Abstract
Joubert syndrome–related disorders (JSRDs) are a group of clinically and genetically heterogeneous conditions that share a midbrain-hindbrain malformation, the molar tooth sign (MTS) visible on brain imaging, with variable neurological, ocular, and renal manifestations. Mutations in the CEP290 gene were recently identified in families with the MTS-related neurological features, many of which showed oculo-renal involvement typical of Senior-Löken syndrome (JSRD-SLS phenotype). Here, we performed comprehensive CEP290-mutation analysis on two nonoverlapping cohorts of JSRD-affected patients with a proven MTS. We identified mutations in 19 of 44 patients with JSRD-SLS. The second cohort consisted of 84 patients representing the spectrum of other JSRD subtypes, with mutations identified in only two patients. The data suggest that CEP290 mutations are frequently encountered and are largely specific to the JSRD-SLS subtype. One patient with mutation displayed complete situs inversus, confirming the clinical and genetic overlap between JSRDs and other ciliopathies.
Joubert syndrome (JS [MIM 213300]) is an autosomal recessive disease presenting with hypotonia, ataxia, neonatal breathing abnormalities, oculomotor apraxia, and psychomotor delay.1,2 The neuroradiological hallmark of JS is a complex midbrain-hindbrain malformation known as the “molar tooth sign” (MTS), which originates from the association of cerebellar vermis hypoplasia or aplasia, horizontally oriented and thickened superior cerebellar peduncles, and a deepened interpeduncular fossa.3 These clinical and neuroradiological features are shared by at least eight distinct syndromes termed “JS-related disorders” (JSRDs), which additionally present with pleiotropic involvement, mainly of the eyes and kidneys.4
The four major subgroups of JSRDs include (1) the classic form (MIM 213300), largely restricted to brain involvement and also occasionally displaying retinopathy and postaxial polydactyly but rarely renal involvement; (2) the oculo-renal form (referred to in this article as “JSRD-SLS”), which associates JS neurological features with the Senior-Löken syndrome (SLS [MIM 266900]) phenotype of nephronophthisis (NPH [MIM 256100]) and retinal dystrophy (either Leber congenital amaurosis [LCA {MIM 204000}] or retinitis pigmentosa); (3) the subgroup with preaxial or mesaxial polydactlyly and orofacial defects, such as lobulated tongue, notched upper lip, or cleft lip/palate, known as the “orofacial-digital type VI” (OFDVI, or Varadi-Papp [MIM 277170]) syndrome; and (4) the subgroup with choroidoretinal coloboma and hepatic fibrosis, referred to as the “cerebellar vermis hypoplasia/aplasia, oligophrenia, ataxia, ocular coloboma, and hepatic fibrosis” (COACH [MIM 216360]) syndrome.
The genetic bases of JSRDs are only partially understood. Two loci, JBTS1/CORS1 and JBTS2/CORS2, have been mapped to chromosomes 9q34.3 and 11p12-q13.3, respectively, in a limited number of families,5–7 whereas mutations in the AHI1 gene have been shown to account for ∼10% of patients with JSRD, who mostly display the classic form of the disease.8–11
Recently, we and others identified pathogenic mutations in the CEP290 gene, which encodes a centrosomal protein, in 13 families with JSRD.12,13 CEP290 protein was localized to the base (centrosome) and stalk of primary cilia, suggesting that JSRDs constitute one of the “cilia-related” group of disorders. This point is strengthened by the clinical overlap between JSRDs and other ciliopathies, including infantile and juvenile NPH (either isolated or associated with retinal dystrophy, which is known as “SLS”), Meckel syndrome (MKS [MIM 249000]), and Bardet-Biedl syndrome (BBS [MIM 209900]).14 Further reinforcing this link, NPHP1 homozygous deletions that are a frequent cause of isolated juvenile NPH and SLS and mutations in MKS3 that are usually responsible for MKS have been detected in rare cases of JSRDs.15–17
Among the 13 families with JSRD with CEP290 mutations reported so far, the majority had JSRD-SLS, in line with the pleiotropic features observed in most ciliopathies. Few patients displayed incomplete phenotypes lacking either renal or retinal involvement and additional signs, such as occipital encephalocele.12,13 Intriguingly, mutations in CEP290 have also been shown to be the most frequent cause of isolated LCA, in the absence of any renal and neurological involvement.18,19
The prevalence of CEP290 mutations among patients with JSRD-SLS, as well as their role in causing other MTS-associated phenotypes and genotype-phenotype correlates, are still unknown. In this study, we sought to address these issues by performing CEP290 mutation analysis in the largest series to date of probands representative of the whole JSRD clinical spectrum.
Subjects and Methods
Patient Ascertainment
Databases located at the IRCCS CSS, Mendel Institute (Rome) (AISJAC database), at the University of California–San Diego (San Diego) (Center for Cerebellar Malformations), and at the JS Foundation were screened for patients with a definite diagnosis of JSRD and neuroradiologically proven MTS. Patients were referred to these two centers from >20 countries on all continents through European and U.S. referral centers. Samples were obtained from referring physicians or through the JS BioBank. Whenever available, detailed clinical data were collected by means of a standardized questionnaire to assess the possible involvement of all organs, including diagnostic testing for ocular, renal, hepatic, and skeletal features.
From these databases, we selected two groups of patients for CEP290 screening. The first cohort consisted of 44 probands with definite or probable JSRD-SLS. Thirty-two patients met the following inclusion criteria for definite JSRD-SLS: (1) presence of MTS, (2) renal signs of NPH (end-stage renal disease [ESRD] and/or typical ultrasound features and/or proof of impaired urinary-concentration ability), and (3) either LCA or progressive retinitis pigmentosa. In this group, we also included 12 children younger than 10 years presenting a cerebello-ocular phenotype with MTS and LCA but with no obvious renal involvement, who had not yet undergone proper urinary-concentration testing or ultrasound examination. In our experience, the risk of a child with LCA within this age group developing a renal disease is >50%; thus, these 12 patients were given diagnoses of “probable JSRD-SLS.”
The second cohort consisted of 84 probands representative of the complete spectrum of the remaining subgroups of JSRDs, including classic JS (n=42), JS plus ocular involvement but without renal involvement by age 10 years (n=21), JS plus NPH or cystic kidney disease without retinal involvement (n=5), COACH syndrome (n=6), and OFDVI syndrome (n=5). Five patients had a proven MTS, but clinical details were not sufficient to assign them to a specific phenotypic subgroup. Informed consent was obtained from all families, and the study was approved by the local ethics committees.
