Abstract
Mutations in the fibrillin-1 (FBN1) gene cause Marfan syndrome (MFS) and have been associated with a wide range of overlapping phenotypes. Clinical care is complicated by variable age at onset and the wide range of severity of aortic features. The factors that modulate phenotypical severity, both among and within families, remain to be determined. The availability of international FBN1 mutation Universal Mutation Database (UMD-FBN1) has allowed us to perform the largest collaborative study ever reported, to investigate the correlation between the FBN1 genotype and the nature and severity of the clinical phenotype. A range of qualitative and quantitative clinical parameters (skeletal, cardiovascular, ophthalmologic, skin, pulmonary, and dural) was compared for different classes of mutation (types and locations) in 1,013 probands with a pathogenic FBN1 mutation. A higher probability of ectopia lentis was found for patients with a missense mutation substituting or producing a cysteine, when compared with other missense mutations. Patients with an FBN1 premature termination codon had a more severe skeletal and skin phenotype than did patients with an inframe mutation. Mutations in exons 24–32 were associated with a more severe and complete phenotype, including younger age at diagnosis of type I fibrillinopathy and higher probability of developing ectopia lentis, ascending aortic dilatation, aortic surgery, mitral valve abnormalities, scoliosis, and shorter survival; the majority of these results were replicated even when cases of neonatal MFS were excluded. These correlations, found between different mutation types and clinical manifestations, might be explained by different underlying genetic mechanisms (dominant negative versus haploinsufficiency) and by consideration of the two main physiological functions of fibrillin-1 (structural versus mediator of TGFβ signalling). Exon 24–32 mutations define a high-risk group for cardiac manifestations associated with severe prognosis at all ages.
Marfan syndrome (MFS [MIM 154700]) is a connective-tissue disorder, with autosomal dominant inheritance and a prevalence of 1 in 5,000–10,000 individuals.1 The cardinal features of MFS involve the ocular, cardiovascular, and skeletal systems.2 The skin, lung, and dura may also be involved. MFS is notable for its variability in age at onset, tissue distribution, and severity of clinical manifestations, both among and within affected families. Because of the high population frequency and the nonspecific nature of many of the clinical findings for MFS, clinical diagnostic criteria for this disorder have been established,3–4 the latest being the Ghent criteria, which superseded the previous so-called Berlin criteria.
Study of the molecular determinants of phenotypical variations in MFS has been possible only since the identification of the causative fibrillin-1 (FBN1) gene5 (MIM 134797). Fibrillin-1 has a modular structure, with 47 repeats of six-cysteine epidermal-growth-factor (EGF)–like motifs, 43 of which are of the calcium-binding (cb) type (cb-EGF).6 Fibrillin-1 monomers associate to form complex extracellular macroaggregates, termed “microfibrils,” which are important for the integrity and homeostasis of both elastic and nonelastic tissues.7–8 The protein also contains seven eight-cysteine motifs, which bear homology to motifs found in the latent transforming-growth-factor beta–binding proteins (TGFβ-BPs), and a proline-rich region. The relationship between FBN1 and TGFβ signaling has been underscored by the identification of mutations in TGFBR2 (MIM 190182) in patients with MFS and Marfan-like conditions9 and in pathological studies in knockin and knockout mice.10 Indeed, fibrillins and TGFβ-BPs constitute a family of structurally related and interacting proteins. In MFS mouse models, deficiency of fibrillin-1 alters matrix sequestration of the large latent complex of TGFβ, rendering the cytokine more prone for activation.10 Recently, a specific fibrillin-1 sequence encoded by exons 44–49 has been shown to regulate the bioavailability of endogenous TGFβ1.11
Two major mutation categories—premature termination codons (PTCs) and inframe mutations—have been reported in the FBN1 gene.12 A total of 559 pathogenic mutations were reported in the last update of the Universal Marfan Database (UMD)–FBN1,12 and most of these are unique to individual families. Two-thirds are missense mutations, the majority of which are cysteine substitutions.
Besides classic MFS, mutations in FBN1 have been associated with a broad spectrum of phenotypes, including neonatal MFS,13 isolated ectopia lentis14 (MIM 129600), isolated ascending aortic aneurysm and dissection,15 isolated skeletal features,16,17 and Weill-Marchesani syndrome18 (MIM 608328). So far, genotype-phenotype correlations in FBN1 have been weak except for the cluster of mutations in exons 24–32 reported in neonatal MFS.13,19–22
Indeed, previous reported studies investigating genotype-phenotype correlations were performed with a maximum of 101 patients.23–27 Those authors compared patients with mutations leading to a PTC versus patients with missense mutations, as well as subjects with missense mutations involving a cysteine versus individuals with other missense mutations. Moreover, they focused on major cardiac, ocular, and skeletal involvement. Differences between the groups, with regard to patients’ age at follow-up, were not taken into account.
Large sets of both clinical and molecular data are needed to study (1) the association between FBN1 mutation type and severity of the disease and (2) the specificity of organ involvement in relation to a mutation type. The international UMD-FBN1, set up in 1995, provides these data for 1,013 probands with known FBN1 mutations who were recruited from specialized MFS clinics all over the world. We report the results of the statistical analysis of these data. These results provide possible clues into pathophysiological processes.
Patients, Material, and Methods
Patients
A total of 1,191 probands who had received a diagnosis of MFS or another type I fibrillinopathy were identified between 1995 and 2005 via the framework of the UMD-FBN112 and participating centers. The inclusion criteria in our study were (1) the presence of a heterozygous pathogenic FBN1 gene mutation and (2) the availability of clinical information.
Overall, 178 patients (15%) had to be excluded from the study (no clinical data available for 129; insufficient data about cardiac, ocular, or skeletal involvement for 44; two different mutations on the same allele for 4; and compound heterozygosity for FBN1 mutations for 1). The 1,013 patients included in our study originated from 38 countries on five continents. The majority (72%) were white Europeans or were of European ancestry, 14% were from North and South America, 8% were from Oceania, 4% were from Asia, and 2% were from Africa. Table 1 summarizes the participation of the different laboratories in the study. Patient age at inclusion ranged from birth to 72 years. The clinical data were collected mainly from standardized questionnaires sent to the referring physician, and a minority were from previous publications that reported sufficient clinical data. The clinical information included a range of qualitative and quantitative clinical parameters, including age at diagnosis of MFS or another type I fibrillinopathy and the presence or absence of clinical features including cardiac, ophthalmologic, skeletal, dermal, pulmonary, and dural manifestations. The age at diagnosis and at surgery for aortic dilatation, ectopia lentis, and scoliosis was also noted. All questionnaires were collected by one individual (L.F.) to rule out duplication of patients in the study. To avoid bias as a result of familial clustering, affected family members of a proband were not included in the analysis.
Table 1. .
Number of Patients Recruited to the Study and Their Laboratory of Origin
| Origin | No. of Patients (Laboratories) |
| Belgium | 167 (1) |
| United Kingdom | 166 (7) |
| Germany | 156 (4) |
| France | 154 (3) |
| United States | 146 (8) |
| Italy | 89 (2) |
| Australia | 80 (1) |
| Asia | 22 (6) |
| Other European countries | 22 (3) |
| Other North and South American countries | 11 (2) |
| Total | 1,013 (37) |
The pathogenic nature of a putative mutation was assessed using recognized criteria. In brief, all nonsense mutations, all deletions or insertions (in or out of frame) were considered pathogenic; for all splice mutations, the wild-type and mutant strength values of the splice sites were compared using genetic algorithms,28–30 and only mutations displaying significant deviation from the norm were included. Missense mutations were considered pathogenic when at least one of the following features was found: (1) de novo missense mutation, (2) missense mutation substituting or creating a cysteine, (3) missense mutation involving a consensus cb residue,21 (4) substitution of glycines implicated in correct domain-domain packing,31 and (5) intrafamilial segregation of a missense mutation involving a conserved amino acid. For 38 mutations not displaying one of the above features, additional data provided by SIFT,32,33 BLOSUM-62,34 and biochemical values (Kristine Yu's Web site) were gathered and analyzed using a new UMD tool (M. Frédéric, C. Boileau, D. Hamroun, M. Lalande, M. Claustres, C. Béroud, G. Collod-Béroud, unpublished data).
