Abstract
We previously reported that pharmacological preconditioning of rabbit hearts with acetylcholine involves activation of phosphatidylinositol 3-kinase (PI3-K) through transactivation of the epidermal growth factor receptor (EGFR). Transactivation is thought to be initiated by cleavage of membrane-bound proheparin-binding EGF-like growth factor (HB-EGF) by a membrane metalloproteinase thus releasing HB-EGF which binds to the EGFR. This pathway leads to redox signaling with the generation of reactive oxygen species (ROS) by mitochondria. We tested whether preconditioning’s physiological triggers, bradykinin and opioid, also signal through the EGFR. Both bradykinin and the synthetic δ-opioid agonist DADLE increased ROS production in isolated cardiomyocytes by approximately 50%. DADLE’s effect was abrogated by either metalloproteinase inhibitor III (MPI) or the diphtheria toxin mutant CRM-197 which blocks heparin-binding EGF shedding indicating that DADLE signals through EGFR transactivation. MPI also blocked DADLE’s infarct-sparing effect in whole hearts. Additionally, blocking Src kinase (a component of the EGFR’s signaling complex) with PP2 or PI3-K with wortmannin blocked DADLE’s effect on cardiomyocyte ROS production and PP2 blocked DADLE’s salvage of ischemic myocardium. Finally, DADLE increased phosphorylation of Akt and extracellular signal-regulated protein kinases (ERK) 1/2 in left ventricular myocardium, and this increase was blocked by the EGFR antagonist AG1478. On the other hand, neither MPI nor CRM-197 prevented bradykinin from increasing ROS production, and MPI did not affect bradykinin’s infarct-sparing effect in intact hearts. Conversely, both PP2 and wortmannin blocked bradykinin’s effect on ROS generation and also aborted bradykinin’s cardioprotective effect in intact hearts. While bradykinin also increased phosphorylation of Akt and ERK in myocardium, that increase was not affected by AG1478. Hence bradykinin, unlike acetylcholine or opioid, does not transactivate EGFR, although all 3 agonists do signal through Src and PI3-K.
Keywords: bradykinin, epidermal growth factor, opioids, preconditioning, transactivation
Ischemic preconditioning is universally recognized as a potent cardioprotective intervention. Many investigators have studied this phenomenon to learn how protection is accomplished in the hope that insights could be extrapolated to the clinical arena. Of the many autacoids released by ischemic myocardium adenosine, bradykinin, and opioids appear to be the principal endogenous triggers of preconditioning. Although all 3 of these agonists bind to surface membrane Gi protein-coupled receptors (GiPCR), the intracellular signaling initiated by each is not identical. For example, the protection from both bradykinin and opioids is dependent on opening of mitochondrial ATP-dependent potassium channels (mitoKATP) and production of reactive oxygen species (ROS) which then act as second messengers to activate protein kinase C (PKC) [1]. However, adenosine bypasses mitoKATP and ROS production, and appears to activate PKC directly through the dissociated G proteins [1]. It is unclear how theses 3 GiPCRs can lead to such diversity of signaling, but these redundant pathways do insure continuing cell protection even after one of the pathways is blocked.
Several years ago we sought to map preconditioning’s trigger pathway. We initially used the muscarinic receptor agonist acetylcholine (ACh) since the M2 receptor is also a GiPCR and protects by similarly opening mitoKATP and releasing ROS [1]. Although ACh is not released by ischemic cells and therefore is not a physiological mediator of preconditioning, we found it to be a convenient agonist with which to work since its receptors are so well characterized. Krieg et al. [2,3] found evidence that ACh signaled through transactivation of the epidermal growth factor receptor (EGFR). The current theory is that receptor binding activates a membrane metalloproteinase (MMP) that in turn cleaves heparin-binding epidermal growth factor-like growth factor (HB-EGF) from a membrane-associated proHB-EGF. The liberated HB-EGF then binds to the ectodomain of the EGF receptor (EGFR) monomer causing it to dimerize with another monomer which transactivates their intrinsic tyrosine kinase activity leading to autophosphorylation of their tyrosine residues. This recruits Src kinase and phosphatidylinositol 3-kinase (PI3-K) forming an active signaling complex. Activation of the latter results in phosphorylation of membrane phospholipids, activation of phosphoinositide-dependent kinases, phosphorylation of Akt and in turn nitric oxide synthase, and production of NO with downstream activation of PKG [4]. The latter then opens mitoKATP with subsequent release of ROS [5,6].
We recently observed that the cardioprotection initiated by the δ-opioid [D-Ala2, D-Leu5]-enkephalin acetate (DADLE) was abrogated by blockade of MMP with the broad-spectrum MP inhibitor III (MPI) [3]. Similarly Cao et al. [7] blocked the ability of Met5-enkephalin, another synthetic δ-opioid agonist, to prolong survival of isolated rabbit cardiomyocytes during simulated ischemia by treating them with AG1478, an EGFR kinase inhibitor. Both observations suggest that δ-opioids also trigger preconditioning through the EGFR. We could find no studies testing whether bradykinin also signals through EGFR transactivation in myocardium. Because of the documented diversity of agonist-induced signaling, we thought it important to establish whether proHB-EGF cleavage and EGFR transactivation occurred with both δ-opioid and bradykinin-stimulated cardioprotection as well. Consequently we tested a number of known blockers of this pathway on both the ability of bradykinin and DADLE to stimulate ROS production in isolated rabbit cardiomyocytes and to limit infarct size in isolated rabbit hearts exposed to ischemia/reperfusion to determine whether or not these receptors signal through the EGFR.
METHODS
This study was performed in accordance with The Guide for the Care and Use of Laboratory Animals (National Academy Press, Washington, DC, 1996).
Adult rabbit myocytes
Rabbit ventricular myocytes were isolated as described previously in detail [8]. Briefly hearts of New Zealand White rabbits were excised and retrogradely perfused with calcium-free Krebs-Henseleit-HEPES buffer containing collagenase (type 2, Worthington, Inc., Lakewood, NJ) (200 U/ml) at 37°C. Viable myocytes were separated by repetitive slow-speed centrifugation and made calcium tolerant by stepwise restoration of calcium in the medium to 1.25 mM. Usually 30–35 million viable, calcium-tolerant cells were extracted per heart. Greater than 65% were rod-shaped.
Immediately after the isolation and separation procedure, cells were plated on laminin coated 24-well plates (Becton Dickinson, Bedford, MA) using creatine (5 mM), L-carnitine (2 mM), and taurine (5 mM) supplemented medium 199 (CCT-medium 199) as described by Piper et al. [9] and Mitcheson et al. [10]. Penicillin (100 U/ml) and streptomycin (100 μg/ml) were added as antibiotics. Cells were stored in incubators at 37°C in air enriched with 5% CO2. A first medium change was performed after 3–4 h; afterwards cells were undisturbed and allowed to equilibrate for at least 18 h.
Experimental design
Each experiment started with a change of medium for 10 min. The medium was then removed and replaced with one containing the drug or drug + blocker (if required) and reduced MitoTracker Red (1 μM) as a dye. This reduced form of the probe is non-fluorescent. When the probe is oxidized by ROS, it then becomes fluorescent. The oxidized product is bound to thiol groups and proteins within mitochondria. After incubation with MitoTracker for 15 min cells were washed twice with fresh MitoTracker-free CCT medium. The wash serves to remove the unbound and thus voltage-dependent pool of dye held in the cells. After washing, fluorescence becomes stable for at least 30 min.
