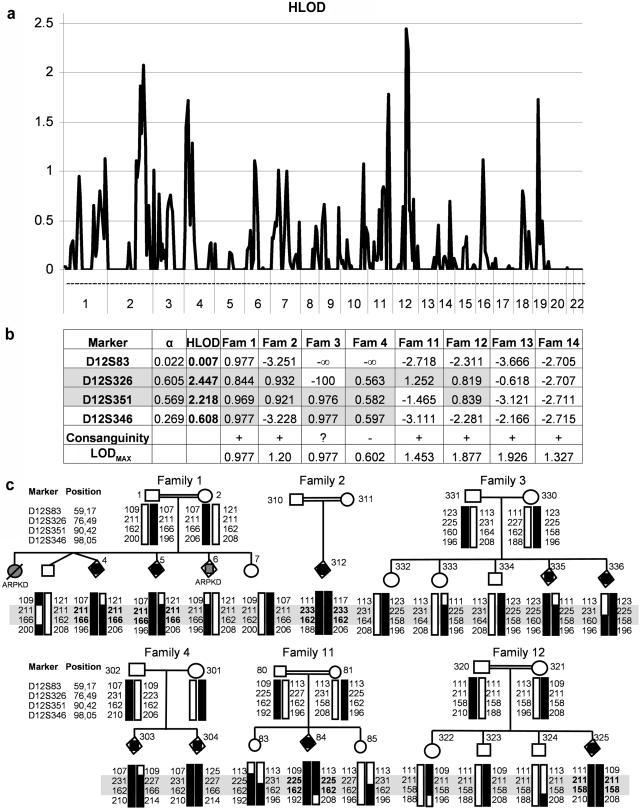
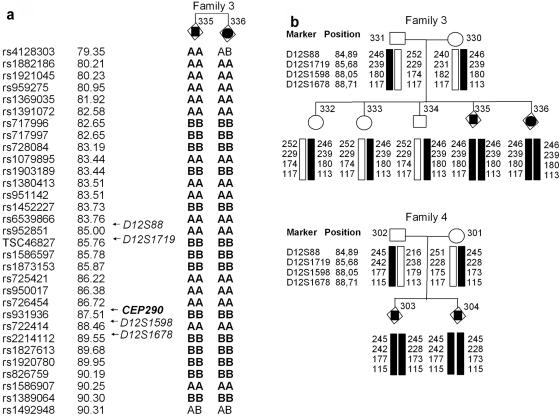
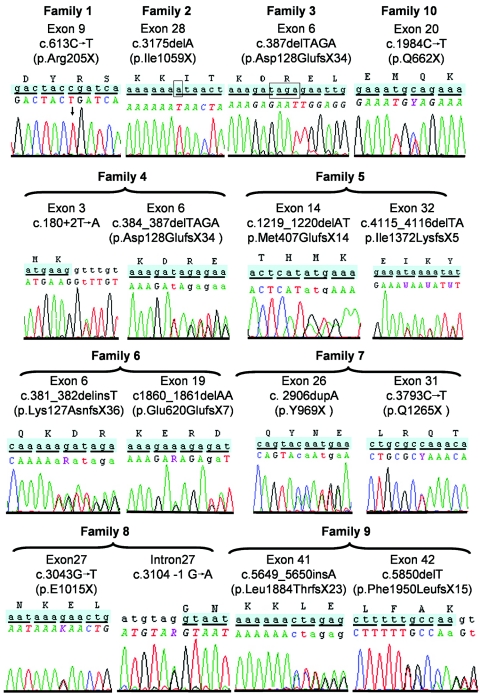
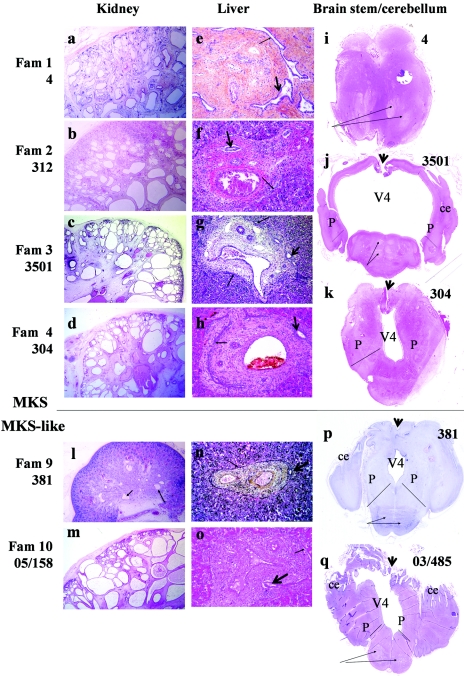
Lekbir Baala
Lekbir Baala
1From the INSERM U781 (L.B.; S. Sivanandamoorthy; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.) and U574 (S. Saunier; R.S.; M.-C.G.), Université René Descartes (L.B.; C.E.; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), Département de Génétique (S.A.; J.M.; C.O.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), and Service Maternité (Y.D.), Hôpital Necker-Enfants Malades, Service de Génétique et d’Embryologie Médicales, Hôpital Armand Trousseau (M.G.), Service d’Anatomie Pathologique, Hôpital Bichat (M.-C.D.), Service de Biologie du Développement, Hôpital Robert Debré (F.M.), and Service d’Anatomopathologie, Hôpital Saint-Vincent de Paul (J.T.), Assistance Publique–Hôpitaux de Paris, Paris; INSERM U535 and Université Paris Sud, Villejuif, France (M.C.-B.; E.G.); West Midlands Regional Genetics, Birmingham Women’s Hospital, Birmingham, United Kingdom (E.R.; F.M.); Service de Génétique, Centre Hospitalo–Universitaire Hôpital Bretonneau, Tours, France (A.T.; C.M.); Département de Pédiatrie et Génétique Médicale et Laboratoire d’Anatomopathologie, Centre Hospitalier Régional Universitaire, Hôpital Morvan, Brest, France (P.P.; P.M.); Génétique Médicale, Centre Hospitalier Intercommunal, Poissy, Saint-Germain-En-Laye, France (J.R.); Department of Human Genetics and Pathology, Hadassah University Hospital, Jerusalem (V.M.; K.M.); Services d’Anatomie Pathologique et Génétique Médicale, Hôpital Hôtel Dieu, Clermont-Ferrand, France (A.M.-B.; C.F.); Service d’Anatomopathologie, Centre Hospitalier Intercommunal de Créteil, Créteil, France (M.S.); and Section of Ophthalmology and Neurosciences, Leeds Institute of Molecular Medicine, St. James’s University Hospital, Leeds, United Kingdom (C.A.J.)