Mutational Analysis
The 128 probands with JSRD were analyzed for CEP290 mutations after a three-step strategy.10 In brief, a denaturing high-performance liquid chromatography (DHPLC)–based analysis was first performed on DNA from one parent of each proband. All exons identified as carrying abnormal elution profiles were sequenced in both directions, and, subsequently, all parental mutations were tested in the affected offspring and, in case of homozygosity, in the other parent as well. Finally, heterozygous patients underwent complete gene analysis by direct sequencing in both directions to identify the second mutation. This strategy allowed DNA from affected children (which is usually scarce and difficult to reobtain) to be preserved and DHPLC limits in identifying homozygous mutations to be overcome. PCRs were performed in a final volume of 25 μl containing 40–80 ng genomic DNA, 1 U AmpliTaq Gold (Applied Biosystems), 15 pmol of each primer, 1.5–2 mM MgCl2, 75 μM of each deoxyribonucleotide triphosphate, and 1× PCR Buffer (Applied Biosystems), through the use of a GeneAmp PCR system 9700 (Applied Biosystems). Samples were run on a Wave DNA Fragment Analysis System (Transgenomics) at column temperatures recommended by Navigator Software version 1.5.4 (Transgenomics). Bidirectional sequencing of purified PCR products (Millipore) was performed by using BigDye chemistry and an ABI 3100 Capillary Array Sequencer (Applied Biosystems). Primers and conditions for PCR and DHPLC are listed in table 1.
Table 1. .
Primers and PCR and DHPLC Conditions for CEP290 Analysis
| Exon(s) | Primer Sequence (5′→3′) | Amplicon Size (bp) |
PCR Annealing Temperature (°C) |
No. of Cycles | DHPLC Oven Temperaturea (°C) |
Buffer B Starting %a |
| 2 | ACCAATAATACTGTGTACCTTG CAGATTGTGACAATTATAGTTG | 289 | 58 | 38 | 53.1/57 | 54.4/52.1 |
| 3 | CAACTATAATTGTCACAATCTG GTTCCACTAATAGCCAAACC | 212 | 58 | 32 | 55.7 | 50.9 |
| 4 | GTGCTTACATTCCAGTATAAAG GTTTAATGAACAAATGGAATTCA | 186 | 58 | 32 | 54.4/58 | 53.4/49 |
| 5 | ACCTTATAATCATGATGGACTC AATAACCATGATTACAATCATCC | 285 | 58 | 32 | 51.8/53.4 | 54.3/54 |
| 6 | TTGTTGACTCATTTGAACCTC AAAAAGCCAGGTAACTTGAAC | 264 | 58 | 32 | 53.2/56.4 | 55.6/54.2 |
| 7 | ACTGCTGAATTTTATCTTCTTC TTAGAAGACTCCAGTCCTGG | 208 | 58 | 32 | 55.7 | 51.2 |
| 8–9 | CAAGATAATATGCATCATTTTCCC ATGAAATTAAAGTTTTTAGGAACC | 472 | 58 | 32 | 55 | 58.7 |
| 10 | AGAGGACACTTATGGCTGCG GTAATGAGATAATATGAAGTCTG | 332 | 56 | 35 | 52.9/54.1 | 56.3/54.6 |
| 11 | CACATATGTAATGTAATGTATCC CTAATAAACGTGTTATAAACCAG | 364 | 56 | 35 | 52.4/55.2 | 55.8/54.7 |
| 12 | GTATCATAAATCTACTAACGGTG ATCGTTCAGAGTTCCAACTG | 284 | 58 | 35 | 54/55.4 | 55.9/55.5 |
| 13 | CTTGTACCCACAAGAAAATATG AGAAAACTCAATATTGACTTGAC | 341 | 56 | 35 | 52.7/54.3 | 55.8/52.8 |
| 14 | TGATTTGAAGGAATAAGTAGTC CTGTGAATGGCAAGAATAATTC | 282 | 58 | 35 | 55/56.1/58 | 57.2/56.1/52 |
| 15 | GTACATTTTCCTTTAGACTTAG ACTTGTAAATCAGGTTGCGC | 311 | 58 | 38 | 54.1/56.6 | 55/52.5 |
| 16–17 | CATTTTTGCAGCTTATTTGAATG ATATCCAGACAACTCACTTATC | 380 | 58 | 38 | 54.8/55.8 | 57.9/56.9 |
| 18 | ATTAAAGTGTTGGAATAGTAGG TATTTTCCTTTACTCTCTTTGC | 218 | 58 | 38 | 55.3 | 50.1 |
| 19 | ATTGATCAAACTTTTCTTAACTTG ACAGAGGTAATTAGGAGTAAAG | 293 | 54 | 35 | 54 | 56.2 |
| 20 | CCAATGATGTCTTTGGTATATG AAATATCTCATCAGAAACTATGG | 340 | 58 | 38 | 54.5/55 | 57.8/57.3 |
| 21 | GTCCATTTTATTTAAAGACAGAC TTAATTCAAGGGGCATTTTCTC | 362 | 58 | 35 | 53.6/57.4 | 56.7/52.9 |
| 22 | TATGGTTGAGGTAAAATTCCTG AGTACTATCTGCATGCTTTGG | 390 | 58 | 38 | 52.6/55.8 | 58.9/54.2 |
| 23 | TAACTTTCCTATAATGTTGTCAG TAAGTTCCTAACAGTAGTTACC | 372 | 54 | 35 | 53.2 | 57.3 |
| 24 | ATACCTCTTGTGTTGAGAAAAC CACAAAGACACATCCATATTAC | 300 | 54 | 35 | 53.3/54.5 | 56.7/54.7 |
| 25 | TATGCAATATTGTACAAAGTAGG TGATACCATCCTATCTTCTGC | 368 | 58 | 38 | 54.5/55.4 | 58.7/57.8 |
| 26 | AAAGTGGCTAGTGCTTGACC TGTTAAATTTATATAAATGCAGGC | 358 | 54 | 35 | 56.3 | 57.9 |
| 27 | AACTGGATTGTGAGTTTTAAGG AGGATTATTCATCTGCCTAAG | 383 | 58 | 38 | 52.7/54.8 | 57.3/53.7 |
| 28 | ACAGCATCTAAAATATCTGAGG AGATCCAGACAAACCACTTAAC | 369 | 58 | 38 | 54.4/56.6 | 57/54.4 |
| 29 | AAGGCCAAGTAAAGAGGATTG TACTACTAAGAATTGTATACCTG | 349 | 58 | 35 | 55/56.9 | 58/55.8 |