Involvement of Different Organ Systems
The proportion of each specific clinical feature or system involved was compared in the different groups of mutation types or locations. The following were each considered as a system: the skeleton, the eye, the heart, the skin, and the dura. The clinical features of each system are listed in table 2. No attempts were made to incorporate dilatation of the pulmonary artery, calcification of the mitral valve annulus, apical blebs, flat cornea and iris, or ciliary muscle hypoplasia in the analyses, since those phenotypes were rarely evaluated. Similarly, the axial globe length had rarely been measured, and the definition of myopia varied widely from center to center. For this reason, myopia of any degree was included. In consequence, the presence or absence of minor ophthalmological involvement could not be assessed. The ages at diagnosis and at surgery were collected for scoliosis, ectopia lentis, and aortic dilatation or dissection. The probability of surgery for ectopia lentis was studied only in the group of patients with ectopia lentis. Similarly, the probability of aortic dissection and surgery for aortic dilatation or dissection was studied only in the group of patients with aortic dilatation. The number of systems involved was also assessed according to the Ghent nosology.4 Patients were classified as having MFS if the diagnostic Ghent nosology criteria were met, including the presence of an FBN1 mutation as a major feature and, in a second step, excluding the presence of an FBN1 mutation as a major feature. All other patients were considered as displaying a type I fibrillinopathy. For the purpose of this study and in the absence of consensus diagnostic features, patients were classified as having neonatal MFS when severe features of MFS, including severe valvular insufficiencies, were present before age 4 wk.
Table 2. .
Frequency of Clinical Features in the Different Systems Involved in MFS and Type I Fibrillinopathies (N=1,013)
| System and Clinical Feature(s) | No. of Events | No. of Available Data |
Percentage |
| Skeletal: | |||
| Arachnodactyly | 751 | 969a | 78 |
| Dolichostenomelia | 522 | 947a | 55 |
| Joint laxity | 600 | 956a | 63 |
| Scoliosis | 508 | 965a | 53 |
| Pectus deformityb | 570 | 962a | 59 |
| Limited elbow extension | 153 | 974a | 16 |
| Protrusio acetabulae | 69 | 298a | 23 |
| Facial dysmorphism | 443 | 913a | 49 |
| High-arched palate | 639 | 932a | 69 |
| Dental malocclusion | 372 | 843a | 44 |
| Pes planus | 402 | 864a | 47 |
| Orthopedic surgery | 113 | 983a | 12 |
| Major skeletal involvement | 327 | 1,013 | 32 |
| Minor skeletal involvement | 564 | 1,013 | 56 |
| Ocular: | |||
| Ectopia lentis | 542 | 1,013 | 54 |
| Myopia | 453 | 865 | 52 |
| Cataract | 39 | 983 | 4 |
| Retinal detachment | 65 | 980 | 7 |
| Glaucoma | 19 | 905 | 2 |
| Surgery for ectopia lentis | 122 | 910 | 13 |
| Other eye surgeries | 43 | 905 | 5 |
| Major eye involvement | 542 | 1,013 | 54 |
| Cardiac: | |||
| Dilatation of the ascending aorta | 775 | 1,013 | 77 |
| Dissection of the ascending aorta | 145 | 1,013 | 14 |
| Dilatation or dissection of the descending or abdominal aorta before age 50 years | 66 | 1,013 | 7 |
| Mitral valve prolapse | 533 | 983 | 54 |
| Mitral regurgitation | 313 | 959 | 33 |
| Aortic insufficiency | 205 | 975 | 21 |
| Aortic surgery | 282 | 1011 | 28 |
| Isolated valvular surgery | 45 | 1004 | 4 |
| Major cardiac involvement | 776 | 1,013 | 77 |
| Minor cardiac involvement | 108 | 1,013 | 11 |
| Skin: | |||
| Striae | 444 | 945 | 47 |
| Herniae | 96 | 988 | 10 |
| Minor skin involvement | 480 | 1,013 | 47 |
| Lung: | |||
| Pneumothorax | 73 | 1,002 | 7 |
| Minor lung involvement | 73 | 1,013 | 7 |
| CNS: | |||
| Dural ectasia | 154 | 292 | 53 |
| Major CNS involvement | 154 | 1,013 | 15 |
Nineteen patients were classified as having minor, major, or neither minor nor major skeletal features, but details about their skeletal manifestations were not available.
Includes pectus excavatum, 246 (26%) of 962; pectus carinatum, 288 (30%) of 962; and undefined pectus malformation.
Mutation Screening
Mutation screening, with the consent of the patient or a guardian, was performed in the 38 different laboratories by use of SSCP analysis, denaturing high-performance liquid chromatography, heteroduplex analysis, long-range RT-PCR, or direct sequencing of RNA extracted from cell lines or of genomic DNA from peripheral-blood samples. PTC mutations were classified as those that would be likely to produce no or a truncated FBN1 protein (frameshifts, stop codons, and out-of-frame splice mutations), whereas inframe mutations were classified as missense mutations, inframe deletions/duplications, or inframe splice mutations. Twenty-nine splice mutations could not be classified and were therefore excluded from the analyses that compared types of mutations.
Statistical Analysis
The frequency of many features of MFS increases with age. Since the patients had different lengths of follow-up, χ2 tests are not appropriate for comparing clinical features between groups. Thus, we used a time-to-event analysis technique to estimate a reliable cumulative probability of observing the different manifestations of MFS. This technique could be applied for the following events: diagnosis of type I fibrillinopathy and diagnosis of scoliosis, ectopia lentis, and/or aortic dilatation or dissection, as well as surgery for these different manifestations, for which the ages at diagnosis were systematically collected. In all time-to-event analyses, the baseline date (time zero) was the date of birth. The time-to-event diagnosis was defined as the interval between the baseline date and the date of first observation of the event. Subjects who did not manifest the studied event during the follow-up course were censored at their last follow-up. Subjects for whom the age at diagnosis of a specific manifestation was not available were excluded from these analyses (a maximum of 4% of patients). The number of observations for each clinical feature is indicated in table 2. The Kaplan-Meier method35 was used to estimate the cumulative probabilities of clinical manifestations of the disease at ages 10, 25, and 40 years, to describe the diagnosis of clinical features over time. Clinical differences among the different mutation groups (different locations or types of mutation) were tested using the nonparametric log-rank test. Overall survival was also described and compared, using the same method, according to the type/location of the mutation. To underline the importance of taking into account the time to diagnosis of clinical features, we compared the results obtained for aortic dilatation using a χ2 test and the time-to-event technique.
