Abstract
Congenital amusia (commonly known as “tone deafness”) is a lifelong impairment of music perception that affects 4% of the population. To estimate whether congenital amusia can be genetically transmitted, its prevalence was quantified by direct auditory testing of 71 members of 9 large families of amusic probands, as well as of 75 members of 10 control families. The results confirm that congenital amusia is expressed by a deficit in processing musical pitch but not musical time and also show that the pitch disorder has a hereditary component. In amusic families, 39% of first-degree relatives have the same cognitive disorder, whereas only 3% have it in the control families. The identification of multiplex families with a high relative risk of experiencing a musical pitch deficit (λs=10.8; 95% confidence interval 8–13.5) enables the mapping of genetic loci for hereditary amusia.
Humans are born with the potential to both speak and make music.1 Thus, it is likely that musical capacity, like language capacity, is coded in the human genome. One powerful means to identify the relevant genes is to search for people who exhibit abnormal behaviors. In the speech domain, such conditions are often termed “specific language impairments” (SLI [MIM 606711]), and a large research effort has been undertaken to understand the origins and varieties of these disorders.2 This research recently made a major step forward with a rapid succession of discoveries that implicate specific genes as causative of abnormal language development3 (MIM 602081 and 605317).
In the musical domain, there is no such comparable effort. Yet music-specific impairments have been reported.4 The condition has been variously termed “note deafness,”5 “tone deafness,”6 “tune deafness,”7 “dysmelodia,”7 and, more recently, “congenital amusia”8 (MIM 191200). All of these terms refer to the same condition, whereby adults who report lifelong difficulties with music exhibit a deficit in detecting pitch changes in melodies. The term “amusia” seems preferable, to acknowledge the possibility that there exist as many forms of congenital amusias as there are forms of acquired amusias that are the consequences of accidental brain damage.9 The term “congenital” means only present from birth; it defines a likely time period but not the etiology. The goal of the present study was to test for the presence of a genetic origin of amusia by studying the family aggregation of congenital amusia.
To date, all documented cases of congenital amusia have been unrelated and have been ascertained by chance via reports in the media and newspaper advertisements. This was how the 46 published cases were recruited since the first documented case,5 with 24 cases from Montreal,10–12 10 cases from Newcastle (United Kingdom),13 11 cases from Kingston (Canada),14 and 1 case from Italy (E. Rusconi, unpublished data). There has not yet been a family study of those cases. The present study is the first systematic search of familial aggregation of congenital amusia.
Such a family-aggregation study is facilitated by the fact that all reports of congenital amusia document a musical disorder that is remarkably similar across cases. All of the reported subjects failed to acquire basic musical abilities, such as normal music perception and music-recognition abilities, despite normal hearing, normal language abilities, and normal intelligence.11 For these individuals, listening to a musical performance is like listening to a foreign speech.5 Congenital amusia appears to be not only specific to the musical domain but also to be monosymptomatic (or nonsyndromic), because there is no concurrent neurodevelopmental disorder such as dyslexia (MIM 127700), autism (MIM 209850), or SLI.
These amusic individuals have a normal understanding of speech and prosody. They can recognize speakers by their voices and can identify all sorts of familiar environmental sounds, such as animal cries. What distinguishes them from unaffected people is their inability to recognize a familiar tune without the aid of the lyrics and their inability to detect out-of-tune singing, including their own.4 They also show little sensitivity to the presence of obviously dissonant chords in classical music,11 a sensitivity that is normally present in infants.15 Most notably, amusic individuals fail to detect “wrong notes” (out-of-scale notes) in conventional but unfamiliar melodies.8,10,11,16 This behavioral failure is diagnostic, because there is no overlap between the distributions of the scores of amusics and controls.11,16 What amusics seem to be lacking are the (implicit) knowledge and procedures required for mapping pitches onto musical scales. This musical-pitch disorder is a clear-cut phenotype that calls for genetic analyses.