1,*,
Sophie Audollent
Sophie Audollent
1From the INSERM U781 (L.B.; S. Sivanandamoorthy; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.) and U574 (S. Saunier; R.S.; M.-C.G.), Université René Descartes (L.B.; C.E.; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), Département de Génétique (S.A.; J.M.; C.O.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), and Service Maternité (Y.D.), Hôpital Necker-Enfants Malades, Service de Génétique et d’Embryologie Médicales, Hôpital Armand Trousseau (M.G.), Service d’Anatomie Pathologique, Hôpital Bichat (M.-C.D.), Service de Biologie du Développement, Hôpital Robert Debré (F.M.), and Service d’Anatomopathologie, Hôpital Saint-Vincent de Paul (J.T.), Assistance Publique–Hôpitaux de Paris, Paris; INSERM U535 and Université Paris Sud, Villejuif, France (M.C.-B.; E.G.); West Midlands Regional Genetics, Birmingham Women’s Hospital, Birmingham, United Kingdom (E.R.; F.M.); Service de Génétique, Centre Hospitalo–Universitaire Hôpital Bretonneau, Tours, France (A.T.; C.M.); Département de Pédiatrie et Génétique Médicale et Laboratoire d’Anatomopathologie, Centre Hospitalier Régional Universitaire, Hôpital Morvan, Brest, France (P.P.; P.M.); Génétique Médicale, Centre Hospitalier Intercommunal, Poissy, Saint-Germain-En-Laye, France (J.R.); Department of Human Genetics and Pathology, Hadassah University Hospital, Jerusalem (V.M.; K.M.); Services d’Anatomie Pathologique et Génétique Médicale, Hôpital Hôtel Dieu, Clermont-Ferrand, France (A.M.-B.; C.F.); Service d’Anatomopathologie, Centre Hospitalier Intercommunal de Créteil, Créteil, France (M.S.); and Section of Ophthalmology and Neurosciences, Leeds Institute of Molecular Medicine, St. James’s University Hospital, Leeds, United Kingdom (C.A.J.)
