Abstract
Exposure of the mammalian host to infective larvae of Schistosoma mansoni causes an acute inflammatory response in the skin and the activation of several cell types of the innate immune response including macrophages. Using an in vitro model of macrophage activation, we show that schistosome larvae possess molecules that directly stimulate both thioglycollate-elicited macrophages (tMφ) and IFNγ-activated tMφ in vitro to produce several cytokines including IL-6, IL-12p40 and IL-10. The parasite-derived molecules are enriched within the material released by the parasite following transformation [0- to 3-h released larval preparation (0-3hRP)] but not within soluble preparations of whole larvae. Cytokine production was maintained in the presence of polymyxin B, confirming that contaminating endotoxin was not responsible. IL-12p40 and IL-10 production was much lower by cells from C3H/HeJ mice, which have defective Toll-like receptor 4 (TLR4), but IL-6 production was unaffected. Experiments using TLR4-/- mice confirmed that IL-12p40 production by tMφ in response to 0-3hRP was partly dependent upon functional TLR4, whereas IL-6 production was entirely independent. In contrast, tMφ from MyD88-/- mice failed to secrete either IL-12p40 or IL-6, underlining a pivotal role of TLR signalling in cytokine production by macrophages in response to stimulation with 0-3hRP. Finally, we show that glycan components of 0-3hRP are required for optimal cytokine production since protease treatment of 0-3hRP had no effect on IL-12p40 production and only a slight effect on IL-6, while sodium meta-periodate treatment almost completely abolished production of both cytokines.
Keywords: IL-6, IL-10, IL-12p40, innate, MyD88
Introduction
The parasitic helminth Schistosoma is the causative agent of the disease schistosomiasis suffered by ∼200 million people worldwide (1). Infective cercariae gain entry into the mammalian host by active penetration of the skin, whereupon they transform into schistosomula that migrate through the dermis and, after several days, exit via the vasculature, or lymphatics (2, 3). Immune-associated events in the epidermis and dermis immediately following parasite exposure are characterized by oedema, the infiltration of mononuclear and polymorphonuclear cells (particularly, macrophages and neutrophils) and the local production of chemokines and cytokines, including IL-6, IL-10 and IL-12p40 (4-7). It is widely believed that this innate response is important in directing the subsequent generation of adaptive responses in the local draining lymph nodes (3, 7). However, the identity of the molecular interactions that occur between parasite larvae, or their released products, and the host’s innate accessory cells are at present unclear.
Immunocompetent accessory cells are some of the first to encounter invading pathogens, such as schistosomes, and recognize a restricted repertoire of microbial molecules, common to many pathogens, through cellular receptors termed pattern recognition receptors (PRRs). Ligation of these PRRs is the trigger for inflammatory cellular events that regulate innate and adaptive immunity, such as cytokine and chemokine production (8). There is also evidence that accessory cells are activated by host ‘danger molecules’ released upon tissue damage (9), which may also bind PRRs (10). Toll-like receptors (TLRs) are an archetypal PRR and play a central role in the induction of the signalling pathways that lead to pro-inflammatory cellular responses (11). These signalling events involve a variety of adapter proteins, with MyD88 being important for signal transduction from a variety of TLRs, including TLR2, TLR4, TLR9, and the IL-1R superfamily (11). Distinct cellular responses can be elicited by the engagement of different TLRs, or by interactions between multiple TLRs, allowing a certain degree of specificity against different pathogens (12). A number of different TLR ligands have been identified and are primarily of viral, bacterial, lower eukaryotic or host origin (11). However, recent observations have extended this identification to include components of higher eukaryotes, such as helminths (13-16).
In this paper, we wished to identify the stage of larval maturation that acts as a source of the molecules responsible for initiating the innate immune response. Consequently, we compared the capacity of live schistosome larvae of different maturation states, and soluble preparations derived from whole larvae, to stimulate cytokine production by macrophages obtained from wild-type (WT) mice and cohorts with genetic deficiencies in TLR4 and MyD88. We demonstrate that the material released by infective larvae upon transformation is rich in molecules that stimulate immune accessory cells via MyD88-dependent TLR4- and non-TLR4-mediated pathways.
Methods
Animals
Female C57Bl/6 strain mice (bred in-house) and C3H/HeN and C3H/HeJ mice (Harlan Seralab, Loughborough, UK) were housed at the University of York. For some experiments TLR4-/- (17) and MyD88-/- (18) mice, on a C57Bl/6 background, (kindly provided by Prof. Akira and Takeda, Osaka University, Japan), were bred and maintained under SPF conditions in the animal unit at the University of Manchester. All animal work was carried out in accordance with the guidelines of the United Kingdom Animals (Scientific Procedures) Act 1986.
Parasites and parasite material
Cercariae of Schistosoma mansoni were shed from Biomphalaria glabrata snails harbouring patent infections, and following concentration by sedimentation on ice for 1 h were washed three times with sterile water. Cercariae were then centrifuged (1000 × g, 5 min) and the larval pellet was frozen at −20°C. Alternatively, cercariae were mechanically transformed using the method described by Ramalho-Pinto et al. (19) to generate larvae for subsequent in vitro culture. In brief, cercariae were mechanically transformed by vortexing for 90 s in RPMI-1640 containing 200 U ml-1 penicillin and 100 μg ml-1 streptomycin (Invitrogen, Paisley, UK) (RPMI-0) and cultured in vitro in RPMI-0 for 3 h at 37°C and 5% CO2 in a humidified incubator (20). The culture supernatant was then removed and the remaining larvae were washed to recover further released material. Pooled supernatants were concentrated 50-fold using centrifugal filter units (Ultrafree-MC with 5-kDa cut-off; Millipore, Watford, UK). As a control, an equivalent volume of RPMI-0 medium containing no parasite material was concentrated using the same method. The 3-h larval heads were isolated from their tails by centrifugation on a 40/70% discontinous Percoll gradient (21), washed seven times in RPMI-0 and then frozen or cultured for a further 18 h in M169 media (20) supplemented with 200 U ml-1 penicillin, 100 μg ml-1 streptomycin and 5% heat-inactivated low-endotoxin FCS (Harlan Seralab). After the requisite culture period, the larvae were washed four times with RPMI-0 to remove all traces of serum proteins and then frozen at −20°C.
The thawed larvae of different maturation stages were sonicated (21 kHz at 6.5 μm amplitude) for 3 min and then centrifuged (100 000 × g) for 1 h to yield a soluble cercarial preparation (SCP), and larvae at 3 h [3-h soluble schistosomula preparation (3hSSP)] and 18 h [18-h soluble schistosomula preparation (18hSSP)]. The concentrated supernatant released by larvae between 0 and 3 h (0-3hRP) and the RPMI-0 control (RPMIc) were treated in a similar way. Protein and endotoxin concentrations of the preparations were determined using the Coomasie Plus-200 assay (Perbio Science UK Ltd, Tattenhall, UK) and Pyrogent Plus® limulus amoebocyte lysate test kit (BioWhittaker, Wokingham, UK), respectively.