| 30 | TAGAAAGTGTACTTAATTGTTCC CCCACTCCCAACATCTAATG | 231 | 58 | 38 | 56.2/58 | 55/52 |
| 31 | AATCTGTGATAACTTTCACTGG TGTTTGCACCACTGAACTCC | 604 | 58 | 35 | 54.7/58 | 61.2/56.5 |
| 32 | CATTATCATCAATGGAGGAATG TAGTCATTTGTGCAATATTCTTG | 603 | 58 | 35 | 53 | 61.7 |
| 33 | CCTGTTATGTGCCTGATGTC TGAGTTAACACTCTAGACTATG | 222 | 58 | 38 | 56.8/58.1 | 55.5/54 |
| 34 | ATCTATGTTTTATCATACAGCTG ATCATTCTATGCATTGCCCTC | 321 | 58 | 38 | 54.6/56.9 | 57.5/54.6 |
| 35 | GCATTTTAAAGGGAAAAAGATAC CACTTTAGGGTAAAATAATATTTAG | 402 | 54 | 37 | 53.5/55.4/57.5 | 58.1/56.1/54.1 |
| 36 | ATATGGAGATACTGTTTCTTCC GCTGAATTTTAATTTACATGGTC | 305 | 58 | 38 | 55.6 | 55.2 |
| 37 | AATATGGAATAAGTATGGCATTG AGCAAACACTTATGTTTATCTTC | 337 | 58 | 38 | 52.4/54.4/57.8 | 58.1/56.1/52.7 |
| 38 | GTGACAGAGTGAGACTGGG ACAACACGGAGATTTATACTAC | 397 | 58 | 38 | 54.8/56.4 | 57.7/56 |
| 39 | ATAGTAGGAAGTAATAAAGCTTG TAGTGAATTCTCTTCCAATAGG | 305 | 58 | 38 | 57.5/59.2 | 55/53 |
| 40 | GTTCCTTTTATCATTGATACTTC AAGTAGAAATAAACTACTACCTC | 352 | 58 | 38 | 52.6/53.8 | 58.6/57.4 |
| 41 | GTGATAGCTTCAGAAAGTTGC CAGAATTAATACAGCCAGGTC | 342 | 58 | 38 | 55.3 | 54.3 |
| 42 | AACATATTTACATATTCTCTAGG TAAAGCTATATAATTTCCAGGTC | 345 | 54 | 38 | 55.2 | 54.4 |
| 43 | TTTGGTTTGGTAATGAGTATGC TTCAATTTCTAGGGGTCAACC | 301 | 58 | 38 | 55/56.6 | 56.8/55.2 |
| 44 | ACACTGAACTTTTCTTTTTTTATC AGATGTAATGCTTTTGGCCAG | 320 | 58 | 38 | 54.4/57.6 | 56.7/52.7 |
| 45 | TATCCAGTATGTCTTTTATGGC ACCATCACCATGATATATTAGG | 329 | 58 | 38 | 53.9/55.7 | 56.4/53.2 |
| 46 | TTTGCCTTTTCTTTTCAATGGC TATCTAAACTTTTCATTTCTGGC | 223 | 58 | 38 | 56.2/57.7 | 53.4/51.9 |
| 47 | TGTTGTATTGTTGGTACTTCG TTAGCCTTGCCTCTCATAAG | 394 | 56 | 35 | 53/54.9 | 57.9/56 |
| 48 | TGGTTTCTAAAACTACTTTGAAG ACTTCCAGTTTTTCCAAGAGG | 296 | 58 | 38 | 53.3/54.4/57.2 | 55.5/54.4/51.6 |
| 49 | TAGAGCCCCAGGTTATTTTTG TGTTCATCAGGAAGAAACCAG | 293 | 58 | 38 | 54.2/56.1/58.5 | 59.5/56.5/53 |
| 50 | TTAGTACAGTTATTTGAACTGAC ACAATGCAAGGAACATCTTGC | 293 | 58 | 38 | 53.7/55.2 | 56.6/53.5 |
| 51 | ACGCTTTGTTAAAAATGTGTATC ATGCTTGTCTCTAGTTGTAGC | 255 | 58 | 38 | 55.5/58.9 | 52.7/46.2 |
| 52 | TCACTAGTTCATAAGAAATGCC AATTCGATTTTACAGGGAGAC | 291 | 58 | 38 | 55.1/56.6 | 56.2/54.2 |
| 53 | CCATTACCTTGAACTCATTCG TAGGATACGTAGTTAAAGATGG | 230 | 58 | 38 | 54.2/55.9 | 54.2/51.5 |
| 54 | ATTCAGGAATACTTTGGCTTTC TTCGGAGAACTGCTTATTTCC | 418 | 58 | 38 | 53.6/55.5 | 57.7/52.9 |
Each value is a different optimized oven temperature, with the corresponding buffer B starting percent for each corresponding temperature at which the amplicon has been analyzed. For some amplicons, to ensure adequate peak separation, we needed to run at a different temperature and buffer B starting percent. In those instances, duplicate values for both are represented.
The common NPHP1 homozygous deletion, the intronic CEP290 mutation recurring in isolated LCA (c.2991+1655A→G), and mutations in the AHI1 gene were excluded in all probands, in accordance with published protocols.10,16,18
Results
CEP290 Mutations
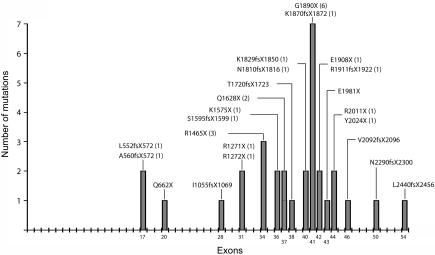
We identified 23 distinct CEP290 mutations in 21 families. Seventeen mutations were novel, whereas six had been reported elsewhere (tables 2 and 3).12,13,19 Six probands were homozygous for CEP290 mutations, and 10 were compound heterozygous, whereas, in 5 probands, only a single mutated allele could be found. All mutations reported were either nonsense or frameshift mutations resulting in a predicted truncated protein. Most mutations (20 of 23) clustered from exon 28 to the end of the gene, with a peak at exon 41 (fig. 1). The most common mutation was G1890X, which was homozygous in three families (two from the United Arab Emirates and one from Turkey) and heterozygous in three others (from Italy, Ireland, and the United Kingdom). The R1465X mutation in exon 34 recurred in three families (from Belgium, Brazil, and the United States), and the Q1628X (exon 37) mutation in two (from the United States and Russia), whereas the remaining 20 mutations were all found in single families (table 2). All mutations segregated with the disease in familial cases. CEP290 nucleotide variants representing either polymorphisms or nucleotide changes of unknown significance are listed in table 4.
