Abstract
β1-integrins are cell surface receptors that participate in sensing the cell’s external environment. We used the Cre-lox system to delete β1-integrin in all lens cells as the lens vesicle transitions into the lens. Adult mice lacking β1-integrin in the lens are microopthalmic due to apoptosis of the lens epithelium and neonatal disintegration of the lens fibers. The first morphological alterations in β1-integrin null lenses are seen at 16.5 dpc when the epithelium becomes disorganized and begins to upregulate the fiber cell markers β- and γ-crystallin, the transcription factors cMaf and Prox1 and down regulate Pax6 levels demonstrating that β1-integrin is essential to maintain the lens epithelial phenotype. Further, β1-integrin null lens epithelial cells upregulate the expression of α-smooth muscle actin and nuclear Smad4 and downregulate Smad6 suggesting that β1-integrin may brake TGFβ family signaling leading to epithelial-mesenchymal transitions in the lens. In contrast, β1-integrin null lens epithelial cells show increased E-cadherin immunoreactivity which supports the proposed role of β1-integrins in mediating complete EMT in response to TGFβ family members. Thus, β1-integrin is required to maintain the lens epithelial phenotype and block inappropriate activation of some aspects of the lens fiber cell differentiation program.
Introduction
The ocular lens consists of two polarized cell types, the lens epithelial cells and lens fiber cells (Piatigorsky, 1981). The lens has been characterized as an inverted epithelium since lens epithelial and fiber cells make apical-apical contacts while the basal surfaces of both cell types contact the lens capsule (Zampighi et al., 2000), a thickened basement membrane that completely surrounds the cellular lens (Parmigiani and McAvoy, 1989). It is well established that contact between the lens epithelium and the capsule is crucial for lens cell survival (Coulombre and Coulombre, 1963; Wormstone et al., 1997) and the maintenance of its epithelial phenotype (Greenburg and Hay, 1982) although the molecular mechanisms involved in this process are not well understood.
Integrins are heterodimeric extracellular matrix (ECM) receptors consisting of one α- and one β-integrin subunit. These receptors allow cells to both attach to and detect the ECM, leading to cell signaling cascades that control such diverse processes as migration, proliferation, cell survival and cellular phenotype (Hynes, 2002; Longhurst and Jennings, 1998). The β1-integrin subunit is able to form functional receptors with the largest diversity of known α-integrins leading to the ability of cells to detect the composition of diverse ECM environments (Hynes, 2002). In the lens, β1-integrin is expressed by both epithelial and fiber cells (Duncan et al., 2000; Menko and Philip, 1995; Wederell et al., 2005), and is a component of the basal fiber cell membrane complex (Bassnett et al., 1999). In cell cultures, function blocking antibodies to β1-integrin attenuate the ability of lens cells to bind to collagen and laminin suggesting that this molecule is important for lens cell-capsule communication (Nishi et al., 1997). However, β1-integrin is also found on the lateral and apical sides of lens cells in regions lacking lens capsule components (Duncan et al., 2000; Menko and Philip, 1995). In the equatorial zone, β1-integrin’s partner, α6, interacts with the IGF receptor and regulates ERK phosphorylation suggesting that integrin-growth factor receptor cross talk is important for lens morphogenesis (Walker and Menko, 1999; Walker et al., 2002).
The expression of β1-integrin is upregulated during epithelial-mesenchymal transitions occurring as a consequence of lens epithelial cell suspension in collagen I gels (Zuk and Hay, 1994) and in response to TGFβ treatment. In fact, it has been proposed that interference with β1-integrin function may be clinically useful to block epithelial-mesenchymal transition of lens cells leading to posterior capsular opacification, a common side effect of modern cataract surgery (Kim et al., 2002; Mathew et al., 2003; Palmade et al., 1994). However, the function of β1-integrin in the lens in vivo is not well understood.
Complete deletion of the β1-integrin gene from mice leads to lethality at the blastocyst stage demonstrating its importance for development (Fassler and Meyer, 1995; Stephens et al., 1995). Conditional inactivation of the β1-integrin gene in embryonic skin keratinocytes leads to defective epithelial proliferation in the absence of increased apoptosis coincident with obvious defects in basement membrane assembly (Brakebusch et al., 2000; Raghavan et al., 2000). Inactivation of the β1-integrin gene in mammary epithelial cells results in defective alveolar morphogenesis associated with a downregulation of cell proliferation, apparently caused by the upregulated expression of p21cip (Li et al., 2005). Further lobuloalveolar differentiation in response to prolactin is also defective in these glands due to defects in Stat5 activation (Naylor et al., 2005). Here we show that removal of the β1-integrin gene from the lens epithelium results in a distinctly different response consisting of the concurrent downregulation of the lens epithelial marker Pax6, upregulation of lens fiber cell transcription factors as well as α-smooth muscle actin in the embryo followed by apoptosis of these cells by birth. These data demonstrate that β1-integrin is important for the maintenance of the lens epithelial phenotype.
Materials and Methods
Animals
All animal experiments described in this article conform to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. B6:129-Itgb1tm1Efu/J mice which harbor an allele of the β1-integrin gene in which exon 3 is flanked by LoxP sites (Raghavan et al., 2000) were obtained from The Jackson Laboratory (Bar Harbor, Maine). MLR10-cre mice which express Cre recombinase in all lens cells from the lens vesicle stage onward (Zhao et al., 2004) were obtained from Michael Robinson (Miami University, Oxford Ohio). All mice were maintained and bred in specific pathogen free conditions at the University of Delaware animal facility under a 14/10 hour light/dark cycle. Embryos are staged by designating the day that the vaginal plug was observed in the dam as 0.5 days post coitum (dpc).
DNA analysis
DNA was isolated from tail biopsies and lenses using the PureGene Tissue and Mouse Tail kit (Gentra Systems, Minneapolis, MN). Mice were genotyped for the presence of the floxed β1-integrin allele by using primers 5’-CGG CTC AAA GCA GAG TGT CAG TC -3’ and 5’-CCA CAA CTT TCC CAG TTA GCT CTC -3’ as recommended by the Jackson Laboratory. Mice were genotyped for the presence of the MRL10-CRE transgene as previously described (Zhao et al., 2004). The extent of exon three deletion of the β1-integrin gene in lens cells was determined by PCR analysis of lens DNA using primers 5’- TGA ATA TGG GCT TGG CAG TTA -3’ and 5’-CCA CAA CTT TCC CAG TTA GCT CTC -3’.
Histological analysis and immunohistochemistry
Animals were euthanized, tissue excised and immersion fixed in Pen-Fix (Richard Allan Scientific, Kalamazoo Michigan) for four hours prior to paraffin embedding. Six micrometer sections were cut and stained by hematoxylin and eosin by standard methods to visualized cellular morphology. These sections were also stained with Periodic Acid-Schiff (PAS) by standard methods to visualize the lens capsule basement membrane. The expression pattern of β-crystallin in the lens was determined by incubating deparafinized sections with rabbit anti-bovine β-crystallin or rabbit anti-bovine γ-crystallin (gifts of Samuel Zigler, National Eye Institute, Bethesda MD) followed by detection with an anti- rabbit Dako Envision horseradish peroxidase kit (Dako Laboratories, Carpinteria, CA) using diaminobenzidine as a substrate.
Immunofluorescence
All fluorescent immunolocalization studies were performed as previously described (Reed et al., 2001). Briefly, tissue was excised, embedded fresh in Optimum Cutting Temperature media (OCT, Tissue Tek, Torrance California), 16 μm thick sections prepared on a cryostat and mounted on ColorFrost plus slides (Fisher Scientific, Hampton New Hampshire). Sections were immersion fixed in ice cold 1:1 acetonemethanol for 10 minutes, blocked in 1% BSA and incubated for one hour at room temperature with the appropriate dilution of primary antibody (see below). Following two, 10 minute washes, unlabeled primary antibodies were detected with the appropriate Alexafluor 568 labeled secondary antibody (Molecular Probes, Eugene Oregon) diluted in blocking buffer containing a 1:2000 dilution of the nucleic acid stain Draq-5 (Biostatus Limited, Leicestershire, United Kingdom). Slides were visualized with a Zeiss LSM 510 Confocal Microscope configured with an Argon/Krypton laser (488 nm and 568 nm excitation lines) and Helium Neon laser (633 nm excitation line) (Carl Zeiss Inc, Göttingen, Germany). All comparisons of staining intensity between specimens were done on sections stained simultaneously and the imaging for each antibody was performed using identical laser power and software settings to ensure validity of intensity comparisons.
A rat monoclonal antibody against β1 integrin (clone MB1.2, MAB1997) was obtained from Chemicon (Temecula, California) and used at a concentration of 5 μg/ml. A FITC conjugated mouse monoclonal antibody to α-smooth muscle actin (clone 1A4, F3777) was obtained from Sigma-Aldrich (St. Louis, Missouri) and used at a concentration of 10 μg/ml. Rat monoclonal antibodies to laminin (clone A5, IgG2a), were obtained from Abcam and used at a concentration of 25 μg/ml. Rabbit polyclonal antibodies to collagen IV (ab6586) were obtained from Abcam and used at a concentration of 5 μg/ml. Rabbit polyclonal antibodies against Pax6 were obtained from Covance (Berkeley California) and used at a 1:150 dilution. Rabbit polyclonal antibodies to Smad6 (90-0400) were obtained from Zymed Laboratories and used at a concentration of 10 μg/ml. Rabbit polyclonal antibodies to Smad-4 (9515) were from Cell Signaling Technologies (Danvers, Massachusetts) and used at a 1:50 dilution. Rabbit polyclonal antibodies against E-cadherin (4065) and connexin 43 (3512) were purchased from Cell Signaling Technologies and used at a 1:50 dilution. Rabbit polyclonal antibodies against cMaf (sc7866) were obtained from Santa Cruz Biotechnology and used at a concentration of 2 μg/ml. Rabbit polyclonal antibodies against aquaporin 0 (AB3071) were obtained from Chemicon and used at a concentration of 1.7 μg/ml. Rabbit polyclonal antibodies against Prox1 were as previously described (Duncan et al., 2002). Rabbit polyclonal antibodies to cleaved caspase 3 (9661) were obtained from Cell Signaling Technology and were used at a 1:50 dilution with an overnight incubation at 4 C.