Independent supportive evidence of the notion that musical-pitch processing might be a good target for phenotype-genotype correlations comes from a recent twin study.17 In the study, MZ and DZ pairs of twins were required to detect anomalous pitches in popular melodies. Genetic-model fitting indicates that the influence of shared genes is more important than shared environments, with a heritability of 70%–80%. Interestingly, this test had been administered to >600 participants in the United Kingdom.7 Of those participants, ∼4% performed as poorly as did 20 adults who considered themselves or were considered by others to be amusic. This suggests that 4% of the population may suffer from a genetically determined defect in perceiving musical pitch. If correct, the prevalence of a musical-pitch deficit should be much higher in the families of amusic individuals.
To test this prediction, we studied the family members of 13 amusic adults, as well as 17 matched and unrelated controls who have been tested in Montreal over the past 6 years.11,18,19 The subjects were initially categorized as either amusic or not amusic, on the basis of their self-declared difficulties with music and their poor global performance on the Montreal Battery of Evaluation of Amusia (MBEA).12 This battery involves six subtests evaluating music perception and memory and takes ∼2 h to complete. Congenital amusia is confirmed if the individual performs 2 SD below the mean MBEA performance of musically intact controls. This is the case for the 13 amusic probands who participated in the present study (see table 1). The only difference between amusic and control probands is that controls did not report musical problems and performed normally on the MBEA. These subjects will be hereafter referred to as “control probands.” All probands had normal intelligence and normal memory, as assessed by standardized tests, and self-reported no history of neurological or psychiatric disorders.
Table 1. .
Characteristics of Tested Subjects
| Findings by Group |
||
| Characteristics | Amusic | Control |
| Probands: | ||
| No. of probands | 13 | 17 |
| Male/female | 6/7 | 5/12 |
| Mean age in years (range) | 59 (34–69) | 56 (46–69) |
| Mean no. of years of education (range) | 17.6 (15–22) | 15 (12–22) |
| MBEAa (range) | 63% (51%–74%) | 88% (81%–94%) |
| Siblings of probands: | ||
| No. of families | 7 | 10 |
| No. of tested siblings | 21 | 22 |
| Male/female | 5/16 | 10/12 |
| Mean age in years (range) | 57 (44–68) | 56 (39–71) |
| Mean no. of years of education (range) | 14 (11–18) | 14 (11–18) |
| Offspring of probands: | ||
| No. of families | 9 | 10 |
| No. of tested offspring | 37 | 36 |
| Male/female | 20/17 | 20/16 |
| Mean age in years (range) | 31 (17–41) | 28 (16–44) |
| Mean no. of years of education (range) | 14 (11–21) | 15 (11–21) |
Global score obtained on the MBEA.12
Each proband was asked to judge whether his or her relatives were amusic, and the proband was then invited to recruit relatives to participate in the present study. Of the 13 families of amusic probands, 9 actively participated in recruiting 21 siblings and 37 offspring, whose musical skills were objectively assessed. From the 10 control families, 22 siblings and 36 offspring took the auditory test. Two participants (one in the control families and one in the amusic families) were excluded because the history indicated an event of brain damage or psychiatric disorder. The general characteristics of the participants are provided in table 1. All participants received a small monetary compensation for their participation and gave their informed consent on an online form before the testing session. The study was conducted with ethical committee approval from the University of Montreal (protocol “Congenital amusia: heritability, neural correlates and plasticity”).