Table 2. .
Genotypes and Phenotypes of Patients with CEP290 Mutations[Note]
| Phenotype |
||||||||||
| Patient | Origin | Consanguinity | Sex | Age (years) |
DNA Alteration | Predicted Protein Alteration | Exon(s) | Eyea | Kidneyb | Otherc |
| With JSRD-SLS: | ||||||||||
| COR083 | Switzerland | No | F | 2 | 5163delT,d homozygous | T1721fsX1723, homozygous | 38 | LCA | NPH | EC |
| COR145 | United Kingdom | No | M | 2 | 1657_1666delA+6031C→T | L552fsX572+R2011X | 17, 44 | LCA | Normal US, UCT NP | … |
| MTI333a | United States | No | M | 3e | 4882C→Td+5610delCAAA | Q1628X+K1870fsX1872 | 37, 41 | LCA | NA | NA |
| MTI333b | United States | No | F | 12 | 4882C→Td+5610delCAAA | Q1628X+K1870fsX1872 | 37, 41 | LCA, RC | NPH (7) | No MR |
| COR109 | France | No | F | 6 | 1682_1683delA+3814C→Td | A560fsX572+R1272X | 17, 31 | LCA | Normal US, UCT NP | … |
| COR003 | Italy | No | F | 6 | 5668G→Td+? | G1890X+? | 41, ? | LCA | NPH | ASD |
| COR084 | Russia | No | F | 6 | 4882C→Td+5941G→T | Q1628X+E1981X | 37, 43 | LCA | NPH | … |
| MTI154 | India | No | F | 6 | 5668G→T,d homozygous | G1890X, homozygous | 41 | RP, VR | NPH | VSD |
| MTI125 | United States | No | F | 6 | 4393C→T+? | R1465X+? | 34, ? | LCA | NPH (6) | … |
| COR004a | Italy | No | F | 17 | 3811C→T+5734delT | R1271X+R1911fsX1922 | 31, 42 | LCA | NPH (13) | … |
| COR004b | Italy | No | M | 7 | 3811C→T+5734delT | R1271X+R1911fsX1922 | 31, 42 | LCA | NPH | … |
| MTI328 | United States | No | M | 8 | 1985A→T+6277delG | Q662X+V2092fsX2096 | 20, 46 | LCA | NPH (4) | … |
| MTI273 | Turkey | Yes | M | 10 | 4786_4790delTAAA, homozygous | S1595fsX1599, homozygous | 36 | LCA | NPH (9) | SI |
| COR125 | United Kingdom | No | F | 10 | 5431_5433delGA+5668G→Td | N1810fsX1816 +G1890X | 40, 41 | LCA | NPH (8) | … |
| MTI487 | Turkey | Yes | F | 10 | 5722G→T, homozygous | E1908X, homozygous | 42 | LCA, RC | US and UCT NP | LT, PP, CM |
| MTI118 | Ireland | No | F | 12 | 3167_3175insAd+5668G→Td | I1055fsX1069+G1890X | 28, 41 | LCA | NPH | … |
| MTI286 | Brazil | No | F | 13 | 4393C→T+? | R1465X+? | 34, ? | LCA | NPH | … |
| MTI111a | Laos | No | M | 13 | 6072C→A+7321dupCTCT | Y2024X+L2440fsX2456 | 44, 54 | LCA | NPH | … |
| MTI111b | Laos | No | F | 28 | 6072C→A+7321dupCTCT | Y2024X+L2440fsX2456 | 44, 54 | NA | NA | NA |
| COR031 | Belgium | Yes | F | 15 | 4393C→T+4723A→Td | R1465X+K1575X | 34, 36 | LCA | NPH | … |
| COR001 | Italy | No | F | 20 | 5489_5493delA+? | K1829fsX1850+? | 40, ? | LCA | NPH (9) | … |
| COR002a | Italy | No | M | 34 | 6870delT+? | N2290fsX2300+? | 50, ? | LCA | NPH (18) | … |
| COR002b | Italy | No | M | 30 | 6870delT+? | N2290fsX2300+? | 50, ? | LCA | NPH (11) | … |
| With JSRD: | ||||||||||
| MTI012 | United Arab Emirates | Yes | M | 8 | 5668G→T,d homozygous | G1890X, homozygous | 41 | Normal | Normal US, UCT NP | … |
| MTI587 | United Arab Emirates | Yes | M | 5 | 5668G→T,d homozygous | G1890X, homozygous | 41 | Normal | NPH | … |
Note.— NA = not available. All patients displayed MTS features, neurological signs typical of JS.
RC = retinal coloboma; RP = retinitis pigmentosa; VR = vision reduction.
NPH includes ESRD (if present, age at onset in years is given in parentheses) and/or typical ultrasound (US) features and/or proof of impaired urinary-concentration ability. UCT = urinary-concentration testing after 1-deamino-8-D-arginine vasopressin challenge; NP = not performed.
EC = encephalocele; MR = mental retardation; ASD = atrial septal defect; VSD = ventricular septal defect; LT = lobulated tongue; PP = postaxial polydactyly; CM = cardiomegaly; SI = complete situs inversus.
The six previously reported mutations (see table 3 for details). All other mutations are novel.
This patient died at age 3 years.
Table 3. .
Recurrent Mutations Identified in the CEP290 Gene
| Phenotype and DNA Mutation |
Predicted Protein Alteration |
Exon | No. of Families | Referencesa |
| JSRD only: | ||||
| 5668G→T | G1890X | 41 | 10 | Valente et al.,12 Sayer et al.,13 PD |
| 4393C→T | R1465X | 34 | 3 | PD |
| LCA only: | ||||
| 2991+1655A→G | C998X | 26 | 44 | den Hollander et al.,18 Perrault et al.19 |
| 5587−1G→C | Splice | 40 | 4 | den Hollander et al.18 |
| 5850delT | F1950fsX1964 | 42 | 3 | den Hollander et al.18 |
| 6604delA | I2203fsX2226 | 48 | 2 | den Hollander et al.18 |
| JSRD and LCA: | ||||
| 4723A→T | K1575X | 36 | 8 | Perrault et al.,19 PD |
| 5163delIT | T1721fsX1723 | 38 | 4 | Perrault et al.,19 PD |
| 3167_3175insA | I1055fsX1069 | 28 | 3 | Sayer et al.,13 Perrault et al.,19 PD |
| 4882C→T | Q1628X | 37 | 3 | Perrault et al.,19 PD |
| 3814C→T | R1272X | 31 | 2 | den Hollander et al.,18 PD |
| 7341–7342insA | L2448fsX2455 | 54 | 2 | Sayer et al.,13 den Hollander et al.18 |
PD = present data.