TUNEL labeling
Tissue was excised, fixed for two hours at room temperature in 4% paraformaldehyde and transferred to 70% ethanol prior to paraffin embedding. Six micrometer sections were prepared and nuclear DNA fragmentation detected by TUNEL staining using the ApopTag Peroxidase in situ Apoptosis Detection kit (Chemicon) following the manufacturer’s directions. Slides were counterstained with 0.5% methyl green to visualize cell nuclei.
Proliferation Assays
Pregnant mice carrying 16.5 dpc embryos were injected intraperitoneally with 100 μg/g body weight of 5-bromo-2’-deoxyuridine (BrdU). Two hours later, the animals were sacrificed, ocular tissue embedded in OCT, and 16 μm thick sections generated on a cryostat. Sections are fixed for 15 minutes in 70% ethanol at -20 C, washed in PBS and treated for two hours with 2N hydrochloric acid at room temperature. The slides were washed twice in PBS, blocked in 1% BSA/PBS for 30 minutes at room temperature and the sections incubated with AlexaFluor 488 labeled monoclonal anti-bromodeoxyuridine (Molecular Probes) diluted to a concentration of 10 μg/ml in PBS containing a 1:1000 dilution of Draq-5 (Biostatus). The fluorescent signal was detected on a confocal microscope as above.
Results
β1 integrin is a major β-integrin subunit expressed in the chicken (Bassnett et al., 1999; Menko and Philip, 1995) and mouse (Duncan et al., 2000) lens. In order to evaluate the function of β1 integrin in the lens in vivo, we mated mice harboring a floxed β1 integrin gene (Raghavan et al., 2000) with mice (MLR10-CRE) which express CRE recombinase in all lens cells starting at 10.5 days post coitum (dpc) (Zhao et al., 2004).
β1-integrin is required for lens development
Adult mice homozygous for the floxed β1 integrin allele who also harbor the MLR10-CRE transgene were microphthalmic (Figure 1A), with little to no lens material present in the eye (data not shown). However, at birth, the eyes from these animals were of relatively normal size, although the lens was profoundly abnormal (Figure 1B). The central epithelium is missing, the lens fibers are highly vacuolated and disintegrating and the remaining equatorial epithelial cells are abnormally elongated (Figure 1B, 8A). The first abnormalities in lens structure are observed at 16.5 dpc and range from mild lens epithelial elongation (Figures 1D, 6B, 6D, 8D) to further elongation of lens epithelial cells associated with the beginning of epithelial cell loss and severe fiber cell abnormalities (Figure 1E,F). Prior to 16.5 dpc, lenses from β1flox/β1flox/CRE mice were morphologically normal (Figure 1H; data not shown) as compared to wildtype (Figure 1G).
Figure 1.
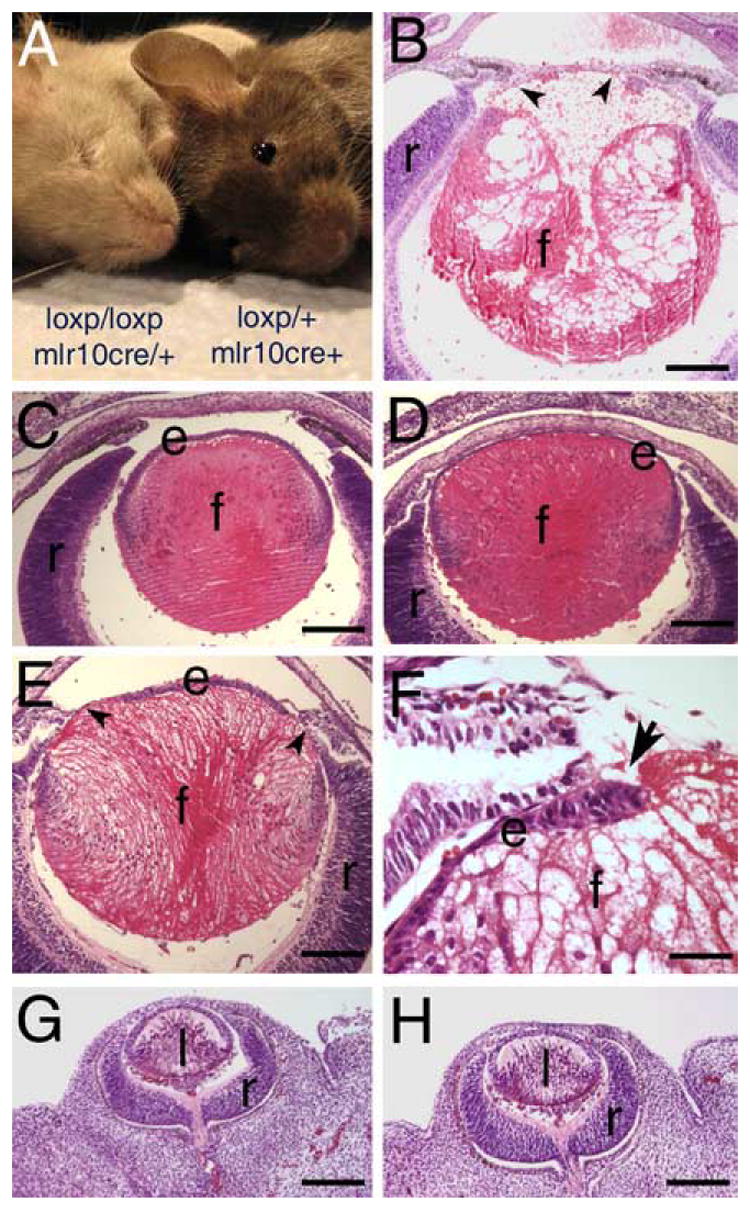
Mice homozygous for a floxed β1-integrin allele carrying MLR10-cre are microophthalmic with lens defects first apparent in late embryonic development. A) Exterior appearance of littermates who are either homozygous or heterozygous for floxed β1-integrin and carry MLR10-cre. B-H) Hematoxylin and eosin stained paraffin sections B) Newborn eye from a mouse homozygous for the floxed β1-integrin allele and carrying MLR10-cre showing loss of the anterior epithelium (arrowheads) and vacuolation of the lens fiber cells. C) Lens from a 16.5 dpc mouse homozygous for the floxed β1-integrin allele but lacking MLR10-CRE showing the normal regular organization of the lens epithelium and fiber cells. D) Eye from a 16.5 dpc mouse homozygous for the floxed β1-integrin allele and carrying MLR10-cre showing mild abnormalities in organization of the lens epithelium and fiber cells. E) Eye from 16.5 dpc mouse homozygous for the floxed β1-integrin allele and carrying MLR10-cre showing further elongation of the lens epithelial cells and regions of lens epithelial cell loss. F) High magnification image of a region of epithelial cell loss (arrow) observed in a 16.5 dpc lens. .Lens from 12.5 dpc mice either heterozygous (G) or homozygous (H) showing no abnormalities in lens morphology. Abbreviations; e, lens epithelium; f, lens fiber cells; r, retina; l- lens. Scale bars: Panels B, C, D, E, G, H-160 μm, Panel F- 40 μm
Figure 8.
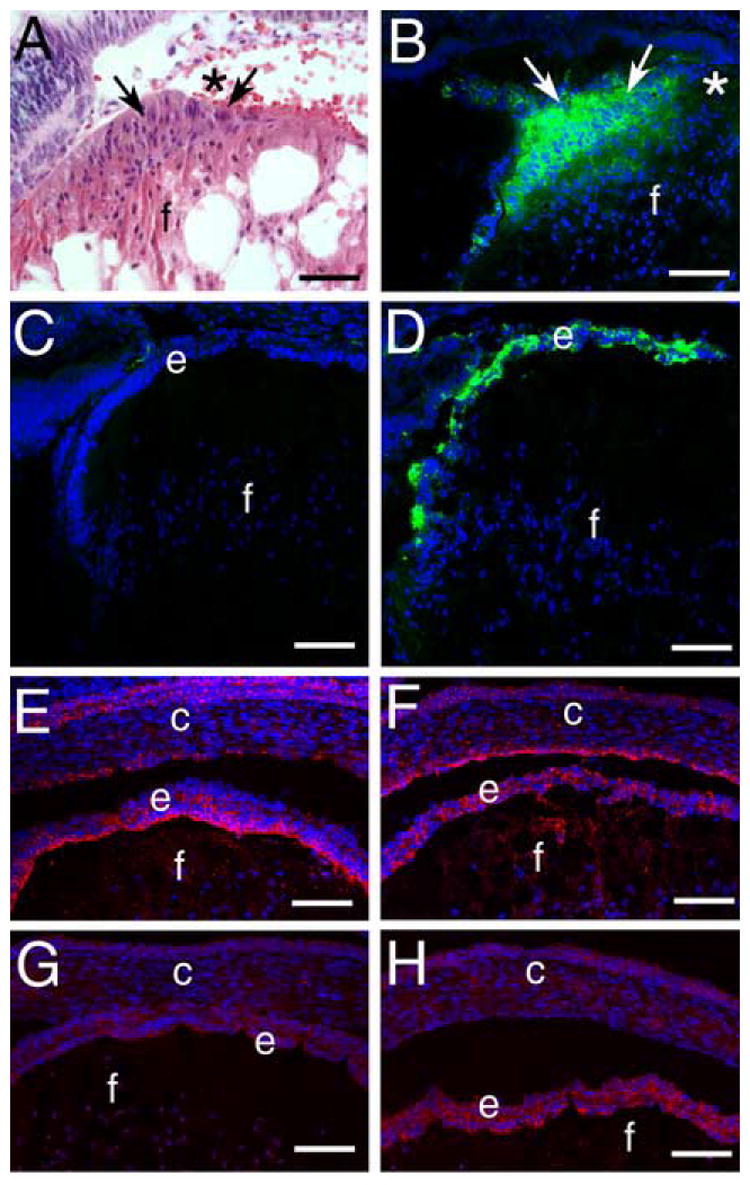
β1-integrin null lens epithelial cells demonstrate some but not all features of epithelial-mesenchymal transition (EMT). A) Hematoxylin and eosin stained paraffin section of the β1-integrin null newborn lens epithelium showing the elongation of these cells (arrows) from their normal flattened cuboidal morphology and the edge of the region of central epithelial cell loss (*). B) Immunodetection of αSMA (green) in the β1-integrin null newborn lens epithelium showing strong staining in the abnormal lens epithelial cells. C) Immunolocalization of αSMA (green) in the 16.5 dpc wildtype lens . D) Immunolocalization of αSMA (green) in the 16.5 dpc β1-integrin null lens imaged under the same conditions as panel C showing αSMA upregulation in the disorganized lens epithelium. E) Immunodetection of connexin 43 (red) in the wildtype 16.5 dpc lens demonstrating its preferred localization at the apical side of the lateral membranes of the lens epithelium. F) Immunodetection of connexin 43 (red) in the β1-integrin null 16.5 dpc lens demonstrating that its distribution is disorganized in the lens epithelium. G) Immunodetection of E-cadherin (red) in the wildtype 16.5 dpc lens demonstrating its expression in the lens epithelium. H) Immunodetection of E-cadherin (red) in the β1-integrin null 16.5 dpc lens demonstrating increased E-cadherin staining in the β1-integrin null lens epithelium. Abbreviations: c-cornea; e-lens epithelium; f-lens fiber cells scale bar: Panel A=30 μm, Panels B-H=77 μm
Figure 6.