Cell culture reagents
LPS (Escherichia coli strain 0111:B4), yeast Zymosan A (Saccharomyces cerevisiae) and polymyxin B (PMB) were obtained from Sigma-Aldrich (Poole, UK). Purified recombinant IFNγ from 211A CHO cell line supernatant was produced in-house.
Isolation of thioglycollate-elicited peritoneal macrophages
Adherent thioglycollate-elicited macrophages (tMφ) were used to directly test the stimulatory activities of different schistosome preparations in vitro since it was not possible to obtain an abundant supply of dermal macrophages ex vivo. Five days following intraperitoneal injection of 0.5 ml sterile 3% Brewers thioglycollate medium (Sigma-Aldrich), peritoneal exudate cells were extracted from sacrificed mice by peritoneal lavage with RPMI-1640 containing 200 U ml-1 penicillin, 100 μg ml-1 streptomycin, 2 mM l-glutamine(Invitrogen) and 10% FCS (Harlan Seralab) (RPMI-10). At this stage, cells from three to four mice were pooled to minimize individual variation. The cells were then washed, re-suspended in RPMI-10 and plated in 96-well tissue culture plates (1-2 × 105 cells per well; Nalge Nunc). After culture for 2 h, non- and semi-adherent cells were removed and discarded from adherent cells by gently washing the monolayer three times with pre-warmed RPMI-10. Resultant adherent tMφ were typically 80-90% F4/80+ as judged by flow cytometry. Adherent tMφ from the different strains of mice were phenotypically similar and recoverable in similar quantities.
Stimulation of tMφ with microbial products and live schistosome larvae
The tMφ monolayer was cultured for 24 h with soluble parasite preparations (SCP, 3hSSP, 18hSSP and 0-3hRP; 0.05-50 μg ml-1), LPS (0.0001-1 μg ml-1) or Zymosan A (0.05-50 μg ml-1) in the presence or absence of PMB (3-10 μg ml-1). Where indicated, tMφ were simultaneously activated with IFNγ (5 U ml-1) to increase sensitivity, particularly in the case of tMφ from C3H/HeN mice. The stimulatory properties of live larvae of different maturation stages were determined by culture of tMφ in the presence of PMB (3 μg ml-1) for 24 h with (i) whole cercariae, (ii) cercariae that had been mechanically transformed immediately prior to culture or (iii) mechanically transformed cercariae that had been cultured in vitro for 3 h followed by removal of the culture supernatant. This un-concentrated 0- to 3-h larval culture supernatant, termed 0-3 h supernatant, was also assayed for its stimulatory properties.
Treatment regimes for 0-3hRP
In experiments to test the contribution of different molecular structures on the stimulatory capacity of parasite material, 0-3hRP was heat treated, digested with proteases or subjected to sodium meta-periodate treatment as described subsequently. First, 0-3hRP was heated to temperatures of 37, 50, 60, 70, 80, 90 or 100°C for 45 min and the protein concentration re-checked. Second, in order to test for protease-sensitive moieties, 0-3hRP was dialysed for 4 h against PBS, pH 2.2 (Slide-A-Lyzer Mini Dialysis Unit with 3.5-kDa cut-off; Perbio Science), and then digested with pepsin (10 μg ml-1 in PBS, pH 2.2; Sigma-Aldrich) overnight at 37°C. Control 0-3hRP was treated similarly but without the addition of proteases. Digested and control samples were then heated at 55°C for 30 min to inactivate the protease, and then dialysed against PBS, pH 7.2, for 4 h. Digestion of 0-3hRP was confirmed by one-dimensional SDS-PAGE of treated and control samples. Third, sodium meta-periodate treatment (22) was used to modify the carbohydrates present in 0-3hRP. Samples of 0-3hRP were dialysed against 50 mM sodium acetate buffer (pH 4.5) for 4 h and treated with sodium meta-periodate (10 mM; Sigma-Aldrich) for 45 min at 25°C in the dark, and the reaction was then stopped with sodium borohydride (50 mM; 30 min, 25°C). Treated 0-3hRP and mock-treated 0-3hRP (as above but without sodium meta-periodate) were then dialysed against several changes of PBS, pH 7.2, overnight. The protein concentrations were determined and adjusted as required. Treated and control 0-3hRP samples (50 μg ml-1) from each of the above three treatment regimes were then used as before to stimulate tMφ, or IFNγ-tMφ where parasite material was limited (i.e. after periodate treatment).
Cytokine detection by ELISA
Supernatants from tMφ cultures were stored at −20°C prior to detection by ELISA of IL-6, IL-10 and IL-12p40 as previously described (6, 7).
Statistics
Comparisons of data within experiments were tested for significance using the Student’s t-test. Significant values are indicated as follows: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, NS = not significant.
Results
Products released by schistosome larvae are potent inducers of cytokine production by tMφ
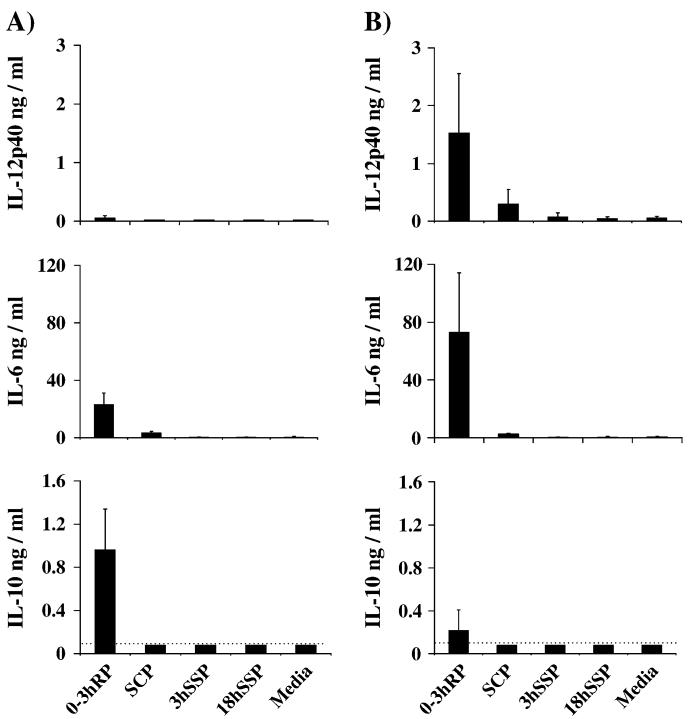
To establish whether skin-stage schistosomes possess innate stimulatory properties, soluble preparations of infective cercariae, in vitro-cultured larvae and the soluble products of mechanically transformed larvae (Table 1) were assayed for their ability to induce cytokine production by tMφ from C3H/HeN mice. High levels of IL-10 and IL-6 were detected in the supernatants of tMφ following 24 h culture with 0-3hRP, whereas little IL-12p40 was detected (Fig. 1A). In order to increase sensitivity, tMφ were also activated with IFNγ (IFNγ-tMφ) prior to co-culture with the different parasite products. Accordingly, IFNγ-tMφ stimulated with 0-3hRP produced much greater levels of IL-12p40 and increased IL-6, but greatly reduced levels of IL-10 (Fig. 1B). In contrast to 0-3hRP, the soluble preparations of whole larvae (i.e. SCP, 3hSSP and 18hSSP) had very limited stimulatory potential, with SCP stimulating only an increase in IL-6 production by tMφ (Fig. 1A), although low levels of both IL-6 and IL-12p40 were induced by SCP in cultures of IFNγ-tMφ (Fig. 1B). Neither 3hSSP nor 18hSSP from skin-stage larvae induced production of IL-10, IL-6 and IL-12p40 by tMφ, or by IFNγ-tMφ (Fig. 1A and B). Similar results were obtained with cells from C57Bl/6 mice, except that greater levels of IL-12p40 were produced by both tMφ and IFNγ-tMφ but again only in response to 0-3hRP (data not shown). Differences in the stimulatory properties of the various parasite preparations were not due to contrasting kinetics of cytokine production, since the level of all cytokines detected from the supernatants of 0-3hRP- and SCP-stimulated cells had reached a peak by 24 h (data not shown).