Figure 1. .
Schematic of distribution of mutations identified in this study across the 54 exons of CEP290. Numbers of patients with mutations are listed in parentheses.
Table 4. .
Polymorphisms and Variants Observed in the CEP290 Gene
| DNA Alteration | Reference SNPa | Predicted Protein Alteration | Exon or Intron | Allele Frequencyb (n=204 Chromosomes) |
| IVS5+36A→G | ss69374911 | … | Intron 5 | Common |
| c.829G→C | ss69374912 | E277Q | Exon 10 | Common |
| c.930A→G | … | V310V | Exon 11 | NS |
| c.1991A→G | … | D664G | Exon 20 | NS |
| IVS20+30delT | … | … | Intron 20 | NS |
| c.2055T→C | ss69374913 | A685A | Exon 21 | Common |
| IVS21+44T→C | ss69374914 | … | Intron 21 | Common |
| c.2268G→A | rs2468255 | S756S | Exon 22 | Common |
| c.2343T→C | … | N781N | Exon 22 | NS |
| c.2512A→G | rs11104738 | K838E | Exon 24 | Common |
| c.2717T→G | rs7970228 | L906W | Exon 25 | NS |
| IVS35+40_46delT | rs11356711 | … | Intron 35 | Common |
| c.4806G→A | … | T1602T | Exon 36 | NS |
| IVS41+25A→C | rs17015438 | … | Intron 41 | Common |
| IVS41+45G→C | ss69374915 | … | Intron 41 | Common |
| IVS47+5_12insT | rs11405846 | … | Intron 47 | Common |
| c.6684T→G | … | N2228K | Exon 49 | NS |
| IVS51−37C→G | ss69374916 | … | Intron 51 | Common |
| IVS53−22T→C | ss69374917 | … | Intron 53 | Common |
“rs” Refers to reference SNP number; “ss” refers to submitter SNP number.
Common = allele frequency >2%. NS = variation found in the parent but not segregating in affected offspring.
Phenotypes Associated with CEP290 Mutations
JSRD-SLS subgroup
CEP290 mutations were identified in 16 (50%) of 32 probands with definite JSRD-SLS and in 3 (25%) of 12 patients with probable JSRD-SLS. All patients had neurological signs typical of JS and a neuroradiologically proven MTS (fig. 2). Fifteen had sporadic cases of disease, whereas four had one affected sib.
Figure 2. .
Brain magnetic resonance imaging results for 12 of 21 probands with mutations, showing the typical midbrain-hindbrain malformation known as the MTS. All panels display axial sections at the pontine or pontomesencephalic junction showing the MTS. A–L, respectively, patients MTI012, MTI286, COR002a, COR083, COR145, COR109, COR084, COR125, MTI333b, MTI154, MTI125, and MTI487.
Among the 16 probands with definite JSRD-SLS, 15 presented with LCA and blindness within the 1st year of life, whereas only 1 (MTI154) had progressive retinitis pigmentosa, with visual reduction documented at age 6 years. One patient (MTI133b) had chorioretinal colobomas in addition to LCA. All patients had signs of infantile or juvenile NPH; the youngest was age 2 years. Eight of them had already developed ESRD at ages ranging between 4 and 18 years. Four patients with sporadic cases presented additional clinical features, including a small occipital encephalocele (COR083), atrial (COR003) or ventricular cardiac septal defect (MTI154), and complete situs inversus (MTI273) (fig. 3).
Figure 3. .
Patient MTI273, homozygous for the S1595fsX1599 mutation in exon 36 of the CEP290 gene and presenting with JSRD-SLS associated with complete situs inversus. Sagittal (A) and axial (B) brain magnetic resonance imaging sections show the MTS malformation. C, Face showing absence of specific dysmorphic features. D, Kidney ultrasound displaying multiple renal cysts and increased echogenicity. E, Chest x-ray showing complete situs inversus, with stomach bubble and heart apex on the patient's right side (R).
Three patients with mutations were given diagnoses of probable JSRD-SLS, since they had LCA but no overt signs of renal involvement by ages 2, 6, and 10 years. However, evaluation of urinary-concentration ability after water deprivation or desmopressin challenge had not been performed, and the presence of an asymptomatic urinary-concentration defect could not be excluded. One patient presented additional clinical signs, including chorioretinal colobomas, postaxial polydactyly, lobulated tongue, and cardiomegaly (MTI487) (table 2).
Other JSRD subgroups
The screening of the CEP290 gene among a second cohort with MTS yielded mutations in only 2 (2%) among 84 probands, one with classic JS (1 of 42) and one with JS plus NPH (1 of 5). No mutations were found among 21 patients with JS plus ocular involvement, 6 with COACH syndrome, 5 with OFDVI syndrome, and 5 with proven MTS but limited clinical data for subgroup assignment.
Between the two patients with mutations, the one classified as having pure JS presented no overt signs of retinal or renal involvement at age 8 years, and results of kidney ultrasound examination were normal. Yet, the possibility that this child might develop a renal disease phenotype with increasing age cannot be ruled out. The second patient with mutation had urinary-concentration defect but normal vision and no signs of retinal involvement at age 5 years. Both patients were homozygous for the recurrent G1980X mutation.
Discussion
In this study, we identified 17 novel CEP290 truncating mutations, raising to 65 the number of known deleterious alterations found in patients with JSRD and/or LCA.12,13,18,19 Most mutations appear to be private, with only 12 mutations recurring in two or more families (table 3). Besides the most common mutation—C998X, which is restricted to LCA—our data show that G1890X represents the second-most frequent CEP290 mutation. Interestingly, this aberration seems to be strictly associated with the JSRD phenotype, since it was never reported in large cohorts of patients with isolated LCA. Yet, other recurrent mutations have been described in families with both JSRD and LCA, making it difficult to establish genotype-phenotype correlates. Of note, whereas mutations causative of isolated LCA appear to be homogeneously distributed throughout the gene,18,19 >80% of mutations identified in families with JSRD cluster in the second half of the gene. We, as well as others,13,19 failed to identify the second mutation in ∼20% of CEP290 families despite extensive sequencing of the whole coding region and canonical splice sites. The possibility that other mutations reside within intronic sequences (as is the case for the recurrent C998X mutation)18 or represent large genomic rearrangements needs to be further explored.