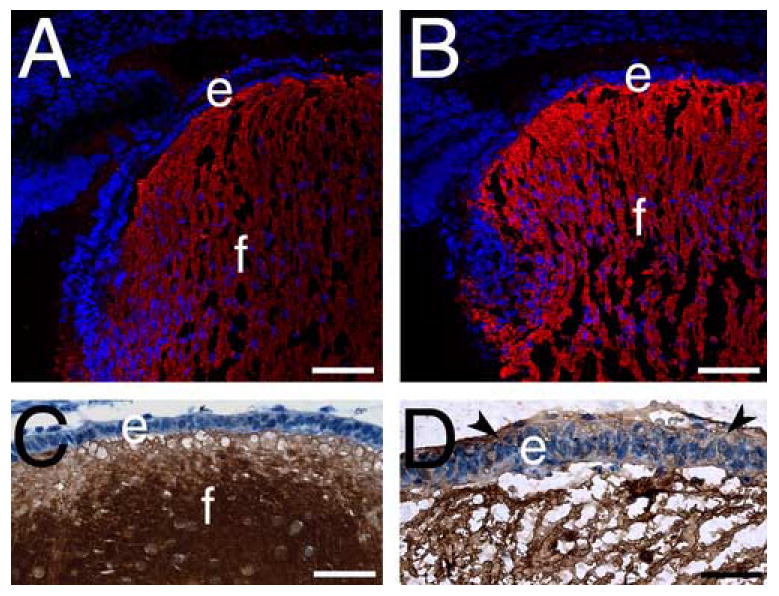
β-crystallin but not aquaporin0 expression is upregulated in 16.5 dpc β1-integrin null lens epithelial cells A,B) Aquaporin 0 (red) immunolocalization in wildtype (A) and β1-integrin (B) null lenses. C, D) β-crystallin (brown) immunolocalization in wildtype (C) and β1-integrin (D) null lenses. Abbreviations. e- lens epithelium, f- lens fibers. Scale Bar: Panels A,B= 77 μm; Panels C,D= 30 μm
In order to establish when β1-integrin protein is lost from the lens, confocal immunofluorescent analysis of β1-integrin expression was performed. At 10.5 dpc, β1-integrin immunoreactivity was detected in lens vesicles from both wildtype (Figure 2A) and β1flox/β1flox/CRE mice (Figure 2B), however, β1-integrin staining was not detected in lens cells from 11.5 dpc β1flox/β1flox/CRE mice (E,F) while β1-integrin protein was detected at both the basal and lateral cell membranes in wildtype lenses at this age (C,D). As expected, β1-integrin protein is detectable throughout the wildtype 12.5 dpc lens (G) while it is absent in both epithelial and fiber cells of the β1flox/β1flox/CRE lens (H) at this age. The efficiency of CRE mediated deletion of exon 3 of the β1-integrin gene in the lens was also assessed by PCR analysis (Figure 3A) of genomic DNA isolated from newborn lenses and found to be essentially complete (Figure 3B).
Figure 2.
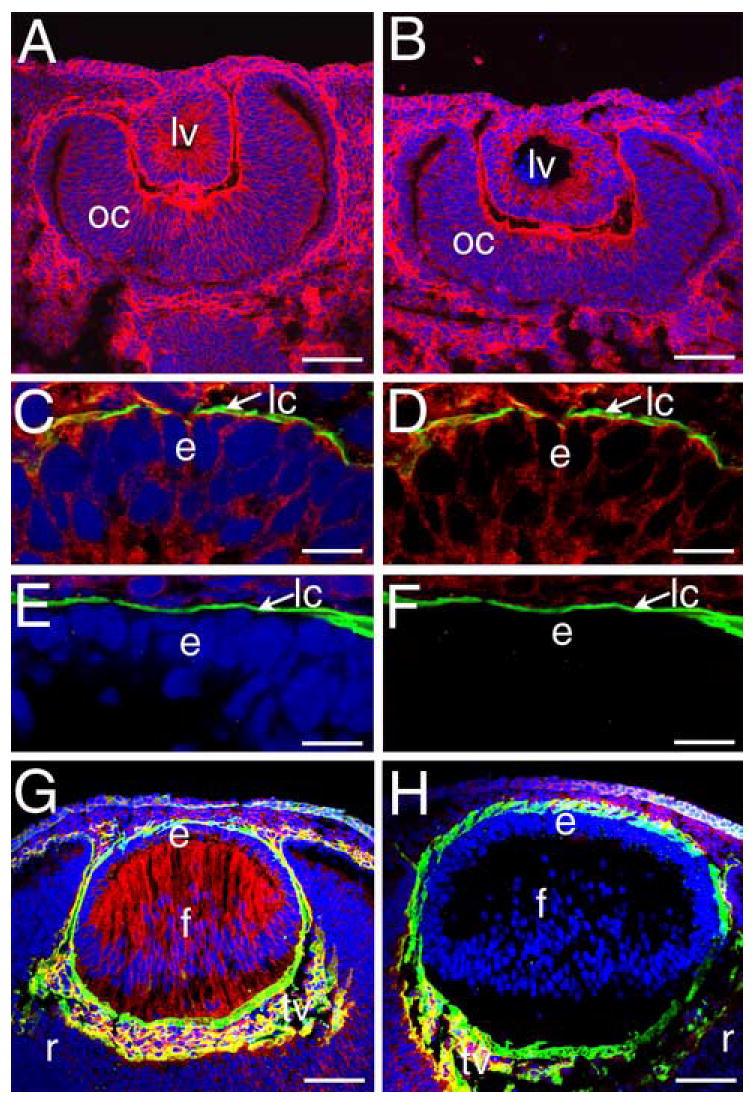
Homozygous flox β1-integrin/MLR10-cre mice lose β1 integrin protein from the lens by 11.5 dpc. A, B) Eyes from 10.5 dpc mice either heterozygous (A) or homozygous (B) for the floxed β1-integrin allele carrying MLR10-cre showing no changes in β1-integrin protein distribution (red) at the lens vesicle stage. C) The anterior lens vesicle of a 11.5 dpc wildtype mouse embryo triple labeled for β1-integrin (red), collagen IV (green) and DNA (blue). Panel D shows the same image as panel C with the DNA channel deleted. E) Anterior lens vesicle from a 11.5 dpc mouse embryo carrying the floxed β1-integrin allele and MLR10-cre triple labeled for β1-integrin (red), collagen IV (green) and DNA (blue). Panel F shows the same image as E with the DNA channel deleted. G) Eye from a wildtype 12.5 dpc mouse embryo showing the normal distribution of β1-integrin (red) in the lens at this stage. H) Eye from a 12.5 dpc mouse embryo homozygous for the floxed β1-integrin allele and carrying MLR10-cre showing the specific loss of β1-integrin protein from all lens cells. Red- β1-integrin, blue- DNA, green collagen IV; Panels A, B, G, H- Bar=77 μm; Panels C-F, Bar= 13 μm Abbreviations: lv- lens vesicle; oc- optic cup; e- lens epithelium; f- lens fibers; r- retina; lc-lens capsule; tv- tunica vasculosa.
Figure 3.
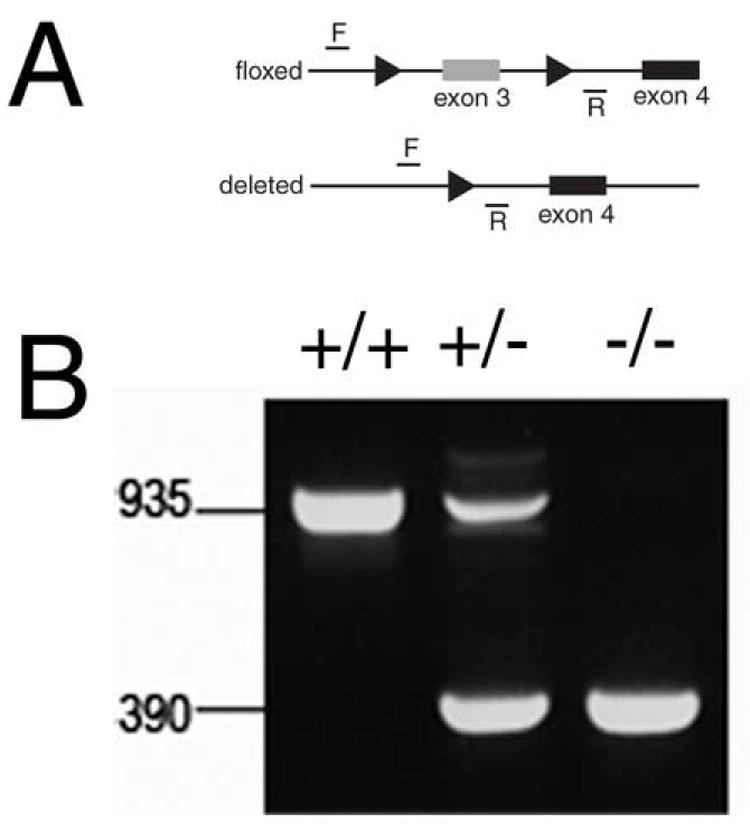
Deletion of exon 3 from the β1-integrin gene using MLR10 CRE is efficient in the lens A) Diagram of the β1-integrin locus showing the postion of the PCR primers used to score the deletion and the presence of the loxP sites (arrowheads). B) PCR analysis of DNA obtained from newborn lenses either wildtype (+/+), heterozygous (+/-) or homozygous (-/-) for the floxed β1-integrin allele demonstrating that β1-integrin deletion is essentially complete.
