Abstract
Retinal stem cells (RSCs) exist as rare pigmented ciliary epithelial cells in adult mammalian eyes. We hypothesized that RSCs are at the top of the retinal cell lineage. Thus, genes expressed early in embryonic development to establish the retinal field in forebrain neuroectoderm may play important roles in RSCs. Pax6, a paired domain and homeodomain-containing transcription factor, is one of the earliest genes expressed in the eye field and is considered a master control gene for retinal and eye development. Here, we demonstrate that Pax6 is enriched in RSCs. Inactivation of Pax6 in vivo results in loss of competent RSCs as assayed by the failure to form clonal RSC spheres from the optic vesicles of conventional Pax6 knockout embryos and from the ciliary epithelial cells of adult Pax6 conditional knockout mice. In vitro clonal inactivation of Pax6 in adult RSCs results in a serious proliferation defect, suggesting that Pax6 is required for the proliferation and expansion of RSCs.
Keywords: retinal stem cell, ciliary epithelia, Pax6
Introduction
Mammalian adult retinal stem cells (RSCs) have been isolated from the pigmented ciliary epithelia (PCE) of mouse (Tropepe et al., 2000) and human eyes (Coles et al., 2004). Although quiescent in vivo, RSCs persist in the PCE and proliferate to form clonal sphere colonies in vitro in the absence of exogenous growth factors, exhibiting the cardinal stem cell properties of self renewal and multipotentiality. However, little is known about the molecular mechanisms underlying the generation, proliferation and differentiation of these RSCs.
In fish and amphibians, many retinal precursor cells are located in the ciliary margin zone (CMZ) and continuously add new neurons and Muller glial cells to the retina in adult animals (Johns, 1977; Wetts and Fraser, 1988). Cells in CMZ are spatially ordered with respect to the development, with stem cells being most peripheral and differentiating retinal progenitor cells (RPCs) more central in the CMZ. Correspondingly, only the early genes in retinal development, e.g Xrx1, Pax6, XSix3, are expressed in the most peripheral stem cell compartment. Genes expressed later in the development in RPCs are expressed more centrally in the CMZ (Perron et al., 1998). The genes, expressed early to establish the eye field, including ET, Rx1, Pax6, Six3, Lhx2, tll and Six6, have been shown to be critical to retinal development (Zuber et al., 2003). Mutations in these genes result in malformation of the retina and eye or no eyes (Andreazzoli et al., 1999; Hanson et al., 1994; Hill et al., 1991; Jordan et al., 1992; Lagutin et al., 2003; Mathers et al., 1997; Porter et al., 1997; Quiring et al., 1994; Yu et al., 2000). Misexpression of these genes can induce ectopic retinal or eye tissues (Andreazzoli et al., 1999; Bernier et al., 2000; Chow et al., 1999; Halder et al., 1995; Loosli et al., 1999; Mathers et al., 1997; Oliver et al., 1996; Zuber et al., 2003). All of these studies suggest that the early expressed eye field genes may have important functions in the regulation of retinal stem cells. However, the roles of these genes in RSCs have not been fully studied, especially in mammalian RSCs.
To study the molecular mechanisms underlying the generation, proliferation and differentiation of mammalian RSCs, we hypothesized that RSCs are at the top of the retinal cell lineage, and that genes expressed early in embryonic development to establish the retinal field may play important roles in RSCs. Pax6, a paired domain and homeodomain containing transcription factor, is one of the earliest genes expressed in the eye field. It is considered a master control gene for retinal and eye development (Gehring, 1996; Ton et al., 1991; Walther and Gruss, 1991). Misexpression of Pax6 induces ectopic eye structures in Drosophila and Xenopus (Chow et al., 1999; Halder et al., 1995). Conversely, loss of function of Pax6 results in aniridia and Peter’s anomaly in humans (Hanson et al., 1994; Jordan et al., 1992), small eye phenotype in mouse (Hill et al., 1991) and eyeless in Drosophila (Quiring et al., 1994). In Pax6 homozygous mutant mice, retinal development arrests at an early primitive optic vesicle stage (Grindley et al., 1995; Hill et al., 1991). Pax6 mutant optic vesicles have reduced proliferation coupled with precocious adoption of a generic neuronal fate, rather than a specific retinal neuron fate (Philips et al., 2005), suggesting that Pax6 regulates the timing of neurogenesis and retinal specific neuron differentiation in the developing retina. In a conditional knockout mouse model, inactivation of Pax6 in the retina restricted retinal progenitor cell (RPC) differentiation entirely to amacrine cells (Marquardt et al., 2001), suggesting that Pax6 is required for the multipotent state of RPCs. These reports have provided evidence for the importance of Pax6 in retinal development. However, the role(s) of Pax6 in RSCs, especially in the adult RSCs, have not been directly illustrated. We employed both the conventional and conditional Pax6 knockout mouse models (Marquardt et al., 2001; St-Onge et al., 1997) to present direct evidence that Pax6 is required for the proliferation and expansion of RSCs.
Experimental Procedures
Animals
The Z/EG reporter mice (Novak et al., 2000) were provided by Drs. Andras Nagy and Corrine Lobe. The Pax6LacZ/LacZ (St-Onge et al., 1996; St-Onge et al., 1997) and α–Cre;Pax6loxP/loxP mice (Marquardt, 2003; Marquardt et al., 2001) kindly were provided by Dr. Peter Gruss.
Immunohistochemistry
Pax6, Syntaxin (HPC-1), Rhodopsin (1D4) and nestin antibodies were purchased from Developmental Hybridoma Bank, Sigma and Chemicon respectively. Immunohistochemistry was performed as previously described (Tropepe et al., 2000).
RNA preparation and RT-PCR
Total RNA was isolated using RNeasy kit (Qiagen). RT-PCR was performed using OneStep RT-PCR kit (Qiagen). Primer sequences: Pax6: Fw1:5′-TCACAGCGGAGTGAATCAGC-3′ and Rev1:5′-TATCGTTGGTACAGACCCCCTC-3′; Fw2:5′-CGGAGTGAATCAGCTTGGT-G-3′ and Rev2:5′-GTTGGTACAGACCCCCTCGG-3′. GAPDH: Fw:5′-TGCACCACCAACTGCTTAGC–3′ and Rev: 5′-TGGATGCAGGGATGATGTTC-3′. 18s rRNA: Fw:5′-GTAACCCGTTGAACCCCATT-3′; Rev: 5′-CCATCCAATCGGTAGTAGCG-3′. 50 ng of total RNA or 1/4 of the RNA from a single sphere was used in the first round Pax6 amplification with Fw1/Rev1. The reaction program was 50 °C for 30 minute for RT, 95 °C for 15 minutes to inactivate RT, followed by 25 cycles of 95°C for 30 seconds, 60 °C for 1 minute, and 72 °C for 1 minute and then 72 °C for 10 minutes. 2 μl of 1:10 dilutant of the first round amplification product was used in Pax6 nested PCR with primers Fw2/Rev2. The primers encompass the alternatively spliced exon5a (Glaser et al., 1992; Ton et al., 1991; Walther and Gruss, 1991). The amplicons are 367 base pairs (bp) (without exon5a) and 409 bp (with exon5a).
