Abstract
Tau in cerebrospinal fluid (CSF) has been proposed as a diagnostic marker for Alzheimer’s disease (AD). This paper presents a new sensitive sandwich ELISA allowing quantitation of tau from 8 μl CSF/well. A human specific monoclonal tau antibody HT7 was used as a capture antibody and a mixture of polyclonal tau antibodies, 92e and R134d was used as reporter antibodies. Tyramide signal amplification (TSA) technology was used in the last step to increase the sensitivity. With this TSA-ELISA, the lowest detection limit for tau was 14.3 pg/ml. Tau levels in CSF were found to be increased in AD patients (807 ± 304 pg/ml, p< 0.001) compared with controls (252 ± 94 pg/ml). Thirty-five of 38 AD cases (92% sensitivity) yielded signals greater than cutoff, while only 1 of 38 control cases (97% specificity) was greater. A highly significant correlation was found between this assay and a commonly used kit, INNOTEST hTAU Antigen.
Keywords: Tau, Cerebrospinal fluid, ELISA
Microtubule associated protein tau is the major protein subunit of paired helical filaments (PHF)/neurofibrillary tangles (NFTs), a histopathological hallmark of Alzheimer’s disease (AD) [9]. Discoveries of abnormal hyperphosphorylation [10] and several-fold increase in levels of tau in AD brain [17] led to the studies of this protein as the disease marker. Total tau and hyperphosphorylated tau in cerebrospinal fluid (CSF) have been shown to correlate with neurofibrillary pathology in AD [7, 20]. Numerous studies have shown the increased CSF tau levels in AD patients [5]. However, tau concentrations in CSF are low and, thus, CSF tau is only quantifiable by sensitive immunoassays. The first report on tau protein concentration in the CSF as a biomarker for AD was published in 1993 [23]. Subsequently, two ELISA methods based on monoclonal antibodies that detect all isoforms of tau, independent of their phosphorylation, were reported [4, 24]. The total tau level in CSF of collectively more than 2500 AD patients and 1400 controls from 36 different studies have been investigated by the most commonly used kit, the “Innogenetics ELISA”. These studies have shown that CSF tau concentrations are about three times higher in patients with AD than in control individuals. The specificity of this assay is 90% and the mean sensitivity to AD is 81%. In the five studies with the other major commercial assay, “Athena” assay, the mean sensitivity was 55%, with similar specificity [5]. The “Innogenetics ELISA” is the most sensitive assay commercially available. This assay which employs monoclonal antibody AT120 as a capture antibody and two biotinylated monoclonal antibodies (BT2 and HT7) mixture as reporter antibodies, reached the lowest detection limit of 59.3 pg/ml using affinity purified tau as a standard and 25 μl/well CSF is needed for quantitation [4].
The combined measure of CSF tau and Aβ42 is known to improve the discrimination of AD from non-AD neurological disorders [14]. Recently, we have shown that AD is subdivided into five subgroups based on CSF levels of tau, Aβ42 and ubiquitin and each subgroup presents a different clinical profile [15]. The establishment of a more sensitive ELISA that needs less amount of CSF and allows the quantitation of several biomarkers from the same sample is required to allow studies on identification of AD subgroups through CSF biomarkers. Many efforts have been made to improve the sensitivity of ELISA since the establishment of conventional ELISA which can detect micro amounts of antigens. Previously, we have reported the sensitive bienzyme-substrate-recycle ELISA by which we can measure total tau and tau phosphorylated at Ser-396/404 from 20 μl CSF/well [13]. In the present study, we have adapted the tyramide signal amplification (TSA) technology involving an analyte-dependent reporter enzyme (horse-radish peroxidase), which catalyzes the deposition of additional reporter molecules (biotin-labeled tyramides) [6]. By combination of this TSA technology, new antibodies and half-area plates, we have developed the most sensitive sandwich ELISA allowing quantitation of tau from 8 μl CSF/well.