In experiments in which the effect of the blockers MPI (3 μM), wortmannin (100 nM), and 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d] pyrimidine (PP2, 1 μM) on ROS production by bradykinin (500 nM) or DADLE (20 nM) was to be examined, the blocker was present in the medium during the 10-min period prior to addition of MitoTracker and the agonist. When CRM-197 (10 μg/ml), a non-toxic mutant of diphtheria toxin, was used, the pretreatment period was 90 min. Previous investigations have documented that the described protocol of a timed incubation followed by washing permits reliable measurement of ROS generation and minimizes any possible influence of changing mitochondrial transmembrane potential [11,12].
Measurement of ROS production
Experiments were designed such that four different conditions were always simultaneously evaluated. Mitochondrial ROS generation was analyzed by measuring the fluorescence of at least 40 individual, rod-shaped cells that were randomly selected within each well. The average fluorescence for the selected cells in each well was computed and compared to the average single cell fluorescence in the respective control well in the same chamber. Thus the treated cells were compared only to untreated cells of the same age and isolated and stained with the same MitoTracker lot. Single cell fluorescence was quantified as described previously [12]. Each set of experiments was repeated five to eight times on different days with cells of different ages. Approximately 200–400 typical rod-shaped cells contributed data for each experiment.
Isolated heart model
As previously described [1], a 2–0 silk suture was passed around a branch of the left coronary artery of New Zealand White rabbits to form a snare by passing the ends of the thread through a small vinyl tube. The heart was rapidly excised, mounted on a Langendorff apparatus by the aortic root, and perfused with oxygenated, warmed Krebs buffer. Perfusion pressure was set at 75 mmHg by adjusting the height of the reservoir. A fluid-filled latex balloon was inserted into the left ventricle and inflated to set an end-diastolic pressure of 5 mmHg. All hearts were allowed to equilibrate for 30 min before the protocols were started.
For the infarct investigations eight groups of hearts were studied. All hearts were subjected to 30 min of regional ischemia and then reperfused for 2 h. Control hearts received no treatment. The second group of hearts was treated with DADLE (20 nM) for 5 min followed by 10 min of washout before the long ischemia. A third group was also treated with DADLE. In addition MPI (3 μM) was added to the perfusate for 15 min starting 5 min before and ending 5 min after DADLE treatment. This protocol allowed 5 min of washout before coronary occlusion. A fourth group of hearts was treated with DADLE and the Src kinase inhibitor PP2 (1 μM). The next group of hearts was treated for 5 min with bradykinin (0.4 μM) followed by 10 min of washout before the 30 min ischemia. The next 3 groups were co-treated with bradykinin as in group 5 and either MPI, PP2, or the PI3-K antagonist wortmannin (100 nM) infused with the same schedule as MPI in group 3.
Infarct size measurement
As previously detailed [1] risk zone was delineated with 1–9 μM green fluorescent microspheres (Duke Scientific Corp., Palo Alto, CA) and infarction with triphenyltetrazolium chloride (TTC) staining. The areas of infarct and risk zone were determined by planimetry of each slice and volumes calculated by multiplying each area by slice thickness and summing them for each heart. Infarct size is expressed as a percentage of the risk zone.
Biochemical measurements
Isolated, buffer-perfused rabbit hearts were treated with either DADLE (20 nM) or bradykinin (0.4 μM) for 5 min. Transmural left ventricular biopsies weighing approximately 25 mg were obtained with a motorized biopsy tool before and at the end of the infusion of the agonist and ejected into liquid nitrogen within 1 s of excision. In 2 additional groups of hearts AG1478 (300 nM) was added to the perfusate for 10 min starting 5 min before either DADLE or bradykinin.
Tissues were prepared as previously described [2]. Electrophoresis of supernatant on 10% SDS-polyacrylamide gel was followed by transfer to nitrocellulose membrane. To identify phosphorylated kinases membranes were probed with monoclonal antibodies for phospho-Akt (Ser 473) and phospho-ERK 1/2 (Thr 202/Thr 204) followed by incubation with a secondary antibody conjugated to horseradish peroxidase. Immunoreactive proteins were detected by enhanced chemiluminescence with LumiGLO. Band densities were determined with Sigmagel software. To test for differences in protein loading and errors introduced during the transfer process each membrane was stained with Ponceau S dye. When a difference in Ponceau staining was apparent the density of the phosphorylated protein was corrected for the difference in protein loading. Samples from each heart were run on the same gel. Because each blot differs in its exposure time, strength of the antibody, and strength of the ECL chemical, it is impossible to compare densities between blots. However we have found that preischemic phosphorylation of Akt and ERK are very reproducible and therefore all band densities are expressed as a percentage of the corresponding baseline sample. Accordingly exposure time for each blot was adjusted to give a density that would allow accurate scanning of all lanes.
Chemicals
All drugs required for cell isolation and culture were purchased from Sigma Chemical (St. Louis, MO). Reduced MitoTracker Red was obtained from Molecular Probes (Eugene, OR), CRM-197, DADLE, wortmannin and Ponceau S from Sigma, MPI and PP2 from Calbiochem (La Jolla, CA), and AG1478 from Alexis Biochemicals (San Diego, CA). Either distilled water or DMSO was used to dissolve the drugs and to prepare stock solutions. The final DMSO concentration was kept below 1%.
Phospho-Akt antibody to Ser 473 (#4251), HRP-linked anti-rabbit IgG antibody, cell lysis buffer, and LumiGLO were purchased from Cell Signaling Technology (Beverly, MA), while anti-ERK 1/2 (#05–481) was obtained from Upstate (Lake Placid, NY).
Data analysis
Fluorescence measurements provide data in arbitrary units (a.u.). To remove the variability caused by different MitoTracker lots, cell age, and environmental conditions, average cell fluorescence was calculated and compared to that of simultaneously studied control cells as described above. Therefore, fluorescence data are provided as a percentage of the respective control (mean ± SEM). To further minimize the possible influence of these variables on the data, ANOVA for repeated measures with Tukey’s post hoc testing was used to evaluate differences in mean fluorescence of the groups within the same experiment. Baseline hemodynamic variables and risk zone and infarct size data amongst groups were compared by one-way ANOVA with Student-Newman-Keuls post hoc testing. Temporal changes in hemodynamics in a given group were evaluated by one-way ANOVA for repeated measures with Tukey’s post hoc testing. Changes in phospho-Akt and phospho-ERK levels were evaluated by Student’s paired t-test. A value of p< 0.05 was considered significant.
RESULTS
Isolated cardiomyocytes
DADLE
Exposing cardiomyocytes to DADLE led to a 55.0 ± 8.8% increase in fluorescence and thus ROS production (Fig. 1). Co-incubation with MPI prevented the DADLE effect on ROS generation, while MPI in the absence of DADLE had no effect on ROS production.
Figure 1.