Embryonic day (E)14 and adult RSC primary culture
The dissection and primary culture were performed as described previously (Tropepe et al., 2000). Briefly, for adult RSC isolation: adult mice were sacrificed by cervical dislocation. Eyes were removed and placed in oxygenated artificial cerebral spinal fluid (aCSF: 124mM NaCl, 5 mM KCl, 1.3 mM MgCl2, 2mM CaCl2, 26 mM NaHCO3, and 10 mM D-glucose, pH 7.4). Each eye was bisected and the ciliary body was isolated as a thin strips and placed in dispase (Invitrogen) for 10 minutes at 37°C, followed by trypsin/hyaluronidase treatment [1.33 mg/mL trypsin (Sigma), 0.67 mg/mL hyaluronidase (Sigma) and 0.2 mg/mL kynurenic acid (Sigma) in aCSF modified with high Mg2+ (3.2 mM MgCl2) and low Ca2+ (0.1 mM CaCl2)] at 37°C for 15 minutes. Ciliary epithelial cells were scraped off from the strips and collected in serum free medium (SFM) with trypsin inhibitor (1 mg/mL. Roche Diagnostics). The cells were triturated 60 times to a single cell suspension. Cells were resuspended in SFM with FGF2 (10 ng/mL) and heparin (2 μg/mL), plated at a low clonal density (10 cells/μL) and incubated at 37°C in a CO2 (5%) incubator (Thermo Electron Co) for 7 days.
For E14 embryos: the eyes were carefully removed and placed in dispase (Invitrogen) at 37°C for 30 seconds. RPE and neural retina (NR) were separated, collected in SFM and triturated about 60 times to achieve a single cell suspension. The cells were spun down and resuspended in SFM with FGF2 (10 ng/mL) and heparin (2 μg/mL). The NR cells were plated at 10 cells/μL. All RPE cells from each animal were plated in 4 wells of a 24-well plate and the total numbers of RSCs were assessed as the total number of RSC spheres formed. In Pax6 homozygous knockout (Pax6LacZ/LacZ) embryos, retinal development was aborted at the primitive optic vesicle stage, which consisted of a thin epithelial sheet, without the thickening of the inner neuroretinal layers and the differentiation of the RPE layer. To evaluate the RSC status in these Pax6LacZ/LacZ embryos, each entire primitive optic vesicle carefully was dissected, dissociated into single cells, and plated in 4 wells of a 24-well plate. Cells were incubated at 37°C in a CO2 (5%) incubator for 7 days.
Three and four independent experiments were performed at the adult and E14 time points, respectively.
Fluorescent Activated Cell Sorting (FACS)
The ciliary epithelial cells of adult Pax6lacZ/wt or wild type littermates were isolated and dissociated into single cells in 200 μL of SFM. The cells were separated into two aliquots; one was treated with 100 μM of a fluorescent β-gal substrate: 5′-chloromethylfluorescein di-β-D-galactopyranoside (CMFDG) (Invitrogen), the other was used as negative control. Both aliquots were incubated at 37 °C for 25′. The cells were spun down and resuspended in 500 μl of SFM+FGF2 (10 ng/ml)+ heparin (2 mg/ml). Propidium iodide (PI) (1.5 μM) was added to assay for live/dead cells. Total live cells were sorted by the green fluorescence from breakdown product of CMFDG, plated at clonal density (<10 cells/μl) and incubated at 37 °C for 1 week.
Retroviral infection
Bi-cistronic pMXIE-Cre-EGFP virus was constructed by inserting a nlsCre cassette in front of the IRES-EGFP in a replication-incompetent retroviral construct pMXIE-EGFP (Hitoshi et al., 2002). Ciliary epithelial cells from adult Pax6flox/flox or Pax6wt/wt animals were isolated and dissociated to single cells and plated in fibronectin-coated 24-well plates at 10 cells/ul. Retrovirus (pMXIE-Cre-EGFP or pMXIE-EGFP) was added 18-24 hours after plating (m.o.i=10). The infected cells (GFP+) were counted under the fluorescent microscope (Olympus) every 24 hours. For differentiation, differentiation medium (SFM+FGF2+heparin+1% Fetal Bovine Serum (FBS)) was added 24 hours after viral infection and was changed every 4 days. After 21 days, cells were fixed with 4% paraformaldehyde. Immunostaining with the amacrine cell marker, HPC-1, or the rod photoreceptor marker, 1D4, was performed.
Results
Pax6 is expressed in the adult retinal stem cell niche- the ciliary epithelia
To study the role of Pax6 in RSCs, we first asked how Pax6 is expressed in the adult RSC niche – the pigmented ciliary epithelia. It has been shown that Pax6 is expressed in the ciliary epithelia and the epithelia of the iris (Marquardt et al., 2001). By immunohistochemistry and confocal microscopy, Pax6 was detected in both layers of the ciliary epithelia (Fig.1A). However, Pax6 is expressed at a much higher level in the outer layer (arrows in Fig.1A), where pigmented RSCs are found (Tropepe et al., 2000), than in the inner layer (arrowheads in Fig.1A). Pax6 expression was further confirmed by RT-PCR on RNA isolated from ciliary epithelia and clonally derived RSC spheres from adult mice (Fig.1B). As controls, Pax6 expression also was assessed and detected in adult neural retina (NR), forebrain neural stem cell (NSC) spheres (Reynolds and Weiss, 1996), embryonic stem (ES) cell-derived NSC spheres (ESDS) (Tropepe et al., 2000; Tropepe et al., 2001) and ES cells. The primers in the RT-PCR assays encompass the alternatively spliced exon5a (Glaser et al., 1992; Ton et al., 1991; Walther and Gruss, 1991). Although both forms of Pax6 transcripts, with or without exon5a (+5a or -5a), were expressed, the ratios of -5a transcript to +5a transcript were different in different tissues (Fig 1C). As in the neural retina, the long transcript with exon5a (+5a) was predominant in RSCs and in the ciliary epithelium (Fig 1 B & C), while the short transcript (-5a) was predominant in NSC spheres. The ESDS and undifferentiated ES cells expressed almost exclusively the short form. The alternatively spliced exon5a encodes a 14 amino acid insertion in the paired domain, which changes the binding specificity of the paired domain (Epstein et al., 1994; Glaser et al., 1992) and may contribute to the different functions of Pax6 in different tissues (Azuma et al., 2005; Dominguez et al., 2004).
Fig. 1. Pax6 is expressed in the adult ciliary epithelia and RSC spheres.
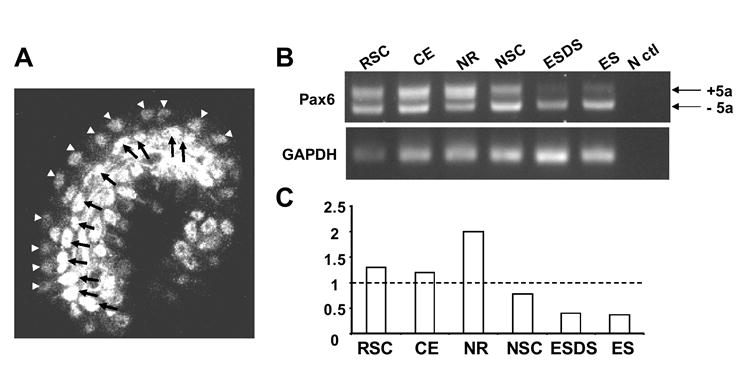
A. confocal image of Pax6 immunostaining of a ciliary process; Anti-Pax6 Monoclonal antibody stains the nuclei of the positive cells. White arrowheads show the less intensely stained the ciliary epithelial cells of the inner layer; black arrows indicate the more intensely stained ciliary epithelial cells in the outer layer. The arrowheads and arrows mark the epithelial cells on one side of the ciliary process. B. RT-PCR of Pax6 gene expression. Pax6 is detected in clonally derived RSC spheres (RSC) and ciliary epithelia (CE) of adult mice. N ctl: negative control (amplification without reverse transcription). Amplicons encompass the alternatively spliced exon 5a (Ton et al., 1991; Walther and Gruss, 1991). The amplification products with or without alternatively spliced exon 5a were labeled as +5a and -5a. C. The ratio of the +5a and −5a transcripts normalized by the control housekeeping gene GAPDH.