For the other features (skeletal features other than scoliosis, skin, lung, and dural involvement) for which the age at diagnosis was not collected, age at last follow-up was the only information available about the time of observation of clinical features. To indirectly take into account the patient’s length of follow-up even in this situation, we adjusted all comparisons of MFS manifestation proportions for the age at last follow-up, categorized into 10-year age groups. These adjusted comparisons were performed using the Mantel-Haenszel (MH) test.36 Since this test is appropriate only if the relationship between the mutation type and the clinical manifestation is similar in the different strata of age at last follow-up, we checked the homogeneity between strata using the Breslow-Day χ2 test of homogeneity.37 If an interaction was observed, results were presented for each category of age at last follow-up. In both analyses, if no information was available for a patient’s given clinical feature, he or she was excluded from the analysis of that specific lesion.
To study the effect of mutation types, we compared (1) patients with a PTC with patients with an inframe mutation, (2) patients with a particular subtype of mutation (nonsense, frameshift, splicing, missense, or inframe deletions/insertions) 2 by 2, and (3) patients with missense mutations eliminating or creating a cysteine. There was no recurrent mutation frequent enough to allow a correlation study regarding the other FBN1 mutations. To study the consequences of mutations in different structural and functional domains, we compared (1) patients with a mutation located in an EGF domain (cb and non-cb) with those with a mutation located in a TGFβ-BP domain; (2) patients with a mutation at the 5′ end of the FBN1 gene (exon 1–21, inclusive) with those with a mutation at the 3′ end of the gene (exon 43–65, inclusive), to take into account the regions involved in the processing of the protein; (3) patients with a mutation located within the so-called neonatal region (exons 24–32) with those with a mutation located in other exons; and (4) patients with a mutation located within the TGFβ1 regulating sequence (exons 44–49) with those with a mutation located in other exons. When locations of mutations were compared, studies were performed with all types of mutations and with missense mutations alone, to exclude a bias due to different types of mutations. Mutations located in exons 24–32 were compared with mutations located elsewhere, with and without the inclusion of neonatal MFS.
SAS software version 9.2 and Stata software version 8 were used for all statistical analyses. Only P values <.001 were considered significant, since multiple tests were performed.
Results
Mutations
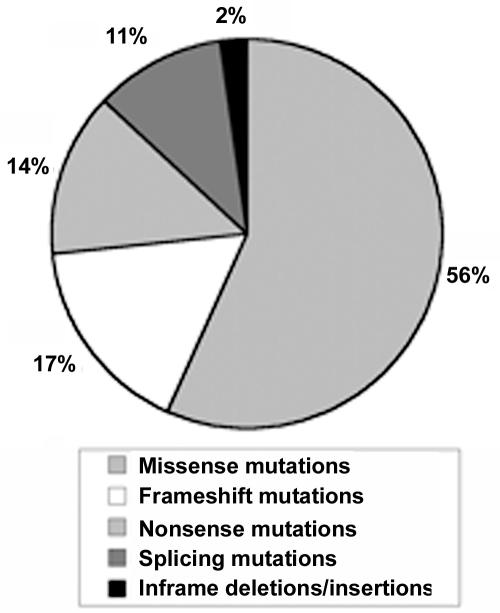
A total of 803 pathogenic mutations were found in 1,013 probands (including 114 recurrent mutations in 324 probands). The distribution of mutations is presented in figure 1. Of the missense mutations, 61% (348 of 573) involved a cysteine (284 replacing and 64 creating a cysteine). Sixty-eight percent (665 of 984) were inframe, whereas 32% (319 of 984) generated a PTC (29 splicing mutations were not classified, since the consequence at the mRNA level could not be determined unambiguously). The majority of mutations were located in an EGF domain (74% [747 of 1,013])—701 of which were located in a cb-EGF domain—and 15% (153 of 1,013) were located in a TGFβ-BP domain. Twenty-nine percent (293 of 1,013) of mutations were found in the 5′ region of the gene, and 379 (37%) were found in the 3′ region of the gene. Twenty percent (198 of 1,013) of mutations were located in exons 24–32. Ten percent (102 of 1,013) of mutations were located in exons 44–49. No major differences in mutation-type categories were detected between laboratories.
Figure 1. .
Types of FBN1 mutations included in the study. Of 1,013, 573 (56%) could be classified as missense mutations, 170 (17%) as frameshift mutations, 137 (14%) as nonsense mutations, 110 (11%) as splicing mutations, and 23 (2%) as inframe deletions or insertions.
Phenotype in the Overall Patient Cohort
Fifty-four percent of patients were males and 46% were females. A family history of MFS was found in 52% of cases. The median age at last follow-up was 29 years (interquartile range [IQR] 15–40 years), including 322 patients aged <18 years (32%). The median age at diagnosis of type I fibrillinopathy was 20 years (IQR 9–34 years). Five percent of patients (n=48) had a neonatal presentation. Overall, at the time of analysis, 61 (6%) had died, 31 (51%) in the context of neonatal MFS, 19 of aortic dissection, 10 during or after aortic surgery, and 1 from a cause unrelated to MFS.
The frequency of manifestations of each organ system in the full cohort of patients is listed in table 2. In particular, of the 1,013 patients, 145 (14%) had dissection of the ascending aorta, 43 (4%) had dissection of the descending aorta, and 30 (3%) had dissection of the abdominal aorta. Protrusio acetabulae and dural ectasia—although they are included in the Ghent nosology—were rarely looked for in our patients (n=298 and n=292, respectively). The majority (89%) of patients could be classified, according to Ghent nosology, as having MFS at their last follow-up, when the presence of an FBN1 mutation was considered a major feature (72% when the presence of an FBN1 mutation was not considered a major feature). Phenotypic differences depending on the sex of the proband were studied for all clinical parameters. Significant differences were found only for the cumulative probability of aortic surgery for patients with aortic dilatation. Indeed, 46% of males had surgery for aortic dilatation before or at age 40 years (99.9% CI 38%–55%) compared with 34% of females (99.9% CI 26%–50%) (P=.0002). A marginally significant result was found for the cumulative probability of ascending aortic dilatation, with a probability of 80% before or at age 40 years in males (99.9% CI 5%–84%) compared with 70% in females (99.9% CI 64%–76%) (P=.0036).
Types of Mutations
Missense mutations substituting or creating a cysteine versus other missense mutations
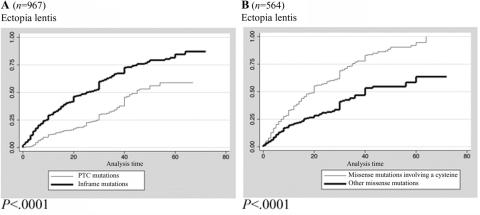
The probability of ectopia lentis was significantly higher with missense mutations involving a cysteine when compared with other missense mutations (log-rank test P<.0001) (fig. 2). The cumulative probability of ectopia lentis diagnosed before or at age 25 years was 59% (99.9% CI 50%–68%) for patients with missense mutations involving a cysteine compared with 32% (99.9% CI 22%–44%) for patients with other missense mutations. Consequently, the percentage of patients with positive Ghent criteria was higher in the group of patients with missense mutations involving a cysteine when compared with other missense mutations (76% vs. 63% when the presence of an FBN1 mutation was not considered as a major feature; MH test P=.0003).
Figure 2. .
Kaplan-Meier analyses for the probability of ectopia lentis diagnoses for patients with different types of mutations. A, Probability of ectopia lentis in PTC versus inframe mutations. The cumulative probability of diagnosis of ectopia lentis before or at age 25 years was 23% (99.9% CI 15%–32%) for patients with PTC mutations (thin line) compared with 50% (99.9% CI 43%–57%) for patients with inframe mutations (thick line) (log-rank test P<.0001). B, Probability of ectopia lentis for patients with missense mutation involving a cysteine versus other missense mutations. The cumulative probability of diagnosis of ectopia lentis before or at age 25 years was 59% (99.9% CI 50%–68%) for patients with missense mutations involving a cysteine (thin line) compared with 32% (99.9% CI 22%–44%) for patients with other missense mutations (thick line) (log-rank test P<.0001).