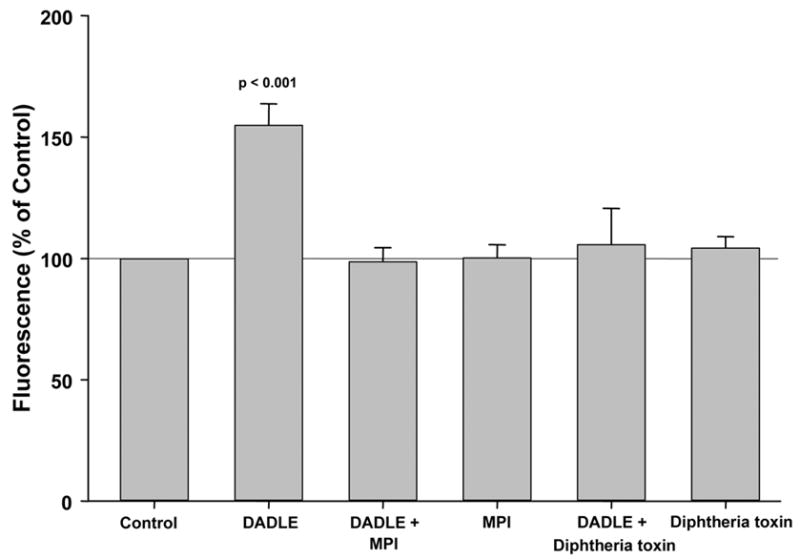
DADLE leads to a significant increase in generation of reactive oxygen species in adult rabbit ventricular myocytes. Co-treatment with either metalloproteinase inhibitor III (MPI) or CRM-197, a mutant diphtheria toxin, completely abolished this action, whereas neither MPI nor CRM-197 alone had any effect. Data are presented as a change in cell fluorescence (mean ± SEM) expressed as a percentage of that of simultaneously studied untreated control cardiomyocytes.
ProHB-EGF is the primary membrane attachment site for diphtheria toxin. The diphtheria toxin mutant, CRM-197, specifically inhibits the mitogenic activity of HB-EGF [13]. To test whether DADLE acts through HB-EGF-dependent activation of EGFR to generate ROS in cardiomyocytes, we measured ROS in cells pretreated with CRM-197 prior to stimulation with DADLE. As expected, the increased ROS production triggered by DADLE was totally abolished by CRM-197 (Fig. 1). CRM-197 on its own had no effect on cell fluorescence.
We next probed the downstream involvement of Src kinase and PI3-K in DADLE’s effect on ROS generation. Again DADLE increased ROS production by more than 50% (Fig. 2). But both PP2 and wortmannin blocked DADLE’s effect on ROS. Neither PP2 nor wortmannin alone had virtually any effect on ROS production.
Figure 2.
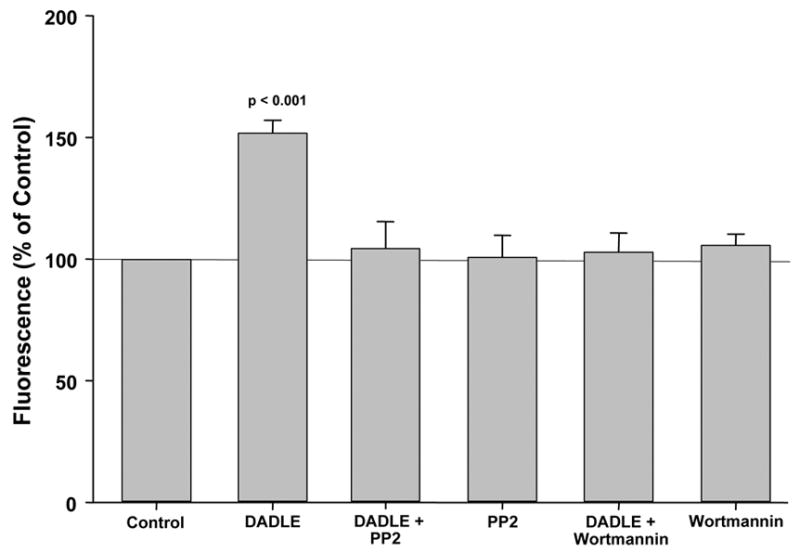
DADLE significantly increased production of reactive oxygen species by adult rabbit ventricular myocytes. This increased production was blocked when cells were additionally exposed to either PP2, an antagonist of Src kinase, or wortmannin, an inhibitor of phosphatidylinositol 3-kinase. Neither PP2 nor wortmannin alone had any influence.
Bradykinin
In parallel studies bradykinin was substituted for DADLE in the above experiments. Bradykinin significantly increased ROS production by 30.5 % (Fig. 3), and surprisingly this increase was not affected by co-treatment with MPI. Furthermore the robust bradykin-induced increase in ROS (53.6 ± 4.5%) was not diminished by CRM-197 either (Fig. 4).
Figure 3.
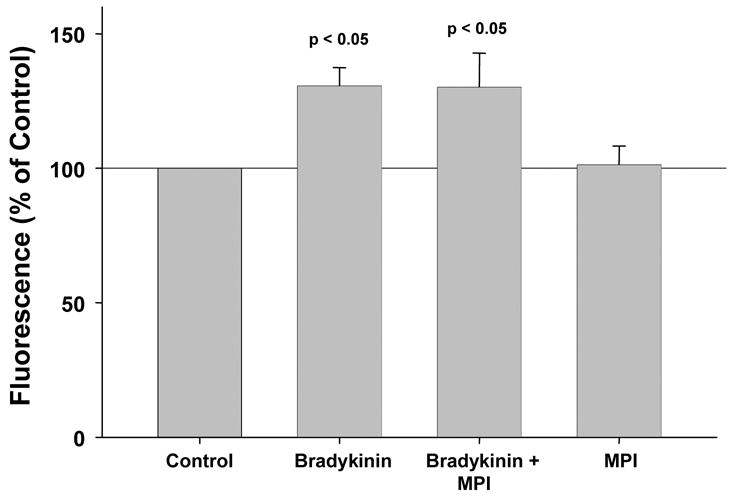
When rabbit cardiomyocytes were exposed to bradykinin, they produced significantly more reactive oxygen species (ROS) than simultaneously studied untreated cells. This increased ROS production was not aborted by metalloproteinase inhibitor III (MPI), casting doubt on transactivation of the epidermal growth factor receptor being part of bradykinin’s trigger pathway. MPI had no independent effect on ROS generation.
Figure 4.
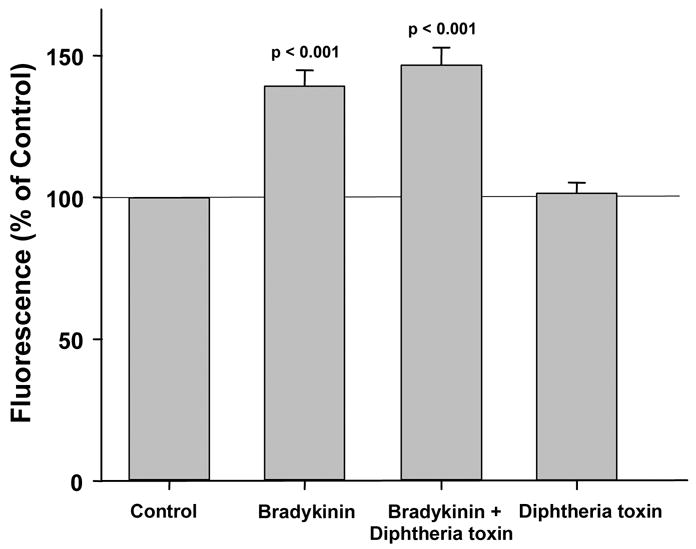
Bradykinin significantly increased production of reactive oxygen species (ROS) by rabbit cardiomyocytes and this effect was not affected by the mutant diphtheria toxin CRM-197. Because the latter prevents cleavage from its inactive precursor of heparin binding epidermal growth factor-like growth factor, an essential component in the transactivation of the epidermal growth factor receptor (EGFR), its failure to limit ROS generation effectively excludes participation of EGFR in signaling triggered by bradykinin. CRM-197 had no independent effect on ROS production.