Selection for Pax6 enriches for retinal stem cells
To further investigate if Pax6 is expressed in RSCs and its role as a possible intrinsic regulating factor for RSCs, we employed the knockout/knockin mouse model (St-Onge et al., 1997), in which the Pax6 gene, from the start codon through the entire paired domain, was replaced by β-galactosidase (β-gal) gene. Therefore, the β-gal expression is under the control of the endogenous Pax6 promoters and can be used as a marker for Pax6 expression. In the heterozygous Pax6LacZ/wt ciliary epithelial cells, the wild type allele provides the Pax6 function, while the mutant allele with the β-gal gene provides a marker for Pax6 expression. Taking advantage of this, the ciliary epithelial cells from adult Pax6lacZ/wt mice were treated with a fluorescent β-gal substrate CMFDG and were sorted into three non-overlapping portions by the intensity of their fluorescence: R1, R2 and R3 (Fig.2 A&B). Subsequent clonal RSC sphere assays showed that cells with low or no Pax6 expression (R1 and R2) did not give rise to any RSC spheres (Fig.2 A&B and table I). Only the cells with high Pax6 expression (R3) gave rise to clonal RSC spheres at a frequency of 1/76, at least a 6.6-fold enrichment of RSCs, compared to the 1/500 frequency seen in non-sorted ciliary epithelial cells (Tropepe et al., 2000). This result suggests that high levels of Pax6 expression are seen in RSCs and that selection for Pax6 enriches for RSCs. The power of enrichment of RSCs by high levels of Pax6 expression might be underestimated here, because the heterozygous Pax6LacZ/wt cells used in this experiment have only one functional Pax6 allele. We have observed a 56% reduction of sphere formation in the adult Pax6LacZ/wt ciliary epithelial cells (fig. 2C) and a similar reduction in E14 embryonic heterozygous RSCs (Fig. 2D), compared to the wild type. Therefore, we predict a higher RSC enrichment in the wild type cells based on their Pax6 expression.
Fig. 2. Pax6 is enriched in retinal stem cells and required for the generation of clonal RSC spheres.
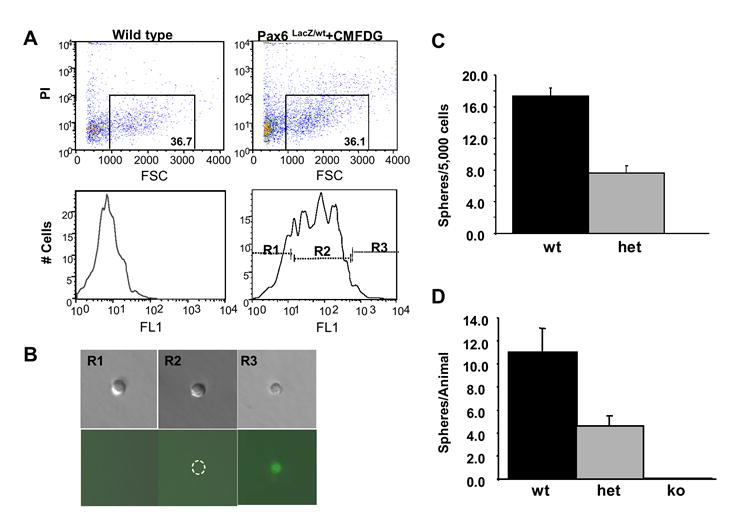
A. (top) Profiles of the non-treated wild type and CMFDG-treated adult Pax6lacZ/wt cells sorted by propidium iodide (PI) and forward scattered count (FSC). The rectangles show the live single cells. The numbers in the rectangles are percentages of live cells. (bottom) Profiles of the live single cells sorted by the CMFDG reaction elicited fluorescence. R1, R2 and R3 indicate the three non-overlapping portions we collected for the clonal RSC sphere assay in table I; B. Examples of the cells from R1, R2 and R3 portions under light microscopy (top) and fluorescent microscopy (bottom). Note that only cells from R3 portion have significant fluorescence elicited from CMFDG/β-galactosidase reaction; the dashed circle in the R2 bottom panel marks the R2 cell with very weak fluorescence; C. Clonal RSC sphere assays on adult Pax6lacZ/wt (het) and wild type (wt) mice; D. Clonal RSC sphere assays on embryonic day 14 (E14) Pax6LacZ mice. ko: Pax6LacZ/LacZ (n=9). het: Pax6LacZ/wt (n=16); wt: wild type littermates (n=13).
Table 1.
Summary of the clonal RSC sphere assays after the FACS sorting
| R1 | R2 | R3 | |
|---|---|---|---|
| Cells Collected | 5089 | 15,396 | 832 |
| Spheres Formed | 0 | 0 | 11 |
| Frequency | 0 | 0 | 1/76 |
Inactivation of Pax6 function in vivo results in loss of competent retinal stem cells at embryonic day 14 (E14)
To study the role of Pax6 in the early development of RSCs, we performed clonal RSC sphere assays on embryonic day (E) 14 Pax6LacZ mice (Fig.2D). At this stage in wild type mice, RSCs reside in the peripheral retinal pigmented epithelium (RPE), which includes the presumptive pigmented ciliary epithelia where the adult RSCs reside (Tropepe et al., 2000). In Pax6LacZ/LacZ embryos, retinal development arrests at the early optic vesicle stage (Grindley et al., 1995; Hill et al., 1991). To evaluate the total RSC population of these embryos, we harvested the entire rudimentary optic vesicles from Pax6LacZ/LacZ embryos and performed clonal RSC sphere assays. No RSC spheres were derived from Pax6LacZ/LacZ optic vesicle cells. In Pax6LacZ/wt heterozygote, we found a 58% reduction in the number of RSC spheres compared to the wild type. These results strongly suggest that loss of Pax6 function led to a complete loss of competent RSCs in Pax6 knockout embryos and that a decreased level of Pax6 in the heterozygote resulted in a reduction in RSC population. Thus, Pax6 is required for the generation of clonal RSC spheres.
The RSC spheres are composed by a few stem cells, but the majority of the cells in the spheres are progenitor cells. The failure to form RSC spheres could be a result of defects in either the stem cells or the progenitor cells or both. At E14, the neural retina contains RPCs, which can proliferate and form primary PRC spheres, which, however, cannot be clonally passaged because of their limited self-renewal ability (Tropepe et al., 2000). A clonal RPC sphere assay on the heterozygote and wild type controls showed a slight, but not significant decrease in the numbers of RPC from the heterozygote when compared to the wild type [3.6+/- 0.7, (n=12) in the heterozygote versus 4.9 +/- 1.0 (n=9) per 5,000 cells in the wild type, p=0.27 (Kruskal-Wallis test)]. This suggests that the effect of inactivation of Pax6 on clonal RSC sphere formation can not be explained fully by its effect on RPCs and that inactivation of Pax6 may have a direct effect on the proliferation of RSCs.