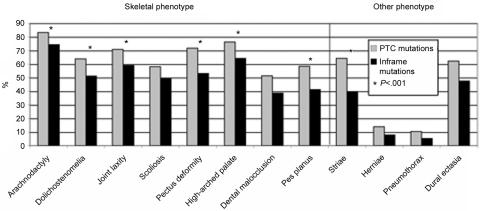
PTC versus inframe mutations.—Patients with a PTC mutation more frequently had major skeletal involvement (40% vs. 28%; MH test P=.0008) with a higher proportion of arachnodactyly, dolichostenomelia, joint hyperlaxity, pectus deformity, high-arched palate, and pes planus. Moreover, a higher frequency of striae distensae (64% vs. 40%; MH test P<.0001) was observed in patients with a PTC mutation (fig. 3). The cumulative probability of a diagnosis of ascending aortic dilatation before or at age 40 years was 77% (99.9% CI 8%–85%) for patients with PTC mutations compared with 74% (99.9% CI 67%–80%) for patients with an inframe mutation (log-rank test P=.7791). Conversely, the cumulative probability of a diagnosis of ectopia lentis and ophthalmologic surgery was significantly lower for patients with PTC mutations compared with patients with an inframe mutation (log-rank test P<.0001 and P=.0001, respectively) (table 3 and figure 2). These results became insignificant for the age at diagnosis of ectopia lentis and ophthalmologic surgery when patients with PTC mutations were compared with patients with missense mutations not involving a cysteine (log-rank test P=.0424 and P=.1020, respectively).
Figure 3. .
Frequency of skeletal, skin, pulmonary, and dural phenotypes in study participants with PTC mutations (gray bars), compared with those with inframe mutations (black bars). An asterisk (*) indicates that differences between groups were statistically significant (MH test P<.001).
Table 3. .
Comparison of Probabilities of Clinical-Feature Diagnosis at a Specific Age for Patients with MFS and Other Type I Fibrillinopathies, According to the Type or Location of FBN1 Mutations[Note]
| Frequency | Probabilities ofClinical FeaturesDiagnosis (%) at Age |
Frequency | Probabilities ofClinical FeaturesDiagnosis (%) at Age |
||||||||
|
n |
(%) |
10 |
25 |
40 |
n |
(%) |
10 |
25 |
40 |
||
| Clinical Feature | PTC Mutations | Inframe Mutations | Pa | ||||||||
| Age at diagnosis of type I fibrillinopathy | 319 | 100.0 | 20.4 | 51.7 | 85.2 | 665 | 100.0 | 32.3 | 61.2 | 86.9 | .0103 |
| Ascending aortic dilatation | 259 | 81.2 | 10.6 | 38.7 | 76.9 | 491 | 73.8 | 20.2 | 43.5 | 73.8 | .7791 |
| Aortic dissection in the population presenting with ascending aortic dilatation | 65 | 25.1 | .0 | 3.6 | 31.1 | 78 | 15.9 | .2 | 5.1 | 22.9 | .2014 |
| Aortic surgery in the population presenting with ascending aortic dilatation | 102 | 39.4 | .0 | 9.4 | 42.0 | 147 | 29.9 | 1.8 | 12.2 | 40.4 | .5301 |
| Survival | 303 |
95.0 |
99.7 |
98.8 |
95.1 |
43 |
6.5 |
95.5 |
94.4 |
93.2 |
.2311 |
| Missense Mutations Involving a Cysteine |
Other Missense Mutations |
||||||||||
| Age at diagnosis of type I fibrillinopathy | 348 | 100.0 | 32.8 | 62.6 | 89.1 | 225 | 100.0 | 28.9 | 57.8 | 82.2 | .0983 |
| Ascending aortic dilatation | 262 | 75.3 | 21.3 | 46.3 | 75.6 | 158 | 70.2 | 17.6 | 37.4 | 69.4 | .0797 |
| Aortic dissection in the population presenting with ascending aortic dilatation | 41 | 15.6 | .0 | 4.6 | 29.9 | 25 | 15.8 | .7 | 5.2 | 10.8 | .4249 |
| Aortic surgery in the population presenting with ascending aortic dilatation | 80 | 30.5 | 1.3 | 14.0 | 47.4 | 49 | 31.0 | 2.7 | 10.4 | 29.0 | .2712 |
| Survival | 330 |
94.8 |
96.5 |
96.1 |
94.5 |
14 |
6.2 |
96.0 |
93.5 |
93.5 |
.6785 |
| Exons 24–32 |
Other Exons |
||||||||||
| Age at diagnosis of type I fibrillinopathy | 198 | 100.0 | 51.0 | 75.8 | 91.9 | 815 | 100.0 | 23.1 | 53.7 | 85.2 | <.0001 |
| Ascending aortic dilatation | 164 | 82.8 | 42.5 | 65.7 | 87.3 | 609 | 74.7 | 11.3 | 36.4 | 72.4 | <.0001 |
| Aortic dissection in the population presenting with ascending aortic dilatation | 21 | 12.8 | .7 | 5.8 | 28.5 | 124 | 20.4 | .2 | 4.3 | 25.7 | .3064 |
| Aortic surgery in the population presenting with ascending aortic dilatation | 52 | 31.7 | 4.7 | 17.5 | 55.2 | 201 | 33.0 | .5 | 9.9 | 38.4 | <.0001 |
| Survival | 159 | 80.3 | 84.5 | 81.1 | 76.1 | 22 | 2.7 | 99.6 | 99.1 | 97.5 | <.0001 |
Note.— All ages are in years. Results are Kaplan-Meier estimates.
Log-rank test P values were for PTC mutations versus inframe mutations, for missense mutations involving a cysteine versus other missense mutations, or for mutations within exons 24–32 versus mutations in other exons.
Other mutation subtypes.—As expected for the results of PTC mutations versus inframe mutations, a lower probability of ectopia lentis was found when nonsense or frameshift mutations were compared with missense mutations (log-rank test P<.0001). No significant difference was found for any manifestation when patients with nonsense mutations were compared with those with frameshift mutations or when patients with missense mutations were compared with those with splicing mutations. A higher probability of ascending aortic dilatation (log-rank test P<.0001) and mitral valve prolapse (log-rank test P=.0007) and a higher frequency of arachnodactyly and joint laxity (MH test P=.0002 and P=.0006, respectively) were found in patients with a mutation eliminating a cysteine than in patients with a mutation creating a cysteine.