Table 1.
Summary of schistosome preparations analysed in this study
| Preparation acronym | Source material: larval developmental stage in vitroa | Equivalent larval position in vivob |
|---|---|---|
| SCP | Infective cercariae | Infective cercariae |
| 3hSSP | 3-h larvae | Epidermis |
| 18hSSP | 18-h larvae | Epidermal/dermal interface |
| 0-3hRP | Molecules released during first 3 h of in vitro culture (×50 concentration) | Molecules released at the onset of infection |
| RPMIcc | Medium control (×50 concentration) | N/A |
Source material in italics are schistosomula or their products following mechanical transformation and culture in vitro for the stated period of time.
Approximate location of larvae if they had matured in vivo.
Equivalent medium control to 0-3hRP.
Fig. 1.
Larval released products but not somatic material stimulate cytokine production by peritoneal macrophages. (A) tMφ and (B) IFNγ-activated tMφ from C3H/HeN mice were cultured overnight with media alone, or larval preparations (50 μg ml-1), and the production of IL-6, IL-12p40 and IL-10 was determined by ELISA. Data are the mean ± SD of two experiments. Dotted lines represent the lower limit of ELISA detection.
Live schistosome larvae have innate stimulatory activity
Since soluble preparations of whole cercariae and in vitro-cultured larvae stimulated little, or no, cytokine production the interaction between tMφ from C3H/HeN mice and live schistosome larvae was further explored. Cercariae stimulated a dose-dependent increase in IL-6 production by tMφ with as few as 100 parasites per well causing a >3-fold increase in IL-6 (Table 2). Cercariae that had been mechanically transformed immediately prior to culture with tMφ stimulated a much greater fold increase in IL-6 compared with non-transformed cercariae when cultured at 50 or 100 larvae per well, although the difference was not apparent when parasites were cultured at a concentration of 200 per well (Table 2). Whereas mechanically transformed cercariae acquired characteristics of skin-stage larvae, such as the loss of tails, shedding of cercarial glycocalyx and release of head gland material, the non-transformed parasites remained intact throughout culture in vitro (data not shown).
Table 2.
Innate stimulatory properties of different development stages of live schistosome larvae and larval released products
| Larval stage/product | Fold increasea in IL-6 production over untreated control by tMφ cultured with larvae (number of parasites per well) |
||
|---|---|---|---|
| 50 | 100 | 200 | |
| Cercariaeb | 1.48 ± 0.67 | 3.39 ± 2.81 | 14.1 ± 8.25 |
| Transformed cercariaeb | 3.71 ± 0.22 | 12.1 ± 3.42 | 14.4 ± 4.99 |
| 0-3 h supernatantc | 0.65 ± 0.49 | 3.58 ± 1.25 | 8.41 ± 2.53 |
| 3-h schistosomulac | 0.65 ± 0.49 | 2.64 ± 2.32 | 10.1 ± 2.56 |
Values are means ± SD of fold increase in IL-6 production by tMφ (2 × 105 per well) cultured for 24 h compared with untreated cells. All cells were cultured in the presence of PMB (3 μg ml-1).
Cercariae obtained from a single shed were left intact or mechanically transformed by vortexing immediately prior to addition to tMφ cultures.
Cercariae were mechanically transformed and cultured in vitro for 3 h. The resulting transformed larval heads and tails were subsequently isolated from the culture supernatant and added to the tMφ cultures. The un-concentrated culture supernatant (0-3 h supernatant) was added to the tMφ cultures in quantities equivalent to that released by the different stated numbers of larvae.
The stimulatory properties of un-concentrated material released by the transformed larvae during the first 3 h of culture post-transformation (0-3 h supernatant) and the 3-h-cultured live larvae devoid of this released material were compared. The 0-3 h supernatant stimulated increased IL-6 production over background levels when cultured at amounts equivalent to that released by 100 and 200 larvae per well (Table 2), and although the actual levels of IL-6 detected were much lower than those stimulated by the concentrated preparation 0-3hRP (cf. Fig. 1), and no IL-10 or IL-12p40 was detected (data not shown), these data demonstrate that the released material was stimulatory at the numbers of parasites conventionally used to infect experimental mice. It was also noted that live 3-h larvae stimulated increased IL-6 production over background levels when cultured at concentrations equivalent to 100 and 200 larvae per well (Table 2), which contrasts with the apparent non-stimulatory nature of 3hSSP (cf. Fig. 1).
Naturally occurring endotoxin of bacterial origin is not a major cause of cytokine production by larval released products
An important consideration of this study was the possible presence in the schistosome preparations of endotoxin (the major active component of which is LPS) that is likely to be a natural contaminant because infective cercariae emerge from the snail intermediate host in a non-sterile environment. Analysis of all parasite preparations using the limulus amoebocyte lysate assay demonstrated that the released larval preparation (0-3hRP) contained low levels of naturally occurring endotoxin contributing the equivalent of 0.0015 ± 0.0003 μg ml-1 LPS to the tMφ culture, whereas only trace levels were detected in the other soluble larval preparations. Therefore, a 2-fold approach was used to determine the stimulatory properties of 0-3hRP in the absence of possible LPS contaminants. This involved, first, using the antibiotic PMB, which blocks stimulation by LPS (23), and second, obtaining macrophages from C3H/HeJ mice that have a natural mutation in the intracellular signalling Toll/IL-1 receptor (TIR) domain of TLR4 (24), through which the majority and most potent types of LPS signal (25).