Polyclonal tau antibodies, 92e to bovine brain tau and R134d to recombinant human brain tau410 (tau 39, three repeat tau with two N terminal inserts) were decribed previously [11, 21]. These antibodies are phosphorylation-independent and detect all isoforms of tau [11, 21]. Recombinant tau, the largest isoform of human tau441 was cloned, expressed, and purified as described previously [1]. Tau protein concentration of the standard was determined by the modified Lowry method [3]. Samples of lumber CSF from living patients, 38 clinically diagnosed AD patients and 38 control subjects (11 healthy, 15 non-demented neurological and 12 dementia with Lewy bodies) were obtained from Göteborg University, Sweden and Ludwig-Maximilian University, Germany. Table 1 illustrates the characteristics of AD patients and control subjects. The use of CSF samples for the present study was approved by the Institutional Review Board of the New York State Institute for Basic Research in Developmental Disabilities. AD subjects fulfilled the National Institute of Neurological and Communicative Disorders and Stroke and Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria of probable AD [19]. Diagnosis of dementia with Lewy bodies was based on McKeith and colleagues’ criteria [18]. All CSF had been stored at −75°C prior to use. The average age of the AD group was 75 ± 7, and the control group was 68 ± 9. These are not age matched sets of samples but neither the AD nor the control CSF samples displayed any correlations with age (r = 0.01, 0.1 respectively).
Table 1.
Characteristics of AD patients and control subjects
| Group | Number (M/F) | Age | MMSE |
|---|---|---|---|
| AD | 38 (25/13) | 75 ± 7 | 22 ± 5 |
| Control | |||
| Healthy | 11 (9/2) | 67 ± 8 | 30 ± 1 |
| NDN | 15 (8/7) | 63 ± 8 | 29 ± 2 |
| DLB | 12 (3/9) | 76 ± 6 | 22 ± 4 |
M, males; F, females; MMSE, mini mental state examination; NDN, non-demented neurological; DLB, dementia with Lewy bodies
A human specific monoclonal tau antibody HT7 (Pierce, Rockford, IL) which recognizes amino acid residues 159–163 was coated at concentration of 2 μg/ml in 0.1 M carbonate buffer, pH 9.5 (50 μl/well) overnight at 4°C on a high binding 96-well half-area plates (Corning Costar, Lowell, MA). After washing three times with 200 μl/well of TBS/0.05% Tween20 (TBST), the plates were blocked by adding 100 μl/well of 2% BSA/TBS and incubated overnight at 4°C. The plates were incubated with 50 μl of an appropriately diluted tau standard or six times diluted CSF samples (diluted with 1% BSA/TBST) for 6 hrs at room temperature. After washing, a mixture of polyclonal tau antibodies, 92e and R134d (each 1:5,000 in 1% BSA/TBST) was added and incubated overnight at 4°C. Peroxidase conjugated antibody to rabbit IgG (Jackson Immuno Research, West Grove, PA) diluted 1:2,000 in 1% BSA/TBST in the amount of 50 μl/well was added and incubated for 1 hr at room temperature. TSA was performed with the ELAST ELISA Amplification System according to the manufacture’s instructions (PerkinElmer, Boston, MA). 3,3′,5,5′-Tetramethylbenzidine Liquid Substrate System (Sigma, St Louis, MO) was used as a substrate of peroxidase and absorption was read at 650 nm. Optical density values obtained from the CSF samples were compared with standard curves generated from known quantities of recombinant tau. For comparisons between two groups, the Mann-Whitney U-test was used. The Sperman correlation coefficient was employed for correlations.