Location of Mutations
Exons 24–32 versus other exons
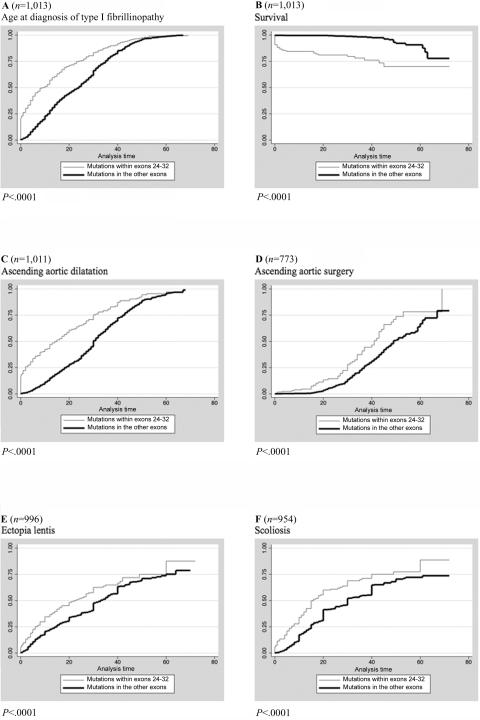
A neonatal onset of the disease was found in 22% (n=42) of patients with a mutation in exons 24–32, compared with 0.6% (n=4) of patients with a mutation in other exons (χ2 test P<.0001). When patients with mutations within exons 24–32 were compared with patients with mutations in the other exons, significant differences were found for joint limitations (34% vs. 11%; MH test P<.0001), scoliosis, ectopia lentis, ascending aortic dilatation, aortic surgery, mitral valve abnormalities (mitral valve prolapse, mitral regurgitation, and/or mitral surgery), younger age at diagnosis of type I fibrillinopathy, and a shorter overall survival (log-rank test P<.001) (table 3 and fig. 4). Indeed, 76% of patients with mutations in exons 24–32 were alive at age 40 years (99.9% CI 61%–87%) compared with 98% (99.9% CI 93%–99%) of patients with mutations located in other exons (log-rank test P<.0001). Moreover, the cumulative probability of ascending aortic dilatation diagnosed before or at age 40 years was 87% (99.9% CI 77%–95%) for patients with a mutation in exons 24–32 compared with 72% (99.9% CI 67%–78%) for patients with a mutation in other exons (log-rank test P<.0001), and the cumulative probability of aortic surgery before or at age 40 years was 55% (99.9% CI 35%–77%) for patients with a mutation in exons 24–32 compared with 38% (99.9% CI 30%–48%) for patients with a mutation in other exons (log-rank test P<.0001). Apart from ectopia lentis, the results were similar when patients with a neonatal onset were excluded. Results were also similar when all types of mutations were included in the analysis and when only missense mutations (except for ectopia lentis) or only missense mutations involving a cysteine were included.
Figure 4. .
Kaplan-Meier analyses for the probability of MFS clinical-features diagnosis for patients with different locations of mutations. A, Age at diagnosis of type I fibrillinopathy with a mutation in exons 24–32 versus in other exons. Fifty percent of patients with a mutation in exons 24–32 (thin line) received a diagnosis at age 9 years (IQR 1–24 years) versus age 24 years (IQR 12–35 years) of patients with a mutation in other exons (thick line) (log-rank test P<.0001). B, Survival of patients with mutations in exons 24–32 versus in other exons. Seventy-six percent of patients with mutations within exons 24–32 (thin line) were alive at age 40 years (99.9%CI 61%–87% years) compared with 98% (99.9% CI 93%–99%) of patients with mutations located in other exons (thick line) (log-rank test P<.0001). C, Probability of diagnosing a dilatation of the ascending aorta for patients with mutations in exons 24–32 versus in other exons. The cumulative probability of diagnosis of ascending aortic dilatation before or at age 40 years was 87% (99.9% CI 77%–95%) for patients with mutations in exons 24–32 (thin line) compared with 72% (99.9% CI 67%–78%) for patients with mutations in other exons (thick line) (log-rank test P<.0001). D, Probability of aortic surgery for patients with mutations in exons 24–32 versus in other exons. The cumulative probability of aortic surgery before or at age 40 years was 55% (99.9% CI 35%–77%) for patients with mutations in exons 24–32 (thin line) compared with 38% (99.9% CI 30%–48%) for patients with mutations in other exons (thick line) (log-rank test P<.0001). E, Probability of ectopia lentis for patients with mutations in exons 24–32 versus in other exons. The cumulative probability of ectopia lentis diagnosis before or at age 25 years was 53% (99.9% CI 39%–67%) for patients with mutations in exons 24–32 (thin line) compared with 38% (99.9% CI 33%–44%) for patients with mutations in other exons (thick line) (log-rank test P=.0003). F, Probability of scoliosis for patients with mutations in exons 24–32 versus in other exons. The cumulative probability of scoliosis diagnosis before or at age 25 years was 61% (99.9% CI 47%–75%) for patients with mutations in exons 24–32 (thin line) compared with 44% (99.9% CI 38%–51%) for patients with mutations in other exons (thick line) (log-rank test P<.0001).
The distribution of types of mutations was significantly different in exons 24–32 from the distribution in the other exons, with an overrepresentation of missense mutations and an underrepresentation of nonsense mutations (table 4) (Fischer test P=.0002). PTC mutations within exons 24–32 were rarely associated with a neonatal MFS presentation (2 [5%] of 43) compared with inframe mutations within this region (41 [95%] of 43). A higher frequency of neonatal presentations was found for mutations in exon 25 when compared with mutations distributed in exons 24–32 (χ2 test P<.0001).
Table 4. .
Types of Mutations Found in Exons 24–32, Compared with Mutations in the Other Exons[Note]
| No. (%) of Mutations in |
||
| Mutation | Exons 24–32 (n=198) |
Other Exons (n=815) |
| Nonsense | 13 (6.6) | 124 (15.2) |
| Frameshift | 27 (13.6) | 143 (17.5) |
| Splicing | 15 (7.6) | 95 (11.7) |
| Missense | 139 (70.2a) | 434 (53.3b) |
| Inframe deletion/insertion | 4 (2.0) | 19 (2.3) |
Note.— Global Fischer exact test for differences was used, according to the location of mutations. P=.0002.
54% involving a cysteine.
63% involving a cysteine.
Exons 44–49 versus other exons
No significant difference was found for any clinical parameter for patients with a mutation located in the specific sequence that regulates the bioavailability of endogenous TGFβ1, compared with those with a mutation located elsewhere.
EGF/TGF β-BP domains
No significant difference was found for any clinical parameter for patients with a mutation located in an EGF domain compared with those with a mutation in a TGFβ-BP domain, nor between patients with a mutation in a cb-EGF domain and those with a mutation in a non–cb-EGF domain. Similar results were found when all types of mutations or only missense mutations were included in the analysis.
5′ versus 3′ mutations
Patients with a mutation located in the 5′ region of the gene had a higher probability of ectopia lentis (log-rank test P=.001). This result was highly significant when only missense mutations were included in the analysis (log-rank test P<.0001), whereas all the other results remained nonsignificant.
Discussion
FBN1 mutations have been associated with a broad spectrum of phenotypes now often called “type I fibrillinopathies,” ranging from single connective-tissue manifestations, such as isolated ectopia lentis, to MFS and lethal neonatal MFS. Every patient with an FBN1 mutation is at risk for developing severe cardiovascular, skeletal, and ophthalmologic complications (L. Faivre, G. Collod-Beroud, B. Loeys, A. Child, C. Binquet, E. Gautier, B. Callewaert, E. Arbustini, K. Mayer, M. Arslan-Kirchner, A. Kiotsekoglou, P. Comeglio, N. Marziliano, D. Halliday, C. Beroud, C. Bonithon-Kopp, M. Claustres, H. Plauchu, P. N. Robinson, L. Adès, J. De Backer, P. Coucke, U. Francke, A. De Paepe, C. Boileau, G. Jondeau, unpublished data). Here, we aimed at identifying the type or location of a given FBN1 mutation that could be associated with the presence of a clinical feature, severity, and/or age at onset. Although no specific manifestation or set of features is pathognomonic for a particular subtype of FBN1 mutation, the occurrence of specific organ involvement differed significantly in some instances.