We then looked at other components of the EGFR’s signaling complex. The Src kinase inhibitor PP2 completely blocked bradykinin’s effect on ROS production (Fig. 5) as did the PI3-K inhibitor wortmannin (Fig. 5).
Figure 5.
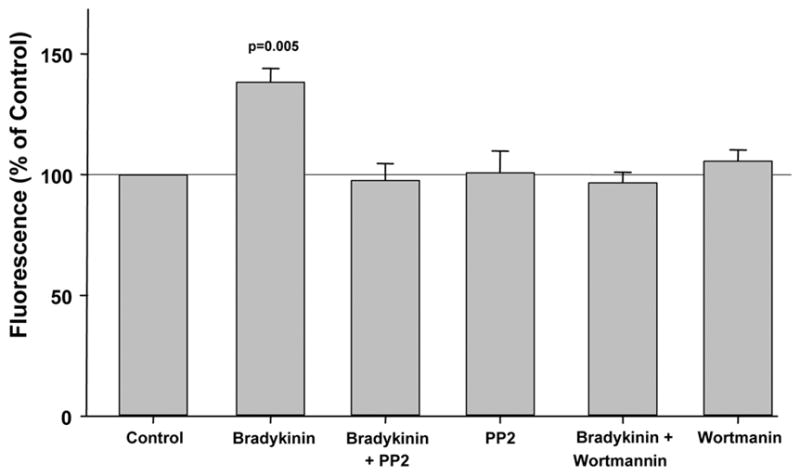
The increased fluorescence and therefore production of reactive oxygen species (ROS) following exposure of rabbit cardiomyocytes to bradykinin were blocked by both PP2 and wortmannin, antagonists of Src kinase and phosphatidylinositol 3-kinase, resp. Neither PP2 nor wortmannin alone had any effect on ROS generation.
Isolated rabbit heart-infarct size
Hemodynamics are presented in Table 1. There were no differences in baseline heart rate, left ventricular developed pressure, or coronary flow among the groups. Wortmannin modestly decreased left ventricular developed pressure and coronary flow. Heart weights were comparable in all groups (Table 2). Risk zone volume was inexplicably smaller in the hearts co-treated with bradykinin and MPI.
Table 1.
Hemodynamics
| Baseline | Drug Treatment | 30 min Occlusion | 30 min Reperfusion | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (bpm) | LVDP (mmHg) | CF (ml/min/g) | HR (bpm) | LVDP (mmHg) | CF (ml/min/g) | HR (bpm) | LVDP (mmHg) | CF (ml/min/g) | HR (bpm) | LVDP (mmHg) | CF (ml/min/g) | |
| Control | 196±3 | 112±4 | 8.8±0.4 | 189±5 | 42±2* | 3.8±0.4* | 192±7 | 74±4* | 6.4±0.5† | |||
| DADLE | 205±8 | 119±2 | 9.2±0.5 | 202±6 | 117±3 | 8.6±0.6 | 197±2 | 56±6* | 4.8±0.4* | 189±4 | 86±7* | 6.8±0.5* |
| DADLE + MPI | 186±6 | 120±2 | 9.5±0.5 | 192±9 | 114±3 | 8.6±0.3‡ | 196±4 | 38±6* | 4.1±0.3* | 204±2 | 78±8* | 6.4±0.4* |
| DADLE + PP2 | 212±6 | 118±3 | 9.4±0.4 | 202±6 | 111±5 | 7.9±0.5 | 202±7 | 61±8* | 4.5±0.7* | 210±5 | 82±6* | 5.4±0.7* |
| bradykinin | 204±4 | 114±2 | 11.1±0.6 | 201±5 | 108±1 | 10.6±0.7 | 190±8 | 46±9* | 5.2±1.1* | 194±7 | 74±2† | 7.3±0.9* |
| bradykinin + MPI | 204±5 | 108±2 | 8.9±0.5 | 206±7 | 96±3 | 8.2±0.4 | 192±12 | 35±5* | 3.4±0.2* | 212±5 | 66±4* | 5.8±0.3* |
| bradykinin + PP2 | 222±9 | 116±3 | 9.0±0.7 | 208±7‡ | 106±7 | 8.3±0.6 | 206±9† | 52±8* | 4.4±0.6* | 208±7‡ | 73±9* | 6.3±0.6* |
| bradykinin + Wort | 200±5 | 109±2 | 7.4±0.2 | 204±4 | 79±10† | 5.6±0.4† | 200±0 | 33±5* | 3.1±0.3* | 197±3 | 52±7* | 3.7±0.4* |
Mean ± SEM
Abbreviations: bradykinin = bradykinin CF = coronary flow DADLE = [D-Ala2, D-Leu5]-enkephalin acetate HR = heart rate LVDP = left ventricular developed pressure MPI = metalloproteinase inhibitor PP2 = 4-amino-5-(4-chlorophenyl)-7(t-butyl)pyrazolo[3,4-d]pyrimidine Wort = wortmannin
Statistical significance of difference between baseline and experimental point:
p<0.001
p<0.01
p<0.05
Table 2.
Infarct size
| N | BW (kg) | HW (g) | Risk Zone (cm3) | Infarct (cm3) | I/R (%) | |
|---|---|---|---|---|---|---|
| Control | 7 | 2.5±0.2 | 8.0±0.6 | 1.43±0.09 | 0.42±0.03 | 29.8±2.4 |
| DADLE | 6 | 2.2±0.1 | 7.3±0.4 | 1.00±0.11 | 0.12±0.03* | 10.9±2.2* |
| DADLE + MPI | 5 | 2.2±0.1 | 6.3±0.3 | 1.11±0.06 | 0.32±0.03 | 28.8±1.8 |
| DADLE + PP2 | 6 | 2.3±0.1 | 7.3±0.3 | 1.22±0.03 | 0.36±0.02 | 29.6±0.8 |
| bradykinin | 7 | 2.3±0.1 | 7.0±1.0 | 1.27±0.15 | 0.18±0.04† | 13.4±1.4‡ |
| bradykinin + MPI | 5 | 2.3±0.0 | 6.9±0.3 | 0.89±0.10‡ | 0.12±0.07* | 12.6±6.9† |
| bradykinin + PP2 | 6 | 2.2±0.0 | 6.8±0.3 | 1.29±0.13 | 0.38±0.05 | 29.0±2.1 |
| bradykinin + Wort | 6 | 2.3±0.0 | 7.0±0.4 | 1.23±0.14 | 0.46±0.06 | 33.4±5.3 |
Mean ± SEM
Abbreviations: see Table 1 BW = body weight HW = heart weight I/R = ratio of infarct size to risk zone volume N = number of animals
Statistical significance of difference between Control and experimental group:
p<0.001
p<0.01
p<0.05
DADLE
Pre-treatment of hearts with DADLE before ischemia/reperfusion was very protective and decreased infarction from 29.8 ± 2.4% of the area at risk in control hearts to 10.9 ± 2.2% (p<0.01) (Table 2 and Fig. 6). Both MPI and PP2 blocked DADLE’s salutary effect. Previous studies have demonstrated lack of independent effect of MPI [14,3] and PP2 [14,3] on infarct size.
Figure 6.