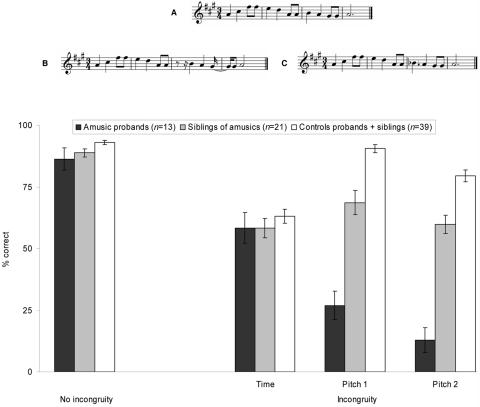
A crucial aspect of a family-aggregation study is the diagnosis of the disorder. The MBEA is valid12 but time consuming. To conduct a large-scale study, we created a test that can be performed independently and remotely via the Internet (Amusia Web site). This online auditory test included the anomalous pitch–detection test used in our prior studies and a control time condition. More specifically, the test comprised 72 melodies that were derived from 12 “original” melodies, as used in the MBEA.12 The melodies were all constructed according to tonal conventions of Western music, in a major mode. They contained 9.6 successive tones, on average, and were computer generated at a tempo of 120 beats/min. The 12 melodies were modified so that the same critical tone was altered either in terms of pitch or time (see fig. 1). The critical tone always fell on the first downbeat in the third bar of the four-bar melody (hence, was metrically stressed) and was 500 ms long. In the incongruous pitch condition, the change consisted of a tone that was outside the key of the melody, hence introducing a “foreign” or “wrong” note. In another condition, the change consisted of a mistuning by half a semitone, hence introducing a “sour” note. The time change consisted of introducing a silence of 5/7 of the beat duration (i.e., 143 ms) directly preceding the critical tone, thereby locally disrupting the meter (i.e., regularity). The melodies were presented with 10 different timbres (e.g., piano, saxophone, clarinet, recorder, harp, strings, or guitar), to make the auditory test more interesting for the participants.
Figure 1. .
Examples from the online auditory test of melody with no incongruity (A), with a time incongruity (B), and with a pitch incongruity (C). The arrow (↓) marks the mistuned pitch, and the flat (♭) marks the out-of-key pitch). Percentage of correct responses is presented below for the corresponding conditions (pitch 1 corresponds to the mistuned pitch and pitch 2 to the out-of-key pitch) in the amusic probands, their siblings, and their matched controls. The error bar represents SE.
The probands were administered the Internet test in our laboratory and were given the computer-access code, to encourage their relatives to participate in the study. All participants were tested with the “out-of-time” condition followed by the “out-of-tune” and “out of key” conditions. In each condition, subjects were presented with 24 melodies (12 congruous and 12 incongruous) one at a time, in a random order. Their task was simply to detect whether an incongruity occurred in each melody and to click a “yes” button whenever they detected an anomaly and a “no” button when they did not detect an incongruity. They received two practice trials before each condition and were provided with feedback after each practice trial. The auditory test lasted 15 min. After the test, the participants completed a detailed questionnaire about their personal history and musical background. At the end of the session, the participants obtained their test scores.
As can be seen in figure 1, the participants of the probands’ generation were fairly accurate, with >86% of correct decisions on average, in deciding that the melodies did not contain an incongruity. However, both the amusic probands and their siblings indicated more false alarms (i.e., errors when there was no incongruity) than did the controls (F(2,70)=3.2; P<.05). More importantly, all participants detected a time incongruity in the melodies with comparable accuracy (P>.05 by planned comparisons). In contrast, the pitch incongruities that were missed by the amusic probands were also missed by approximately half of their siblings but were easily detected by the controls. This distinct pattern of results is supported by a significant interaction between type of incongruity (time, mistuned, or out of key) and group (controls, siblings of amusics, or amusic probands; F(4,70)=22.0; P<.001). These test results highlight the significance of testing for the presence of a musical-pitch deficit in the families of amusic individuals and confirms the specificity of the disorder.16,18 Moreover, the consistency of the results obtained across testing conditions in amusic probands validates the sensitivity of the online auditory-testing procedure, which in turn provides a convenient tool for identifying further cases.