The mechanism by which mutations in FBN1 result in disease is unclear, since the biochemical pathway of fibrillin-1 assembly into microfibrils is still poorly understood and since the role of fibrillin-1 in TGFβ signaling has only recently emerged. A dominant negative model was first proposed,38–39 in which the mutant monomer disrupts assembly of normal fibrillin-1 into microfibrils or is itself misincorporated into the microfibril. Recent studies have given evidence of a critical contribution of haploinsufficiency in the pathogenesis of MFS.40 Different effects on trafficking have also been demonstrated, with some mutations acting as dominant negative and others as haploinsufficient.41 Here, our data suggest that both genetic mechanisms are involved and that their tissue distribution may differ.
The first striking result of this study is the strong correlation found between ectopia lentis and the presence of a mutation affecting a cysteine residue, confirming earlier conclusions on a smaller sample.24–27,42 It is noteworthy that phenotypes strongly overlapping with type I fibrillinopathies are associated with mutations in TGFBR1/2 and thus altered TGFβ signaling. However, the main feature of these phenotypes, as compared with type I fibrillinopathies, is the almost consistent absence of ocular involvement.43 Thus, it could be speculated that the functional aspect of fibrillin-1 that is altered in patients with ectopia lentis is not involved in TGFβ signaling but is a structural function in the extracellular matrix. Our data suggest that correct cysteine localization and disulfide bonding play an important role in the structural integrity of the suspensory ligaments of the lens, itself relying on the structural function of fibrillin-1 in this organ.44 Also, in the subgroup of patients with a mutation affecting a cysteine residue, we found a significantly higher probability of ascending aortic dilatation and mitral valve prolapse and a higher frequency of arachnodactyly and joint laxity, when comparing patients with a mutation eliminating a cysteine with those with a mutation creating a cysteine. Therefore, it seems that the disappearance of a conserved cysteine residue implicated in a disulfide bond leads to a more severe disorganization of a given module than does the introduction of a new and supernumerary cysteine residue.
The second striking result of our study is the strong correlation between FBN1 PTC mutation and severe skeletal and skin phenotypes. Contrary to what is described in the preceding paragraph, mutations in TGFBR1/2 are associated with skeletal and sometimes skin alterations highly comparable to those found in patients with FBN1. Thus, it is expected that a function or pathway common to fibrillin-1 and the TGFβ type 1/2 receptors is altered in these patients. It could be speculated that haploinsufficiency for fibrillin-1 in bone and skin has a stronger effect on the TGFβ signaling function of the protein than on its structural function—and thus that, in bone growth, fibrillin-1 acts as a mediator of TGFβ signaling. The different correlations found for skeletal and skin manifestations on the one hand versus the ocular system on the other hand might then be explained by differences in the composition and function of fibrillin-rich microfibrils in different organs and further underscore the complexity of the composition of microfibrils and their interactions within tissues. Patients with a mutation in exons 24–32 had a more severe and complete phenotype, including younger age at diagnosis and higher probability of scoliosis, ectopia lentis, ascending aortic dilatation, aortic surgery, mitral valve abnormalities, and shorter survival. However, patients with aortic dilatation and a mutation in exons 24–32 did not present a higher probability of aortic dissection than did patients with aortic dilatation and a mutation located elsewhere. These data can be explained in part by a higher probability of aortic surgery in such patients. It can also be postulated that, because of the general severity of the phenotype, type I fibrillinopathy was diagnosed before the occurrence of aortic dissection in patients with a mutation in exons 24–32. Since the majority of these results were replicated even when neonatal MFS cases were excluded, we conclude that patients with a mutation in exons 24–32 have a poorer prognosis, with earlier onset of morbidity than in patients with a mutation located elsewhere. The presence of a mutation in this region appears to be the best indicator of early-onset aortic risk. More than a “neonatal region,” it should be considered a “severe region.” This result can be explained by the role of these exons in the central stretch of contiguous EGF-like domains and their importance for alignment and stability of the 10-nm microfibrils in the extracellular matrix.45 The distribution of the mutation types in exons 24–32 is different from the distribution found in other exons of the gene. Mutations leading to PTC are significantly underrepresented, contrasting with an overrepresentation of inframe mutations. In particular, nonsense mutations have never to our knowledge been described in association with neonatal MFS, and this observation may be of importance in elucidating the pathogenetic role of the exon 24–32 region.
In this study, we sought to avoid the main bias inherent in our study design. Clinical/molecular correlations are complicated by a wide age range in individuals. In younger patients, the clinical phenotype and symptoms may not be fully developed. Indeed, incorrect significant results can be obtained when χ2 tests are used, and the use of the Kaplan-Meier approach allowed us to take into account the heterogeneity of the length of follow-up among groups, as well as the young median age of the patients in the cohort. Since age at lesion onset cannot be assessed for MFS, notably for aortic dilatation, we used the ages at which each main clinical manifestation was discovered. We considered only probands, to minimize the possible influence of early medical interventions in relatives (earlier monitoring and earlier preventive therapy with β-blockers) and to avoid overrepresentation of a mutation. Finally, we also excluded patients (4%) for whom no information about one of the major systems of MFS (cardiac, ocular, and skeletal system) was available. The low number of excluded patients had no significant impact on our results but provided a homogeneous study population, whatever the clinical feature investigated. However, it should be noted that the majority of patients were of European origin, thus the conclusions may not be totally applicable to all ethnic groups. Another aspect of the proband collection is the high frequency of de novo mutations. It is now well documented that the yield in mutation screening is highest in probands displaying an MFS phenotype diagnosed using the Ghent nosology.24 Furthermore, molecular confirmation of an apparent de novo event is important for genetic counseling in at-risk relatives. Therefore, the important number of de novo mutations found in the probands does not reveal a higher-than-reported mutation rate but reflects practices and screening results from the contributing centers worldwide.
A few studies have addressed the question of a genotype-phenotype correlation in FBN1 carriers. Only five included a sufficient number of patients (101, 93, 57, 81 and 76 patients)23–27 to draw conclusions. A simple χ2 approach was used, and probands as well as their affected family members were considered in two of the five series. Authors mainly compared the different phenotypes related to PTC mutations with those of cysteine substitutions. A significantly higher frequency of ectopia lentis associated with cysteine substitutions when compared with PTC mutations was a consistent finding. A tendency toward a more severe skeletal phenotype23–24 and a more severe cardiac phenotype23,27 in the PTC group was discussed, but with inconsistency in significant results. This could be explained partly by the small sample sizes of the populations studied. The protein phenotypes were studied only by Schrijver et al.23 A preponderance of probands with PTC mutations had markedly reduced extracellular fibrillin deposition with reduced synthesis, whereas individuals with cysteine substitutions had normal levels of fibrillin synthesis and markedly reduced matrix deposition. Genotype-phenotype correlations in type I fibrillinopathies have also been complicated by clinical heterogeneity among individuals with the same mutation, within and among families.46–48 The type or location of a mutation alone cannot explain these variations. The existence of genetic or environmental modifiers, as well as the susceptibility of microfibrillar matrices to proteolytic degradation, or intrafamilial variation in FBN1 expression have been postulated.40,49
In conclusion, our results show a strong correlation between ectopia lentis and the presence of a mutation affecting a cysteine residue, whatever its location within the protein. Conversely, PTC mutations are associated with severe skeletal and skin phenotypes. These correlations found between different mutation types and clinical manifestations may indicate different underlying pathophysiologic mechanisms, both genetic (dominant negative vs. haploinsufficiency) and functional (structural function of fibrillin-1 vs. mediator of TGFβ signaling). Finally, we show that the location of a mutation within the exon 24–32 region is associated with a severe prognosis, not only in newborns but at all ages. However, we believe that these results cannot be used for individual prognosis but show that aortic monitoring is warranted in every patient with an FBN1 mutation.