Pax6 is required for RSC sphere formation in adult animals
The Pax6LacZ/LacZ conventional knockout mice die soon after birth. Therefore, to directly study the role of Pax6 in RSCs in adult mice, we employed the α-Cre; Pax6loxP/loxP conditional knockout mouse model (Marquardt et al., 2001), in which the endogenous Pax6 gene was replaced by a modified Pax6 allele, Pax6loxP, in which exons 4-6 are flanked by two loxP sites. The expression of Cre recombinase is under the control of the Pax6 retinal specific regulatory element, α promoter (Kammandel et al., 1999), which targets the expression of Pax6 to the peripheral neuroretina, ciliary epithelia and the iris. A RSC sphere assay on the adult conditional knockout mice (Fig.3A) revealed that there were 23% (p<0.05) and 72% (p<0.05) decreases in the numbers of clonal RSC spheres derived from the heterozygote and conditional knockout, respectively, compared to wild type This suggests that inactivation of Pax6 results in a negative effect on the competence of RSCs to form RSC spheres and possibly the maintenance of RSCs in adult animals.
Fig. 3. Pax6 is required for RSC sphere formation in adult animals.
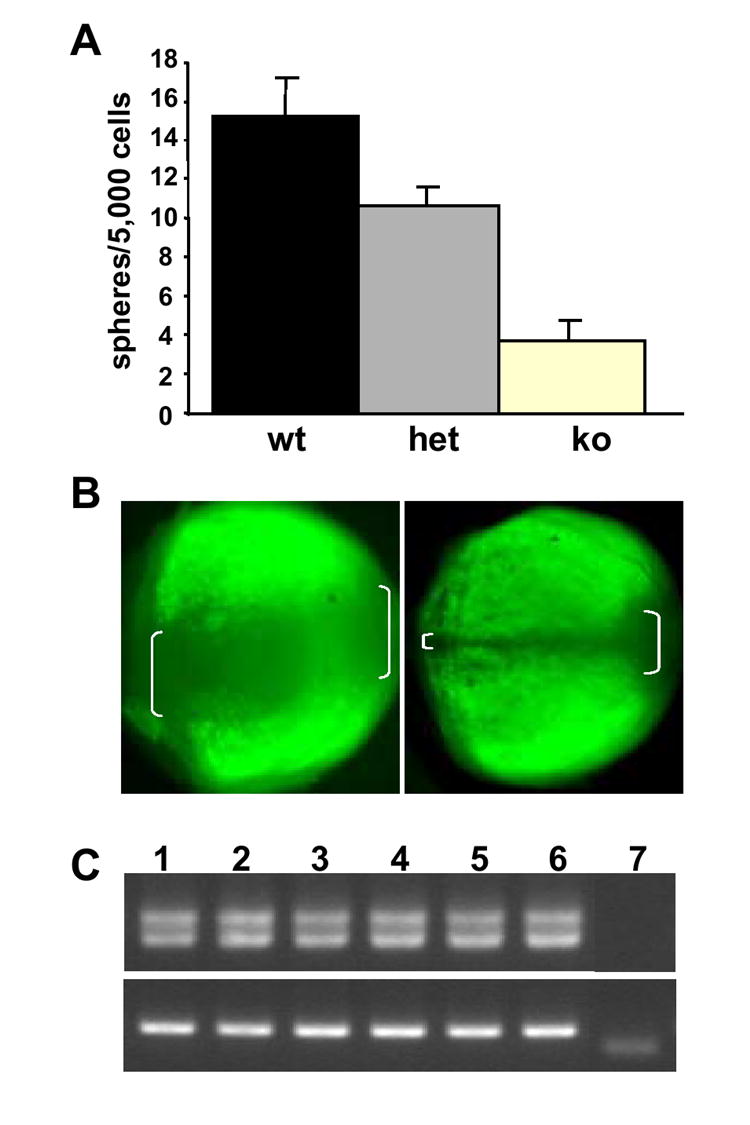
A. RSC sphere assays in the Pax6 conditional knockout mouse model (Marquardt et al., 2001). ko: α-Cre +/-; Pax6loxP/loxP (n=4). het: α-Cre +/-; Pax6loxP/wt (n=4). wt: α-Cre -/-; Pax6wt/wt (n=4). B. whole mount fluorescent images of adult α-Cre, Z/EG mouse eyes showing the α-promoter-driven expression has gaps (marked by white brackets) as seen from dorsal (left) and ventral (right) views of the retina. Anterior is on the left. C. RT-PCR of Pax6 on the clonal RSC spheres derived from adult α-Cre; Pax6loxP/loxP ciliary epithelial cells. The same primers, encompassing the alternatively spliced exon 5a were used. 1: wild type single RSC sphere; 2-6: RSC spheres derived from α-Cre; Pax6loxP/loxP CE cells, 7: negative control (amplification without reverse transcription).
It has been shown that the α-promoter activity has a dorsal expressional gap at E13.5 (Kammandel et al., 1999; Marquardt et al., 2001). If this expression gap persists in the adult mice, Cre recombinase in the α-Cre transgenic animals would fail to be expressed in the gap region, and hence, Pax6 function wouldn’t be inactivated in the gap. Therefore, we hypothesized that the spheres from the α-Cre;Pax6loxP/loxP mice might have been derived from the RSCs that escaped Cre excision. To confirm the α-promoter controlled expression in adult mice, we generated α-Cre;Z/EG double transgenic mice. The Z/EG line (Novak et al., 2000) is a Cre reporter line: Cre excision removes the lacZ gene and activates the expression of the second reporter, enhanced green fluorescent protein (EGFP). The α-Cre;Z/EG double transgenic mice showed that the α promoter not only has the dorsal expression gap, but also a ventral gap in the adult mice that extended into the adult peripheral retina and ciliary epithelia (Fig. 3B). To further confirm our hypothesis that the residual spheres from the α-Cre;Pax6loxP/loxP mice were derived from the RSCs that have escaped Cre excision, we performed RT-PCR on the RNA samples from these spheres. Indeed, all of the spheres derived from the α-Cre;Pax6loxP/loxP mice were actually Pax6 positive (figure 3C). Therefore, no RSC spheres were derived from true Pax6 knockout ciliary epithelial cells in adult mice, suggesting that Pax6 is required for clonal RSC sphere formation.
Inactivation of Pax6 inhibits the proliferation of retinal stem cells
To study the mechanism(s) of Pax6 on the maintenance of RSCs, we infected Pax6loxP/loxP and wild type adult ciliary epithelial cells in vitro with a bi-cistronic Cre-EGFP retrovirus (pMXIE-Cre-EGFP) (Fig.4A), which expresses both Cre recombinase and EGFP in the infected cells. Since, only RSCs in the adult ciliary epithelial cells divide and only the dividing cells can be infected with retrovirus, we could use EGFP expression to trace the infected clonal RSCs in which Pax6 function is inactivated by the Cre recombinase. Four days after viral infection, the infected cells still retained a retinal precursor phenotype (Fig. 4B). However, the average number of progeny in each GFP positive RSC clone derived from a single RSC was reduced by 65% in Cre infected Pax6loxP/loxP clones compared to the Cre infected wild type (Pax6wt/wt) RSC clones (Fig.4C). 91% of the Cre virus-infected Pax6loxP/loxP RSC clones had only one or two cells/clone (Fig.4D). Only one of these Pax6-/- clones had 4 GFP-positive cells, and none of the clones contained more than 4 GFP positive cells. In contrast, 43% of the wild type infected clones had more than 4 progeny, including 2 clones (~7.6%) with 10 or more GFP positive cells. We did not observe decreases in RSC clone size when using the control retrovirus (pMXIE-EGFP) in Pax6loxP/loxP or Pax6wt/wt RSCs (4.8+/- 0.6 and 4.1 +/- 0.5 cells/clone, respectively). The Cre-EGFP virus-infected RSCs survived as well as the control virus (pMXIE-EGFP) infected cells. Indeed, we observed more clones in the pMXIE-Cre-GFP infected Pax6loxP/loxP cells than in the wild type cells (total numbers of clones observed: in wild type cells: n=35 with the Cre-EGFP virus and n=65 with the EGFP virus; in Pax6loxP/loxP cells: n=55 with the Cre-EGFP virus and n=26 with the EGFP virus). Therefore, the smaller number of cells per clone in pMXIE-Cre-EGFP infected Pax6 mutant RSCs cannot be due to more cell death of RSCs. The best explanation for this result is that inactivation of Pax6 inhibits the proliferation of RSCs.