1,*,
Jéléna Martinovic
Jéléna Martinovic
1From the INSERM U781 (L.B.; S. Sivanandamoorthy; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.) and U574 (S. Saunier; R.S.; M.-C.G.), Université René Descartes (L.B.; C.E.; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), Département de Génétique (S.A.; J.M.; C.O.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), and Service Maternité (Y.D.), Hôpital Necker-Enfants Malades, Service de Génétique et d’Embryologie Médicales, Hôpital Armand Trousseau (M.G.), Service d’Anatomie Pathologique, Hôpital Bichat (M.-C.D.), Service de Biologie du Développement, Hôpital Robert Debré (F.M.), and Service d’Anatomopathologie, Hôpital Saint-Vincent de Paul (J.T.), Assistance Publique–Hôpitaux de Paris, Paris; INSERM U535 and Université Paris Sud, Villejuif, France (M.C.-B.; E.G.); West Midlands Regional Genetics, Birmingham Women’s Hospital, Birmingham, United Kingdom (E.R.; F.M.); Service de Génétique, Centre Hospitalo–Universitaire Hôpital Bretonneau, Tours, France (A.T.; C.M.); Département de Pédiatrie et Génétique Médicale et Laboratoire d’Anatomopathologie, Centre Hospitalier Régional Universitaire, Hôpital Morvan, Brest, France (P.P.; P.M.); Génétique Médicale, Centre Hospitalier Intercommunal, Poissy, Saint-Germain-En-Laye, France (J.R.); Department of Human Genetics and Pathology, Hadassah University Hospital, Jerusalem (V.M.; K.M.); Services d’Anatomie Pathologique et Génétique Médicale, Hôpital Hôtel Dieu, Clermont-Ferrand, France (A.M.-B.; C.F.); Service d’Anatomopathologie, Centre Hospitalier Intercommunal de Créteil, Créteil, France (M.S.); and Section of Ophthalmology and Neurosciences, Leeds Institute of Molecular Medicine, St. James’s University Hospital, Leeds, United Kingdom (C.A.J.)
1,
Catherine Ozilou
Catherine Ozilou
1From the INSERM U781 (L.B.; S. Sivanandamoorthy; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.) and U574 (S. Saunier; R.S.; M.-C.G.), Université René Descartes (L.B.; C.E.; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), Département de Génétique (S.A.; J.M.; C.O.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), and Service Maternité (Y.D.), Hôpital Necker-Enfants Malades, Service de Génétique et d’Embryologie Médicales, Hôpital Armand Trousseau (M.G.), Service d’Anatomie Pathologique, Hôpital Bichat (M.-C.D.), Service de Biologie du Développement, Hôpital Robert Debré (F.M.), and Service d’Anatomopathologie, Hôpital Saint-Vincent de Paul (J.T.), Assistance Publique–Hôpitaux de Paris, Paris; INSERM U535 and Université Paris Sud, Villejuif, France (M.C.-B.; E.G.); West Midlands Regional Genetics, Birmingham Women’s Hospital, Birmingham, United Kingdom (E.R.; F.M.); Service de Génétique, Centre Hospitalo–Universitaire Hôpital Bretonneau, Tours, France (A.T.; C.M.); Département de Pédiatrie et Génétique Médicale et Laboratoire d’Anatomopathologie, Centre Hospitalier Régional Universitaire, Hôpital Morvan, Brest, France (P.P.; P.M.); Génétique Médicale, Centre Hospitalier Intercommunal, Poissy, Saint-Germain-En-Laye, France (J.R.); Department of Human Genetics and Pathology, Hadassah University Hospital, Jerusalem (V.M.; K.M.); Services d’Anatomie Pathologique et Génétique Médicale, Hôpital Hôtel Dieu, Clermont-Ferrand, France (A.M.-B.; C.F.); Service d’Anatomopathologie, Centre Hospitalier Intercommunal de Créteil, Créteil, France (M.S.); and Section of Ophthalmology and Neurosciences, Leeds Institute of Molecular Medicine, St. James’s University Hospital, Leeds, United Kingdom (C.A.J.)