The presence of PMB had little or no effect upon the levels of IL-10 and IL-6 produced by C3H/HeN tMφ when stimulated with 0-3hRP (Fig. 2A). These concentrations of PMB also had no effect on cell viability, or upon the stimulatory properties of other known microbial stimuli, such as Zymosan A (data not shown). In contrast, PMB dramatically inhibited stimulation by LPS, with a 90-fold decrease in IL-6 production and complete abrogation of IL-10 production (Fig. 2A). The serotype of LPS was selected for its highly stimulatory properties (26; data not shown) and was used at a concentration that had at least twice the endotoxin activity calculated to be contributed to the culture by 0-3hRP, to ensure that its potency was equal to, or higher than, the possible endotoxin contamination within this parasite preparation. In some experiments, tMφ were also stimulated with IFNγ to increase sensitivity and again PMB had no effect upon the levels of IL-12p40 and IL-6 produced by cells from C3H/HeN mice stimulated with 0-3hRP but completely ablated the stimulatory properties of LPS (Fig. 2B). Similar data were obtained with both tMφ and IFNγ-tMφ from C57Bl/6 mice (data not shown). Combined, the data above provide strong evidence that the stimulatory properties of 0-3hRP are due to schistosome factors and not bacterial LPS contamination. However, as a precaution, all further assays of schistosome products were performed in the presence of PMB (3 μg ml-1), which was sufficient to inhibit up to 0.01 μg ml-1 LPS (data not shown).
Fig. 2.
Stimulatory properties of larval released products are independent of endotoxin yet have both TLR4-dependent and -independent components. (A, C) tMφ and (B, D) IFNγ-activated tMφ from C3H/HeN or C3H/HeJ mice were cultured overnight with media alone, 0-3hRP (50 μg ml-1) or LPS (0.001 μg ml-1), in the presence of 0 (black bars) or 10 (white bars) μg ml-1 PMB. In these assays, 0-3hRP contributed the equivalent of 0.0005 μg ml-1 endotoxin to the cell culture. Supernatants from triplicate wells were pooled and analysed by ELISA for the production of IL-6, IL-10 and IL-12p40. Data are the mean ± SD of two experiments. Dotted lines represent the lower limit of ELISA detection.
As expected, tMφ from C3H/HeJ mice failed to produce detectable levels of IL-10 upon exposure to LPS, and produced 23-fold less IL-6 than their C3H/HeN counterparts (Fig. 2C). Furthermore, IFNγ-tMφ from C3H/HeJ mice produced 80-fold less IL-12p40 and 60-fold less IL-6 than their C3H/HeN counterparts upon exposure to LPS (Fig. 2D). In contrast, 0-3hRP stimulated IL-10 and IL-6 production by tMφ (Fig. 2C), and IL-12p40 and IL-6 production by IFNγ-tMφ (Fig. 2D) from C3H/HeJ mice, even in the presence of PMB. However, there was diversity in the requirement for functional TLR4 in the cytokine response to 0-3hRP. In this respect, C3H/HeJ tMφ produced 4-fold less IL-10 than C3H/HeN cells, but similar levels of IL-6 when stimulated with 0-3hRP in the presence of PMB (Fig. 2A and C). Furthermore, C3H/HeJ IFNγ-tMφ produced 2.7-fold less IL-12p40 than C3H/HeN cells after culture with 0-3hRP, but only 1.8-fold less IL-6 (Fig. 2B and D). That 0-3hRP stimulated less IL-10 and IL-12p40 but similar amounts of IL-6 by both tMφ and IFNγ-tMφ from C3H/HeJ compared with C3H/HeN counterparts suggests that 0-3hRP acts upon Mφ through both TLR4-dependent and -independent pathways, and that these pathways have a differential role in the resulting cytokine profile.
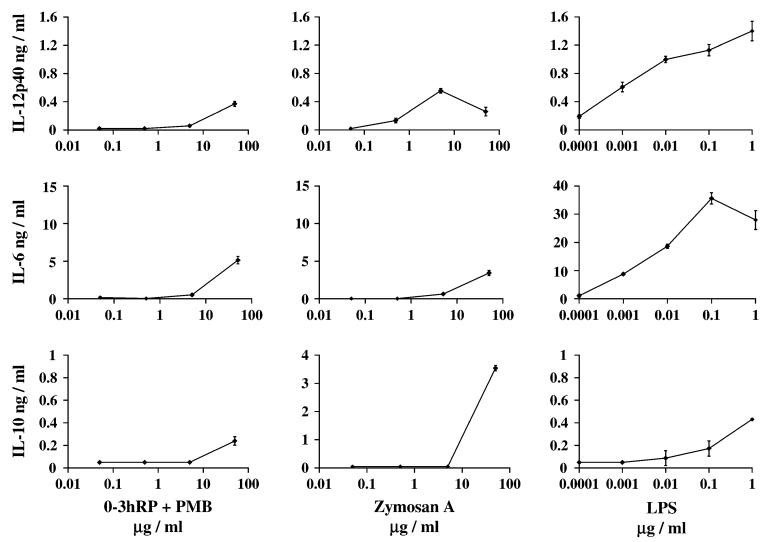
0-3hRP stimulates a different cytokine profile to classic microbe-derived stimuli
0-3hRP is unique among our soluble parasite preparations in its ability to stimulate Mφ cytokine production. To characterize the response to 0-3hRP and the pathways through which it may signal, its stimulatory properties were compared with LPS (E. coli) and Zymosan A (yeast) known to signal predominantly through TLR4 (24) and TLR2 (27), respectively. It is important to note that direct comparisons of potency of these stimuli are difficult due to the molecular heterogeneity of 0-3hRP and Zymosan, and our present lack of knowledge of the molar concentrations of their active components. Nevertheless, the overall profile of cytokine production by tMφ differed qualitatively in response to Zymosan A and 0-3hRP, such that the ratio of IL-12p40 to IL-6 was markedly different (Fig. 3). In this respect, titration of these microbial stimuli demonstrated that 0-3hRP was a weaker inducer of IL-12p40 production relative to Zymosan A, but was a more potent inducer of IL-6. Conversely, LPS stimulated high levels of both these cytokines but relatively little IL-10 (Fig. 3).
Fig. 3.
Profiles of cytokine production by tMφ from C57Bl/6 mice in response to 0-3hRP compared with other microbe-derived stimuli. tMφ were cultured with 0-3hRP (0.05-50 μg ml-1) plus PMB (3 μg ml-1), LPS (0.0001-1 μg ml-1) or Zymosan A (0.05-50 μg ml-1), and the production of IL-12p40, IL-6 and IL-10 was analysed by ELISA. Data are presented as the mean ± SD of three wells, and are representative of two to three experiments.
0-3hRP contains ligands for multiple PRRs that are dependent upon signalling via MyD88
Having shown that macrophages from C3H/HeJ mice produce less IL-12p40 and IL-10 than cells from C3H/HeN mice when cultured with 0-3hRP (Fig. 2), we used tMφ from TLR4-/- mice (17) to clarify the role of this receptor in the recognition of the larval released molecules. To control for differences in basal states of activation by tMφ from WT and TLR4-/- mice, cytokine production was expressed as a percentage of that stimulated by TLR4-independent Zymosan A (27). The 2-fold greater cytokine production by WT over TLR4-/- tMφ in response to Zymosan A was not due to endotoxin contamination because addition of PMB had no effect (data not shown), illustrating that tMφ from TLR4-/- mice most likely have a lower activation state. TLR4-/- tMφ produced little, or no, increase in IL-12p40 or IL-6 upon stimulation with LPS, whereas abundant cytokine production by WT tMφ was detected, confirming that TLR4 is essential for the recognition of LPS (Fig. 4A). In contrast, there was a dichotomy in the requirement for TLR4 in the production of IL-6 and IL-12p40 in response to 0-3hRP. As such, TLR4-/- tMφ stimulated with 0-3hRP produced significantly (P < 0.001) less IL-12p40 than WT cells relative to Zymosan A; yet, there was no difference in the amounts of IL-6 (Fig. 4A), reinforcing our observations made using C3H/HeN and C3H/HeJ cells (Fig. 2).