Fig. 4. Inactivation of Pax6 inhibits the proliferation of RSCs in vitro.
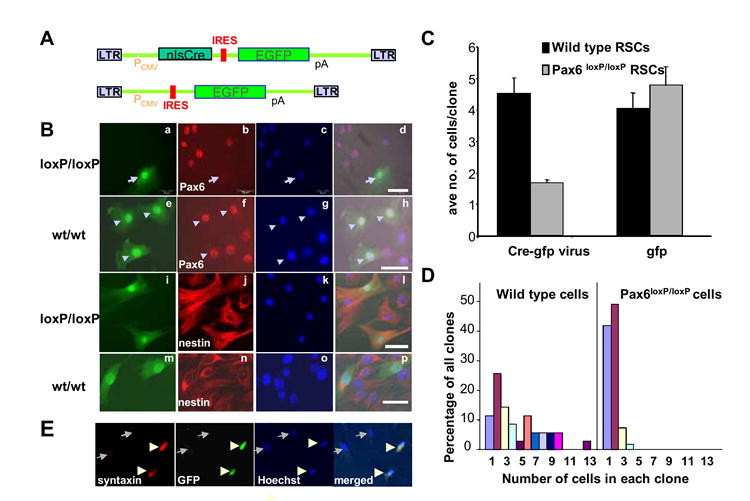
A. pMXIE-Cre-EGFP (top) and pMXIE-EGFP (bottom) retroviral constructs. PCMV: CMV promoter; nlsCre: Cre recombinase with a nuclear localization signal (nls); EGFP: enhanced green fluorescent protein; IRES: internal ribosomal entry site; pA: polyA signal. B. Cre-EGFP retroviral infection of adult Pax6loxP/loxP and wild type RSCs. a-h: Pax6 is inactivated in the Cre-EGFP infected Pax6loxP/loxP RSCs (arrows in a-d), but not the wild type RSCs (arrowheads in e-h). a,e: GFP channel showing the infected cells; b,f: Pax6 immunostaining; c,g: Hoechst nuclear staining; d,h: merged images. i-p: RSCs infected with Cre-EGFP retrovirus retain progenitor identity with neural progenitor marker nestin expression. i,m: GFP channel showing the infected cells; j,n: immunostaining of nestin. k,o: Hoechst nuclear staining; l,p: merged images. GFP expression marks the infected RSCs and their progeny. C: Average number of progeny in each clone derived from single viral infected adult RSCs 4 days after retroviral infection in vitro. Data represent means +/- SEMs. D. Distribution of different sized clones derived from single virus-infected RSCs 4 days after Cre-EGFP retroviral infection of wild type or Pax6loxP/loxP ciliary epithelial cells. E. Immunohistochemistry of differentiated RSCs from Pax6loxP/loxP animals 21 day after pMXIE-Cre-EGFP viral infection with syntaxin monoclonal antibody. Arrowheads showed two infected cells, which are expressing the amacrine cell marker syntaxin. Arrows pointed two non-infected cells, which were syntaxin negative.
Previously, Marquardt et al showed that Pax6 inactivation results in the exclusive generation of amacrine cells in the retina (Marquardt et al., 2001). To determine the function of Pax6 on fate determination in adult RSCs and their progeny, we differentiated retrovirus-infected RSC colonies on laminin coated slides. The results showed that inactivation of Pax6 by retroviral expression of Cre-EGFP in Pax6loxP/loxP RSCs resulted in differentiation almost exclusively into amacrine cells using syntaxin as a marker (97.2 +/- 2.8% versus 12.8 +/- 1.1% in wild type infected RSC colonies) (Table 2 and figure 4E), consistent with the previous observation by Marquardt et al in α–Cre;Pax6loxP/loxP animals in vivo. In contrast, only 0.95 +/- 0.9% of infected cells are rhodopsin (1D4) positive photoreceptors amongst the Pax6loxP/loxP cells compared to 21.8% +/- 1.7% in wild type infected RSC colonies. Our data suggest that inactivation of Pax6 produces two effects: inhibiting the proliferation of RSCs, and separately, directing the differentiation of RSC progeny to an amacrine cell fate.
Table 2.
Differentiation assays on the pMXIE-Cre-EGFP retroviral infected RSCs
| Cell type | Pax6Flox/flox | Pax6wt/wt |
|---|---|---|
| Amacrine cells (syntaxin positive) | 97.2±2.8 | 12.8±1.1 |
| Rod photoreceptors (1D4 positive) | 0.95±0.9 | 21.8±1.7 |
Discussion
Mammalian adult RSCs exist as rare pigmented ciliary epithelial cells. They are quiescent in vivo, but they persist through their adult lives, maintaining their capability to self renew and differentiate into mature retinal cell types (Coles et al., 2004; Tropepe et al., 2000). Previously, we demonstrated that the numbers of retinal progenitors negatively regulate the numbers of RSCs by a cell non-autonomous mechanism in vivo (Coles et al., 2006; Tropepe et al., 2000). Here, we reveal directly that Pax6 is required for the proliferation and thus the expansion of RSCs. In vivo, this serious proliferation defect in Pax6-/- RSCs would preclude the expansion of the first RSCs by symmetrical divisions in the embryonic optic cup, as well as inhibit the expansion of the retinal progenitor populations through asymmetric proliferation of RSCs.
Our result showed that Pax6 is highly expressed in the retinal stem cell niche- the pigmented ciliary epithelia. Previously, Pax6 has been shown to be expressed in the ciliary epithelia (Das et al., 2005; Marquardt et al., 2001). By immunostaining and confocal microscopy, the present report reveals that Pax6 expression is not equal in the two layers of the ciliary epithelia (Fig 1). Pax6 is expressed at a much higher level in the outer layer than the inner layer of the ciliary epithelia. The inner layer of the ciliary epithelia is nonpigmented and is continuous with the neural retina, the outer layer is pigmented and continuous with the RPE. It is the outer pigmented layer where the RSCs reside (Tropepe et al., 2000). Therefore, the results suggest that high level Pax6 expression co-exists with RSCs in the outer pigmented ciliary epithelia. Our FACS sorting and subsequent clonal sphere assay further showed that only the cells with the highest levels of Pax6 expression can give rise to RSC spheres, suggesting that Pax6 is highly enriched in RSCs and that high levels of Pax6 expression are hallmarks of the retinal stem cells.