A standard curve was constructed with different concentration of recombinant tau in the range of 15.6 to 1000 pg/ml. The curve was linear from 15.6 to 250 pg/ml of recombinant tau (Fig. 1). The lowest detection limit for tau with this sensitive sandwich ELISA, TSA-ELISA was calculated as the mean of 12 determinations of the sample diluent plus 4 standard deviations and was determined to be 14.3 pg/ml. The intra-assay coefficient of variation determined by triplicate of 20 CSF samples averaged 4.9%. In order to examine reproducibility, 4 CSF samples were analyzed in each of two experiments. After normalization, the inter-assay coefficient of variation averaged 5.8%. Tau levels in CSF were determined with the newly developed TSA-ELISA. Six times diluted CSF samples were used so that signals obtained were in the linear range and above the lowest detection limit. The results obtained from 38 AD and 38 control CSF samples showed that tau levels in CSF were significantly higher in AD cases (807 ± 304 pg/ml, p< 0.001) as compared with control cases (252 ± 94 pg/ml). Individual values are given in Fig. 2. A cutoff value of 414 pg/ml was derived between AD and control signals based on the mean of the control samples plus 1.72 standard deviations. Three of 38 AD cases fell below this cutoff of 92% sensitivity, while 1 of 38 control case was above the cutoff resulting in specificity of 97%. Analysis of the same CSF samples by a commonly used kit, INNOTEST hTAU Antigen (Innogenetics, Ghent, Belgium) revealed similar values. A highly significant correlation was found between the values obtained from the TSA-ELISA and the INNOTEST hTAU Antigen (Fig. 3). The sensitivity and specificity from the INNOTEST hTAU Antigen were 82% and 95%, respectively. The sensitivity of the diagnostic value from the TSA-ELISA was 10% higher than the INNOTEST hTAU Antigen.
Fig. 1.

Standard curve. The curve is linear from 15.6 to 250 pg/ml of recombinant human brain tau, tau441.
Fig. 2.

CSF levels of tau determined by a new sensitive sandwich ELISA using TSA, the TSA-ELISA. Dotted line represents the cutoff point, estimated as the mean of the control samples plus 1.72 standard deviations (414 pg/ml).
Fig. 3.
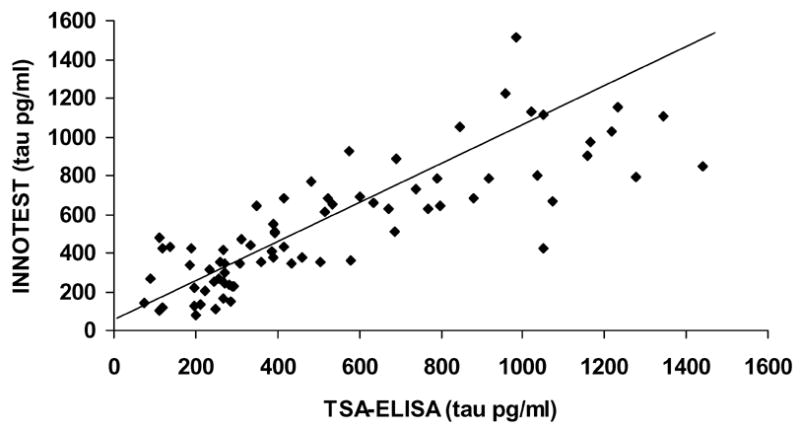
Correlation between the TSA-ELISA and the INNOTEST hTAU Antigen assay (n = 76, r = 0.84, p< 0.001).
CSF tau levels were not increased in dementia with Lewy bodies (DLB) patients (269 ± 68 pg/ml) with the TSA-ELISA. This result is consistent with our previous study using the INNOTEST hTAU Antigen [15] and other studies [8, 16] and is compatible with the pathologic features that numerous senile plaques but few NFTs are observed in the DLB brain [12]. Although it is still controversial whether CSF tau is useful in discriminating AD from DLB because some studies reported that CSF tau levels were increased in DLB patients [2, 22], our results suggest that CSF tau is useful in discriminating AD from DLB. We compared the TSA-ELISA with the bienzyme-substrate-recycle ELISA which we have reported previously [13] and confirmed that the TSA-ELISA could provide several analytical advantages over the bienzyme-substrate-recycle ELISA. The TSA-ELISA showed better sensitivity and reproducibility than the bienzyme-substrate-recycle ELISA. The TSA-ELISA could also reduce the number of handling steps. In conclusion, we have developed an ultrasensitive sandwich ELISA allowing quantitation of tau from the 8 μl CSF/well. This assay reached the lowest detection limit of 14.3 pg/ml using recombinant tau as a standard. Tau levels in CSF were found to be increased in AD patients (807 ± 304 pg/ml, p< 0.001) compared with controls (252 ± 94 pg/ml) using this assay. A highly significant correlation was found between this assay and a commonly used kit, INNOTEST hTAU Antigen. The sensitivity and specificity of the diagnostic value from this assay were 92% and 97%, respectively. This assay might be more sensitive for AD diagnosis than the other total tau ELISAs based on the reported sensitivities from previous studies [5, 13]. However, a large number of independent studies in the future can only establish the exact value of the new assay.