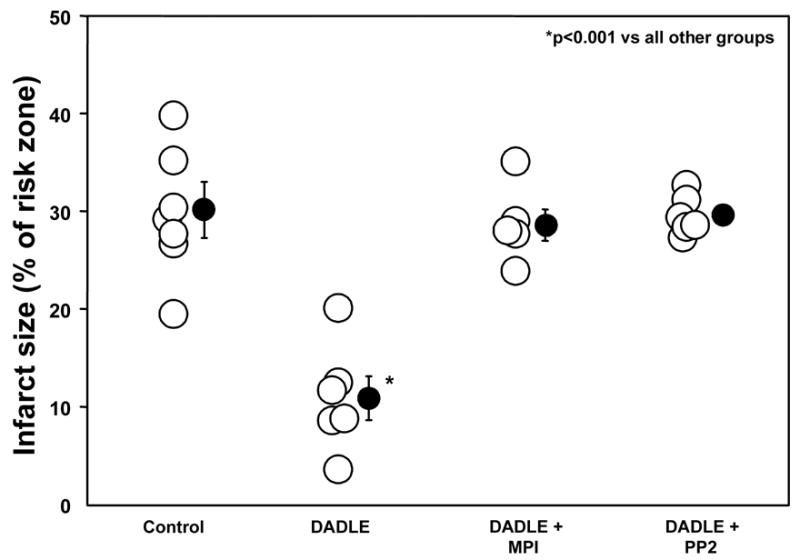
Effect of DADLE, metalloproteinase inhibitor III (MPI), and PP2 on infarct size expressed as a percentage of the risk zone in isolated rabbit hearts. Open circles represent individual data points, while closed circles represent group averages with SEM. DADLE significantly decreased infarction following a 30-min coronary occlusion/2-h reperfusion, and this salutary effect was aborted by both MPI and the Src kinase antagonist PP2. These results support involvement of transactivation of the epidermal growth factor receptor in the intracellular signaling triggered by DADLE.
Bradykinin
Bradykinin also had a marked cardioprotective effect (13.4 ± 1.4% infarction, p<0.01 vs Control) (Table 2 and Fig. 7). Whereas MPI had no effect on this protection (12.6 ± 6.9% infarction), both PP2 and wortmannin aborted the protective effect. A previous study demonstrated that wortmannin has no effect on infarct size in untreated hearts [15]. Because of the smaller risk zone volume in one of the bradykinin groups, infarct size was plotted against risk zone volume in all groups and regression lines for each relationship compared (data not shown). Regression lines for all protected groups (DADLE, bradykinin, bradykinin + MPI) were significantly different from those for unprotected groups (Control, DADLE + MPI, DADLE + PP2, bradykinin + PP2, bradykinin + wortmannin).
Figure 7.
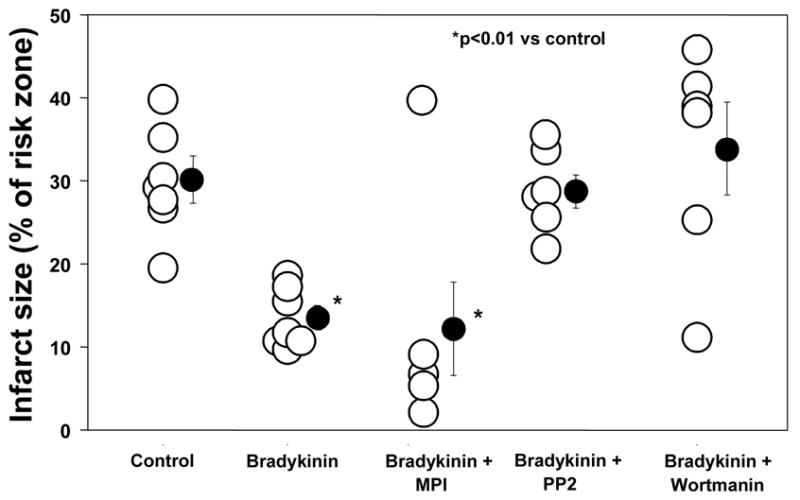
Infarct size as a percentage of the risk zone following a 30-min coronary occlusion/2-h reperfusion in isolated rabbit hearts. Open circles represent individual data points, while closed circles represent group averages with SEM. Bradykinin salvaged ischemic myocardium. Metalloproteinase inhibitor III (MPI) had no effect on bradykinin’s cardioprotective effect suggesting cleavage of heparin binding epidermal growth factor-like growth factor and therefore subsequent transactivation of the epidermal growth factor receptor were not essential for bradykinin’s protective effect. These results distinguish bradykinin from DADLE. However, both PP2 and wortmannin, Src kinase and phosphatidylinositol 3-kinase antagonists, resp., did block bradykinin’s salutary effect implicating these kinases in the downstream signaling cascade. The latter observations suggest bradykinin and DADLE do have signaling steps in common.
Isolated rabbit heart-phospho-Akt and phospho-ERK
Both DADLE (n=4) and bradykinin (n=4) elicited significant increases in Akt phosphorylation (Fig. 8), although the response following DADLE was more robust. The EGFR antagonist AG1478 effectively blocked any response to DADLE. Although there was a trend for AG1478 to also lower the level of Akt phosphorylation with bradykinin, it was not significant. Apparent differences in baseline phosphorylation in the blots reflect differing exposure times between blots rather than any differences in baseline phosphorylation.
Figure 8.
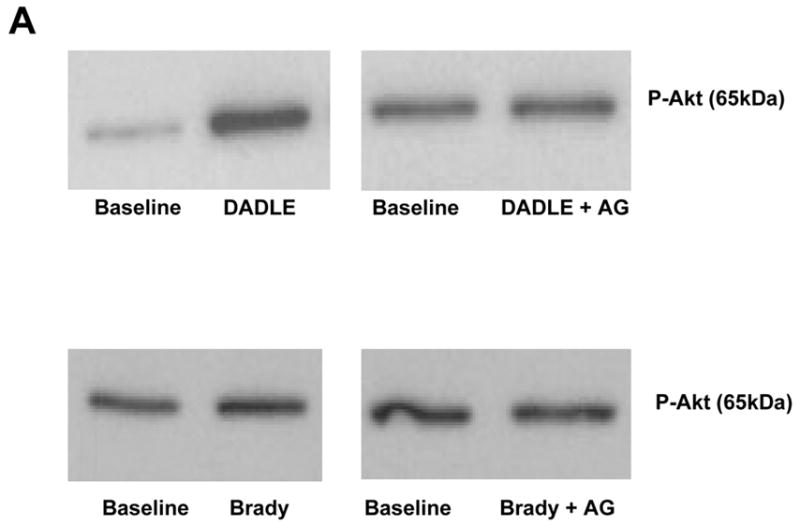
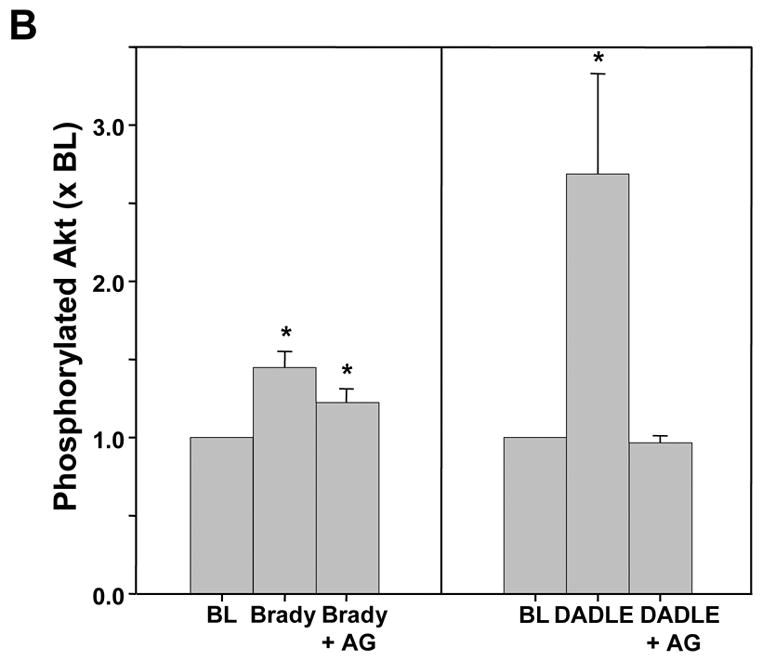
A. Representative western blot of phosphorylated Akt in myocardial samples before and after exposure to either bradykinin (Brady) or DADLE. The additional effect of the EGFR antagonist AG1478 (AG) on the change in phosphorylation induced by the agonist is demonstrated. All samples from each heart were run on the same blot. Differences in the baseline densities derive from differing exposure times of the individual blots rather than any differences in baseline phosphorylation. Because it is impossible to compare western blot data between blots all data are normalized to the baseline (BL) sample for each heart. B.Effect of bradykinin (Brady) and DADLE on phosphorylation of Akt determined by a phospho-specific antibody in serial biopsies from isolated rabbit hearts with and without simultaneous exposure to AG1478 (AG). *p<0.05 vs baseline
Additionally both DADLE (n=5) and bradykinin (n=4) also produced significant increases in phosphorylation of ERK 1/2 (p44/p42) (Fig. 9). As seen with Akt the response to DADLE was nearly double that to bradykinin. AG1478 completely blocked DADLE’s effect on ERK phosphorylation, whereas bradykinin’s effect was minimally diminished. Because of the greater variability, the level of phosphorylated ERK 2 after AG1478 was neither significantly greater than that at baseline nor was it smaller than that with bradykinin alone.