Loss of β1-integrin from the lens does not lead to loss of the lens capsule
The lens capsule is a thickened basement membrane synthesized by the lens cells that it encloses (Rafferty and Goossens, 1978). Since β1-integrins have been implicated in basement membrane synthesis and assembly (Li et al., 2002; Lohikangas et al., 2001), we investigated the integrity of this structure in the β1-integrin null lens. Immunostaining for collagen IV (Figure 4A,B) and laminin (Figure 4C,D), as well as periodic acid-Schiff staining (Figure 4E, F) demonstrated that the lens capsule is intact around the β1-integrin null lens (Figure 4B, D, F) and has a composition grossly similar to wildtype (Figure 4A, C, E). Notably, the anterior lens capsule is even detectable in regions that have already lost the lens epithelium (Figure 4F) suggesting that β1-integrins are not necessary for maintenance of the lens capsule.
Figure 4.
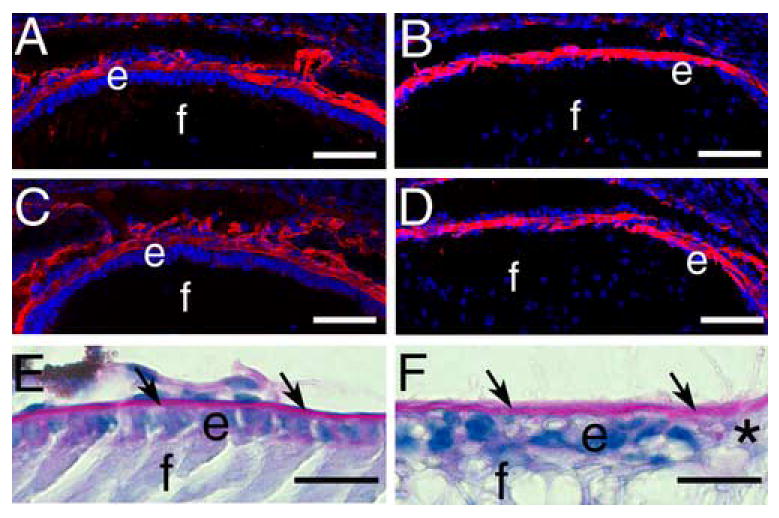
β1-integrin null lenses retain the lens capsule A, B) Immunofluorescent staining of collagen IV (red) in the wildtype (A) and β1-integrin null (B) 16.5 dpc lens capsule C, D) Immunofluorescent staining of laminin (red) in the wildtype (C) and β1-integrin null (D) 16.5 dpc lens capsule. E, F) Periodic acid-Schiff staining (pink) of the newborn lens capsule (arrows) from wildtype (E) and β1-integrin null (F)mouse lenses showing that the lens capsule is retained even in areas denuded of lens epithelial cells (*). Abbreviations; e-lens epithelium; f- lens fiber cells. Panels A-D: scale bar= 77 μm. Panels E, F: scale bar =20 μm.
β1-integrin null lens epithelial cells are lost from the lens by apoptosis
Since the epithelium is lost from β1flox/β1flox/CRE lenses, we next sought to determine whether this is a function of cell proliferation or apoptosis. BrDu incorporation assays revealed no obvious difference in the number of lens epithelial cells actively synthesizing DNA between wildtype (Figure 5A) and β1flox/β1flox/CRE (Figure 5B) lenses. In contrast, TUNEL labeling revealed a small number of β1-integrin null lens epithelial cells with fragmented DNA at 16.5 dpc (data not shown) while newborn β1flox/β1flox/CRE mice exhibited many apoptotic nuclei in the cells directly adjacent to the region of lens epithelial cell loss (Figure 5D). In contrast, few to no TUNEL positive cells are detected in the lens epithelium of wildtype mice at either age (Figure 5C, data not shown). In order to confirm that this reflects the activation of a classical apoptotic response, β1flox/β1flox/CRE lenses were stained for the cleaved form of caspase 3 responsible for apoptotic responses (Boatright and Salvesen, 2003). At 16.5 dpc, active caspase 3 was not detected in lenses lacking β1-integrin (data not shown), however, newborn lenses lacking β1-integrin exhibited appreciable cleaved caspase3 staining in both the remaining lens epithelial adjacent to the region of epithelial cell loss (Figure 5F) and fiber cells (data not shown), while no cleaved caspase 3 positive cells were found in the wildtype newborn lens (Figure 5E).
Figure 5.
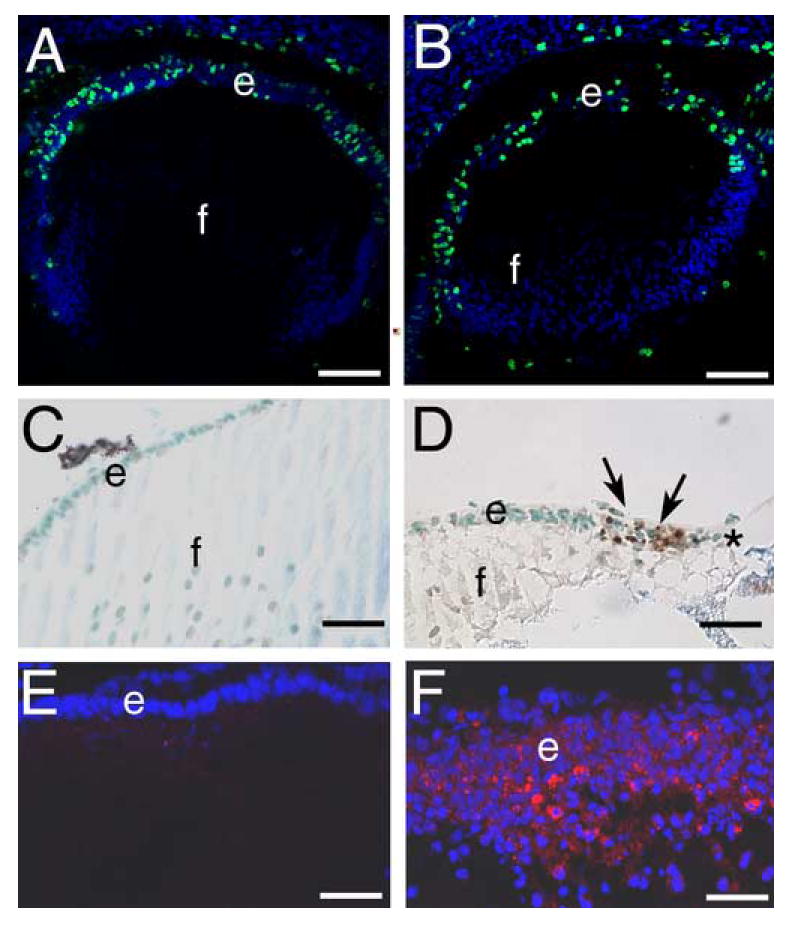
β1-integrin null lens epithelial cells are lost by birth due to elevated levels of apoptosis A, B) 16.5 dpc embryonic lenses stained for cells actively synthesizing DNA (green) using the 5-bromodeoxyuridine assay. Note that wildtype (A) and β1-integrin null (B) lens epithelia have qualitatively similar levels of cell proliferation. C, D) newborn lenses stained for apoptotic cells (brown) using TUNEL assay. Note that wildtype lenses (C) do not exhibit TUNEL positive lens epithelial cells while numerous TUNEL positive cells (arrows) are found in β1-integrin null lenses (D), especially in regions of lens epithelial cell loss (*). E, F) Newborn lens epithelial cells stained for cleaved caspase3 (Asp175). Note that wildtype lenses have little to no detectable cleaved caspase 3 (E) while numerous β1-integrin null lens epithelial cells are cleaved caspase 3 positive. Abbreviations: e- lens epithelium; f- lens fibers. Panels A,B: scale bar= 77 μm, C,D=30 μm, E,F=38 μm.
Some lens fiber cell markers are upregulated in β1-integrin null lens epithelial cells
Next, it was of interest to determine whether lenses lacking β1-integrin still maintain molecular markers associated with the lens lineage. First, we investigated the expression pattern of the lens fiber cell markers aquaporin 0/Mip (Shiels et al., 1991), β-crystallin and γ-crystallin (Chen et al., 2002). Aquaporin 0, a membrane protein important for lens function (Francis et al., 2000; Shiels et al., 1991), is normally found exclusively in lens fiber cells in the wildtype lens (Figure 6A) and this distribution was unchanged in the β1-integrin null lens (Figure 6B). In contrast, moderate amounts of ectopic β- and γ-crystallin protein were detected in β1-integrin null lens epithelial cells (Figure 6D, γ-crystallin data not shown) while these proteins were not detected in epithelial cells in wildtype lenses (Figure 6C, γ-crystallin data not shown).
Pax6 is downregulated while cMaf and Prox1 are upregulated in β1-integrin null lens epithelial cells
Pax6 is a paired/homeodomain transcription factor which is essential for early lens development (Ashery-Padan et al., 2000) and the maintenance of the lens epithelial phenotype (Collinson et al., 2001; Lovicu et al., 2004b). Further, Pax6 is a transcriptional repressor of the βB1-crystallin promoter (Duncan et al., 1998; Duncan et al., 2004) and β-crystallins are elevated in β1-integrin null lens epithelial cells (Figure 6D). Thus, we investigated Pax6 expression in the β1-integrin null lens. In the normal lens, Pax6 is found predominately in the lens epithelium, and its expression levels decrease during lens fiber cell differentiation (Figure 7A, (Duncan et al., 2000; Li et al., 1994)). In contrast, Pax6 levels are drastically decreased in the β1-integrin null lens epithelium (Figure 7B).
Figure 7.