Acknowledgments
We thank Dr. Ezzat El-Akkad for purification of recombinant tau and Janet Murphy for secretarial assistance. These studies were supported in part by the New York State Office of Mental Retardation and Developmental Disabilities, National Institutes of Health grant AG028538 and a grant IIRG-06-25836 from Alzheimer’s Association, Chicago, IL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alonso AD, Zaidi T, Novak M, Barra HS, Grundke-Iqbal I, Iqbal K. Interaction of tau isoforms with Alzheimer’s disease abnormally hyperphosphorylated tau and in vitro phosphorylation into the disease-like protein. J Biol Chem. 2001;276:37967–37973. doi: 10.1074/jbc.M105365200. [DOI] [PubMed] [Google Scholar]
- 2.Arai H, Morikawa Y, Higuchi M, Matsui T, Clark CM, Miura M, Machida N, Lee VM, Trojanowski JQ, Sasaki H. Cerebrospinal fluid tau levels in neurodegenerative diseases with distinct tau-related pathology. Biochem Biophys Res Commun. 1997;236:262–264. doi: 10.1006/bbrc.1997.6908. [DOI] [PubMed] [Google Scholar]
- 3.Bensadoun A, Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976;70:241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- 4.Blennow K, Wallin A, Agren H, Spenger C, Siegfried J, Vanmechelen E. Tau protein in cerebrospinal fluid: a biochemical marker for axonal degeneration in Alzheimer disease? Mol Chem Neuropathol. 1995;26:231–245. doi: 10.1007/BF02815140. [DOI] [PubMed] [Google Scholar]
- 5.Blennow K, Hampel H. CSF markers for incipient Alzheimer’s disease. Lancet Neurol. 2003;2:605–613. doi: 10.1016/s1474-4422(03)00530-1. [DOI] [PubMed] [Google Scholar]
- 6.Bobrow MN, Harris TD, Shaughnessy KJ, Litt GJ. Catalyzed reporter deposition, a novel method of signal amplification. Application to immunoassays. J Immunol Methods. 1989;125:279–285. doi: 10.1016/0022-1759(89)90104-x. [DOI] [PubMed] [Google Scholar]
- 7.Buerger K, Ewers M, Pirttila T, Zinkowski R, Alafuzoff I, Teipel SJ, DeBernardis J, Kerkman D, McCulloch C, Soininen H, Hampel H. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer’s disease. Brain. 2006;129:3035–3041. doi: 10.1093/brain/awl269. [DOI] [PubMed] [Google Scholar]
- 8.Gomez-Tortosa E, Gonzalo I, Fanjul S, Sainz MJ, Cantarero S, Cemillan C, Yebenes JG, del Ser T. Cerebrospinal fluid markers in dementia with lewy bodies compared with Alzheimer disease. Arch Neurol. 2003;60:1218–1222. doi: 10.1001/archneur.60.9.1218. [DOI] [PubMed] [Google Scholar]
- 9.Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986;261:6084–6089. [PubMed] [Google Scholar]
- 10.Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grundke-Iqbal I, Vorbrodt AW, Iqbal K, Tung YC, Wang GP, Wisniewski HM. Microtubule-associated polypeptides tau are altered in Alzheimer paired helical filaments. Brain Res. 1988;464:43–52. doi: 10.1016/0169-328x(88)90017-4. [DOI] [PubMed] [Google Scholar]