Clonal RSC sphere assays on both the embryonic retina of the conventional knockout mice and adult retina of the conditional knockout mice revealed that Pax6 expression is not only a hallmark of the retinal stem cells, but also that Pax6 is required for their stemness or competence to form RSC spheres (Fig 2D & 3A). In Pax6 homozygous knockout (Pax6LacZ/LacZ) embryos, retinal development is arrested at a primitive optic vesicle stage. Although some RPE markers are expressed in these primitive optic vesicles, no fully differentiated RPE is developed in the Pax6 knockout embryo (Grindley et al., 1996; Baumer et al., 2003; Philips et al, 2005). However, the mere absence of the pigment could not be the reason for the loss of RSC competence. Previously, we have shown that similar numbers of RSC spheres can be derived from pigmented and non-pigmented animals (Tropepe et al., 2000).
Furthermore, the experiments employing in vitro inactivation of Pax6 by the Cre-EGFP retroviral infection showed that the underlying mechanism is a requirement for Pax6 in the proliferation and expansion of retinal stem cells (Fig 4). Our in vitro inactivation of Pax6 experiment supports that the inability of adult retinal stem cells to form spheres is the result of a decrease in their ability to proliferate. Previously, Marquardt et al showed a reduced proliferation of RPCs in the Pax6 conditional knockout retina at E12.5-18.5 (Marquardt et al., 2001). While we were preparing this manuscript, Philips et al (Philips et al., 2005) also reported reduced proliferation of RPCs in Pax6-/- optic vesicle at E10.5. We believe that Pax6 plays roles in both the proliferation and differentiation of RSCs and RPCs. Our present study is the first report on the function of Pax6 in retinal stem cells and our results uncovered a novel aspect of Pax6 function that it plays important roles in the proliferation of retinal stem cells, other than in the retinal progenitor cells.
Our finding that Pax6 is required for the expansion of RSCs contrasts with the function of Pax6 in the cortical neural stem cells, where inactivation of Pax6 results in increased proliferation of neural stem cells and overexpression of Pax6 in cortical precursors led to decreased proliferation (Estivill-Torrus et al., 2002; Haubst et al., 2004; Heins et al., 2002). However, in neural progenitor cells, Pax6 appeared to have similar functions as in retinal progenitors (Philips et al., 2005) to promotes proliferation by preventing the progenitor cells from exiting the cell cycles and premature differentiation (Quinn et al., 2006). Pax6 carries multiple functional domains. It is suggested that the selective use of the paired domain (PD and PD5a) and homeodomain of the gene may contribute to the region-specific differences of Pax6 function (Dominguez et al., 2004; Haubst et al., 2004; Heins et al., 2002). It appears that both the paired domain and the homeodomain are important for the regulation of proliferation and cell fate determination in the retina (Favor et al., 2001; Haubst et al., 2004), while the homeodomain plays no role in these functions in the telencephalon (Haubst et al., 2004). The two different isoforms of paired domains, PD and PD5a, also impose different effects to the overall function of Pax6 in different tissues. With the insertion of exon 5a, PD5a appears to enhance the proliferation rather than the cell fate specification in both the telencephalon in mouse (Haubst et al., 2004) and the eyes in vertebrates (Azuma et al., 2005; Singh et al., 2002) and in Drosophila (Dominguez et al., 2004). The relative predominant expression of the +5a transcript encoding PD5a in the RSCs and retina (Fig 1) may, to some extent, explain the proliferative deficits we observed in the RSCs in the mouse models, because both isoforms (PD & PD5a) of the Pax6 are inactivated in these mouse models (Marquardt et al., 2001; St-Onge et al., 1997). The cross-interactions between PD/PD5a (Epstein et al., 1994; Epstein et al., 1994), homeodomain (Mishra et al., 2002; Singh et al., 2000) and the C terminus of Pax6 protein (Singh et al., 2001; Singh et al., 2002) modulate the overall functions of Pax6 in different tissue at different developmental stages. More detailed studies on these cross-interactions and the target genes of Pax6 and Pax6(+5a) will be critical to the full understanding of the different functions of Pax6 in the regulation of the proliferation and cell fate determination in different tissues.
In our in vitro inactivation experiment, under stem cell culture condition (non-differentiating), the RSCs with Pax6 inactivated by the Cre recombinase retain retinal precursor phenotype and continue to express nestin. Under differentiating condition for 21 days, however, they differentiated almost exclusively to an amacrine cell phenotype characterized by the expression of amacrine cell specific marker, syntaxin, consistent with the observation by Marquardt et al (2001) in the conditional knockout retina that inactivation of Pax6 result in loss of the multipotency of the RPCs in the developing neural retina, biasing the differentiation of RPCs almost exclusively to amacrine cells.
In Sey/Sey (Pax6-/-) embryos, most of the eye field transcription factors, e.g. Rx1, Six3, Six6 and Lhx2 are expressed in the rudimentary optic vesicle (Jean et al., 1999; Mathers et al., 1997; Oliver et al., 1995; Porter et al., 1997). Other reports show that retinal markers, such as Tyrp2, Mitf and CHX10 are expressed (Baumer et al., 2003; Grindley et al., 1995) in Pax6 mutants, arguing that RSCs and progenitor cells can be formed in the absence of Pax6. The present data suggest that even if the first RSCs are generated in the embryonic optic vesicle, the presence of a serious proliferation deficit in Pax6-/- RSC will limit the expansion of the RSC pool by decreasing symmetrical divisions and limit the increase of the RPC pool by decreasing the asymmetrical division of RSCs.
The present study reveals that an intrinsic factor, Pax6 is required for and acts cell autonomously in retinal stem cells. Other early genes involved in eye field specification, e.g. ET, Rx1,Six6 Six3, Lhx2 and tll (Hollemann et al., 1998; Jean et al., 1999; Li et al., 1997; Mathers et al., 1997; Oliver et al., 1995; Porter et al., 1997; Zuber et al., 2003) also may play important roles in retinal stem cells. It will be important to study the different roles and interactions of these early genes in the generation, proliferation and differentiation of RSCs. Furthermore, searching for the upstream and downstream targets of these key players regulating RSCs will open new directions in the improved manipulation of these stem cells and their use in the treatment of retinal degeneration.