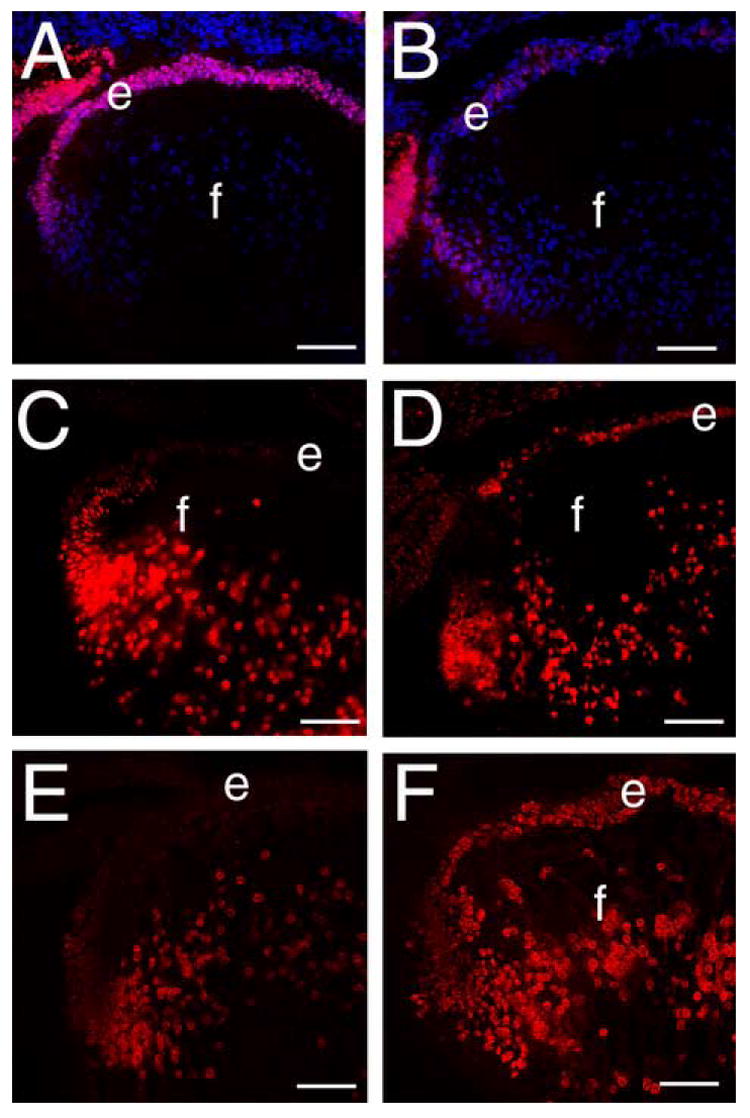
β1-integrin null lens epithelial cells show downregulated Pax6 and upregulated cMaf and Prox1 expression at 16.5 dpc A, B Pax6 (red) immunolocalization in wildtype (A) and β1-integrin (B) null lenses, C,D) cMaf (red) immunolocalization in wildtype (C) and β1-integrin (D) null lenses. E, F) Prox1 (red) immunolocalization in wildtype (E) and β1-integrin (F) null lenses. Abbreviations: e-lens epithelium; f- lens fibers. Blue in panel A,B is DNA staining All scale bars= 77 μm
We next investigated the expression pattern of two transcription factors required for lens fiber cell differentiation, cMaf (Kawauchi et al., 1999) and Prox1 (Wigle et al., 1999), in the β1-integrin null lens. In the wildtype lens, cMaf (Figure 7C) and Prox1 (Figure 7E) are both found at relatively low levels in lens epithelial cells while their levels upregulate at the transition zone and are maintained at high levels in lens fiber cells. In contrast, intense cMaf (Figure 7D) and Prox1 (Figure 7E) immunostaining are detected in the β1-integrin null lens epithelium suggesting that they are responsible for the inappropriate activation of β-crystallin expression (Cui et al., 2004) in β1-integrin null lens epithelial cells.
Loss of β1-integrin from the lens results in upregulation of α-smooth muscle actin (αSMA) and E-cadherin expression
Since the inappropriate lens epithelial cell elongation (Figure 8A), upregulation of some fiber cell markers, followed by apoptosis was similar to that reported previously in transgenic mice overexpressing autoactivating TGFβ in lens fiber cells (Flugel-Koch et al., 2002; Lovicu et al., 2004b), we then investigated whether α-smooth muscle actin (αSMA), the most widely used marker of lens epithelial-mesenchymal transition (de Iongh et al., 2005), was upregulated in the β1-integrin null lens. In the wildtype newborn lens, αSMA was not detected under the conditions used (data not shown). In contrast, intense αSMA immunoreactivity was detected in the remaining lens epithelial cells of lenses lacking β1-integrin (Figure 8B). The elevated expression of αSMA is also detected throughout the lens epithelium at 16.5 dpc (Figure 8D) while αSMA protein was not detected in the wildtype lens under these conditions (Figure 8C). Elevations in αSMA protein levels were not detected in the lens at earlier developmental times (data not shown).
At 16.5 dpc, lens epithelial cells normally express the gap junction protein connexin 43, and this protein is found to be concentrated at the lateral membranes close to the apical end of the cells (Figure 8E, (Gao and Spray, 1998)). However, this distribution is lost from lens epithelial cells lacking β1-integrin and instead connexin 43 is found evenly distributed along the cell membrane (Figure 8F). In contrast, E-cadherin staining is more intense in 16.5 dpc lens epithelial cells lacking β1-integrin (Figure 8H) as compared to the wildtype lens (Figure 8G).
Smad6 protein is lost from the β1-integrin null lens and nuclear Smad4 levels increase
In the adult lens, TGFβ signaling is recognized as a major potentiator of epithelial-mesenchymal transition (de Iongh et al., 2005). The canonical TGFβ signaling pathway is mediated via R-Smad interaction with SMAD4 and translocation of this complex into the nucleus (ten Dijke and Hill, 2004). In other tissues, this pathway has been found to be antagonized by inhibitory SMADs which both block R-Smad activation by TGFβ receptors and target R-Smads for degradation (Park, 2005). In the wildtype 16.5 dpc lens, the inhibitory Smad, Smad 6, is found throughout the lens epithelium (Figure 9A) while only low levels of nuclear Smad4 (Figure 9E) are detected. In contrast, β1-integrin null lenses have nearly undetectable levels of Smad 6 in the lens epithelium (Figure 9C) while nuclear Smad4 levels are elevated (Figure 9F). These data indicate β1-integrin signaling is important to prevent inappropriate activation of Smad signaling pathways in the lens epithelium.
Figure 9.
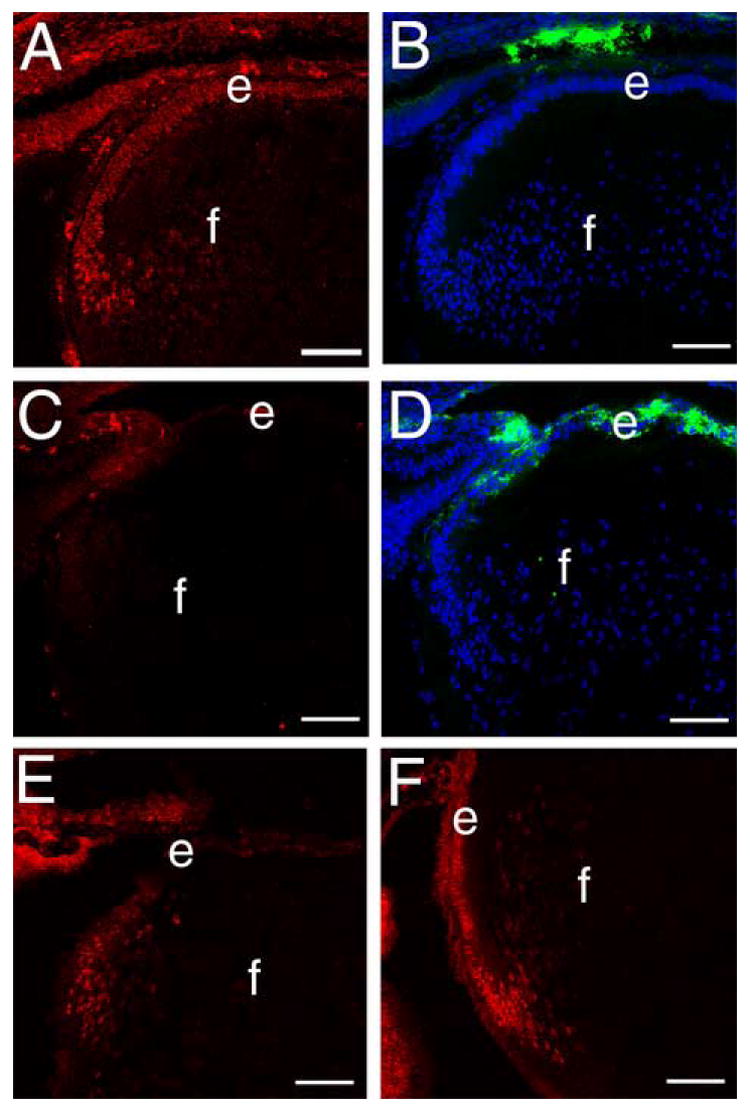
Smad6 expression is downregulated and Smad4 levels are upregulated in β1-integrin null lens epithelial cells A) Distribution of Smad6 (red) in the wildtype 16.5 dpc lens. B) the same section shown in A stained for DNA (blue) and αSMA (green). C) Distribution of Smad6 (red) in the β1-integrin null 16.5 dpc lens showing the downregulation of Smad6 levels in the lens epithelium D) the same section shown in C stained for DNA (blue) and αSMA (green) showing the upregulation of αSMA levels in a lens with reduced Smad6 levels. E) the distribution of Smad4 (red) in the wildtype 16.5 dpc lens demonstrating that this protein is predominately located in the nuclei of lens cells undergoing the transition between epithelial and fiber cells. F) The distribution of Smad4 (red) in the β1-integrin null 16.5 dpc lens showing that nuclear Smad4 levels are levated in β1-integrin null lens epithelial cells. Abbreviations: e-lens epithelium; f- lens fibers. All scale bars =77 μm
Discussion
This report investigates the phenotypic and molecular consequences of deleting exon 3 of the β1-integrin gene from the embryonic lens. Recombination of a floxed β1-integrin allele mediated by MLR10-Cre resulted in the loss of β1-integrin protein from the lens by 11.5 dpc coincident with primary lens fiber cell elongation. The first morphological consequences of this deletion are alterations in lens epithelial cell shape and fiber cell organization at 16.5 dpc associated with the downregulation of Pax6 expression, upregulation of fiber cell preferred transcription factors, a redistribution of connexin 43 protein and increased E-cadherin and α-smooth muscle actin (SMA) staining. By birth, the central epithelium is absent due to increased amounts of apoptosis as measured by both TUNEL and cleaved caspase 3 staining. Investigation of pathways which could be controlling the increased amounts of SMA revealed that β1-integrin null lens epithelial cells have increased amounts of nuclear SMAD4 and reduced amounts of SMAD6. In aggregate, these results lead us to propose that β1-integrin cross talks with pathways controlling fiber cell differentiation, epithelial-mesenchymal transition and cell survival in the lens.