We found that CEP290 is a major causative gene of definite JSRD-SLS, with demonstrable deleterious mutations detected in half of the patients in this subgroup. The renal disease phenotype was characterized mostly by juvenile NPH, with development of renal failure toward the end of the 1st decade or early in the 2nd decade. However, the age at onset of renal failure was found to be extremely variable, with the youngest patient aged 4 years. Thus, patients with CEP290 mutations can also develop the infantile form of NPH with rapid progression toward renal failure within the first few years of life.
We also identified mutations in three children with retinal blindness but no overt clinical signs of NPH by age 10 years (probable JSRD-SLS). It will be crucial to follow up these patients to evaluate whether they will develop renal disease later in life, to define the prognostic value of CEP290 mutations in predicting NPH. This issue carries important implications for genetics counseling of families and for the setup of early interventional strategies aimed at delaying the progression toward renal failure and minimizing complications such as growth retardation or bone disease.
The ocular phenotype observed in patients with JSRD-SLS is usually characterized by LCA. Yet, patients with rarer cases show no detectable defects in vision within the 1st year of life but later develop a progressive phenotype of retinitis pigmentosa, usually with some residual sight. By screening patients with other non–JSRD-SLS phenotypes, we identified pathogenic mutations in two children with preserved vision and normal ophthalmologic testing at ages 5 and 8 years (MTI012 and MTI587, respectively). Intriguingly, we noticed that these two patients, as well as the single child with JSRD-SLS with retinitis pigmentosa who was not blind at age 6 years (MTI154), were the only three to be homozygous for the recurrent G1890X mutation in exon 41. This correlation holds true also for patients described elsewhere. In fact, of nine patients homozygous for this mutation, only one was reported to have LCA,12,13 leading us to speculate that this truncating mutation, through unknown mechanisms, might spare the retina. Conversely, other truncating and splice-site mutations, including the recurrent C998X, are invariably associated with isolated LCA (table 3), and the rd16 mouse, which carries an inframe 298-aa deletion of CEP290, shows a pure retinal degeneration phenotype.18–20 Therefore, there appear to be specific mutations that show predilection for one organ system over another. We excluded the possibility of alternative splicing of implicated exons as a mechanism of this selective organ sparing, because they are present in all mRNAs represented in the UCSC Genome Browser. This observation has not been reported elsewhere for any known genetic disease, to our knowledge, and warrants functional studies of these mutant proteins in specific cell and animal models.
Among the 84 patients with other JSRD subtypes, we identified no mutations other than the ones in the 2 patients without retinopathy. Therefore, it appears that the clinical spectrum of CEP290 mutations is largely restricted to the JSRD-SLS phenotype. It is interesting to note that one patient with mutation with probable JSRD-SLS (MTI487) also presented additional clinical features reminiscent of the OFDVI and COACH subgroups, such as lobulated tongue, postaxial polydactyly of the hands and feet, and bilateral chorioretinal colobomas. Although a definite diagnosis of one of these syndromes could not be made with the current classification system, the clinical spectrum of MTS-associated features does require further delineation.
In some patients with JSRD-SLS with CEP290 mutations, we observed additional peculiar clinical features, expanding the phenotypic spectrum to clearly overlap with other ciliopathies. The first example is the presence in three patients (COR083 and two patients in the work of Sayer et al.13) of occipital (meningo)encephalocele. This neural-tube defect represents a constant feature of MKS, which has been recently shown to be allelic to JSRD at the MKS3 gene.17 The second example is patient MTI273, a 10-year-old Turkish boy presenting a JSRD-SLS phenotype associated with complete situs inversus. Although this observation may be coincidental, this is unlikely because of the rarity of both conditions and the report of similar phenotypes within the spectrum of ciliopathies. Mutations in NPHP2, which encodes inversin, cause infantile NPH occasionally associated with retinal dystrophy or with situs inversus.21,22 Similarly, randomization of left-right body-axis symmetry was reported in 4% of Finnish patients with MKS23 and in a BBS-affected family with mutations in BBS8.24 Of note, two other patients with CEP290 mutations in our cohort (COR03 and MTI154) presented with congenital heart malformations (atrial and ventricular septal defect, respectively) that are often part of the clinical spectrum of abnormalities observed in situs inversus.
The study of animal models with disrupted ciliary structure or function has highlighted a critical role for cilia in regulating specific developmental pathways of neurulation and left-right axis determination. In mouse embryos lacking KIF3B, a protein essential for intraflagellar transport (IFT) and ciliogenesis, the consequent lack of twirling nodal cilia led to a complex developmental phenotype of growth retardation, randomized left-right axis asymmetry, and neural-tube formation defects.25 Knockout mice for distinct IFT proteins display neural-tube defects that are consequent to disruption of the sonic hedgehog signaling pathway,26–28 whereas a deletion of inversin in mouse or knockdown in zebrafish resulted in renal cystic phenotype with laterality defects.21,29
Since these proteins share several domains and have been demonstrated to act in large protein-protein networks,14 it will be intriguing to investigate how CEP290 possibly interacts with other ciliary proteins to regulate basic developmental mechanisms and sensory functions in multiple tissues.
Acknowledgments
This work was supported by grants from the U.S. National Institute of Neurological Disease and Stroke, the Italian Ministry of Health (Ricerca Corrente 2007; Ricerca Finalizzata 2005 Progetto Malattie Rare grant number 526/A36), the Fondazione Pierfranco e Luisa Mariani Organizzazione Non Lucrativa di Utilita Sociale, the March of Dimes, the Burroughs Wellcome Fund Award in Translational Research, and the National Institutes of Health.