Fig. 4.
Differential requirement for TLR4 and MyD88 in the signalling pathway stimulated by 0-3hRP. (A) tMφ from C57Bl/6 (open bars) and TLR4-/- (black bars) mice or (B) C57Bl/6 (open bars) and MyD88-/- (hatched bars) mice were cultured overnight with media alone, 0-3hRP (50 μg ml-1) plus PMB (3 μg ml-1), LPS (0.01 μg ml-1) or Zymosan A (5 μg ml-1). Supernatants were removed and analysed by ELISA for the production of IL-12p40 and IL-6. (A) Cytokine production is displayed as a percentage of the amount produced by the respective cells when cultured with Zymosan A. (B) Alternatively, cytokine production is displayed as the actual concentration detected in the culture supernatants. Data are presented as the mean ± SD of three wells.
Since MyD88 is a key adapter molecule in the downstream signalling of many TLRs (28, 29) and is essential for the induction of most TLR4-dependent cellular responses, such as the production of IL-6 (18), we wanted to determine its role in the activation of tMφ by 0-3hRP. LPS and Zymosan A are known to induce MyD88-dependent and -independent cellular responses (18, 30, 31), and in our assay, both stimulated significantly (P < 0.01 and P < 0.001, respectively) increased IL-12p40 production by tMφ from MyD88-/- mice compared with cells cultured with media alone, although the levels were much lower than those of WT cohorts (P < 0.001) (Fig. 4B). In contrast, IL-12p40 production by tMφ in response to 0-3hRP was totally dependent upon MyD88, with cells from MyD88-/- mice failing to produce any increase in IL-12p40 over cells cultured with media alone (Fig. 4B). IL-6 production in response to all three microbe-derived stimuli was entirely MyD88 dependent (Fig. 4B).
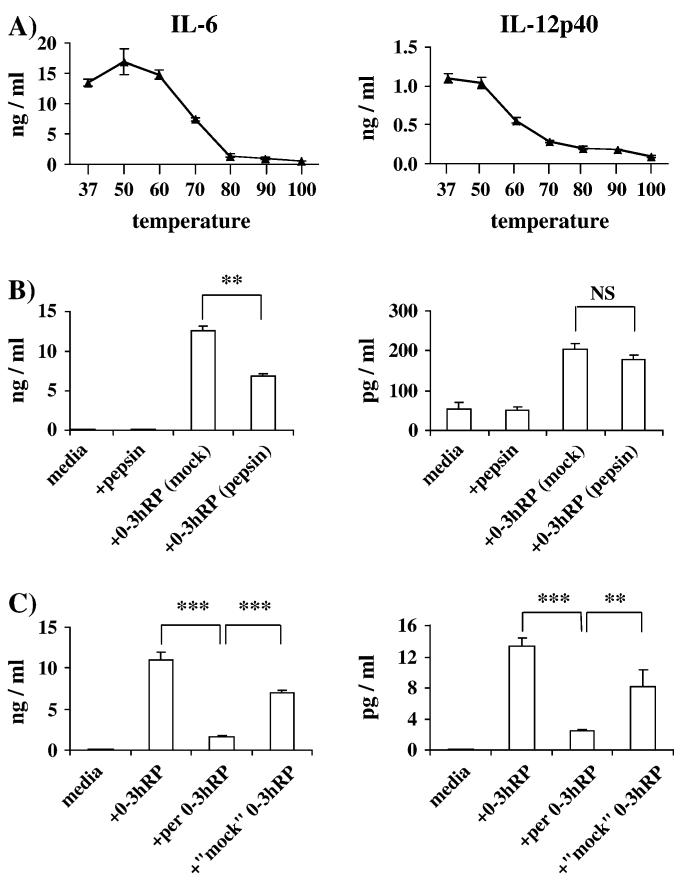
The cytokine-stimulating activity of 0-3hRP is heat labile but dependent upon the presence of glycans
To make an initial biochemical analysis of the stimulatory components within 0-3hRP, we first assessed their sensitivity to heat inactivation. The elements in 0-3hRP responsible for stimulating tMφ production of IL-12p40 and IL-6 were heat stable at temperatures up to 50-60°C (Fig. 5A). However, exposure of 0-3hRP to higher temperatures dramatically decreased its ability to stimulate production of these cytokines, with >90% reduction following heat inactivation at 100°C (Fig. 5A). In separate experiments, treatment of 0-3hRP with either pepsin (Fig. 5B) or trypsin (data not shown) had no significant effect on the ability of stimulated tMφ to produce IL-12p40, although the production of IL-6 was reduced by up to 40% (P < 0.01). Finally, treatment of 0-3hRP with sodium meta-periodate significantly reduced by >90% the secretion of IL-12p40 and IL-6 (both P < 0.001) by IFNγ-tMφ (Fig. 5C). Although ‘mock’ periodate treatment of 0-3hRP also slightly reduced production of both IL-6 and IL-12p40, sodium meta-periodate treatment further reduced the production of IL-6 (P < 0.001) and IL-12p40, by almost 80 and 70% (P < 0.01), respectively, compared with this control (Fig. 5C).
Fig. 5.
The cytokine stimulatory components of 0-3hRP are heat labile, resistant to protease treatment but sensitive to periodate treatment. (A, B) tMφ or (C) IFNγ-tMφ from C57Bl/6 mice were cultured with batches of (A) 0-3hRP previously incubated at temperatures ranging from 37 to 100°C, (B) 0-3hRP treated with pepsin (10 μg ml-1) or (C) 0-3hRP treated with sodium meta-periodate, all in the presence of PMB (3 μg ml-1). Data are means ± SEM of three or more culture supernatants and are representative of a minimum of two experiments.
Discussion
The acute cutaneous inflammatory response induced by penetrating larvae of S. mansoni is well documented (e.g. 6, 7), although the parasites have also developed several strategies for regulating the extent of inflammation (reviewed in 3, 32). Nevertheless, the molecular interactions between parasite and host that initiate the inflammatory response remain poorly characterized.