Late embryonic lens development is profoundly abnormal in the β1-integrin null lens
In the lens, β1-integrins have been implicated in the attachment of lens cells to the capsule (Bassnett et al., 1999; Nishi et al., 1997), and have been proposed to be essential for lens differentiation (Menko et al., 1998). Here we show that loss of β1-integrin protein from the lens vesicle coincident with primary fiber cell elongation does not result in obvious morphological defects in the embryonic-early fetal lens. As morphogenesis proceeds through fetal development into the postnatal period in mice, the expression profile of cellular receptors (Srinivasan et al., 1998), transcription factors (Min et al., 2004) and structural proteins (Carper et al., 1986; Kelley et al., 2002; Sinha et al., 2001) changes dramatically in the lens. Similarly, as lenses lacking β1-integrin protein enter late fetal development, the first morphological alterations are observed. At 16.5 dpc, the regular cuboidal organization of the lens epithelium is lost and by birth, most of the lens epithelium is absent and the remaining epithelial cells are elongated demonstrating an important role for β1-integrin in late fetal lens epithelial cells. Further defects are also detected in the lens fiber cells including cell disorganization, vacuole formation and finally postnatal liquefaction of the remaining lens material. While this phenotype may suggest that β1-integrin is also important for lens fiber cell function, it is also possible that these phenotypes are a secondary response to the loss of the lens epithelium since it is known that fiber cells communicate extensively with the lens epithelium in vivo (Bassnett et al., 1994; Rae et al., 1996). Definitive determination of β1-integrin function in lens fiber cells awaits the analysis of specific deletion of β1-integrin in lens fiber cells, leaving lens epithelial expression intact. It also should be noted that our data does not exclude the possibility that β1-integrin is essential for the early morphogenesis of the lens since β1-integrin expression is intact in the lens of homozygous flox β1-integrin/MLR10-cre mice during lens placode formation and lens vesicle closure. The role of β1-integrin in the lens placode and pit can be explored using a CRE strain active during early lens morphogenesis such as LE-CRE (Ashery-Padan et al., 2000).
Lens epithelial cells from β1-integrin null mice exhibit some but not all features of epithelial-mesenchyme transition and lens fiber cell differentiation
The morphology of lens epithelial cells lacking β1-integrin is reminiscent of the disruption of epithelial organization associated with epithelial-mesenchymal transition of these cells following injury (Saika et al., 2004) and/or exposure to inappropriate extracellular signals (de Iongh et al., 2005). Expression studies of the most common diagnostic marker for lens EMT (Marcantonio and Vrensen, 1999), α-smooth muscle actin, revealed that β1-integrin null lens epithelial cells upregulate the expression of this protein at 16.5dpc as the normal apical-lateral distribution of connexin43 (Gao and Spray, 1998) is lost, consistent with the proposition that these cells are undergoing epithelial-mesenchymal transition (de Iongh et al., 2005). Further, these lens epithelial cells inappropriately downregulate Pax6 expression, which has been proposed to be necessary for EMT in the lens epithelium (Lovicu et al., 2004b). However, the β1-integrin null lens does not have all molecular features of EMT since neither rt-PCR nor immunostaining detected increases in collagen I or fibronectin expression (data not shown) and β1-integrin null epithelial lens cells exhibited elevated, not reduced staining of the epithelial marker E-cadherin. Interestingly, it was recently reported that β1-integrin clustering upon matrix binding decreases E-cadherin levels by activating MMP-9 and inducing the shedding of the E-cadherin ectodomain in ovarian cancer cells (Symowicz et al., 2007) which suggests that the enhanced E-cadherin staining observed in β1-integrin null lenses could result from reduced ectodomain shedding. However, it is still possible that the levels of intact E-cadherin are not being modulated in the β1-integrin null lens and instead the increased staining observed reflects a change in E-cadherin conformation resulting from the loss of β1-integrin.
These observations are consistent with two competing functions of β1-integrin in the lens. The upregulation of αSMA expression and the redistribution of connexin 43 in β1-integrin null lenses suggests that β1-integrin signaling serves as a block to these EMT responses under basal conditions in the lens epithelium. In contrast, the expression of several β1-binding α-integrins are upregulated in lens cells undergoing EMT (Barbour et al., 2004; Zuk and Hay, 1994) and EMT responses are blocked in lens cells treated with β1-integrin function blocking antibodies (Zuk and Hay, 1994) leading to the idea that integrins are necessary for a complete EMT response (de Iongh et al., 2005). This is consistent with the report that transfection of lens cells with active ILK, a mediator of β1-integrin signaling, leads to downregulation of E-cadherin (de Iongh et al., 2005), while E- cadherin levels appear enhanced in β1-integrin deficient lens epithelial cells. It is also possible that a portion of the phenotype observed results from an excess of α-integrin subunits that either can not find a β-integrin partner or which form inappropriate heterodimers due to changes in the ratio of α- to β-integrin subunits.
Loss of the lens epithelium from β1-integrin null lenses results from elevated levels of apoptosis, not decreased amounts of proliferation
Cell adhesion to extracellular matrices via β1-integrins has long been proposed to protect cells from apoptosis/anoikis by signaling to cell survival pathways (Frisch and Ruoslahti, 1997). However, conditional deletion of the β1-integrin gene from either skin keratinocytes , hair follicles (Brakebusch et al., 2000; Raghavan et al., 2000), or luminal mammary epithelial cells (Li et al., 2005; Naylor et al., 2005) does not result in increased apoptosis in vivo even following cell detachment from the underlying basement membrane. Instead, these cells have profound reductions in cell proliferation rates. In contrast, relatively normal amounts of cell proliferation are detected in β1-integrin null lens epithelial cells in vivo, instead, the loss of the lens epithelium at birth is mediated by increased amounts of apoptosis. Notably though, this increased apoptosis does not appear to be associated with classical anoikis since histological analyses found that β1-integrin null lens epithelial cells remain attached to the lens capsule.
In the lens, EMT associated with injury (Saika et al., 2002), anterior subcapsular cataract (Font and Brownstein, 1974) or TGFβ treatment (Maruno et al., 2002) are associated with significant levels of apoptosis which has been compared to the myofibroblast apoptosis that occurs as the granulation region of a healed epithelial wound resolves into a scar (Maruno et al., 2002). Since EMT markers are induced in the lens as a result of β1-integrin deletion prior to the onset of apoptosis, it is likely that the observed epithelial apoptosis is a secondary event related to the induction of EMT pathways in the lens instead of being a direct effect of β1-integrin loss.
β1-integrin null lens epithelial cells exhibit altered Smad pathway components
Adult lenses overexpressing an autoactivating form TGFβ1 in fiber cells under the control of a weak promoter develop anterior subcapsular cataracts associated with upregulation of α-SMA, β-crystallin and collagen I protein levels, downregulation of E-cadherin and connexin43 expression and increased cell proliferation (de Iongh et al., 2005; Lovicu et al., 2004a; Srinivasan et al., 1998). In contrast, lenses from mice expressing higher levels of autoactivating TGFβ1 begin expressing α-smooth muscle actin in the lens epithelium by 16.5 dpc and lose the lens epithelium by birth due to apoptosis (Flugel-Koch et al., 2002). This difference in response is probably attributable to the relative insensitivity of the fetal mouse lens to TGFβ due to very low levels of TGFβ receptor expression as compared to the adult lens (Srinivasan et al., 1998). Notably, the morphology and dynamics of α-SMA expression in the β1-integrin null newborn lens is very similar to lenses expressing high levels of TGFβ leading to the hypothesis that β1-integrin deletion removes a block to TGFβ family member induced EMT responses in the embryonic lens.
Binding of TGFβ family members to their receptors leads to the phosphorylation of a receptor regulated Smad (R-Smad) leading to its dimerization with the signaling mediator, Smad4 and the translocation of this complex to the nucleus (ten Dijke and Hill, 2004). The activated Smad complex then interacts with other transcription factors which either results in the activation of genes involved in the EMT response such as α-smooth muscle actin (Hu et al., 2003) or the degradation of transcriptional repressors (Moustakas et al., 2001). This pathway can be negatively regulated by inhibitory Smads (Smad6 or 7) which can block R-Smad phosphorylation and lead to ubiquitin regulated degradation of both activated TGFβ receptors and R-Smads (Park, 2005). Our current data suggest that inappropriate activation of the Smad pathway is a likely explanation for the induction of SMA expression in the β1-integrin null lens since we detected increased amounts of nuclear Smad4 in β1-integrin null lens epithelial cells. While increased activation of TGFβ family member receptors is a possible explanation for this observation, our current data suggests that increased levels of nuclear Smad4 are a result of decreased amounts of Smad6 in the β1-integrin null lens. This hypothesis is consistent with prior reports of downregulated I-Smad expression in various fibrotic diseases (Dong et al., 2002; Yu et al., 2006).
Overall though, the function of β1-integrins in the lens is likely to be complex. Integrins serve as both structural linkers between the extracellular matrix and the cytoskeleton and cell signaling molecules that regulate cell behavior and identity (Belkin and Stepp, 2000; Heino, 2000; Wiesner et al., 2005). Notably, it was recently reported that short term disassembly of actin stress fibers in the lens epithelium was sufficient to induce lens fiber cell differentiation but sustained stress fiber disassembly results in lens epithelial cell apoptosis (Weber and Menko, 2006) suggesting a potential mechanism regulating the phenotypes in the β1-integrin null lens.. Alternatively, the loss of β1-integrin is expected to result in the alteration of many cell signaling cascades including those utilizing FAK, Rho GTPases, ERK, and ILK (Cohen and Guan, 2005; Grashoff et al., 2004; Longhurst and Jennings, 1998). Further experiments are necessary to determine the precise signaling cascades that β1-integrins control in the lens.
Conclusions
Our data demonstrate the β1-integrin is essential for the maintenance of the lens epithelium, preventing the expression of both lens fiber cell and myofibroblast markers and ensuring lens epithelial cell survival. Since prior work has shown that β1-integrin expression is upregulated by TGFβ in the lens and β1-integrin function is required for lens EMT in vitro, our data fits with proposed pathways in which increased β1-integrin engagement during EMT results in the loss of E-cadherin expression. However, the response of the lens to β1-integrin deletion is novel compared to the consequences of β1-integrin deletion in other epithelial tissues suggesting that the lens uses β1-integrin activated pathways in different ways than the skin or mammary epithelium.