Other members of the International JSRD Study Group are Padraic Grattan-Smith (Sydney, Australia); Richard Leventer (Victoria, Australia); Andreas Janecke (Innsbruck, Austria); Rudy Van Coster (Ghent, Belgium); Karin Dias, Carla Moco, and Ana Moreira (Porto Alegre, Brazil); Chong Ae Kim (São Paulo); Gustavo Maegawa (Toronto); Ghada M. H. Abdel-Salam, Alice Abdel-Aleem, and Maha S. Zaki (Cairo, Egypt); Itxaso Marti and Susana Quijano-Roy (Garches, France); Pascale de Lonlay and Alain Verloes (Paris); Renaud Touraine (St. Etienne, France); Michel Koenig, Clotilde Lagier-Tourenne, and Jean Messer (Strasbourg, France); Heike Philippi (Mainz, Germany); Sofia Kitsiou Tzeli (Athens, Greece); Saevar Halldorsson, Jonina Johannsdottir, and Peter Ludvigsson (Reykjavik); Alex Magee (Belfast); Dorit Lev and Marina Michelson (Holon, Israel); Bruria Ben-Zeev (Ramat-Gan, Israel); Rita Fischetto and Mattia Gentile (Bari, Italy); Silvia Battaglia and Lucio Giordano (Brescia, Italy); Loredana Boccone (Cagliari, Italy); Martino Ruggieri (Catania, Italy); Stefania Bigoni and Alessandra Ferlini (Ferrara, Italy); Maria Alice Donati and Elena Procopio (Florence, Italy); Gianluca Caridi, Francesca Faravelli, and Gianmarco Ghiggeri (Genoa, Italy); Silvana Briuglia and Gaetano Tortorella (Messina, Italy); Stefano D’Arrigo, Chiara Pantaleoni, Daria Riva, and Graziella Uziel (Milan, Italy); Anna Maria Laverda and Alberto Permunian (Padova, Italy); Stefania Bova (Pavia, Italy); Roberta Battini (Pisa, Italy); Maria Roberta Cilio, Marilù Di Sabato, Francesco Emma, Vincenzo Leuzzi, and Pasquale Parisi (Rome); Alessandro Simonati (Verona, Italy); Asma A. Al-Tawari, Laila Bastaki, and Ahmad Aqeel (Kuwait City); Mirjam M. de Jong (Groningen, The Netherlands); Roshan Koul and Anna Rajab (Muscat, Oman); Matloob Azam (Islamabad, Pakistan); Clara Barbot (Oporto, Portugal); Berta Rodriguez (La Coruna, Spain); Ignacio Pascual-Castroviejo (Madrid); Sinan Comu (Istanbul); Mustafa Akcakus (Kayseri, Turkey); David Nicholl (Birmingham, United Kingdom); C. Geoffrey Woods (Cambridge, United Kingdom); Christopher Bennett and Jane Hurst (Leeds, United Kingdom); Christopher A. Walsh (Boston); Saunder Bernes (Mesa, AZ); Henry Sanchez (Fremont, CA); Aldon E. Clark (Laguna Niguel, CA); Clement Donahue (San Francisco); Jin Hahn and Terence D. Sanger (Stanford, CA); Tomas E. Gallager (Manoa, HI); William B. Dobyns (Chicago); Cynthia Daugherty (Bangor, ME); Kalpathy S. Krishnamoorthy and Dean Sarco (Boston); Trudy McKanna (Grand Rapids, MI); Joanne Milisa (Albuquerque, NM); Wendy K. Chung, Darryl C. De Vivo, Hillary Raynes, and Romaine Schubert (New York); Alison Seward (Columbus, OH); David G. Brooks (Philadelphia); Amy Goldstein (Pittsburg, PA); James Caldwell and Eco Finsecke (Tulsa, OK); Bernard L. Maria (Charleston, SC); Kenton Holden (Mt. Pleasant, SC); Robert P. Cruse (Houston, TX); and Kathryn J. Swoboda (Salt Lake City, UT).
Web Resources
Accession numbers and URLs for data presented herein are as follows:
- AISJAC, http://www.aisjac.com
- Center for Cerebellar Malformations, http://www.ccm.ucsd.edu
- JS BioBank, http://www.joubertsyndrome.org/BioBank.asp
- JS Foundation, http://www.joubertsyndrome.org/RegCoordinators.asp
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for JS, JSRD, SLS, NPH, LCA, OFDVI, COACH, MKS, and BBS)
- Standardized clinical questionnaire for JSRD evaluation, http://www.ccm.ucsd.edu/PatientQuestionnaire2004.pdf
- UCSC Genome Browser, http://www.genome.ucsc.edu/
References
- 1.Joubert M, Eisenring JJ, Robb JP, Andermann F (1969) Familial agenesis of the cerebellar vermis: a syndrome of episodic hyperpnea, abnormal eye movements, ataxia, and retardation. Neurology 19:813–825 [DOI] [PubMed] [Google Scholar]
- 2.Boltshauser E, Isler W (1977) Joubert syndrome: episodic hyperpnea, abnormal eye movements, retardation and ataxia, associated with dysplasia of the cerebellar vermis. Neuropadiatrie 8:57–66 [DOI] [PubMed] [Google Scholar]
- 3.Maria BL, Hoang KB, Tusa RJ, Mancuso AA, Hamed LM, Quisling RG, Hove MT, Fennell EB, Booth-Jones M, Ringdahl DM, et al (1997) “Joubert syndrome” revisited: key ocular motor signs with magnetic resonance imaging correlation. J Child Neurol 12:423–430 [DOI] [PubMed] [Google Scholar]
- 4.Gleeson JG, Keeler LC, Parisi MA, Marsh SE, Chance PF, Glass IA, Graham JM Jr, Maria BL, Barkovich AJ, Dobyns WB (2004) Molar tooth sign of the midbrain-hindbrain junction: occurrence in multiple distinct syndromes. Am J Med Genet A 125:125–134 10.1002/ajmg.a.20437 [DOI] [PubMed] [Google Scholar]
- 5.Saar K, Al-Gazali L, Sztriha L, Rueschendorf F, Nur-E-Kamal M, Reis A, Bayoumi R (1999) Homozygosity mapping in families with Joubert syndrome identifies a locus on chromosome 9q34.3 and evidence for genetic heterogeneity. Am J Hum Genet 65:1666–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keeler LC, Marsh SE, Leeflang EP, Woods CG, Sztriha L, Al-Gazali L, Gururaj A, Gleeson JG (2003) Linkage analysis in families with Joubert syndrome plus oculo-renal involvement identifies the CORS2 locus on chromosome 11p12-q13.3. Am J Hum Genet 73:656–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valente EM, Salpietro DC, Brancati F, Bertini E, Galluccio T, Tortorella G, Briuglia S, Dallapiccola B (2003) Description, nomenclature, and mapping of a novel cerebello-renal syndrome with the molar tooth malformation. Am J Hum Genet 73:663–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dixon-Salazar T, Silhavy JL, Marsh SE, Louie CM, Scott LC, Gururaj A, Al-Gazali L, Al-Tawari AA, Kayserili H, Sztriha L, et al (2004) Mutations in the AHI1 gene, encoding Jouberin, cause Joubert syndrome with cortical polymicrogyria. Am J Hum Genet 75:979–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferland RJ, Eyaid W, Collura RV, Tully LD, Hill RS, Al-Nouri D, Al-Rumayyan A, Topcu M, Gascon G, Bodell A, et al (2004) Abnormal cerebellar development and axonal decussation due to mutations in AHI1 in Joubert syndrome. Nat Genet 36:1008–1013 10.1038/ng1419 [DOI] [PubMed] [Google Scholar]