The reference range used to establish the amusic threshold was determined from the scores obtained by the 39 members of the control families. The threshold was set at 2 SD (SD 7.3%) from the control mean (88.8%) averaged over the two pitch conditions (comprising 24 melodies with no incongruity and 24 melodies with either a mistuned or an out-of-key pitch). Accordingly, 9 (8 females) of the 21 tested siblings of amusic probands were considered “affected,” whereas 12 (8 females) were unaffected (see table 2). Many affected siblings originated from the same family. In fact, five amusic probands had at least one positive-tested family member, whereas two had none. Of the controls, 2 of 22 tested siblings scored below the cut-off score and were classified as “affected” (table 2). Hence, 2 of 10 control probands had a positive-tested family member. Thus, amusic probands are significantly more likely to have a test-confirmed positive family history than are controls (P⩽.01 by Fisher's exact test).
Table 2. .
Amusia in Siblings of Amusic Probands and in Siblings of Controls (Excluding Probands)
| No. of Siblings of Probands |
No. of Siblings of Controls |
|||||||
| Determined | n | Affected | Unaffected | Unknown | n | Affected | Unaffected | Unknown |
| By reporta | 57 | 39 | 15 | 3 | 52 | 2 | 41 | 9 |
| By test | 21 | 9 | 12 | … | 22 | 2 | 20 | … |
When the proband was unsure whether a relative was amusic, the relative was classified as “unknown.”
To quantify family aggregation, the sibling relative risk was computed under the assumption of single ascertainment according to λs, defined as the risk to siblings of probands over the population prevalence for a specific disease or trait.20 The sibling relative risk λs for congenital amusia was calculated to be 10.8 (95% CI 8–13.5) on the basis of the siblings of seven amusic probands and a population prevalence of 4%.7 The recurrence risk is slightly higher if we also consider the probands’ reports (table 2). However, amusic probands were not very accurate in their evaluation. The rate of agreement between reports and behavioral scores of the tested siblings was 58% correct (with 11 correct vs. 8 incorrect diagnoses). Concordance was higher (77.3%) in control families (with 17 correct vs. 5 incorrect diagnoses). These results show how important objective testing is in the music domain. Genetic studies of musical abilities cannot rely merely on subject reports, as is often the case in SLI, dyslexia, and congenital prosopagnosia.
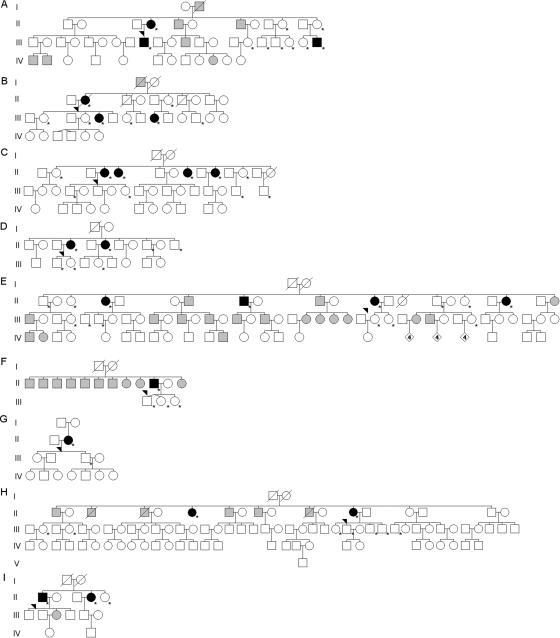
It is worth emphasizing that congenital amusia does not result from a peculiar family environment. In that case, all siblings would be impaired. If we exclude the amusic proband who was an only child, in each tested family, there was at least one sibling who was not affected (see the pedigrees in fig. 2). Thus, the musical-pitch disorder cannot be attributed exclusively to the family environment. A more important environmental factor is the amount of musical experience. In principle, amusic individuals must be less musically oriented than the general population. This musical attitude finds some support in the responses to the questionnaire. Among the 24 affected (as confirmed by test) individuals who completed the questionnaire, 58% (14 of 24) had music lessons early in life, whereas 75% (36 of 48) of the unaffected individuals reported having had music lessons. Moreover, the majority of both the affected and unaffected individuals declared that they listened to music occasionally. However, a third of the affected individuals (8 of 24) reported that they never listened to music or did so rarely, whereas this response was less frequent in unaffected listeners (12.7% [6 of 48]). Finally, there were fewer (amateur) musicians among family members of the affected individuals (10 of 24) compared with the unaffected participants (31 of 49; χ2=15.11; P<.001). In summary, music was less present, although by no means absent, in the environment of the amusic subjects than in that of musically intact individuals.