1,
Marie-Claude Babron
Marie-Claude Babron
1From the INSERM U781 (L.B.; S. Sivanandamoorthy; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.) and U574 (S. Saunier; R.S.; M.-C.G.), Université René Descartes (L.B.; C.E.; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), Département de Génétique (S.A.; J.M.; C.O.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), and Service Maternité (Y.D.), Hôpital Necker-Enfants Malades, Service de Génétique et d’Embryologie Médicales, Hôpital Armand Trousseau (M.G.), Service d’Anatomie Pathologique, Hôpital Bichat (M.-C.D.), Service de Biologie du Développement, Hôpital Robert Debré (F.M.), and Service d’Anatomopathologie, Hôpital Saint-Vincent de Paul (J.T.), Assistance Publique–Hôpitaux de Paris, Paris; INSERM U535 and Université Paris Sud, Villejuif, France (M.C.-B.; E.G.); West Midlands Regional Genetics, Birmingham Women’s Hospital, Birmingham, United Kingdom (E.R.; F.M.); Service de Génétique, Centre Hospitalo–Universitaire Hôpital Bretonneau, Tours, France (A.T.; C.M.); Département de Pédiatrie et Génétique Médicale et Laboratoire d’Anatomopathologie, Centre Hospitalier Régional Universitaire, Hôpital Morvan, Brest, France (P.P.; P.M.); Génétique Médicale, Centre Hospitalier Intercommunal, Poissy, Saint-Germain-En-Laye, France (J.R.); Department of Human Genetics and Pathology, Hadassah University Hospital, Jerusalem (V.M.; K.M.); Services d’Anatomie Pathologique et Génétique Médicale, Hôpital Hôtel Dieu, Clermont-Ferrand, France (A.M.-B.; C.F.); Service d’Anatomopathologie, Centre Hospitalier Intercommunal de Créteil, Créteil, France (M.S.); and Section of Ophthalmology and Neurosciences, Leeds Institute of Molecular Medicine, St. James’s University Hospital, Leeds, United Kingdom (C.A.J.)
1,
Sivanthiny Sivanandamoorthy
Sivanthiny Sivanandamoorthy
1From the INSERM U781 (L.B.; S. Sivanandamoorthy; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.) and U574 (S. Saunier; R.S.; M.-C.G.), Université René Descartes (L.B.; C.E.; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), Département de Génétique (S.A.; J.M.; C.O.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), and Service Maternité (Y.D.), Hôpital Necker-Enfants Malades, Service de Génétique et d’Embryologie Médicales, Hôpital Armand Trousseau (M.G.), Service d’Anatomie Pathologique, Hôpital Bichat (M.-C.D.), Service de Biologie du Développement, Hôpital Robert Debré (F.M.), and Service d’Anatomopathologie, Hôpital Saint-Vincent de Paul (J.T.), Assistance Publique–Hôpitaux de Paris, Paris; INSERM U535 and Université Paris Sud, Villejuif, France (M.C.-B.; E.G.); West Midlands Regional Genetics, Birmingham Women’s Hospital, Birmingham, United Kingdom (E.R.; F.M.); Service de Génétique, Centre Hospitalo–Universitaire Hôpital Bretonneau, Tours, France (A.T.; C.M.); Département de Pédiatrie et Génétique Médicale et Laboratoire d’Anatomopathologie, Centre Hospitalier Régional Universitaire, Hôpital Morvan, Brest, France (P.P.; P.M.); Génétique Médicale, Centre Hospitalier Intercommunal, Poissy, Saint-Germain-En-Laye, France (J.R.); Department of Human Genetics and Pathology, Hadassah University Hospital, Jerusalem (V.M.; K.M.); Services d’Anatomie Pathologique et Génétique Médicale, Hôpital Hôtel Dieu, Clermont-Ferrand, France (A.M.-B.; C.F.); Service d’Anatomopathologie, Centre Hospitalier Intercommunal de Créteil, Créteil, France (M.S.); and Section of Ophthalmology and Neurosciences, Leeds Institute of Molecular Medicine, St. James’s University Hospital, Leeds, United Kingdom (C.A.J.)