- 10.Valente EM, Brancati F, Silhavy JL, Castori M, Marsh SE, Barrano G, Bertini E, Boltshauser E, Zaki MS, Abdel-Aleem A, et al (2006) AHI1 gene mutations cause specific forms of Joubert syndrome-related disorders. Ann Neurol 59:527–534 10.1002/ana.20749 [DOI] [PubMed] [Google Scholar]
- 11.Parisi MA, Doherty D, Eckert ML, Shaw DW, Ozyurek H, Aysun S, Giray O, Al Swaid A, Al Shahwan S, Dohayan N, et al (2006) AHI1 mutations cause both retinal dystrophy and renal cystic disease in Joubert syndrome. J Med Genet 43:334–339 10.1136/jmg.2005.036608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valente EM, Silhavy JL, Brancati F, Barrano G, Krishnaswami SR, Castori M, Lancaster MA, Boltshauser E, Boccone L, Al-Gazali L, et al (2006) Mutations in CEP290, which encodes a centrosomal protein, cause pleiotropic forms of Joubert syndrome. Nat Genet 38:623–625 10.1038/ng1805 [DOI] [PubMed] [Google Scholar]
- 13.Sayer JA, Otto EA, O’Toole JF, Nurnberg G, Kennedy MA, Becker C, Hennies HC, Helou J, Attanasio M, Fausett BV, et al (2006) The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat Genet 38:674–681 10.1038/ng1786 [DOI] [PubMed] [Google Scholar]
- 14.Hildebrandt F, Otto E (2005) Cilia and centrosomes: a unifying pathogenic concept for cystic kidney disease? Nat Rev Genet 6:928–940 [DOI] [PubMed] [Google Scholar]
- 15.Parisi MA, Bennett CL, Eckert ML, Dobyns WB, Gleeson JG, Shaw DW, McDonald R, Eddy A, Chance PF, Glass IA (2004) The NPHP1 gene deletion associated with juvenile nephronophthisis is present in a subset of individuals with Joubert syndrome. Am J Hum Genet 75:82–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castori M, Valente EM, Donati MA, Salvi S, Fazzi E, Procopio E, Galluccio T, Emma F, Dallapiccola B, Bertini E (2005) NPHP1 gene deletion is a rare cause of Joubert syndrome related disorders. J Med Genet 42:e9 10.1136/jmg.2004.027375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baala L, Romano S, Khaddour R, Saunier S, Smith UM, Audollent S, Ozilou C, Faivre L, Laurent N, Foliguet B, et al (2007) The Meckel-Gruber syndrome gene, MKS3, is mutated in Joubert syndrome. Am J Hum Genet 80:186–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.den Hollander AI, Koenekoop RK, Yzer S, Lopez I, Arends ML, Voesenek KE, Zonneveld MN, Strom TM, Meitinger T, Brunner HG, et al (2006) Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. Am J Hum Genet 79:556–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perrault I, Delphin N, Hanein S, Gerber S, Dufier JL, Roche O, Defoort-Dhellemmes S, Dollfus H, Fazzi E, Munnich A, et al (2007) Spectrum of NPHP6/CEP290 mutations in Leber congenital amaurosis and delineation of the associated phenotype. Hum Mutat 28:416 10.1002/humu.9485 [DOI] [PubMed] [Google Scholar]
- 20.Chang B, Khanna H, Hawes N, Jimeno D, He S, Lillo C, Parapuram SK, Cheng H, Scott A, Hurd RE, et al (2006) In-frame deletion in a novel centrosomal/ciliary protein CEP290/NPHP6 perturbs its interaction with RPGR and results in early-onset retinal degeneration in the rd16 mouse. Hum Mol Genet 15:1847–1857 10.1093/hmg/ddl107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Otto EA, Schermer B, Obara T, O’Toole JF, Hiller KS, Mueller AM, Ruf RG, Hoefele J, Beekmann F, Landau D, et al (2003) Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nat Genet 34:413–420 10.1038/ng1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Toole JF, Otto EA, Frishberg Y, Hildebrandt F (2006) Retinitis pigmentosa and renal failure in a patient with mutations in INVS. Nephrol Dial Transplant 21:1989–1991 10.1093/ndt/gfl088 [DOI] [PubMed] [Google Scholar]
- 23.Salonen R (1984) The Meckel syndrome: clinicopathological findings in 67 patients. Am J Med Genet 18:671–689 10.1002/ajmg.1320180414 [DOI] [PubMed] [Google Scholar]
- 24.Ansley SJ, Badano JL, Blacque OE, Hill J, Hoskins BE, Leitch CC, Kim JC, Ross AJ, Eichers ER, Teslovich TM, et al (2003) Basal body dysfunction is a likely cause of pleiotropic Bardet-Biedl syndrome. Nature 425:628–633 10.1038/nature02030 [DOI] [PubMed] [Google Scholar]
- 25.Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N (1998) Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell 95:829–837 10.1016/S0092-8674(00)81705-5 [DOI] [PubMed] [Google Scholar]
- 26.Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV (2003) Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 426:83–87 10.1038/nature02061 [DOI] [PubMed] [Google Scholar]
- 27.Marszalek JR, Ruiz-Lozano P, Roberts E, Chien KR, Goldstein LS (1999) Situs inversus and embryonic ciliary morphogenesis defects in mouse mutants lacking the KIF3A subunit of kinesin-II. Proc Natl Acad Sci USA 96:5043–5048 10.1073/pnas.96.9.5043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takeda S, Yonekawa Y, Tanaka Y, Okada Y, Nonaka S, Hirokawa N (1999) Left-right asymmetry and kinesin superfamily protein KIF3A: new insights in determination of laterality and mesoderm induction by kif3A−/− mice analysis. J Cell Biol 145:825–836 10.1083/jcb.145.4.825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morgan D, Turnpenny L, Goodship J, Dai W, Majumder K, Matthews L, Gardner A, Schuster G, Vien L, Harrison W, et al (1998) Inversin, a novel gene in the vertebrate left-right axis pathway, is partially deleted in the inv mouse. Nat Genet 20:149–156 10.1038/2450 [DOI] [PubMed] [Google Scholar]