Figure 9.
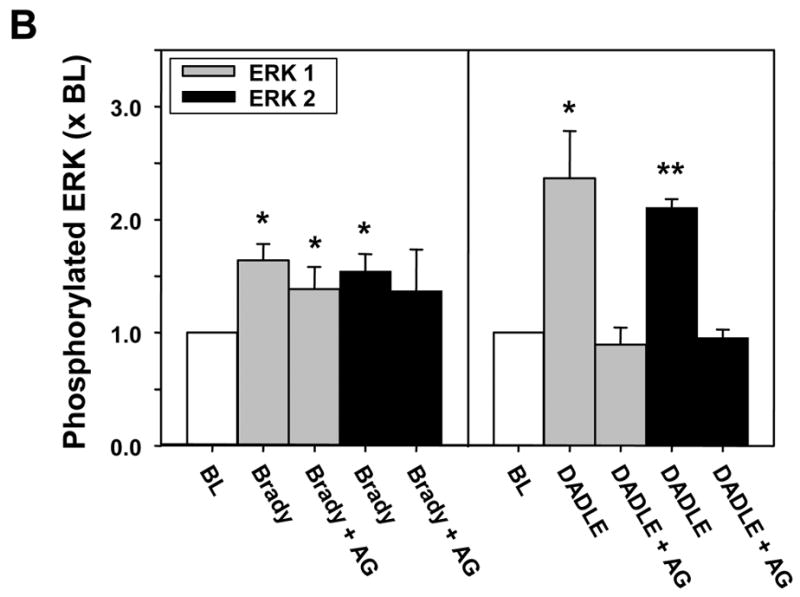
A. Representative western blot of phosphorylated ERK 1/2 (p44/p42) in myocardial samples before and after exposure to either bradykinin (Brady) or DADLE. The additional effect of the EGFR antagonist AG1478 (AG) on the change in phosphorylation induced by the agonist is demonstrated. All samples from each heart were run on the same blot. Differences in the baseline densities derive from differing exposure times of the individual blots rather than any differences in baseline phosphorylation. Because it is impossible to compare western blot data between blots all data are normalized to the baseline (BL) sample for each heart. B. Effect of bradykinin (Brady) and DADLE on phosphorylation of ERK 1/2 determined by a phospho-specific antibody in serial biopsies from isolated rabbit hearts with and without simultaneous exposure to AG1478 (AG). All data are normalized to the baseline (BL) sample for each heart. *p<0.025 **p<0.001 vs baseline
DISCUSSION
Signal transduction in preconditioning is thought to have two phases: the trigger phase occurring before ischemia and the mediator phase occurring during and after ischemia. The former involves in sequential order: binding of bradykinin and opioid to GiPCRs, activation of PI3-K, phosphorylation of Akt, generation of nitric oxide, activation of PKG, opening of mitoKATP, and generation of ROS. Redox signaling by the ROS is then thought to activate PKC which we believe to be the defining event that puts the cell into the preconditioned state. Adenosine A1 receptors were found to activate PKC through a pathway that bypasses the redox signaling. In the present study we provide further evidence that opioid activates PI3-K through the EGFR similar to what was seen with muscarinic receptors. On the other hand we could find no evidence that bradykinin uses the EGFR to activate PI3-K.
We originally identified involvement of the EGFR in preconditioning’s trigger pathway using pharmacological preconditioning with ACh. While all of our evidence was indirect it was substantial. We first observed that ACh triggered Akt phosphorylation in rabbit hearts [2]. As expected that increase was dependent on PI3-K, but unexpectedly it could also be blocked by genistein, a tyrosine kinase blocker, and by the Src kinase blocker PP2. Src kinase is known to be a component in EGFR’s signaling complex. That suggested EGFR transactivation, which is known to be coupled to muscarinic receptors in other tissues [16,17,18]. Accordingly the selective EGFR antagonist AG1478 blocked the pathway. We also saw phosphorylation of tyrosine groups on the EGFR. Unfortunately AG1478 cannot be used in infarction studies in rabbit hearts because it is a direct preconditioning agent [3]. It also generates mitochondrial ROS in cardiomyocytes [3]. That forced us to look for more indirect evidence of EGFR involvement. Prenzel and colleagues [17] proposed that ACh activates the EGFR by triggering metalloproteinase-induced shedding of HB-EGF from a membrane bound precursor. We therefore tested whether a metalloproteinase inhibitor could block ACh’s protection and found that it could [3]. We also found that CRM-197 blocked ROS production in cardiomyocytes. Unfortunately CRM-197 is too expensive to use in whole hearts. The next obvious question was whether either of the physiological triggers of preconditioning, opioid or bradykinin, also uses the EGFR for signaling. The pathways used by the two receptors to trigger preconditioning beyond PI3-K appear to be identical to that used by ACh: all 3 use NOS, PKG, mitoKATP and redox signaling to activate PKC [1,19,4]. Furthermore Cao et al. [7] found that AG1478 could block a δ-opioid agonist’s protection in a cardiomyocyte model of ischemic injury suggesting that the opioid receptors do indeed couple through the EGFR. Our present results confirm their conclusion. Surprisingly we could find no evidence of EGFR involvement in bradykinin’s triggering. Nonetheless downstream signaling steps involving Src kinase and PI3-K are intact. Therefore, we conclude that the bradykinin signal rejoins the pathway used by opioid and ACh at a point distal to the EGFR.