Acknowledgments
This work was supported by grants from NIH, the Canadian Institute of Health Research, the Canadian Genetic Diseases Network, and the Stem Cell Network of Canada.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proofbefore it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreazzoli M, Gestri G, Angeloni D, Menna E, Barsacchi G. Role of Xrx1 in Xenopus eye and anterior brain development. Development. 1999;126:2451–60. doi: 10.1242/dev.126.11.2451. [DOI] [PubMed] [Google Scholar]
- Azuma N, Tadokoro K, Asaka A, Yamada M, Yamaguchi Y, Handa H, Matsushima S, Watanabe T, Kohsaka S, Kida Y, Shiraishi T, Ogura T, Shimamura K, Nakafuku M. The Pax6 isoform bearing an alternative spliced exon promotes the development of the neural retinal structure. Hum Mol Genet. 2005;14:735–45. doi: 10.1093/hmg/ddi069. [DOI] [PubMed] [Google Scholar]
- Baumer N, Marquardt T, Stoykova A, Spieler D, Treichel D, Ashery-Padan R, Gruss P. Retinal pigmented epithelium determination requires the redundant activities of Pax2 and Pax6. Development. 2003;130:2903–15. doi: 10.1242/dev.00450. [DOI] [PubMed] [Google Scholar]
- Bernier G, Panitz F, Zhou X, Hollemann T, Gruss P, Pieler T. Expanded retina territory by midbrain transformation upon overexpression of Six6 (Optx2) in Xenopus embryos. Mech Dev. 2000;93:59–69. doi: 10.1016/s0925-4773(00)00271-9. [DOI] [PubMed] [Google Scholar]
- Chow RL, Altmann CR, Lang RA, Hemmati-Brivanlou A. Pax6 induces ectopic eyes in a vertebrate. Development. 1999;126:4213–22. doi: 10.1242/dev.126.19.4213. [DOI] [PubMed] [Google Scholar]
- Coles BL, Angenieux B, Inoue T, Del Rio-Tsonis K, Spence JR, McInnes RR, Arsenijevic Y, van der Kooy D. Facile isolation and the characterization of human retinal stem cells. Proc Natl Acad Sci U S A. 2004;101:15772–7. doi: 10.1073/pnas.0401596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles BL, Horsford DJ, McInnes RR, van der Kooy D. Loss of retinal progenitor cells leads to an increase in the retinal stem cell population in vivo. Eur J Neurosci. 2006;23:75–82. doi: 10.1111/j.1460-9568.2005.04537.x. [DOI] [PubMed] [Google Scholar]
- Das AV, James J, Rahnenfuhrer J, Thoreson WB, Bhattacharya S, Zhao X, Ahmad I. Retinal properties and potential of the adult mammalian ciliary epithelium stem cells. Vision Res. 2005;45:1653–66. doi: 10.1016/j.visres.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Dominguez M, Ferres-Marco D, Gutierrez-Avino FJ, Speicher SA, Beneyto M. Growth and specification of the eye are controlled independently by Eyegone and Eyeless in Drosophila melanogaster. Nat Genet. 2004;36:31–9. doi: 10.1038/ng1281. [DOI] [PubMed] [Google Scholar]
- Epstein J, Cai J, Glaser T, Jepeal L, Maas R. Identification of a Pax paired domain recognition sequence and evidence for DNA-dependent conformational changes. J Biol Chem. 1994;269:8355–61. [PubMed] [Google Scholar]
- Epstein JA, Glaser T, Cai J, Jepeal L, Walton DS, Maas RL. Two independent and interactive DNA-binding subdomains of the Pax6 paired domain are regulated by alternative splicing. Genes Dev. 1994;8:2022–34. doi: 10.1101/gad.8.17.2022. [DOI] [PubMed] [Google Scholar]
- Estivill-Torrus G, Pearson H, van Heyningen V, Price DJ, Rashbass P. Pax6 is required to regulate the cell cycle and the rate of progression from symmetrical to asymmetrical division in mammalian cortical progenitors. Development. 2002;129:455–66. doi: 10.1242/dev.129.2.455. [DOI] [PubMed] [Google Scholar]
- Favor J, Peters H, Hermann T, Schmahl W, Chatterjee B, Neuhauser-Klaus A, Sandulache R. Molecular characterization of Pax6(2Neu) through Pax6(10Neu): an extension of the Pax6 allelic series and the identification of two possible hypomorph alleles in the mouse Mus musculus. Genetics. 2001;159:1689–700. doi: 10.1093/genetics/159.4.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ. The master control gene for morphogenesis and evolution of the eye. Genes Cells. 1996;1:11–5. doi: 10.1046/j.1365-2443.1996.11011.x. [DOI] [PubMed] [Google Scholar]
- Glaser T, Walton DS, Maas RL. Genomic structure, evolutionary conservation and aniridia mutations in the human PAX6 gene. Nat Genet. 1992;2:232–9. doi: 10.1038/ng1192-232. [DOI] [PubMed] [Google Scholar]
- Grindley JC, Davidson DR, Hill RE. The role of Pax-6 in eye and nasal development. Development. 1995;121:1433–42. doi: 10.1242/dev.121.5.1433. [DOI] [PubMed] [Google Scholar]
- Halder G, Callaerts P, Gehring WJ. Induction of ectopic eyes by targeted expression of the eyeless gene in Drosophila. Science. 1995;267:1788–92. doi: 10.1126/science.7892602. [DOI] [PubMed] [Google Scholar]
- Hanson IM, Fletcher JM, Jordan T, Brown A, Taylor D, Adams RJ, Punnett HH, van Heyningen V. Mutations at the PAX6 locus are found in heterogeneous anterior segment malformations including Peters’ anomaly. Nat Genet. 1994;6:168–73. doi: 10.1038/ng0294-168. [DOI] [PubMed] [Google Scholar]
- Haubst N, Berger J, Radjendirane V, Graw J, Favor J, Saunders GF, Stoykova A, Gotz M. Molecular dissection of Pax6 function: the specific roles of the paired domain and homeodomain in brain development. Development. 2004;131:6131–40. doi: 10.1242/dev.01524. [DOI] [PubMed] [Google Scholar]
- Heins N, Malatesta P, Cecconi F, Nakafuku M, Tucker KL, Hack MA, Chapouton P, Barde YA, Gotz M. Glial cells generate neurons: the role of the transcription factor Pax6. Nat Neurosci. 2002;5:308–15. doi: 10.1038/nn828. [DOI] [PubMed] [Google Scholar]
- Hill RE, Favor J, Hogan BL, Ton CC, Saunders GF, Hanson IM, Prosser J, Jordan T, Hastie ND, van Heyningen V. Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature. 1991;354:522–5. doi: 10.1038/354522a0. [DOI] [PubMed] [Google Scholar]
- Hitoshi S, Alexson T, Tropepe V, Donoviel D, Elia AJ, Nye JS, Conlon RA, Mak TW, Bernstein A, van der Kooy D. Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes Dev. 2002;16:846–58. doi: 10.1101/gad.975202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollemann T, Bellefroid E, Pieler T. The Xenopus homologue of the Drosophila gene tailless has a function in early eye development. Development. 1998;125:2425–32. doi: 10.1242/dev.125.13.2425. [DOI] [PubMed] [Google Scholar]
- Jean D, Bernier G, Gruss P. Six6 (Optx2) is a novel murine Six3-related homeobox gene that demarcates the presumptive pituitary/hypothalamic axis and the ventral optic stalk. Mech Dev. 1999;84:31–40. doi: 10.1016/s0925-4773(99)00068-4. [DOI] [PubMed] [Google Scholar]
- Johns PR. Growth of the adult goldfish eye. III. Source of the new retinal cells. J Comp Neurol. 1977;176:343–57. doi: 10.1002/cne.901760304. [DOI] [PubMed] [Google Scholar]
- Jordan T, Hanson I, Zaletayev D, Hodgson S, Prosser J, Seawright A, Hastie N, van Heyningen V. The human PAX6 gene is mutated in two patients with aniridia. Nat Genet. 1992;1:328–32. doi: 10.1038/ng0892-328. [DOI] [PubMed] [Google Scholar]