1,
Sophie Saunier
Sophie Saunier
1From the INSERM U781 (L.B.; S. Sivanandamoorthy; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.) and U574 (S. Saunier; R.S.; M.-C.G.), Université René Descartes (L.B.; C.E.; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), Département de Génétique (S.A.; J.M.; C.O.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), and Service Maternité (Y.D.), Hôpital Necker-Enfants Malades, Service de Génétique et d’Embryologie Médicales, Hôpital Armand Trousseau (M.G.), Service d’Anatomie Pathologique, Hôpital Bichat (M.-C.D.), Service de Biologie du Développement, Hôpital Robert Debré (F.M.), and Service d’Anatomopathologie, Hôpital Saint-Vincent de Paul (J.T.), Assistance Publique–Hôpitaux de Paris, Paris; INSERM U535 and Université Paris Sud, Villejuif, France (M.C.-B.; E.G.); West Midlands Regional Genetics, Birmingham Women’s Hospital, Birmingham, United Kingdom (E.R.; F.M.); Service de Génétique, Centre Hospitalo–Universitaire Hôpital Bretonneau, Tours, France (A.T.; C.M.); Département de Pédiatrie et Génétique Médicale et Laboratoire d’Anatomopathologie, Centre Hospitalier Régional Universitaire, Hôpital Morvan, Brest, France (P.P.; P.M.); Génétique Médicale, Centre Hospitalier Intercommunal, Poissy, Saint-Germain-En-Laye, France (J.R.); Department of Human Genetics and Pathology, Hadassah University Hospital, Jerusalem (V.M.; K.M.); Services d’Anatomie Pathologique et Génétique Médicale, Hôpital Hôtel Dieu, Clermont-Ferrand, France (A.M.-B.; C.F.); Service d’Anatomopathologie, Centre Hospitalier Intercommunal de Créteil, Créteil, France (M.S.); and Section of Ophthalmology and Neurosciences, Leeds Institute of Molecular Medicine, St. James’s University Hospital, Leeds, United Kingdom (C.A.J.)
1,
Rémi Salomon
Rémi Salomon
1From the INSERM U781 (L.B.; S. Sivanandamoorthy; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.) and U574 (S. Saunier; R.S.; M.-C.G.), Université René Descartes (L.B.; C.E.; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), Département de Génétique (S.A.; J.M.; C.O.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), and Service Maternité (Y.D.), Hôpital Necker-Enfants Malades, Service de Génétique et d’Embryologie Médicales, Hôpital Armand Trousseau (M.G.), Service d’Anatomie Pathologique, Hôpital Bichat (M.-C.D.), Service de Biologie du Développement, Hôpital Robert Debré (F.M.), and Service d’Anatomopathologie, Hôpital Saint-Vincent de Paul (J.T.), Assistance Publique–Hôpitaux de Paris, Paris; INSERM U535 and Université Paris Sud, Villejuif, France (M.C.-B.; E.G.); West Midlands Regional Genetics, Birmingham Women’s Hospital, Birmingham, United Kingdom (E.R.; F.M.); Service de Génétique, Centre Hospitalo–Universitaire Hôpital Bretonneau, Tours, France (A.T.; C.M.); Département de Pédiatrie et Génétique Médicale et Laboratoire d’Anatomopathologie, Centre Hospitalier Régional Universitaire, Hôpital Morvan, Brest, France (P.P.; P.M.); Génétique Médicale, Centre Hospitalier Intercommunal, Poissy, Saint-Germain-En-Laye, France (J.R.); Department of Human Genetics and Pathology, Hadassah University Hospital, Jerusalem (V.M.; K.M.); Services d’Anatomie Pathologique et Génétique Médicale, Hôpital Hôtel Dieu, Clermont-Ferrand, France (A.M.-B.; C.F.); Service d’Anatomopathologie, Centre Hospitalier Intercommunal de Créteil, Créteil, France (M.S.); and Section of Ophthalmology and Neurosciences, Leeds Institute of Molecular Medicine, St. James’s University Hospital, Leeds, United Kingdom (C.A.J.)