How solid is the evidence that bradykinin does not signal through the EGFR? MPI, a hydroxamate-based inhibitor of metalloproteinases, is known to block MMP-1,-2, and-13 with IC50 <10nM, MMP-7 with IC50 = 10–100nM, and MMP-3 with IC50 = 135 nM. It is not known which metalloproteinase is responsible for HB-EGF cleavage [17]. Suzuki et al. [20] originally proposed that MMP-3 was the responsible metalloproteinase, but Hao et al. [21] identified MMP-7 as a major HB-EGF sheddase. Increasing information suggests that a unique group of metalloproteases called a disintegrin and metalloprotease (ADAM) which includes several members, specifically ADAM 9, 10, 12, and 17, supports EGFR transactivation by initiating shedding of HB-EGF [22,23,24,25,26,27,28]. Although HB-EGF shedding appears to be critical for transactivation to initiate several biologic processes in cardiac tissue, specific inhibitors which can distinguish between the various MMPs or even designate an ADAM as the responsible protease are not available. Thus bradykinin could still be activating a metalloproteinase or metalloprotease that was not blocked by MPI. On the other hand, the failure of CRM-197 to block bradykinin’s effect in isolated cardiomyocytes strongly argues against EGFR dependence in the pathway. Furthermore, the failure of AG1478, an EGFR antagonist, to block the ability of bradykinin to increase Akt and ERK phosphorylation is additional evidence that this GiPCR does not signal through EGFR. Because bradykinin-induced phosphorylation of ERK 2 and Akt appeared to be attenuated following exposure of the heart to AG1478, the data could suggest that some of bradykinin’s signaling was through the EGFR in addition to an alternate coupling. Unfortunately the statistics are not strong enough to confirm that a true attenuation had occurred. Our data also do not exclude involvement of a different membrane growth factor receptor such as the platelet-derived growth factor receptor that can transactivate and recruit Src kinase to form a signaling module that can then activate PI3-K.
Prior examinations of the effect of AG1478 on bradykinin’s ability to activate and phosphorylate downstream MAPK and specifically ERK1/2 have yielded mixed results. In agreement with our observations Graness et al. [29] observed that AG1478 did not affect bradykinin’s activaton of MAPK in A431 cells. On the other hand, Adomeit [30] in COS-7 cells, Yang [31] in rat vascular smooth muscle cells, and Mukhin [32] in mIMCD-3 cells reported that AG1473 inhibited bradykinin-induced activation of MAPK. Thus bradykinin’s effect on EGFR and transactivation may be tissue-and cell-specific. Our experiments are the first in myocardium and demonstrate that bradykinin causes phosphorylation of Akt and ERK1/2 without the involvement of EGFR.
PP2 blocked ROS production in isolated cardiomyocytes and cardioprotection in intact hearts by both bradykinin and DADLE, suggesting involvement of Src kinase. The latter is presumably downstream of EGFR in the case of DADLE, whereas its activation by bradykinin is effected in the absence of EGFR occupancy. It is noteworthy that Fryer et al. [33] were unable to block DADLE’s infarct-sparing effect in in vivo rats with PP2. However, their chosen dose of PP2 was based on the agent’s known IC50 in an in vitro system, and, therefore, might not have accounted for factors encountered in intact animals such as protein binding. Since only one dose of PP2 was used and no other parameter of adequacy of PP2 blockade was monitored, it is possible that the amount injected was insufficient to block Src kinase in the heart. Of interest Audet et al. [34] have noted the stimulatory effect on ERK phosphorylation of the δ-opioid receptor ligand TICP in HEK293s cells was blocked by PP2, whereas PP2 paradoxically increased ERK phosphorylation triggered by a second δ-opioid receptor ligand DPDPE. Therefore, the PP2 response may also be tissue-and cell-specific.
It is unknown how two GiPCR agonists, bradykinin and DADLE, cleaving a common Gi protein can produce such diversity of signaling. But from a teleological viewpoint this diversity would be expected to increase the survival advantage of the organism. We have now demonstrated at least 3 variations of signaling during the trigger phase (adenosine, ACh-opioid and bradykinin), and the differences ensure a high probability of cardioprotection even after blockade of a single signaling moiety.
Acknowledgments
This work was supported in part by grants HL-20648 and HL-50688 from the Heart, Lung and Blood Institute of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cohen MV, Yang X-M, Liu GS, Heusch G, Downey JM. Acetylcholine, bradykinin, opioids, and phenylephrine, but not adenosine, trigger preconditioning by generating free radicals and opening mitochondrial KATP channels. Circ Res. 2001;89:273–278. doi: 10.1161/hh1501.094266. [DOI] [PubMed] [Google Scholar]
- 2.Krieg T, Qin Q, McIntosh EC, Cohen MV, Downey JM. ACh and adenosine activate PI3-kinase in rabbit hearts through transactivation of receptor tyrosine kinases. Am J Physiol. 2002;283:H2322–H2330. doi: 10.1152/ajpheart.00474.2002. [DOI] [PubMed] [Google Scholar]
- 3.Krieg T, Cui L, Qin Q, Cohen MV, Downey JM. Mitochondrial ROS generation following acetylcholine-induced EGF receptor transactivation requires metalloproteinase cleavage of proHB-EGF. J Mol Cell Cardiol. 2004;36:435–443. doi: 10.1016/j.yjmcc.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Oldenburg O, Qin Q, Krieg T, Yang X-M, Philipp S, Critz SD, et al. Bradykinin induces mitochondrial ROS generation via NO, cGMP, PKG, and mitoKATP channel opening and leads to cardioprotection. Am J Physiol. 2004;286:H468–H476. doi: 10.1152/ajpheart.00360.2003. [DOI] [PubMed] [Google Scholar]
- 5.Pain T, Yang X-M, Critz SD, Yue Y, Nakano A, Liu GS, et al. Opening of mitochondrial KATP channels triggers the preconditioned state by generating free radicals. Circ Res. 2000;87:460–466. doi: 10.1161/01.res.87.6.460. [DOI] [PubMed] [Google Scholar]
- 6.Costa ADT, Garlid KD, West IC, Lincoln TM, Downey JM, Cohen MV, et al. Protein kinase G transmits the cardioprotective signal from cytosol to mitochondria. Circ Res. 2005;97:329–336. doi: 10.1161/01.RES.0000178451.08719.5b. [DOI] [PubMed] [Google Scholar]
- 7.Cao Z, Liu L, Van Winkle DM. Met5-enkephalin-induced cardioprotection occurs via transactivation of EGFR and activation of PI3K. Am J Physiol. 2005;288:H1955–H1964. doi: 10.1152/ajpheart.00256.2004. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong S, Downey JM, Ganote CE. Preconditioning of isolated rabbit cardiomyocytes: induction by metabolic stress and blockade by the adenosine antagonist SPT and calphostin C, a protein kinase C inhibitor. Cardiovasc Res. 1994;28:72–77. doi: 10.1093/cvr/28.1.72. [DOI] [PubMed] [Google Scholar]
- 9.Piper HM, Probst I, Schwartz P, Hütter FJ, Spieckermann PG. Culturing of calcium stable adult cardiac myocytes. J Mol Cell Cardiol. 1982;14:397–412. doi: 10.1016/0022-2828(82)90171-7. [DOI] [PubMed] [Google Scholar]
