. doi: 10.1097/01.ju.0000049032.38743.c7. [DOI] [PubMed] [Google Scholar]
- 5.Barth PJ, Gerharz EW, Ramaswamy A, Riedmiller H. The influence of lymph node counts on the detection of pelvic lymph node metastasis in prostate cancer. Pathol Res Pract. 1999;195:633. doi: 10.1016/S0344-0338(99)80128-9. [DOI] [PubMed] [Google Scholar]
- 6.Daneshmand S, Quek ML, Stein JP, Lieskovsky G, Cai J, Pinski J, et al. Prognosis of patients with lymph node positive prostate cancer following radical prostatectomy: long-term results. J Urol. 2004;172:2252. doi: 10.1097/01.ju.0000143448.04161.cc. [DOI] [PubMed] [Google Scholar]
- 7.Cheng L, Zincke H, Blute ML, Bergstralh EJ, Scherer B, Bostwick DG. Risk of prostate carcinoma death in patients with lymph node metastasis. Cancer. 2001;91:66. doi: 10.1002/1097-0142(20010101)91:1<66::aid-cncr9>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 8.Joseph NE, Sigurdson ER, Hanlon AL, Wang H, Mayer RJ, MacDonald JS, et al. Accuracy of determining nodal negativity in colorectal cancer on the basis of the number of nodes retrieved on resection. Ann Surg Oncol. 2003;10:213. doi: 10.1245/aso.2003.03.059. [DOI] [PubMed] [Google Scholar]
- 9.Iyer RV, Hanlon A, Fowble B, Freedman G, Nicolaou N, Anderson P, et al. Accuracy of the extent of axillary nodal positivity related to primary tumor size, number of involved nodes, and number of nodes examined. Int J Radiat Oncol Biol Phys. 2000;47:1177. doi: 10.1016/s0360-3016(00)00574-5. [DOI] [PubMed] [Google Scholar]
- 10.Gajra A, Newman N, Gamble GP, Kohman LJ, Graziano SL. Effect of number of lymph nodes sampled on outcome in patients with stage I non-small-cell lung cancer. J Clin Oncol. 2003;21:1029. doi: 10.1200/JCO.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Herr HW, Bochner BH, Dalbagni G, Donat SM, Reuter VE, Bajorin DF. Impact of the number of lymph nodes retrieved on outcome in patients with muscle invasive bladder cancer. J Urol. 2002;167:1295. [PubMed] [Google Scholar]
- 12.Clark T, Parekh DJ, Cookson MS, Chang SS, Smith ER, Jr, Wells N, et al. Randomized prospective evaluation of extended versus limited lymph node dissection in patients with clinically localized prostate cancer. J Urol. 2003;169:145. doi: 10.1016/S0022-5347(05)64055-4. [DOI] [PubMed] [Google Scholar]
- 13.Meng MV, Carroll PR. When is pelvic lymph node dissection necessary before radical prostatectomy? A decision analysis. J Urol. 2000;164:1235. [PubMed] [Google Scholar]
- 14.Kattan MW, Eastham JA, Stapleton AM, Wheeler TM, Scardino PT. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst. 1998;90:766. doi: 10.1093/jnci/90.10.766. [DOI] [PubMed] [Google Scholar]
- 15.Heidenreich A, Varga Z, Von Knobloch R. Extended pelvic lymphadenectomy in patients undergoing radical prostatectomy: high incidence of lymph node metastasis. J Urol. 2002;167:1681. [PubMed] [Google Scholar]
- 16.Weingartner K, Ramaswamy A, Bittinger A, Gerharz EW, Voge D, Riedmiller H. Anatomical basis for pelvic lymphadenectomy in prostate cancer: results of an autopsy study and implications for the clinic. J Urol. 1996;156:1969. doi: 10.1016/s0022-5347(01)65406-5. [DOI] [PubMed] [Google Scholar]
- 17.Bochner BH, Herr HW, Reuter VE. Impact of separate versus en bloc pelvic lymph node dissection on the number of lymph nodes retrieved in cystectomy specimens. J Urol. 2001;166:2295. [PubMed] [Google Scholar]
- 18.Palapattu GS, Allaf ME, Trock BJ, Epstein JI, Walsh PC. Prostate specific antigen progression in men with lymph node metastases following radical prostatectomy: results of long-term followup. J Urol. 2004;172:1860. doi: 10.1097/01.ju.0000139886.25848.4a. [DOI] [PubMed] [Google Scholar]
- 19.Zincke H. Bilateral pelvic lymphadenectomy and radical retropubic prostatectomy for stage C or D1 adenocarcinoma of the prostate: possible beneficial effect of adjuvant treatment. NCI Monogr. 1988;9:109. [PubMed] [Google Scholar]
- 20.Messing EM, Manola J, Sarosdy M, Wilding G, Crawford ED, Trump D. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N Engl J Med. 1999;341:1781. doi: 10.1056/NEJM199912093412401. [DOI] [PubMed] [Google Scholar]