Acknowledgments
We thank I. Kaitila (Helsinki), P. Khau Van Kien (Montpellier), S. Davies (Cardiff), and T. Uyeda (Irosaki, Japan) for their participation in the study. We also thank C. Bonaïti (Villejuif, France) for her helpful comments in the statistical-analyses design. This work was supported by a grant from the French ministry of health (PHRC 2004), GIS maladies rares 2004, Bourse de la Société Française de Cardiologie, Fédération Française de Cardiologie 2005, and ANR-05-PCOD-014 from the Agence Nationale pour la Recherche. B.C. and B.L.L. are, respectively, a research fellow and a senior clinical investigator of the Fund for Scientific Research–Flanders.
Web Resources
The URLs for data presented herein are as follows:
- Kristine Yu's Web site, http://cmgm.stanford.edu/biochem218/Projects%202001/Yu.pdf (for paper entitled “Theoretical Determination of Amino Acid Substitution Groups Based on Qulaitiative Physicochemical Properties”)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for MFS, FBN1, TGFBR2, isolated ectopia lentis, and Weill-Marchesani syndrome)
- UMD, http://www.umd.be/
References
- 1.Pyeritz RE (1993) Marfan syndrome: current and future clinical and genetic management of cardiovascular manifestations. Semin Thorac Cardiovasc Surg 5:11–16 [PubMed] [Google Scholar]
- 2.Judge DP, Dietz HC (2005) Marfan’s syndrome. Lancet 366:1965–1976 10.1016/S0140-6736(05)67789-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beighton P, de Paepe A, Danks D, Finidori G, Gedde-Dahl T, Goodman R, Hall JG, Hollister DW, Horton W, McKusick VA, et al (1988) International nosology of heritable disorders of connective tissue, Berlin, 1986. Am J Med Genet 29:581–594 10.1002/ajmg.1320290316 [DOI] [PubMed] [Google Scholar]
- 4.De Paepe A, Devereux RB, Dietz HC, Hennekam RC, Pyeritz RE (1996) Revised diagnostic criteria for the Marfan syndrome. Am J Med Genet 62:417–426 [DOI] [PubMed] [Google Scholar]
- 5.Dietz HC, Cutting GR, Pyeritz RE, Maslen CL, Sakai LY, Corson GM, Puffenberger EG, Hamosh A, Nanthakumar EJ, Curristin SM, et al (1991) Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature 352:337–339 10.1038/352337a0 [DOI] [PubMed] [Google Scholar]
- 6.Handford PA (2000) Fibrillin-1, a calcium binding protein of extracellular matrix. Biochim Biophys Acta 1498:84–90 10.1016/S0167-4889(00)00085-9 [DOI] [PubMed] [Google Scholar]
- 7.Sakai LY, Keene DR, Engvall E (1986) Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils. J Cell Biol 103:2499–2509 10.1083/jcb.103.6.2499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pereira L, Andrikopoulos K, Tian J, Lee SY, Keene DR, Ono R, Reinhardt DP, Sakai LY, Biery NJ, Bunton T, et al (1997) Targeting of the gene encoding fibrillin-1 recapitulates the vascular aspect of Marfan syndrome. Nat Genet 17:218–222 10.1038/ng1097-218 [DOI] [PubMed] [Google Scholar]
- 9.Mizuguchi T, Collod-Beroud G, Akiyama T, Abifadel M, Harada N, Morisaki T, Allard D, Varret M, Claustres M, Morisaki H, et al (2004) Heterozygous TGFBR2 mutations in Marfan syndrome. Nat Genet 36:855–860 10.1038/ng1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC (2003) Dysregulation of TGF-β activation contributes to pathogenesis in Marfan syndrome. Nat Genet 33:407–411 10.1038/ng1116 [DOI] [PubMed] [Google Scholar]
- 11.Chaudhry SS, Cain SA, Morgan A, Dallas SL, Shuttleworth CA, Kielty CM (2007) Fibrillin-1 regulates the bioavailability of TGFβ1. J Cell Biol 176:355–367 10.1083/jcb.200608167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collod-Beroud G, Le Bourdelles S, Ades L, Ala-Kokko L, Booms P, Boxer M, Child A, Comeglio P, De Paepe A, Hyland JC, et al (2003) Update of the UMD-FBN1 mutation database and creation of an FBN1 polymorphism database. Hum Mutat 22:199–208 10.1002/humu.10249 [DOI] [PubMed] [Google Scholar]
- 13.Booms P, Cisler J, Mathews KR, Godfrey M, Tiecke F, Kaufmann UC, Vetter U, Hagemeier C, Robinson PN (1999) Novel exon skipping mutation in the fibrillin-1 gene: two “hot spots” for the neonatal Marfan syndrome. Clin Genet 55:110–117 10.1034/j.1399-0004.1999.550207.x [DOI] [PubMed] [Google Scholar]
- 14.Lonnqvist L, Child A, Kainulainen K, Davidson R, Puhakka L, Peltonen L (1994) A novel mutation of the fibrillin gene causing ectopia lentis. Genomics 19:573–576 10.1006/geno.1994.1110 [DOI] [PubMed] [Google Scholar]
- 15.Milewicz DM, Michael K, Fisher N, Coselli JS, Markello T, Biddinger A (1996) Fibrillin-1 (FBN1) mutations in patients with thoracic aortic aneurysms. Circulation 94:2708–2711 [DOI] [PubMed] [Google Scholar]
- 16.Hayward C, Porteous ME, Brock DJ (1994) A novel mutation in the fibrillin gene (FBN1) in familial arachnodactyly. Mol Cell Probes 8:325–327 10.1006/mcpr.1994.1045 [DOI] [PubMed] [Google Scholar]
- 17.Milewicz DM, Grossfield J, Cao SN, Kielty C, Covitz W, Jewett T (1995) A mutation in FBN1 disrupts profibrillin processing and results in isolated skeletal features of the Marfan syndrome. J Clin Invest 95:2373–2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faivre L, Gorlin RJ, Wirtz MK, Godfrey M, Dagoneau N, Samples JR, Le Merrer M, Collod-Beroud G, Boileau C, Munnich A, et al (2003) In frame fibrillin-1 gene deletion in autosomal dominant Weill-Marchesani syndrome. J Med Genet 40:34–36 10.1136/jmg.40.1.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu W, Qian C, Comeau K, Brenn T, Furthmayr H, Francke U (1996) Mutant fibrillin-1 monomers lacking EGF-like domains disrupt microfibril assembly and cause severe Marfan syndrome. Hum Molec Genet 5:1581–1587 10.1093/hmg/5.10.1581 [DOI] [PubMed] [Google Scholar]
- 20.Putnam EA, Cho M, Zinn AB, Towbin JA, Byers PH, Milewicz DM (1996) Delineation of the Marfan phenotype associated with mutations in exons 23–32 of the FBN1 gene. Am J Med Genet 62:233–242 [DOI] [PubMed] [Google Scholar]
- 21.Dietz HC, Pyeritz RE (1995) Mutations in the human gene for fibrillin-1 (FBN1) in the Marfan syndrome and related disorders. Hum Mol Genet 4:1799–1809 [DOI] [PubMed] [Google Scholar]
- 22.Robinson PN, Booms P, Katzke S, Ladewig M, Neumann L, Palz M, Pregla R, Tiecke F, Rosenberg T (2002) Mutations of FBN1 and genotype-phenotype correlations in Marfan syndrome and related fibrillinopathies. Hum Mutat 20:153–161 10.1002/humu.10113 [DOI] [PubMed] [Google Scholar]