1,
Marie Gonzales
Marie Gonzales
1From the INSERM U781 (L.B.; S. Sivanandamoorthy; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.) and U574 (S. Saunier; R.S.; M.-C.G.), Université René Descartes (L.B.; C.E.; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), Département de Génétique (S.A.; J.M.; C.O.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), and Service Maternité (Y.D.), Hôpital Necker-Enfants Malades, Service de Génétique et d’Embryologie Médicales, Hôpital Armand Trousseau (M.G.), Service d’Anatomie Pathologique, Hôpital Bichat (M.-C.D.), Service de Biologie du Développement, Hôpital Robert Debré (F.M.), and Service d’Anatomopathologie, Hôpital Saint-Vincent de Paul (J.T.), Assistance Publique–Hôpitaux de Paris, Paris; INSERM U535 and Université Paris Sud, Villejuif, France (M.C.-B.; E.G.); West Midlands Regional Genetics, Birmingham Women’s Hospital, Birmingham, United Kingdom (E.R.; F.M.); Service de Génétique, Centre Hospitalo–Universitaire Hôpital Bretonneau, Tours, France (A.T.; C.M.); Département de Pédiatrie et Génétique Médicale et Laboratoire d’Anatomopathologie, Centre Hospitalier Régional Universitaire, Hôpital Morvan, Brest, France (P.P.; P.M.); Génétique Médicale, Centre Hospitalier Intercommunal, Poissy, Saint-Germain-En-Laye, France (J.R.); Department of Human Genetics and Pathology, Hadassah University Hospital, Jerusalem (V.M.; K.M.); Services d’Anatomie Pathologique et Génétique Médicale, Hôpital Hôtel Dieu, Clermont-Ferrand, France (A.M.-B.; C.F.); Service d’Anatomopathologie, Centre Hospitalier Intercommunal de Créteil, Créteil, France (M.S.); and Section of Ophthalmology and Neurosciences, Leeds Institute of Molecular Medicine, St. James’s University Hospital, Leeds, United Kingdom (C.A.J.)
1,
Eleanor Rattenberry
Eleanor Rattenberry
1From the INSERM U781 (L.B.; S. Sivanandamoorthy; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.) and U574 (S. Saunier; R.S.; M.-C.G.), Université René Descartes (L.B.; C.E.; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), Département de Génétique (S.A.; J.M.; C.O.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), and Service Maternité (Y.D.), Hôpital Necker-Enfants Malades, Service de Génétique et d’Embryologie Médicales, Hôpital Armand Trousseau (M.G.), Service d’Anatomie Pathologique, Hôpital Bichat (M.-C.D.), Service de Biologie du Développement, Hôpital Robert Debré (F.M.), and Service d’Anatomopathologie, Hôpital Saint-Vincent de Paul (J.T.), Assistance Publique–Hôpitaux de Paris, Paris; INSERM U535 and Université Paris Sud, Villejuif, France (M.C.-B.; E.G.); West Midlands Regional Genetics, Birmingham Women’s Hospital, Birmingham, United Kingdom (E.R.; F.M.); Service de Génétique, Centre Hospitalo–Universitaire Hôpital Bretonneau, Tours, France (A.T.; C.M.); Département de Pédiatrie et Génétique Médicale et Laboratoire d’Anatomopathologie, Centre Hospitalier Régional Universitaire, Hôpital Morvan, Brest, France (P.P.; P.M.); Génétique Médicale, Centre Hospitalier Intercommunal, Poissy, Saint-Germain-En-Laye, France (J.R.); Department of Human Genetics and Pathology, Hadassah University Hospital, Jerusalem (V.M.; K.M.); Services d’Anatomie Pathologique et Génétique Médicale, Hôpital Hôtel Dieu, Clermont-Ferrand, France (A.M.-B.; C.F.); Service d’Anatomopathologie, Centre Hospitalier Intercommunal de Créteil, Créteil, France (M.S.); and Section of Ophthalmology and Neurosciences, Leeds Institute of Molecular Medicine, St. James’s University Hospital, Leeds, United Kingdom (C.A.J.)