- 12.Hansen LA, Masliah E, Galasko D, Terry RD. Plaque-only Alzheimer disease is usually the lewy body variant, and vice versa. J Neuropathol Exp Neurol. 1993;52:648–654. doi: 10.1097/00005072-199311000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Hu YY, He SS, Wang X, Duan QH, Grundke-Iqbal I, Iqbal K, Wang J. Levels of nonphosphorylated and phosphorylated tau in cerebrospinal fluid of Alzheimer’s disease patients: an ultrasensitive bienzyme-substrate-recycle enzyme-linked immunosorbent assay. Am J Pathol. 2002;160:1269–1278. doi: 10.1016/S0002-9440(10)62554-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hulstaert F, Blennow K, Ivanoiu A, Schoonderwaldt HC, Riemenschneider M, De Deyn PP, Bancher C, Cras P, Wiltfang J, Mehta PD, Iqbal K, Pottel H, Vanmechelen E, Vanderstichele H. Improved discrimination of AD patients using beta-amyloid(1–42) and tau levels in CSF. Neurology. 1999;52:1555–1562. doi: 10.1212/wnl.52.8.1555. [DOI] [PubMed] [Google Scholar]
- 15.Iqbal K, Flory M, Khatoon S, Soininen H, Pirttila T, Lehtovirta M, Alafuzoff I, Blennow K, Andreasen N, Vanmechelen E, Grundke-Iqbal I. Subgroups of Alzheimer’s disease based on cerebrospinal fluid molecular markers. Ann Neurol. 2005;58:748–757. doi: 10.1002/ana.20639. [DOI] [PubMed] [Google Scholar]
- 16.Kanemaru K, Kameda N, Yamanouchi H. Decreased CSF amyloid beta42 and normal tau levels in dementia with Lewy bodies. Neurology. 2000;54:1875–1876. doi: 10.1212/wnl.54.9.1875. [DOI] [PubMed] [Google Scholar]
- 17.Khatoon S, Grundke-Iqbal I, Iqbal K. Brain levels of microtubule-associated protein tau are elevated in Alzheimer’s disease: a radioimmuno-slot-blot assay for nanograms of the protein. J Neurochem. 1992;59:750–753. doi: 10.1111/j.1471-4159.1992.tb09432.x. [DOI] [PubMed] [Google Scholar]
- 18.McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe J, Mirra SS, Byrne EJ, Lennox G, Quinn NP, Edwardson JA, Ince PG, Bergeron C, Burns A, Miller BL, Lovestone S, Collerton D, Jansen EN, Ballard C, de Vos RA, Wilcock GK, Jellinger KA, Perry RH. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 19.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 20.Tapiola T, Overmyer M, Lehtovirta M, Helisalmi S, Ramberg J, Alafuzoff I, Riekkinen P, Sr, Soininen H. The level of cerebrospinal fluid tau correlates with neurofibrillary tangles in Alzheimer’s disease. Neuroreport. 1997;8:3961–3963. doi: 10.1097/00001756-199712220-00022. [DOI] [PubMed] [Google Scholar]
- 21.Tatebayashi Y, Iqbal K, Grundke-Iqbal I. Dynamic regulation of expression and phosphorylation of tau by fibroblast growth factor-2 in neural progenitor cells from adult rat hippocampus. J Neurosci. 1999;19:5245–5254. doi: 10.1523/JNEUROSCI.19-13-05245.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tschampa HJ, Schulz-Schaeffer W, Wiltfang J, Poser S, Otto M, Neumann M, Kretzschmar HA. Decreased CSF amyloid beta42 and normal tau levels in dementia with Lewy bodies. Neurology. 2001;56:576. doi: 10.1212/wnl.56.4.576. [DOI] [PubMed] [Google Scholar]
- 23.Vandermeeren M, Mercken M, Vanmechelen E, Six J, van de Voorde A, Martin JJ, Cras P. Detection of tau proteins in normal and Alzheimer’s disease cerebrospinal fluid with a sensitive sandwich enzyme-linked immunosorbent assay. J Neurochem. 1993;61:1828–1834. doi: 10.1111/j.1471-4159.1993.tb09823.x. [DOI] [PubMed] [Google Scholar]
- 24.Vigo-Pelfrey C, Seubert P, Barbour R, Blomquist C, Lee M, Lee D, Coria F, Chang L, Miller B, Lieberburg I, Schenk D. Elevation of microtubule-associated protein tau in the cerebrospinal fluid of patients with Alzheimer’s disease. Neurology. 1995;45:788–93. doi: 10.1212/wnl.45.4.788. [DOI] [PubMed] [Google Scholar]