- 10.Mitcheson JS, Hancox JC, Levi AJ. Cultured adult cardiac myocytes: future applications, culture methods, morphological and electrophysiological properties. Cardiovasc Res. 1998;39:280–300. doi: 10.1016/s0008-6363(98)00128-x. [DOI] [PubMed] [Google Scholar]
- 11.Oldenburg O, Qin Q, Sharma AR, Cohen MV, Downey JM, Benoit JN. Acetylcholine leads to free radical production dependent on KATP channels, Gi proteins, phosphatidylinositol 3-kinase and tyrosine kinase. Cardiovasc Res. 2002;55:544–552. doi: 10.1016/s0008-6363(02)00332-2. [DOI] [PubMed] [Google Scholar]
- 12.Krenz M, Oldenburg O, Wimpee H, Cohen MV, Garlid KD, Critz SD, et al. Opening of ATP-sensitive potassium channels causes generation of free radicals in vascular smooth muscle cells. Basic Res Cardiol. 2002;97:365–373. doi: 10.1007/s003950200045. [DOI] [PubMed] [Google Scholar]
- 13.Mitamura T, Higashiyama S, Taniguchi N, Klagsbrun M, Mekada E. Diphtheria toxin binds to the epidermal growth factor (EGF)-like domain of human heparin-binding EGF-like growth factor/diphtheria toxin receptor and inhibits specifically its mitogenic activity. J Biol Chem. 1995;270:1015–1019. doi: 10.1074/jbc.270.3.1015. [DOI] [PubMed] [Google Scholar]
- 14.Qin Q, Downey JM, Cohen MV. Acetylcholine but not adenosine triggers preconditioning through PI3-kinase and a tyrosine kinase. Am J Physiol. 2003;284:H727–H734. doi: 10.1152/ajpheart.00476.2002. [DOI] [PubMed] [Google Scholar]
- 15.Baines CP, Wang L, Cohen MV, Downey JM. Myocardial protection by insulin is dependent on phosphatidylinositol 3-kinase but not protein kinase C or KATP channels in the isolated rabbit heart. Basic Res Cardiol. 1999;94:188–198. doi: 10.1007/s003950050142. [DOI] [PubMed] [Google Scholar]
- 16.Keely SJ, Uribe JM, Barrett KE. Carbachol stimulates transactivation of epidermal growth factor receptor and mitogen-activated protein kinase in T84 cells. Implications for carbachol-stimulated chloride secretion. J Biol Chem. 1998;273:27111–27117. doi: 10.1074/jbc.273.42.27111. [DOI] [PubMed] [Google Scholar]
- 17.Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, et al. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402:884–888. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- 18.Cheng K, Zimniak P, Raufman J-P. Transactivation of the epidermal growth factor receptor mediates cholinergic agonist-induced proliferation of H508 human colon cancer cells. Cancer Res. 2003;63:6744–6750. [PubMed] [Google Scholar]
- 19.Oldenburg O, Cohen MV, Yellon DM, Downey JM. Mitochondrial KATP channels: role in cardioprotection. Cardiovasc Res. 2002;55:429–437. doi: 10.1016/s0008-6363(02)00439-x. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki M, Raab G, Moses MA, Fernandez CA, Klagsbrun M. Matrix metalloproteinase-3 releases active heparin-binding EGF-like growth factor by cleavage at a specific juxtamembrane site. J Biol Chem. 1997;272:31730–31737. doi: 10.1074/jbc.272.50.31730. [DOI] [PubMed] [Google Scholar]
- 21.Hao L, Du M, Lopez-Campistrous A, Fernandez-Patron C. Agonist-induced activation of matrix metalloproteinase-7 promotes vasoconstriction through the epidermal growth factor-receptor pathway. Circ Res. 2004;94:68–76. doi: 10.1161/01.RES.0000109413.57726.91. [DOI] [PubMed] [Google Scholar]
- 22.Izumi Y, Hirata M, Hasuwa H, Iwamoto R, Umata T, Miyado K, et al. A metalloprotease-disintegrin, MDC9/meltrin-γ/ADAM9 and PKCδ are involved in TPA-induced ectodomain shedding of membrane-anchored heparin-binding EGF-like growth factor. EMBO J. 1998;17:7260–7272. doi: 10.1093/emboj/17.24.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weskamp G, Cai H, Brodie TA, Higashyama S, Manova K, Ludwig T, et al. Mice lacking the metalloprotease-disintegrin MDC9 (ADAM9) have no evident major abnormalities during development or adult life. Mol Cell Biol. 2002;22:1537–1544. doi: 10.1128/mcb.22.5.1537-1544.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan Y, Shirakabe K, Werb Z. The metalloprotease Kuzbanian (ADAM10) mediates the transactivation of EGF receptor by G protein-coupled receptors. J Cell Biol. 2002;158:221–226. doi: 10.1083/jcb.200112026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asakura M, Kitakaze M, Takashima S, Liao Y, Ishikura F, Yoshinaka T, et al. Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: metalloproteinase inhibitors as a new therapy. Nat Med. 2002;8:35–40. doi: 10.1038/nm0102-35. [DOI] [PubMed] [Google Scholar]
- 26.Sahin U, Weskamp G, Kelly K, Zhou H-M, Higashiyama S, Peschon J, et al. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J Cell Biol. 2004;164:769–779. doi: 10.1083/jcb.200307137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mifune M, Ohtsu H, Suzuki H, Nakashima H, Brailoiu E, Dun NJ, et al. G protein coupling and second messenger generation are indispensable for metalloprotease-dependent, heparin-binding epidermal growth factor shedding through angiotensin II type-1 receptor. J Biol Chem. 2005;280:26592–26599. doi: 10.1074/jbc.M502906200. [DOI] [PubMed] [Google Scholar]
- 28.Higashiyama S, Nanba D. ADAM-mediated ectodomain shedding of HB-EGF in receptor cross-talk. Biochim Biophys Acta. 2005;1751:110–117. doi: 10.1016/j.bbapap.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Graness A, Hanke S, Boehmer FD, Presek P, Liebmann C. Protein-tyrosine-phosphatase-mediated epidermal growth factor (EGF) receptor transinactivation and EGF receptor-independent stimulation of mitogen-activated protein kinase by bradykinin in A431 cells. Biochem J. 2000;347:441–447. doi: 10.1042/0264-6021:3470441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adomeit A, Graness A, Gross S, Seedorf K, Wetzker R, Liebmann C. Bradykinin B2 receptor-mediated mitogen-activated protein kinase activation in COS-7 cells requires dual signaling via both protein kinase C pathway and epidermal growth factor receptor transactivation. Mol Cell Biol. 1999;19:5289–5297. doi: 10.1128/mcb.19.8.5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang C-M, Lin M-I, Hsieh H-L, Sun C-C, Ma Y-H, Hsiao L-D. Bradykinin-induced p42/p44 MAPK phosphorylation and cell proliferation via Src, EGF receptors, and PI3-K/Akt in vascular smooth muscle cells. J Cell Physiol. 2005;203:538–546. doi: 10.1002/jcp.20250. [DOI] [PubMed] [Google Scholar]
- 32.Mukhin YV, Garnovsky EA, Ullian ME, Garnovskaya MN. Bradykinin B2 receptor activates extracellular signal-regulated protein kinase in mIMCD-3 cells via epidermal growth factor receptor transactivation. J Pharmacol Exp Ther. 2003;304:968–977. doi: 10.1124/jpet.102.043943. [DOI] [PubMed] [Google Scholar]
- 33.Fryer RM, Wang Y, Hsu AK, Nagase H, Gross GJ. Dependence of δ1-opioid receptor-induced cardioprotection on a tyrosine kinase-dependent but not a Src-dependent pathway. J Pharmacol Exp Ther. 2001;299:477–482. [PubMed] [Google Scholar]
- 34.Audet N, Paquin-Gobeil M, Landry-Paquet O, Schiller PW, Piñeyro G. Internalization and Src activity regulate the time course of ERK activation by delta opioid receptor ligands. J Biol Chem. 2005;280:7808–7816. doi: 10.1074/jbc.M411695200. [DOI] [PubMed] [Google Scholar]