Acknowledgments
Funding: National Eye Institute grant EY015279 to MKD and INBRE program grant P20 RR16472 supporting the University of Delaware Core Imaging facility.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashery-Padan R, Marquardt T, Zhou X, Gruss P. Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev. 2000;14:2701–11. doi: 10.1101/gad.184000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour W, Saika S, Miyamoto T, Ohkawa K, Utsunomiya H, Ohnishi Y. Expression patterns of beta1-related alpha integrin subunits in murine lens during embryonic development and wound healing. Curr Eye Res. 2004;29:1–10. doi: 10.1080/02713680490513137. [DOI] [PubMed] [Google Scholar]
- Bassnett S, Kuszak JR, Reinisch L, Brown HG, Beebe DC. Intercellular communication between epithelial and fiber cells of the eye lens. J Cell Sci. 1994;107(Pt 4):799–811. doi: 10.1242/jcs.107.4.799. [DOI] [PubMed] [Google Scholar]
- Bassnett S, Missey H, Vucemilo I. Molecular architecture of the lens fiber cell basal membrane complex. J Cell Sci. 1999;112:2155–2165. doi: 10.1242/jcs.112.13.2155. [DOI] [PubMed] [Google Scholar]
- Belkin AM, Stepp MA. Integrins as receptors for laminins. Microsc Res Tech. 2000;51:280–301. doi: 10.1002/1097-0029(20001101)51:3<280::AID-JEMT7>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Boatright KM, Salvesen GS. Mechanisms of caspase activation. Curr Opin Cell Biol. 2003;15:725–31. doi: 10.1016/j.ceb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Brakebusch C, Grose R, Quondamatteo F, Ramirez A, Jorcano JL, Pirro A, Svensson M, Herken R, Sasaki T, Timpl R, Werner S, Fassler R. Skin and hair follicle integrity is crucially dependent on beta 1 integrin expression on keratinocytes. Embo J. 2000;19:3990–4003. doi: 10.1093/emboj/19.15.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carper D, Smith-Gill SJ, Kinoshita JH. Immunocytochemical localization of the 27Kbeta-crystallin polypeptide in the mouse lens duringdevelopment using a specific monoclonal antibody:implications for cataract formation in the Philly mouse. Dev Biol. 1986;113:104–109. doi: 10.1016/0012-1606(86)90112-0. [DOI] [PubMed] [Google Scholar]
- Chen Q, Ash JD, Branton P, Fromm L, Overbeek PA. Inhibition of crystallin expression and induction of apoptosis by lens-specific E1A expression in transgenic mice. Oncogene. 2002;21:1028–37. doi: 10.1038/sj.onc.1205050. [DOI] [PubMed] [Google Scholar]
- Cohen LA, Guan JL. Mechanisms of focal adhesion kinase regulation. Curr Cancer Drug Targets. 2005;5:629–43. doi: 10.2174/156800905774932798. [DOI] [PubMed] [Google Scholar]
- Collinson JM, Quinn JC, Buchanan MA, Kaufman MH, Wedden SE, West JD, Hill RE. Primary defects in the lens underlie complex anterior segment abnormalities of the Pax6 heterozygous eye. Proc Natl Acad Sci U S A. 2001;98:9688–93. doi: 10.1073/pnas.161144098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombre J, Coulombre A. Lens development: fiber elongation and lens orientation. Science. 1963;142:1489–1494. doi: 10.1126/science.142.3598.1489. [DOI] [PubMed] [Google Scholar]
- Cui W, Tomarev SI, Piatigorsky J, Chepelinsky AB, Duncan MK. Mafs, Prox1 and Pax6 can regulate chicken beta B1-cystallin gene expression. J Biol Chem. 2004 doi: 10.1074/jbc.M312414200. [DOI] [PubMed] [Google Scholar]
- de Iongh RU, Wederell E, Lovicu FJ, McAvoy JW. Transforming growth factor-beta-induced epithelial-mesenchymal transition in the lens: a model for cataract formation. Cells Tissues Organs. 2005;179:43–55. doi: 10.1159/000084508. [DOI] [PubMed] [Google Scholar]
- Dong C, Zhu S, Wang T, Yoon W, Li Z, Alvarez RJ, ten Dijke P, White B, Wigley FM, Goldschmidt-Clermont PJ. Deficient Smad7 expression: a putative molecular defect in scleroderma. Proc Natl Acad Sci U S A. 2002;99:3908–13. doi: 10.1073/pnas.062010399. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Duncan MK, Cui W, Oh D-J, Tomarev SI. Prox1 is differentially localized during lens development. Mech Dev. 2002;112:195–198. doi: 10.1016/s0925-4773(01)00645-1. [DOI] [PubMed] [Google Scholar]
- Duncan MK, Haynes JI, II, Cvekl A, Piatigorsky J. Dual roles for Pax-6: a transcriptional repressor of lens fiber-cell specific β-crystallin genes. Mol Cell Biol. 1998;18:5579–5586. doi: 10.1128/mcb.18.9.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan MK, Kozmik Z, Cveklova K, Piatigorsky J, Cvekl A. Overexpression of Pax-6 (5a) in lens fiber cells results in cataract and upregulation of α5β1 integrin expression. J Cell Sci. 2000;113:3173–3185. doi: 10.1242/jcs.113.18.3173. [DOI] [PubMed] [Google Scholar]
- Duncan MK, Xie L, David LL, Robinson ML, Taube JR, Cui W, Reneker LW. Ectopic Pax6 expression disturbs lens fiber cell differentiation. Invest Ophthalmol Vis Sci. 2004;45:3589–98. doi: 10.1167/iovs.04-0151. [DOI] [PubMed] [Google Scholar]
- Fassler R, Meyer M. Consequences of lack of beta 1 integrin gene expression in mice. Genes Dev. 1995;9:1896–908. doi: 10.1101/gad.9.15.1896. [DOI] [PubMed] [Google Scholar]
- Flugel-Koch C, Ohlmann A, Piatigorsky J, Tamm ER. Disruption of anterior segment development by TGF-beta1 overexpression in the eyes of transgenic mice. Dev Dyn. 2002;225:111–25. doi: 10.1002/dvdy.10144. [DOI] [PubMed] [Google Scholar]
- Font RL, Brownstein S. A light and electron microscopic study of anterior subcapsular cataracts. Am J Ophthalmol. 1974;78:972–84. doi: 10.1016/0002-9394(74)90811-3. [DOI] [PubMed] [Google Scholar]
- Francis P, Chung JJ, Yasui M, Berry V, Moore A, Wyatt MK, Wistow G, Bhattacharya SS, Agre P. Functional impairment of lens aquaporin in two families with dominantly inherited cataracts. Hum Mol Genet. 2000;9:2329–34. doi: 10.1093/oxfordjournals.hmg.a018925. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol. 1997;9:701–6. doi: 10.1016/s0955-0674(97)80124-x. [DOI] [PubMed] [Google Scholar]
- Gao Y, Spray DC. Structural changes in lenses of mice lacking the gap junction protein connexin43. Invest Ophthalmol Vis Sci. 1998;39:1198–209. [PubMed] [Google Scholar]
- Garcia CM, Kwon GP, Beebe DC. alpha-Smooth muscle actin is constitutively expressed in the lens epithelial cells of several species. Exp Eye Res. 2006;83:999–1001. doi: 10.1016/j.exer.2006.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grashoff C, Thievessen I, Lorenz K, Ussar S, Fassler R. Integrin-linked kinase: integrin’s mysterious partner. Curr Opin Cell Biol. 2004;16:565–71. doi: 10.1016/j.ceb.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Greenburg G, Hay ED. Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. J Cell Biol. 1982;95:333–9. doi: 10.1083/jcb.95.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heino J. The collagen receptor integrins have distinct ligand recognition and signaling functions. Matrix Biol. 2000;19:319–23. doi: 10.1016/s0945-053x(00)00076-7. [DOI] [PubMed] [Google Scholar]
- Hu B, Wu Z, Phan SH. Smad3 mediates transforming growth factor-beta-induced alpha-smooth muscle actin expression. Am J Respir Cell Mol Biol. 2003;29:397–404. doi: 10.1165/rcmb.2003-0063OC. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Kawauchi S, Takahashi S, Nakajima O, Ogino H, Morita M, Nishizawa M, Yasuda K, Yamamoto M. Regulation of lens fiber cell differentiation by transcription factor c-Maf. J Biol Chem. 1999;274:19254–60. doi: 10.1074/jbc.274.27.19254. [DOI] [PubMed] [Google Scholar]
- Kelley PB, Sado Y, Duncan MK. Expression of Collagen IV subtypes in the developing lens capsule. Matrix Biol. 2002;21:415–423. doi: 10.1016/s0945-053x(02)00014-8. [DOI] [PubMed] [Google Scholar]
- Kim JT, Lee do H, Chung KH, Kang IC, Kim DS, Joo CK. Inhibitory effects of salmosin, a disintegrin, on posterior capsular opacification in vitro and in vivo. Exp Eye Res. 2002;74:585–94. doi: 10.1006/exer.2001.1150. [DOI] [PubMed] [Google Scholar]
- Li H-S, Yang J-M, Jacobson RD, Pasko D, Sundin O. Pax-6 is first expressed in a region of ectoderm anterior to the early neural plate: implications for stepwise determination of the lens. Dev Biol. 1994;162:181–194. doi: 10.1006/dbio.1994.1077. [DOI] [PubMed] [Google Scholar]
- Li N, Zhang Y, Naylor MJ, Schatzmann F, Maurer F, Wintermantel T, Schuetz G, Mueller U, Streuli CH, Hynes NE. Beta1 integrins regulate mammary gland proliferation and maintain the integrity of mammary alveoli. Embo J. 2005;24:1942–53. doi: 10.1038/sj.emboj.7600674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Harrison D, Carbonetto S, Fassler R, Smyth N, Edgar D, Yurchenco PD. Matrix assembly, regulation, and survival functions of laminin and its receptors in embryonic stem cell differentiation. J Cell Biol. 2002;157:1279–90. doi: 10.1083/jcb.200203073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohikangas L, Gullberg D, Johansson S. Assembly of laminin polymers is dependent on beta1-integrins. Exp Cell Res. 2001;265:135–44. doi: 10.1006/excr.2001.5170. [DOI] [PubMed] [Google Scholar]