- Kammandel B, Chowdhury K, Stoykova A, Aparicio S, Brenner S, Gruss P. Distinct cis-essential modules direct the time-space pattern of the Pax6 gene activity. Dev Biol. 1999;205:79–97. doi: 10.1006/dbio.1998.9128. [DOI] [PubMed] [Google Scholar]
- Lagutin OV, Zhu CC, Kobayashi D, Topczewski J, Shimamura K, Puelles L, Russell HR, McKinnon PJ, Solnica-Krezel L, Oliver G. Six3 repression of Wnt signaling in the anterior neuroectoderm is essential for vertebrate forebrain development. Genes Dev. 2003;17:368–79. doi: 10.1101/gad.1059403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Tierney C, Wen L, Wu JY, Rao Y. A single morphogenetic field gives rise to two retina primordia under the influence of the prechordal plate. Development. 1997;124:603–15. doi: 10.1242/dev.124.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loosli F, Winkler S, Wittbrodt J. Six3 overexpression initiates the formation of ectopic retina. Genes Dev. 1999;13:649–54. doi: 10.1101/gad.13.6.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt T. Transcriptional control of neuronal diversification in the retina. Prog Retin Eye Res. 2003;22:567–77. doi: 10.1016/s1350-9462(03)00036-3. [DOI] [PubMed] [Google Scholar]
- Marquardt T, Ashery-Padan R, Andrejewski N, Scardigli R, Guillemot F, Gruss P. Pax6 is required for the multipotent state of retinal progenitor cells. Cell. 2001;105:43–55. doi: 10.1016/s0092-8674(01)00295-1. [DOI] [PubMed] [Google Scholar]
- Mathers PH, Grinberg A, Mahon KA, Jamrich M. The Rx homeobox gene is essential for vertebrate eye development. Nature. 1997;387:603–7. doi: 10.1038/42475. [DOI] [PubMed] [Google Scholar]
- Mishra R, Gorlov IP, Chao LY, Singh S, Saunders GF. PAX6, paired domain influences sequence recognition by the homeodomain. J Biol Chem. 2002;277:49488–94. doi: 10.1074/jbc.M206478200. [DOI] [PubMed] [Google Scholar]
- Novak A, Guo C, Yang W, Nagy A, Lobe CG. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–55. [PubMed] [Google Scholar]
- Oliver G, Loosli F, Koster R, Wittbrodt J, Gruss P. Ectopic lens induction in fish in response to the murine homeobox gene Six3. Mech Dev. 1996;60:233–9. doi: 10.1016/s0925-4773(96)00632-6. [DOI] [PubMed] [Google Scholar]
- Oliver G, Mailhos A, Wehr R, Copeland NG, Jenkins NA, Gruss P. Six3, a murine homologue of the sine oculis gene, demarcates the most anterior border of the developing neural plate and is expressed during eye development. Development. 1995;121:4045–55. doi: 10.1242/dev.121.12.4045. [DOI] [PubMed] [Google Scholar]
- Perron M, Kanekar S, Vetter ML, Harris WA. The genetic sequence of retinal development in the ciliary margin of the Xenopus eye. Dev Biol. 1998;199:185–200. doi: 10.1006/dbio.1998.8939. [DOI] [PubMed] [Google Scholar]
- Philips GT, Stair CN, Young Lee H, Wroblewski E, Berberoglu MA, Brown NL, Mastick GS. Precocious retinal neurons: Pax6 controls timing of differentiation and determination of cell type. Dev Biol. 2005;279:308–21. doi: 10.1016/j.ydbio.2004.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter FD, Drago J, Xu Y, Cheema SS, Wassif C, Huang SP, Lee E, Grinberg A, Massalas JS, Bodine D, Alt F, Westphal H. Lhx2, a LIM homeobox gene, is required for eye, forebrain, and definitive erythrocyte development. Development. 1997;124:2935–44. doi: 10.1242/dev.124.15.2935. [DOI] [PubMed] [Google Scholar]
- Quinn JC, Molinek M, Martynoga BS, Zaki PA, Faedo A, Bulfone A, Hevner RF, West JD, Price DJ. Pax6 controls cerebral cortical cell number by regulating exit from the cell cycle and specifies cortical cell identity by a cell autonomous mechanism. Dev Biol. 2006 doi: 10.1016/j.ydbio.2006.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiring R, Walldorf U, Kloter U, Gehring WJ. Homology of the eyeless gene of Drosophila to the Small eye gene in mice and Aniridia in humans. Science. 1994;265:785–9. doi: 10.1126/science.7914031. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev Biol. 1996;175:1–13. doi: 10.1006/dbio.1996.0090. [DOI] [PubMed] [Google Scholar]
- Singh S, Chao LY, Mishra R, Davies J, Saunders GF. Missense mutation at the C-terminus of PAX6 negatively modulates homeodomain function. Hum Mol Genet. 2001;10:911–8. doi: 10.1093/hmg/10.9.911. [DOI] [PubMed] [Google Scholar]
- Singh S, Mishra R, Arango NA, Deng JM, Behringer RR, Saunders GF. Iris hypoplasia in mice that lack the alternatively spliced Pax6(5a) isoform. Proc Natl Acad Sci U S A. 2002;99:6812–5. doi: 10.1073/pnas.102691299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Stellrecht CM, Tang HK, Saunders GF. Modulation of PAX6 homeodomain function by the paired domain. J Biol Chem. 2000;275:17306–13. doi: 10.1074/jbc.M000359200. [DOI] [PubMed] [Google Scholar]
- St-Onge L, Furth PA, Gruss P. Temporal control of the Cre recombinase in transgenic mice by a tetracycline responsive promoter. Nucleic Acids Res. 1996;24:3875–7. doi: 10.1093/nar/24.19.3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Onge L, Sosa-Pineda B, Chowdhury K, Mansouri A, Gruss P. Pax6 is required for differentiation of glucagon-producing alpha-cells in mouse pancreas. Nature. 1997;387:406–9. doi: 10.1038/387406a0. [DOI] [PubMed] [Google Scholar]
- Ton CC, Hirvonen H, Miwa H, Weil MM, Monaghan P, Jordan T, van Heyningen V, Hastie ND, Meijers-Heijboer H, Drechsler M, et al. Positional cloning and characterization of a paired box- and homeobox-containing gene from the aniridia region. Cell. 1991;67:1059–74. doi: 10.1016/0092-8674(91)90284-6. [DOI] [PubMed] [Google Scholar]
- Tropepe V, Coles BL, Chiasson BJ, Horsford DJ, Elia AJ, McInnes RR, van der Kooy D. Retinal stem cells in the adult mammalian eye. Science. 2000;287:2032–6. doi: 10.1126/science.287.5460.2032. [DOI] [PubMed] [Google Scholar]
- Tropepe V, Hitoshi S, Sirard C, Mak TW, Rossant J, van der Kooy D. Direct neural fate specification from embryonic stem cells: a primitive mammalian neural stem cell stage acquired through a default mechanism. Neuron. 2001;30:65–78. doi: 10.1016/s0896-6273(01)00263-x. [DOI] [PubMed] [Google Scholar]
- Walther C, Gruss P. Pax-6, a murine paired box gene, is expressed in the developing CNS. Development. 1991;113:1435–49. doi: 10.1242/dev.113.4.1435. [DOI] [PubMed] [Google Scholar]
- Wetts R, Fraser SE. Multipotent precursors can give rise to all major cell types of the frog retina. Science. 1988;239:1142–5. doi: 10.1126/science.2449732. [DOI] [PubMed] [Google Scholar]
- Yu RT, Chiang MY, Tanabe T, Kobayashi M, Yasuda K, Evans RM, Umesono K. The orphan nuclear receptor Tlx regulates Pax2 and is essential for vision. Proc Natl Acad Sci U S A. 2000;97:2621–5. doi: 10.1073/pnas.050566897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber ME, Gestri G, Viczian AS, Barsacchi G, Harris WA. Specification of the vertebrate eye by a network of eye field transcription factors. Development. 2003;130:5155–67. doi: 10.1242/dev.00723. [DOI] [PubMed] [Google Scholar]