Figure 2. .
Pedigrees of all tested families from the amusic probands (arrow heads). An asterisk (*) indicates evaluation by test. Black symbols indicate amusic individuals, as determined by test; white symbols indicate nonamusic individuals, as determined by test; and gray symbols indicate amusic individuals by report. Pedigrees shown in panels A and B involve the four amusic offspring.
The next generation was more musical. The 37 tested offspring from amusic families and the 36 tested offspring from the control families had considerably more musical experience than did their parents. This is the reason we examined them separately. Most (65 of 73) offspring had music lessons during childhood, and 53 of 73 still played the instrument at the time of testing. Such musicianship is as high in the amusics’ offspring as in the controls’ offspring (with 26 and 27, respectively, who were playing music in each group). This considerable musical experience may explain the lower incidence of detectable amusia in the younger generation. Only 4 of the 73 offspring can be considered amusic, because they obtained a pitch score below the cut-off (corresponding to 2 SD [6.9%] below the mean [89.8%] obtained by the control offspring on the pitch test). This incidence of congenital amusia among the amusic offspring (11% [4 of 37]) matches the 11% of amusic cases identified among students in a prior study, with use of the MBEA.14 However, these students had less musical experience (4 of 11 had music lessons) than did the present sample. Thus, the lower incidence of amusia in the younger generation might be better explained by a cohort effect than by music education. Indeed, music in most environments has become ubiquitous over the past few decades. Thus, musical stimulation and experience must be considerably greater for the generation of the offspring than for their parents, given that it probably started early in life, when the offspring brains were more plastic.
This cohort effect may explain why heritability is not very high in offspring. Despite the fact that all four amusic offspring are from families of amusic probands, only two have an amusic mother (see fig. 2A and 2B). If we consider that 21 offspring have one amusic parent confirmed by test, the relative risk, λ, is 2.3 (95% CI 0–5) with a population prevalence of 4%. Yet the two amusic offspring from an amusic mother are both musically trained and declare that they still play music. Therefore, despite early and continuous musical practice, the predispositions of congenital amusia are still discernible in these two individuals.
Altogether, the test results support the view that congenital amusia is a heritable disorder. The same disorder is expressed in 39% of first-degree relatives in amusic families, whereas it is present in only 3% of control families (table 3). This incidence of amusia, associated with λs=10.8 and λ=2.3, is in the same order of magnitude as the heritability of language impairments. The reported incidence of positive family history of SLI in the families of probands has a range of 24%–78%, whereas, in control families, it has a range of 3%–46%.21 Thus, the relative risk for siblings, λs, lies between 1.7 and 8 for spoken-language disorders. In the musical domain, similar estimates have been obtained in a totally different condition that concerns rare musicians who have absolute pitch (MIM 159300). This ability, which involves naming isolated pitches without a reference (see the review by Zatorre22), also aggregates in families, with λs between 7.823 and 12.2.24 Absolute-pitch ability appears under the guidance of genetic factors,24 although gene loci have not yet been identified. Thus, the present estimate of relative risk of congenital amusia (λs=10.8) seems in line with the influence of genetic factors, as documented for other cognitive functions.
Table 3. .
Proportion of First-Degree Relatives Classified as Amusic by Test in Families of Amusic Probands and in Families of Controls
| No./Total (%) Amusic |
||||
| Group | Probands | Siblings | Offspring | All Family Members |
| Amusic | 9/9 | 9/21 (43) | 2/21a (10) | 20/51 (39) |
| Control | 0/10 | 2/22 (9) | 0/36 (0) | 2/68 (3) |
Corresponds to the 21 offspring who have one parent confirmed by test to be amusic.