In this study, we show that molecules released from schistosome larvae are recognized directly by the innate immune system resulting in both pro- and anti-inflammatory cytokine production by macrophages. The stimulatory molecules are most abundant within the material released by larvae upon transformation. It is likely that the highly stimulatory properties of 0-3hRP and the apparent lack of equivalent components in the soluble preparations of whole larvae (i.e. 3hSSP and 18hSSP) reflect the physiological context in which the innate immune system has evolved to recognize molecules released by invading schistosomes. In this respect, molecules released by the parasite as it penetrates the skin are readily visible to innate accessory cells (5, 7), and contrast with the somatic antigens that form the dominant constituents of the soluble schiotosomula preparations (33). The molecular composition of 0-3hRP is currently being defined using a proteomic approach (Curwen and Wilson, in preparation) but it comprises a heterogenous mixture of protein constituents, probably originating from the post- and pre-acetabular glands (34), rich in proteases that aid parasite penetration of the skin (35, 36). It is unlikely to contain more than just a small number of membrane molecules (released via membrane turnover during in vitro culture, and subsequently solubilized by sonication) but may contain a quantity of glycans originating from the protective glycocalyx that is shed by the transforming larvae (37). Although 0-3hRP is a subset of the molecules contained in SCP (soluble cercariae), it is diluted by abundant somatic components that are unlikely to be good PRR ligands, thus accounting for the limited stimulatory nature of SCP. The accessibility of molecules expressed upon the surface of skin-stage larvae (i.e. transmembrane molecules) may provide another way for parasites to interact with innate accessory cells and could explain the stimulatory capacity of live 3-h larvae compared with the equivalent soluble preparation (3hSSP), in which membrane-bound material would likely be diluted by somatic constituents as previously noted.
One problem experienced by studies attempting to characterize pathogen-associated molecules with innate stimulatory properties is the potential interference by low levels of naturally occurring endotoxin (38). However, we provide definitive evidence that the stimulatory properties of 0-3hRP are due to molecules of schistosome origin and not LPS-like contaminants. For example, the greatly increased stimulatory capacity of transformed compared with non-transformed cercariae demonstrates that the material released by transforming larvae directly stimulates macrophages and is not of microbial origin since both groups of parasites were derived from the same pool and would contain equal quantities of any possible microbial contaminants. 0-3hRP also retained the majority of its stimulatory properties in the presence of endotoxin-neutralizing PMB, stimulated cytokine production by tMφ from C3H/HeJ mice that lack the functional receptor for LPS endotoxin and was heat labile. Further indirect evidence of the existence of schistosome PRR ligands is provided by our recent observation that 0-3hRP induces a novel maturation phenotype of dendritic cells compared with classic microbial products such as endotoxin and yeast glycans (39).
The initial trigger for cytokine production by accessory cells is thought to be the direct or indirect ligation of specific TLRs or other PRRs by pathogen products, resulting in translocation of transcription factors such as nuclear factor-κβ to the nucleus. Comparing responses of tMφ from C3H/HeJ mice with those of C3H/HeN mice in the presence of PMB, we show that parasite-derived factors released by schistosome larvae act partly through a TLR4-dependent pathway to induce production of a limited repertoire of cytokines. The mutation in TLR4 of C3H/HeJ mice occurs in the intracellular signalling TIR domain, allowing the receptor to potentially retain a function through its extracellular domain (24). We verified this observation using tMφ from mice with a targeted disruption of the TLR4 gene (17). The similarity of the response in C3H/HeJ and TLR4-/- tMφ versus their TLR+/+ controls suggests that the function of this receptor in the recognition of 0-3hRP is solely dependent upon events that follow signalling through the intracellular TIR domain, unlike its role in the recognition of the helminth molecule ES-62 (16). Our observations affirm the promiscuity of TLR4, adding to the growing list of ligands identified for this receptor. Indeed, a schistosome egg glycan containing the Lewis X moiety was recently shown to be a TLR4 ligand with potent immunoregulatory function (14, 40). Since glycans containing the Lewis moiety are found within material released by cercariae (41-43), they represent likely candidates for the TLR4 ligands within 0-3hRP. However, most interesting was our finding that some of the stimulatory properties (i.e. induction of IL-6) of 0-3hRP were independent of TLR4, and may explain the apparent discrepancy in the phenotypic diversity that C3H/HeJ and C3H/HeN mice display to different parameters of infection. In this respect, the TLR4 gene does not appear to have a substantial effect on protective immunity and local cytokine production by dermal exudate cells induced by vaccination with irradiated larvae (7), although it is reported to have a role in the activation of larvicidal mechanisms of peritoneal macrophages towards skin-stage schistosomula in vitro (44).
Given the complexity of the material released by transforming schistosomes, it is perhaps not surprising that there are multiple schistosome-derived ligands of PRRs within 0-3hRP. The complete dependence of the cytokine response induced by 0-3hRP upon the adapter molecule MyD88 suggests that the TLR4-independent schistosome PRR ligands signal through other TLRs. Possible candidates include TLR2 and TLR3 since a glycolipid from the adult stage and double-stranded RNA from the schistosome egg signal through these receptors, respectively (13, 15). In this respect, the heat sensitivity of 0-3hRP suggests the presence of protein-associated stimulatory compounds. However, since protease treatment (with either pepsin or trypsin) did not substantially diminish the stimulatory nature of 0-3hRP (albeit IL-6 is slightly affected), and because periodate treatment virtually abolished both IL-6 and IL-12p40 production, it would seem most likely that the major stimulatory component of 0-3hRP resides within glycan structures. Moreover, since glycan structures by themselves would not be expected to be sensitive to heat treatment, it might be predicted that the glycans are linked to a protein structure that confers stimulatory capacity on the glycan components. Alternatively, the presence of a virulence factor in 0-3hRP that acts through the MyD88-dependent IL-1R α-chain cannot be discounted. Together, our observations underline the importance of a more detailed characterization of the biochemical nature of the stimulatory components of 0-3hRP.
Many innate accessory cells including Mφ individually tailor responses to specific types of microbe (45). The signalling events that control the diversity of accessory responses are the object of intense study, and are believed to be mediated by the different TLRs (11, 46) and the adapter proteins they recruit (12). While our observations of LPS and Zymosan A support the view that TLR4 agonists preferentially induce production of IL-6 compared with TLR2 ligands (47), 0-3hRP also preferentially stimulates IL-6 production compared with the TLR2 ligand Zymosan A (Fig. 3), but in a TLR4-independent manner (Figs 2 and 4A), demonstrating that signalling via PRRs other than TLR4 can also preferentially induce IL-6 production. Furthermore, we show that diverse signalling pathways can be activated downstream of an individual TLR depending upon the ligand, since the schistosome ligands of TLR4 contributed little to IL-6 production and more to the production of IL-12p40 and IL-10, whereas high IL-6, IL-12p40 and IL-10 production resulting from stimulation with LPS was completely dependent upon TLR4. One explanation for the dichotomy in TLR4 function in our study is that the signalling pathway controlling preferential production of IL-6 is shared by multiple receptors involved in the recognition of 0-3hRP, and represents a semi-redundant system, whereas control of IL-12p40 and IL-10 production by TLR4 is non-redundant. However, we favour the hypothesis that diverse signalling pathways may result from an individual TLR through its association with different accessory PRRs and the signalling adapter molecules they recruit (12, 14). One candidate accessory PRR that may be involved is the macrophage mannose receptor, since we have preliminary data showing ligands of this receptor within 0-3hRP (unpublished results). Differential expression of PRRs by accessory cells also presents an attractive explanation for the contrasting response of tMφ to 0-3hRP compared with the limited response of dendritic cell (39).