- Longhurst CM, Jennings LK. Integrin-mediated signal transduction. Cell Mol Life Sci. 1998;54:514–26. doi: 10.1007/s000180050180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovicu FJ, Ang S, Chorazyczewska M, McAvoy JW. Deregulation of lens epithelial cell proliferation and differentiation during the development of TGFbeta-induced anterior subcapsular cataract. Dev Neurosci. 2004a;26:446–55. doi: 10.1159/000082286. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, Steven P, Saika S, McAvoy JW. Aberrant lens fiber differentiation in anterior subcapsular cataract formation: a process dependent on reduced levels of Pax6. Invest Ophthalmol Vis Sci. 2004b;45:1946–53. doi: 10.1167/iovs.03-1206. [DOI] [PubMed] [Google Scholar]
- Marcantonio JM, Vrensen GF. Cell biology of posterior capsular opacification. Eye. 1999;13(Pt 3b):484–8. doi: 10.1038/eye.1999.126. [DOI] [PubMed] [Google Scholar]
- Maruno KA, Lovicu FJ, Chamberlain CG, McAvoy JW. Apoptosis is a feature of TGF beta-induced cataract. Clin Exp Optom. 2002;85:76–82. doi: 10.1111/j.1444-0938.2002.tb03012.x. [DOI] [PubMed] [Google Scholar]
- Mathew MR, McLean SM, Murray SB, Bennett HG, Webb LA, Esakowitz L. Expression of CD18, CD49b, CD49c and CD49e on lens anterior capsules in human cataracts. Eye. 2003;17:473–7. doi: 10.1038/sj.eye.6700380. [DOI] [PubMed] [Google Scholar]
- Menko AS, Philip NJ. Beta 1 integrins in epithelial tissues: a unique distribution in the lens. Exp Cell Res. 1995;218:516–521. doi: 10.1006/excr.1995.1186. [DOI] [PubMed] [Google Scholar]
- Menko S, Philp N, Veneziale B, Walker J. Integrins and development: how might these receptors regulate differentiation of the lens. Ann NY Acad Sci. 1998;842:36–41. doi: 10.1111/j.1749-6632.1998.tb09629.x. [DOI] [PubMed] [Google Scholar]
- Min JN, Zhang Y, Moskophidis D, Mivechi NF. Unique contribution of heat shock transcription factor 4 in ocular lens development and fiber cell differentiation. Genesis. 2004;40:205–17. doi: 10.1002/gene.20087. [DOI] [PubMed] [Google Scholar]
- Moustakas A, Souchelnytskyi S, Heldin CH. Smad regulation in TGF-beta signal transduction. J Cell Sci. 2001;114:4359–69. doi: 10.1242/jcs.114.24.4359. [DOI] [PubMed] [Google Scholar]
- Naylor MJ, Li N, Cheung J, Lowe ET, Lambert E, Marlow R, Wang P, Schatzmann F, Wintermantel T, Schuetz G, Clarke AR, Mueller U, Hynes NE, Streuli CH. Ablation of beta1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. J Cell Biol. 2005;171:717–28. doi: 10.1083/jcb.200503144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi O, Nishi K, Akaishi T, Shirasawa E. Detection of cell adhesion molecules in lens epithelial cells of human cataracts. Invest Ophthalmol Vis Sci. 1997;38:579–85. [PubMed] [Google Scholar]
- Palmade F, Sechoy-Chambon O, Regnouf de Vains JB, Coquelet C, Bonne C. Inhibition of cell adhesion to lens capsule by LCM 1910, an RGD-derived peptide. J Ocul Pharmacol. 1994;10:623–32. doi: 10.1089/jop.1994.10.623. [DOI] [PubMed] [Google Scholar]
- Park SH. Fine tuning and cross-talking of TGF-beta signal by inhibitory Smads. J Biochem Mol Biol. 2005;38:9–16. doi: 10.5483/bmbrep.2005.38.1.009. [DOI] [PubMed] [Google Scholar]
- Parmigiani CM, McAvoy JW. A morphometric analysis of the development of the rat lens capsule. Curr Eye Res. 1989;8:1271–1277. doi: 10.3109/02713688909013906. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J. Lens differentiation in vertebrates: A review of cellular and molecular features. Differentiation. 1981;19:134–152. doi: 10.1111/j.1432-0436.1981.tb01141.x. [DOI] [PubMed] [Google Scholar]
- Rae JL, Bartling C, Rae J, Mathias RT. Dye transfer between cells of the lens. J Membr Biol. 1996;150:89–103. doi: 10.1007/s002329900033. [DOI] [PubMed] [Google Scholar]
- Rafferty N, Goossens W. Growth and Aging of the Lens Capsule. Growth. 1978;42:375–89. [Google Scholar]
- Raghavan S, Bauer C, Mundschau G, Li Q, Fuchs E. Conditional ablation of beta1 integrin in skin. Severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J Cell Biol. 2000;150:1149–60. doi: 10.1083/jcb.150.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed NA, Oh DJ, Czymmek KJ, Duncan MK. An immunohistochemical method for the detection of proteins in the vertebrate lens. J Immunol Methods. 2001;253:243–52. doi: 10.1016/s0022-1759(01)00374-x. [DOI] [PubMed] [Google Scholar]
- Saika S, Kono-Saika S, Ohnishi Y, Sato M, Muragaki Y, Ooshima A, Flanders KC, Yoo J, Anzano M, Liu CY, Kao WW, Roberts AB. Smad3 signaling is required for epithelial-mesenchymal transition of lens epithelium after injury. Am J Pathol. 2004;164:651–63. doi: 10.1016/S0002-9440(10)63153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saika S, Miyamoto T, Ishida I, Ohnishi Y, Ooshima A. Lens epithelial cell death after cataract surgery. J Cataract Refract Surg. 2002;28:1452–6. doi: 10.1016/s0886-3350(02)01223-3. [DOI] [PubMed] [Google Scholar]
- Shiels A, Griffin CS, Muggleton-Harris AL. Immunochemical comparison of the major intrinsic protein of eye-lens fibre cell membranes in mice with hereditary cataracts. Biochim Biophys Acta. 1991;1097:318–24. doi: 10.1016/0925-4439(91)90087-p. [DOI] [PubMed] [Google Scholar]
- Sinha D, Wyatt MK, Sarra R, Jaworski C, Slingsby C, Thaung C, Pannell L, Robison WG, Favor J, Lyon M, Wistow G. A temperature-sensitive mutation of Crygs in the murine Opj cataract. J Biol Chem. 2001;276:9308–15. doi: 10.1074/jbc.M010583200. [DOI] [PubMed] [Google Scholar]
- Srinivasan Y, Lovicu FJ, Overbeek PA. Lens-specific expression of transforming growth factor beta1 in transgenic mice causes anterior subcapsular cataracts. J Clin Invest. 1998;101:625–34. doi: 10.1172/JCI1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens LE, Sutherland AE, Klimanskaya IV, Andrieux A, Meneses J, Pedersen RA, Damsky CH. Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 1995;9:1883–95. doi: 10.1101/gad.9.15.1883. [DOI] [PubMed] [Google Scholar]
- Symowicz J, Adley BP, Gleason KJ, Johnson JJ, Ghosh S, Fishman DA, Hudson LG, Stack MS. Engagement of collagen-binding integrins promotes matrix metalloproteinase-9-dependent e-cadherin ectodomain shedding in ovarian carcinoma cells. Cancer Res. 2007;67:2030–9. doi: 10.1158/0008-5472.CAN-06-2808. [DOI] [PubMed] [Google Scholar]
- ten Dijke P, Hill CS. New insights into TGF-beta-Smad signalling. Trends Biochem Sci. 2004;29:265–73. doi: 10.1016/j.tibs.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Walker JL, Menko AS. α6 Integrin is regulated with lens cell differentiation by linkage to the cytoskeleton and isoform switching. Dev Biol. 1999;210:497–511. doi: 10.1006/dbio.1999.9277. [DOI] [PubMed] [Google Scholar]
- Walker JL, Zhang L, Zhou J, Woolkalis MJ, Menko AS. Role for alpha 6 integrin during lens development: Evidence for signaling through IGF-1R and ERK. Dev Dyn. 2002;223:273–84. doi: 10.1002/dvdy.10050. [DOI] [PubMed] [Google Scholar]
- Weber GF, Menko AS. Actin filament organization regulates the induction of lens cell differentiation and survival. Dev Biol. 2006;295:714–29. doi: 10.1016/j.ydbio.2006.03.056. [DOI] [PubMed] [Google Scholar]
- Wederell ED, Brown H, O’Connor M, Chamberlain CG, McAvoy JW, de Iongh RU. Laminin-binding integrins in rat lens morphogenesis and their regulation during fibre differentiation. Exp Eye Res. 2005;81:326–39. doi: 10.1016/j.exer.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Wiesner S, Legate KR, Fassler R. Integrin-actin interactions. Cell Mol Life Sci. 2005;62:1081–99. doi: 10.1007/s00018-005-4522-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigle JT, Chowdhury k, Gruss P, Oliver G. Prox1 function is crucial for mouse lens-fibre elongation. Nat Genet. 1999;21:318–322. doi: 10.1038/6844. [DOI] [PubMed] [Google Scholar]
- Wormstone IM, Liu CS, Rakic JM, Marcantonio JM, Vrensen GF, Duncan G. Human lens epithelial cell proliferation in a protein-free medium. Invest Ophthalmol Vis Sci. 1997;38:396–404. [PubMed] [Google Scholar]
- Yu H, Bock O, Bayat A, Ferguson MW, Mrowietz U. Decreased expression of inhibitory SMAD6 and SMAD7 in keloid scarring. J Plast Reconstr Aesthet Surg. 2006;59:221–9. doi: 10.1016/j.bjps.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Zampighi GA, Eskandari S, Kreman M. Epithelial organization of the mammalian lens. Exp Eye Res. 2000;71:415–35. doi: 10.1006/exer.2000.0895. [DOI] [PubMed] [Google Scholar]
- Zhao H, Yang Y, Rizo CM, Overbeek PA, Robinson ML. Insertion of a Pax6 consensus binding site into the alphaA-crystallin promoter acts as a lens epithelial cell enhancer in transgenic mice. Invest Ophthalmol Vis Sci. 2004;45:1930–9. doi: 10.1167/iovs.03-0856. [DOI] [PubMed] [Google Scholar]
- Zuk A, Hay ED. Expression of beta 1 integrins changes during transformation of avian lens epithelium to mesenchyme in collagen gels. Dev Dyn. 1994;201:378–93. doi: 10.1002/aja.1002010409. [DOI] [PubMed] [Google Scholar]