The low relative risk of the offspring (λ=2.3) compared with their parents suggests that congenital amusia can be less penetrant when the musical environment is enriched. This result highlights the role of experience-dependent tuning of the musical-pitch system. Music processing, like most complex cognitive systems, owes its ultimate functional properties both to the genetic prewiring and to experience-based plasticity. Genetic analyses of congenital amusia may help to understand this interplay between predispositions and plasticity.
However, genes do not specify cognitive functions. Genes influence brain development. Thus, the next step in the genetic analysis of congenital amusia is to identify the genes and to relate these genes to the neuroanatomical anomalies found in the brain of the amusic probands. The amusic brain seems to suffer from impoverished communication in a right-hemisphere–based network involving the inferior frontal cortex (BA 47) and the right auditory cortex (BA 22).19,25 Compared with controls, amusics have less white matter in the right inferior frontal cortex,19 whereas they have thicker cortex in the same right inferior frontal area and the right auditory area.25 These anomalies, which point to abnormal connectivity, neuronal migration, or proliferation, are consistent with malformations during cortical development. These are exactly what genes are coding for.3
In conclusion, congenital amusia is likely to be influenced by several genes that interact, both with each other and with the environment, to produce an overall susceptibility to the development of the disorder (i.e., a complex disorder). Its clear-cut behavioral expression (phenotype) and the identification of a few multiplex families with amusic difficulties facilitates the search for the responsible genes.26
Acknowledgments
We are very grateful to the amusic and control individuals for their continued cooperation. We thank Benoît Gagnon, Aliette Lochy, and Jean Gautier, for the pilot testing of the Internet test, and Sylvie Provost and Amina Barhdadi, for assistance with data analyses. This work was supported by a grant from the Canadian Institutes of Health Research (to I.P.).
Web Resources
The URLs for data presented herein are as follows:
- Amusia, http://www.brams.umontreal.ca/amusia-demo/ (for test examples and the full questionnaire used in the online auditory test)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for SLI, abnormal language development, congenital amusia, dyslexia, autism, and absolute pitch)
References
- 1.Mithen SJ (2005) The singing Neanderthals: the origins of music, language, mind and body. Weidenfeld and Nicolson, London [Google Scholar]
- 2.Bishop DVM, Snowling MJ (2004) Developmental dyslexia and specific language impairment: same or different? Psychol Bull 130:858–886 10.1037/0033-2909.130.6.858 [DOI] [PubMed] [Google Scholar]
- 3.Fisher S, Francks C (2006) Genes, cognition and dyslexia: learning to read the genome. Trends Cogn Sci 10:250–257 10.1016/j.tics.2006.04.003 [DOI] [PubMed] [Google Scholar]
- 4.Peretz I, Hyde K (2003) What is specific to music processing? Insights from congenital amusia. Trends Cogn Sci 7:362–367 10.1016/S1364-6613(03)00150-5 [DOI] [PubMed] [Google Scholar]
- 5.Allen G (1878) Note-deafness. Mind 10:157–167 10.1093/mind/os-3.10.157 [DOI] [Google Scholar]
- 6.Fry DB (1948) An experimental study of tone deafness. Speech: 1–7 [Google Scholar]
- 7.Kalmus H, Fry DB (1980) On tune deafness (dysmelodia): frequency, development, genetics and musical background. Ann Hum Genet 43:369–382 [DOI] [PubMed] [Google Scholar]