The data presented in this study contribute greatly to our understanding of the host-parasite interactions that follow exposure to infective schistosome larvae. We clearly demonstrate the existence of schistosome-derived factors that stimulate innate accessory cells through MyD88-dependent receptors, including TLR4, to produce a repertoire of pro- and anti-inflammatory cytokines similar to that stimulated by classical microbial products. As an innate organ, the skin is the major site for immune recognition of the invading parasite, and our findings warrant further investigation into the potentially parasite-beneficial or host-protective effects exerted by the recognition of these parasite factors.
Acknowledgements
We thank the staff of the University of York Animal Unit, Ann Bamford (University of York) for maintenance of the parasite life cycle and Prof. R. Grencis and A. Bancroft for allowing the use of mice and facilities at the University of Manchester, UK. We also thank Prof. S. Akira and Y. Takeda, Osaka University, Japan, for permission to use TLR4-/- and MyD88-/- mice. This work was supported by a Wellcome Trust University Fellowship to A.P.M. (grant 056213); S.J.J. and J.P.H. were supported by PhD studentships from the Biotechnology and Biological Sciences Research Council (BBSRC). J.P.H. received additional industrial support from GlaxoSmithKline UK. S.F.-B. was funded by BBSRC grant no. BBS/B/08531.
Abbreviations
- 0-3 h
un-concentrated 0- to 3-h larval culture supernatant supernatant
- 0-3hRP
0- to 3-h released larval preparation (consisting of concentrated 0- to 3-h larval culture supernatant)
- 3hSSP
3-h soluble schistosomula preparation
- 18hSSP
18-h soluble schistosomula preparation
- PMB
polymyxin B
- PRR
pattern recognition receptor
- RPMIc
RPMI-0 control
- SCP
soluble cercarial preparation
- TIR
Toll/IL-1 receptor
- TLR
Toll-like receptor
- tMφ
thioglycollate-elicited macrophage
- WT
wild type
References
- 1.Available at http://www.who.int/tdr/dw/schisto2004.htm
- 2.McKerrow JH, Salter J. Invasion of skin by Schistosoma cercariae. Trends Parasitol. 2002;18:193. doi: 10.1016/s1471-4922(02)02309-7. [DOI] [PubMed] [Google Scholar]
- 3.Mountford AP, Trottein F. Schistosomes in the skin: a balance between immune priming and regulation. Trends Parasitol. 2004;20:221. doi: 10.1016/j.pt.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Incani RN, McLaren DJ. Histopathological and ultrastructural studies of cutaneous reactions elicited in naive and chronically infected mice by invading schistosomula of Schistosoma mansoni. Int. J. Parasitol. 1984;14:259. doi: 10.1016/0020-7519(84)90077-8. [DOI] [PubMed] [Google Scholar]
- 5.Riengrojpitak S, Anderson S, Wilson RA. Induction of immunity to Schistosoma mansoni: interaction of schistosomula with accessory leucocytes in murine skin and draining lymph nodes. Parasitology. 1998;117:301. doi: 10.1017/s0031182098003187. [DOI] [PubMed] [Google Scholar]
- 6.Hogg KG, Kumkate S, Mountford AP. IL-10 regulates early IL-12-mediated immune responses induced by the radiation-attenuated schistosome vaccine. Int. Immunol. 2003;15:1451. doi: 10.1093/intimm/dxg142. [DOI] [PubMed] [Google Scholar]
- 7.Hogg KG, Kumkate S, Anderson S, Mountford AP. Interleukin-12 p40 secretion by cutaneous CD11c+ and F4/80+ cells is a major feature of the innate immune response in mice that develop Th1-mediated protective immunity to Schistosoma mansoni. Infect. Immun. 2003;71:3563. doi: 10.1128/IAI.71.6.3563-3571.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medzhitov R, Janeway CA., Jr. Innate immunity: impact on the adaptive immune response. Curr. Opin. Immunol. 1997;9:4. doi: 10.1016/s0952-7915(97)80152-5. [DOI] [PubMed] [Google Scholar]
- 9.Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr. Opin. Immunol. 2001;13:114. doi: 10.1016/s0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- 10.Wallin RP, Lundqvist A, More SH, von Bonin A, Kiessling R, Ljunggren HG. Heat-shock proteins as activators of the innate immune system. Trends Immunol. 2002;23:130. doi: 10.1016/s1471-4906(01)02168-8. [DOI] [PubMed] [Google Scholar]
- 11.Akira S, Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4:499. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 12.Underhill DM, Ozinsky A. Toll-like receptors: key mediators of microbe detection. Curr. Opin. Immunol. 2002;14:103. doi: 10.1016/s0952-7915(01)00304-1. [DOI] [PubMed] [Google Scholar]
- 13.van der Kleij D, Latz E, Brouwers JF, et al. A novel host-parasite lipid cross-talk. Schistosomal lyso-phosphatidylserine activates Toll-like receptor 2 and affects immune polarization. J. Biol. Chem. 2002;277:48122. doi: 10.1074/jbc.M206941200. [DOI] [PubMed] [Google Scholar]
- 14.Thomas PG, Carter MR, Atochina O, et al. Maturation of dendritic cell 2 phenotype by a helminth glycan uses a Toll-like receptor 4-dependent mechanism. J. Immunol. 2003;171:5837. doi: 10.4049/jimmunol.171.11.5837. [DOI] [PubMed] [Google Scholar]
- 15.Aksoy E, Zouain CS, Vanhoutte F, et al. Double-stranded RNAs from the helminth parasite schistosoma activate TLR3 in dendritic cells. J. Biol. Chem. 2005;280:277. doi: 10.1074/jbc.M411223200. [DOI] [PubMed] [Google Scholar]
- 16.Goodridge HS, Marshall FA, Else KJ, et al. Immunomodulation via novel use of TLR4 by the filarial nematode phosphorylcholine-containing secreted product, ES-62. J. Immunol. 2005;174:284. doi: 10.4049/jimmunol.174.1.284. [DOI] [PubMed] [Google Scholar]
- 17.Hoshino K, Takeuchi O, Kawai T, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 1999;162:3749. [PubMed] [Google Scholar]
- 18.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 19.Ramalho-Pinto FJ, Gazzinelli G, Howells RE, Mota-Santos TA, Figueiredo EA, Pellegrino J. Schistosoma mansoni: defined system for stepwise transformation of cercaria to schistosomule in vitro. Exp. Parasitol. 1974;36:360. doi: 10.1016/0014-4894(74)90076-9. [DOI] [PubMed] [Google Scholar]
- 20.Harrop R, Wilson RA. Protein synthesis and release by cultured schistosomula of Schistosoma mansoni. Parasitology. 1993;107:265. doi: 10.1017/s0031182000079245. [DOI] [PubMed] [Google Scholar]