1,
Chantal Esculpavit
Chantal Esculpavit
1From the INSERM U781 (L.B.; S. Sivanandamoorthy; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.) and U574 (S. Saunier; R.S.; M.-C.G.), Université René Descartes (L.B.; C.E.; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), Département de Génétique (S.A.; J.M.; C.O.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), and Service Maternité (Y.D.), Hôpital Necker-Enfants Malades, Service de Génétique et d’Embryologie Médicales, Hôpital Armand Trousseau (M.G.), Service d’Anatomie Pathologique, Hôpital Bichat (M.-C.D.), Service de Biologie du Développement, Hôpital Robert Debré (F.M.), and Service d’Anatomopathologie, Hôpital Saint-Vincent de Paul (J.T.), Assistance Publique–Hôpitaux de Paris, Paris; INSERM U535 and Université Paris Sud, Villejuif, France (M.C.-B.; E.G.); West Midlands Regional Genetics, Birmingham Women’s Hospital, Birmingham, United Kingdom (E.R.; F.M.); Service de Génétique, Centre Hospitalo–Universitaire Hôpital Bretonneau, Tours, France (A.T.; C.M.); Département de Pédiatrie et Génétique Médicale et Laboratoire d’Anatomopathologie, Centre Hospitalier Régional Universitaire, Hôpital Morvan, Brest, France (P.P.; P.M.); Génétique Médicale, Centre Hospitalier Intercommunal, Poissy, Saint-Germain-En-Laye, France (J.R.); Department of Human Genetics and Pathology, Hadassah University Hospital, Jerusalem (V.M.; K.M.); Services d’Anatomie Pathologique et Génétique Médicale, Hôpital Hôtel Dieu, Clermont-Ferrand, France (A.M.-B.; C.F.); Service d’Anatomopathologie, Centre Hospitalier Intercommunal de Créteil, Créteil, France (M.S.); and Section of Ophthalmology and Neurosciences, Leeds Institute of Molecular Medicine, St. James’s University Hospital, Leeds, United Kingdom (C.A.J.)
1,
Annick Toutain
Annick Toutain
1From the INSERM U781 (L.B.; S. Sivanandamoorthy; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.) and U574 (S. Saunier; R.S.; M.-C.G.), Université René Descartes (L.B.; C.E.; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), Département de Génétique (S.A.; J.M.; C.O.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), and Service Maternité (Y.D.), Hôpital Necker-Enfants Malades, Service de Génétique et d’Embryologie Médicales, Hôpital Armand Trousseau (M.G.), Service d’Anatomie Pathologique, Hôpital Bichat (M.-C.D.), Service de Biologie du Développement, Hôpital Robert Debré (F.M.), and Service d’Anatomopathologie, Hôpital Saint-Vincent de Paul (J.T.), Assistance Publique–Hôpitaux de Paris, Paris; INSERM U535 and Université Paris Sud, Villejuif, France (M.C.-B.; E.G.); West Midlands Regional Genetics, Birmingham Women’s Hospital, Birmingham, United Kingdom (E.R.; F.M.); Service de Génétique, Centre Hospitalo–Universitaire Hôpital Bretonneau, Tours, France (A.T.; C.M.); Département de Pédiatrie et Génétique Médicale et Laboratoire d’Anatomopathologie, Centre Hospitalier Régional Universitaire, Hôpital Morvan, Brest, France (P.P.; P.M.); Génétique Médicale, Centre Hospitalier Intercommunal, Poissy, Saint-Germain-En-Laye, France (J.R.); Department of Human Genetics and Pathology, Hadassah University Hospital, Jerusalem (V.M.; K.M.); Services d’Anatomie Pathologique et Génétique Médicale, Hôpital Hôtel Dieu, Clermont-Ferrand, France (A.M.-B.; C.F.); Service d’Anatomopathologie, Centre Hospitalier Intercommunal de Créteil, Créteil, France (M.S.); and Section of Ophthalmology and Neurosciences, Leeds Institute of Molecular Medicine, St. James’s University Hospital, Leeds, United Kingdom (C.A.J.)