- 8.Peretz I (2001) Brain specialization for music: new evidence from congenital amusia. Ann NY Acad Sci 930:153–165 [PubMed] [Google Scholar]
- 9.Stewart L, von Kriegstein K, Warren JD, Griffiths TD (2006) Music and the brain: disorders of music listening. Brain 129:2533–2553 10.1093/brain/awl171 [DOI] [PubMed] [Google Scholar]
- 10.Peretz I, Ayotte J, Zatorre RJ, Mehler J, Ahad P, Penhune VB, Jutras B (2002) Congenital amusia: a disorder of fine-grained pitch discrimination. Neuron 33:185–191 10.1016/S0896-6273(01)00580-3 [DOI] [PubMed] [Google Scholar]
- 11.Ayotte J, Peretz I, Hyde K (2002) Congenital amusia: a group study of adults afflicted with a music-specific disorder. Brain 125:238–251 10.1093/brain/awf028 [DOI] [PubMed] [Google Scholar]
- 12.Peretz I, Champod A-S, Hyde KL (2003) Varieties of musical disorders: the Montreal battery of evaluation of amusia. Ann NY Acad Sci 999:58–75 10.1196/annals.1284.006 [DOI] [PubMed] [Google Scholar]
- 13.Foxton JM, Dean JL, Gee R, Peretz I, Griffiths T (2004) Characterization of deficits in pitch perception underlying tone-deafness. Brain 127:801–810 10.1093/brain/awh105 [DOI] [PubMed] [Google Scholar]
- 14.Cuddy L, Balkwill L-L, Peretz I, Holden R (2005) Musical difficulties are rare: a study of tone-deafness among university students. Ann NY Acad Sci 1060: 311–324 10.1196/annals.1360.026 [DOI] [PubMed] [Google Scholar]
- 15.Zentner MR, Kagan J (1996) Perception of music by infants. Nature 383:29 10.1038/383029a0 [DOI] [PubMed] [Google Scholar]
- 16.Hyde KL, Peretz I (2005) Congenital amusia: impaired musical pitch but intact musical time. In: Syka J, Merzenich M (eds) Plasticity and signal representation in the auditory system. Springer, New York, pp 291–296 [Google Scholar]
- 17.Drayna D, Manichaikul A, de Lange M, Snieder H, Spector T (2001) Genetic correlates of musical pitch recognition in humans. Science 291:1969–1972 10.1126/science.291.5510.1969 [DOI] [PubMed] [Google Scholar]
- 18.Hyde KL, Peretz I (2004) Brains that are out of tune but in time. Psychol Sci 15:356–360 10.1111/j.0956-7976.2004.00683.x [DOI] [PubMed] [Google Scholar]
- 19.Hyde KL, Zatorre R, Griffiths TD, Lerch J, Peretz I (2006) Morphometry of the amusic brain: a two-site study. Brain 129:2562–2570 10.1093/brain/awl204 [DOI] [PubMed] [Google Scholar]
- 20.Risch N (1990) Linkage strategies for genetically complex traits. I. Multilocus models. Am J Hum Genet 46:222–228 [PMC free article] [PubMed] [Google Scholar]
- 21.Stromswold K (1998) Genetics of spoken language disorders. Hum Biol 70:297–324 [PubMed] [Google Scholar]
- 22.Zatorre R (2003) Absolute pitch: a model for understanding the influence of genes and development on neural and cognitive function. Nat Neurosci 6:692–695 10.1038/nn1085 [DOI] [PubMed] [Google Scholar]
- 23.Baharloo S, Service SK, Risch N, Gitschier J, Freimer NB (2000) Familial aggregation of absolute pitch. Am J Hum Genet 67:755–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gregersen PK, Kowalsky E, Kohn N, Marvin EW (2001). Early childhood music education and predisposition to absolute pitch: teasing apart genes and environment. Am J Med Genet 98:280–282 [DOI] [PubMed] [Google Scholar]
- 25.Hyde K, Lerch J, Zatorre R, Griffiths T, Peretz I (2006) Cortical thickness in congenital amusia: when less is better than more. NeuroImage 31,1, Abst 411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Risch N (2000) Searching for genetic determinants in the new millennium. Nature 405:847–856 10.1038/35015718 [DOI] [PubMed] [Google Scholar]