- 21.Mountford AP, Harrop R, Wilson RA. Antigens derived from lung-stage larvae of Schistosoma mansoni are efficient stimulators of proliferation and gamma interferon secretion by lymphocytes from mice vaccinated with attenuated larvae. Infect. Immun. 1995;63:1980. doi: 10.1128/iai.63.5.1980-1986.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okano M, Satoskar AR, Nishizaki K, Abe M, Harn DA., Jr. Induction of Th2 responses and IgE is largely due to carbohydrates functioning as adjuvants on Schistosoma mansoni egg antigens. J. Immunol. 1999;163:6712. [PubMed] [Google Scholar]
- 23.Iwagaki A, Porro M, Pollack M. Influence of synthetic antiendotoxin peptides on lipopolysaccharide (LPS) recognition and LPS-induced proinflammatory cytokine responses by cells expressing membrane-bound CD14. Infect. Immun. 2000;68:1655. doi: 10.1128/iai.68.3.1655-1663.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 25.Netea MG, van Deuren M, Kullberg BJ, Cavaillon JM, Van der Meer JW. Does the shape of lipid A determine the interaction of LPS with Toll-like receptors? Trends Immunol. 2002;23:135. doi: 10.1016/s1471-4906(01)02169-x. [DOI] [PubMed] [Google Scholar]
- 26.Luchi M, Morrison DC. Comparable endotoxic properties of lipopolysaccharides are manifest in diverse clinical isolates of gram-negative bacteria. Infect. Immun. 2000;68:1899. doi: 10.1128/iai.68.4.1899-1904.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Underhill DM, Ozinsky A, Hajjar AM, et al. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401:811. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- 28.Muzio M, Ni J, Feng P, Dixit VM. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science. 1997;278:1612. doi: 10.1126/science.278.5343.1612. [DOI] [PubMed] [Google Scholar]
- 29.Medzhitov R, Preston-Hurlburt P, Kopp E, et al. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol. Cell. 1998;2:253. doi: 10.1016/s1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- 30.Edwards AD, Manickasingham SP, Sporri R, et al. Microbial recognition via Toll-like receptor-dependent and -independent pathways determines the cytokine response of murine dendritic cell subsets to CD40 triggering. J. Immunol. 2002;169:3652. doi: 10.4049/jimmunol.169.7.3652. [DOI] [PubMed] [Google Scholar]
- 31.Kaisho T, Hoshino K, Iwabe T, Takeuchi O, Yasui T, Akira S. Endotoxin can induce MyD88-deficient dendritic cells to support T(h)2 cell differentiation. Int. Immunol. 2002;14:695. doi: 10.1093/intimm/dxf039. [DOI] [PubMed] [Google Scholar]
- 32.Jenkins SJ, Hewitson JP, Jenkins GR, Mountford AP. Modulation of the host’s response by schistosome larvae. Parasite Immunol. 2005 doi: 10.1111/j.1365-3024.2005.00789.x. in press.
- 33.Curwen RS, Ashton PD, Johnston DA, Wilson RA. The Schistosoma mansoni soluble proteome: a comparison across four life-cycle stages. Mol. Biochem. Parasitol. 2004;138:57. doi: 10.1016/j.molbiopara.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 34.Stirewalt MA. Schistosoma mansoni: cercaria to schistosomule. Adv. Parasitol. 1974;12:115. doi: 10.1016/s0065-308x(08)60388-7. [DOI] [PubMed] [Google Scholar]
- 35.Salter JP, Lim KC, Hansell E, Hsieh I, McKerrow JH. Schistosome invasion of the human skin and degradation of dermal elastin are mediated by a single serine protease. J. Biol. Chem. 2000;275:38667. doi: 10.1074/jbc.M006997200. [DOI] [PubMed] [Google Scholar]
- 36.Salter JP, Choe Y, Albrecht H, et al. Cercarial elastase is encoded by a functionally conserved gene family across multiple species of schistosomes. J. Biol. Chem. 2002;277:24618. doi: 10.1074/jbc.M202364200. [DOI] [PubMed] [Google Scholar]
- 37.Samuelson JC, Caulfield JP. Loss of covalently labeled glycoproteins and glycolipids from the surface of newly transformed schistosomula of Schistosoma mansoni. J. Cell Biol. 1982;94:363. doi: 10.1083/jcb.94.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akira S. Mammalian Toll-like receptors. Curr. Opin. Immunol. 2003;15:1. doi: 10.1016/s0952-7915(02)00013-4. [DOI] [PubMed] [Google Scholar]
- 39.Jenkins SJ, Mountford AP. Dendritic cells activated with products released by schistosome larvae drive Th2-type immune responses, which can be inhibited by manipulation of CD40 co-stimulation. Infect. Immun. 2005;73:395. doi: 10.1128/IAI.73.1.395-402.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okano M, Satoskar AR, Nishizaki K, Harn DA. Lacto-N-fucopentaose III found on Schistosoma mansoni egg antigens functions as adjuvant for proteins by inducing Th2-type response. J. Immunol. 2001;167:442. doi: 10.4049/jimmunol.167.1.442. [DOI] [PubMed] [Google Scholar]
- 41.Robijn ML, Wuhrer M, Kornelis D, Deelder AM, Geyer R, Hokke CH. Mapping fucosylated epitopes on glycoproteins and glycolipids of Schistosoma mansoni cercariae, adult worms and eggs. Parasitology. 2005;130:67. doi: 10.1017/s0031182004006390. [DOI] [PubMed] [Google Scholar]
- 42.Huang HH, Tsai PL, Khoo KH. Selective expression of different fucosylated epitopes on two distinct sets of Schistosoma mansoni cercarial O-glycans: identification of a novel core type and Lewis X structure. Glycobiology. 2001;11:395. doi: 10.1093/glycob/11.5.395. [DOI] [PubMed] [Google Scholar]
- 43.Wuhrer M, Dennis RD, Doenhoff MJ, Lochnit G, Geyer R. Schistosoma mansoni cercarial glycolipids are dominated by Lewis X and pseudo-Lewis Y structures. Glycobiology. 2000;10:89. doi: 10.1093/glycob/10.1.89. [DOI] [PubMed] [Google Scholar]
- 44.James SL, Skamene E, Meltzer MS. Macrophages as effector cells of protective immunity in murine schistosomiasis. V. Variation in macrophage schistosomulacidal and tumoricidal activities among mouse strains and correlation with resistance to reinfection. J. Immunol. 1983;131:948. [PubMed] [Google Scholar]
- 45.Nau GJ, Richmond JFL, Schlesinger A, Jennings EG, Lander ES, Young RA. Human macrophage activation programs induced by bacterial pathogens. Proc. Natl Acad. Sci. USA. 2002;99:1503. doi: 10.1073/pnas.022649799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Re F, Strominger JL. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J. Biol. Chem. 2001;276:37692. doi: 10.1074/jbc.M105927200. [DOI] [PubMed] [Google Scholar]
- 47.Schilling D, Thomas K, Nixdorff K, Vogel SN, Fenton MJ. Toll-like receptor 4 and Toll-IL-1 receptor domain-containing adapter protein (TIRAP)/myeloid differentiation protein 88 adapter-like (Mal) contribute to maximal IL-6 expression in macrophages. J. Immunol. 2002;169:5874. doi: 10.4049/jimmunol.169.10.5874. [DOI] [PubMed] [Google Scholar]