- 23.Schrijver I, Liu W, Odom R, Brenn T, Oefner P, Furthmayr H, Francke U (2002) Premature termination mutations in FBN1: distinct effects on differential allelic expression and on protein and clinical phenotypes. Am J Hum Genet 71:223–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loeys B, De Backer J, Van Acker P, Wettinck K, Pals G, Nuytinck L, Coucke P, De Paepe A (2004) Comprehensive molecular screening of the FBN1 gene favors locus homogeneity of classical Marfan syndrome. Hum Mutat 24:140–146 10.1002/humu.20070 [DOI] [PubMed] [Google Scholar]
- 25.Biggin A, Holman K, Brett M, Bennetts B, Adès L (2004) Detection of thirty novel FBN1 mutations in patients with Marfan syndrome or a related fibrillinopathy. Hum Mutat 23:99 10.1002/humu.9207 [DOI] [PubMed] [Google Scholar]
- 26.Arbustini E, Grasso M, Ansaldi S, Malattia C, Pilotto A, Porcu E, Disabella E, Marziliano N, Pisani A, Lanzarini L, et al (2005) Identification of sixty-two novel and twelve known FBN1 mutations in eighty-one unrelated probands with Marfan syndrome and other fibrillinopathies. Hum Mutat 26:494 10.1002/humu.9377 [DOI] [PubMed] [Google Scholar]
- 27.Rommel K, Karck M, Haverich A, von Kodolitsch Y, Rybczynski M, Muller G, Singh KK, Schmidtke J, Arslan-Kirchner M (2005) Identification of 29 novel and nine recurrent fibrillin-1 (FBN1) mutations and genotype-phenotype correlations in 76 patients with Marfan syndrome. Hum Mutat 26:529–539 10.1002/humu.20239 [DOI] [PubMed] [Google Scholar]
- 28.Shapiro MB, Senapathy P (1987) RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression. Nucleic Acids Res 15:7155–7174 10.1093/nar/15.17.7155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Senapathy P, Shapiro MB, Harris NL (1990) Splice junctions, branch point sites, and exons: sequence statistics, identification, and applications to genome project. Methods Enzymol 183:252–278 [DOI] [PubMed] [Google Scholar]
- 30.Beroud C, Hamroun D, Collod-Beroud G, Boileau C, Soussi T, Claustres M (2005) UMD (Universal Mutation Database): 2005 update. Hum Mutat 26:184–191 10.1002/humu.20210 [DOI] [PubMed] [Google Scholar]
- 31.Downing A, Knott V, Werner J, Cardy C, Campbell ID, Handford PA (1996) Solution structure of a pair of calcium-binding epidermal growth factor-like domains: implications for the Marfan syndrome and other genetic disorders. Cell 85:597–605 10.1016/S0092-8674(00)81259-3 [DOI] [PubMed] [Google Scholar]
- 32.Ng PC, Henikoff S (2001) Predicting deleterious amino acid substitutions. Genome Res 11:863–874 10.1101/gr.176601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ng PC, Henikoff S (2003) SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res 31:3812–3814 10.1093/nar/gkg509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henikoff S, Henikoff JG (1992) Amino acid substitution matrices from protein blocks. Proc Natl Acad Sci USA 89:10915–10919 10.1073/pnas.89.22.10915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaplan E, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481 10.2307/2281868 [DOI] [Google Scholar]
- 36.Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22:719–748 [PubMed] [Google Scholar]
- 37.Breslow N, Day N (1987) Statistical methods in cancer research: the design and analyses of cohort studies. Vol 2. International Agency for Research on Cancer Scientific Publications, Lyon [PubMed] [Google Scholar]
- 38.Dietz HC, McIntosh I, Sakai LY, Corson GM, Chalberg SC, Pyeritz RE, Francomano CA (1993) Four novel FBN1 mutations: significance for mutant transcript level and EGF-like domain calcium binding in the pathogenesis of Marfan syndrome. Genomics 17:468–475 10.1006/geno.1993.1349 [DOI] [PubMed] [Google Scholar]
- 39.Eldadah ZA, Brenn T, Furthmayr H, Dietz HC (1995) Expression of a mutant human fibrillin allele upon a normal human or murine genetic background recapitulates a Marfan cellular phenotype. J Clin Invest 95:874–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Judge DP, Biery NJ, Keene DR, Geubtner J, Myers L, Huso DL, Sakai LY, Dietz HC (2004) Evidence for a critical contribution of haploinsufficiency in the complex pathogenesis of Marfan syndrome. J Clin Invest 114:172–181 10.1172/JCI200420641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whiteman P, Handford PA (2003) Effective secretion of recombinant fragments of fibrillin-1: implications of protein misfolding for the pathogenesis of Marfan syndrome and related disorders. Hum Mol Genet 12:727–737 10.1093/hmg/ddg081 [DOI] [PubMed] [Google Scholar]
- 42.Schrijver I, Liu W, Brenn T, Furthmayr H, Francke U (1999) Cysteine substitutions in epidermal growth factor-like domains of fibrillin-1: effects on biochemical and clinical phenotypes. Am J Hum Genet 65:1007–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loeys BL, Schwarze U, Holm T, Callewaert BL, Thomas GH, Pannu H, De Backer JF, Oswald GL, Symoens S, Manouvrier S, et al (2006) Aneurysm syndromes caused by mutations in the TGF-β receptor. N Engl J Med 355:788–798 10.1056/NEJMoa055695 [DOI] [PubMed] [Google Scholar]
- 44.Nemet AY, Assia EI, Apple DJ, Barequet IS (2006) Current concepts of ocular manifestations in Marfan syndrome. Surv Ophthalmol 51:561–575 10.1016/j.survophthal.2006.08.008 [DOI] [PubMed] [Google Scholar]
- 45.Lönnqvist L, Karttunen L, Rantamäki T, Kielty C, Raghunath M, Peltonen L (1996) A point mutation creating an extra N-glycosylation site in fibrillin-1 results in neonatal Marfan syndrome. Genomics 36:468–475 10.1006/geno.1996.0492 [DOI] [PubMed] [Google Scholar]
- 46.Katzke S, Booms P, Tiecke F, Palz M, Pletschacher A, Turkmen S, Neumann LM, Pregla R, Leitner C, Schramm C, et al (2002) TGGE screening of the entire FBN1 coding sequence in 126 individuals with Marfan syndrome and related fibrillinopathies. Hum Mutat 20:197–208 10.1002/humu.10112 [DOI] [PubMed] [Google Scholar]
- 47.Korkko J, Kaitila I, Lonnqvist L, Peltonen L, Ala-Kokko L (2002) Sensitivity of conformation sensitive gel electrophoresis in detecting mutations in Marfan syndrome and related conditions. J Med Genet 39:34–41 10.1136/jmg.39.1.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loeys B, Nuytinck L, Delvaux I, De Bie S, De Paepe A (2001) Genotype and phenotype analysis of 171 patients referred for molecular study of the fibrillin-1 gene FBN1 because of suspected Marfan syndrome. Arch Intern Med 161:2447–2454 10.1001/archinte.161.20.2447 [DOI] [PubMed] [Google Scholar]
- 49.Hutchinson S, Furger A, Halliday D, Judge DP, Jefferson A, Dietz HC, Firth H, Handford PA (2003) Allelic variation in normal human FBN1 expression in a family with Marfan syndrome: a potential modifier of phenotype? Hum Mol Genet 12:2269–2276 10.1093/hmg/ddg241 [DOI] [PubMed] [Google Scholar]