1,
Claude Moraine
Claude Moraine
1From the INSERM U781 (L.B.; S. Sivanandamoorthy; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.) and U574 (S. Saunier; R.S.; M.-C.G.), Université René Descartes (L.B.; C.E.; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), Département de Génétique (S.A.; J.M.; C.O.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), and Service Maternité (Y.D.), Hôpital Necker-Enfants Malades, Service de Génétique et d’Embryologie Médicales, Hôpital Armand Trousseau (M.G.), Service d’Anatomie Pathologique, Hôpital Bichat (M.-C.D.), Service de Biologie du Développement, Hôpital Robert Debré (F.M.), and Service d’Anatomopathologie, Hôpital Saint-Vincent de Paul (J.T.), Assistance Publique–Hôpitaux de Paris, Paris; INSERM U535 and Université Paris Sud, Villejuif, France (M.C.-B.; E.G.); West Midlands Regional Genetics, Birmingham Women’s Hospital, Birmingham, United Kingdom (E.R.; F.M.); Service de Génétique, Centre Hospitalo–Universitaire Hôpital Bretonneau, Tours, France (A.T.; C.M.); Département de Pédiatrie et Génétique Médicale et Laboratoire d’Anatomopathologie, Centre Hospitalier Régional Universitaire, Hôpital Morvan, Brest, France (P.P.; P.M.); Génétique Médicale, Centre Hospitalier Intercommunal, Poissy, Saint-Germain-En-Laye, France (J.R.); Department of Human Genetics and Pathology, Hadassah University Hospital, Jerusalem (V.M.; K.M.); Services d’Anatomie Pathologique et Génétique Médicale, Hôpital Hôtel Dieu, Clermont-Ferrand, France (A.M.-B.; C.F.); Service d’Anatomopathologie, Centre Hospitalier Intercommunal de Créteil, Créteil, France (M.S.); and Section of Ophthalmology and Neurosciences, Leeds Institute of Molecular Medicine, St. James’s University Hospital, Leeds, United Kingdom (C.A.J.)
1,
Philippe Parent
Philippe Parent
1From the INSERM U781 (L.B.; S. Sivanandamoorthy; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.) and U574 (S. Saunier; R.S.; M.-C.G.), Université René Descartes (L.B.; C.E.; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), Département de Génétique (S.A.; J.M.; C.O.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), and Service Maternité (Y.D.), Hôpital Necker-Enfants Malades, Service de Génétique et d’Embryologie Médicales, Hôpital Armand Trousseau (M.G.), Service d’Anatomie Pathologique, Hôpital Bichat (M.-C.D.), Service de Biologie du Développement, Hôpital Robert Debré (F.M.), and Service d’Anatomopathologie, Hôpital Saint-Vincent de Paul (J.T.), Assistance Publique–Hôpitaux de Paris, Paris; INSERM U535 and Université Paris Sud, Villejuif, France (M.C.-B.; E.G.); West Midlands Regional Genetics, Birmingham Women’s Hospital, Birmingham, United Kingdom (E.R.; F.M.); Service de Génétique, Centre Hospitalo–Universitaire Hôpital Bretonneau, Tours, France (A.T.; C.M.); Département de Pédiatrie et Génétique Médicale et Laboratoire d’Anatomopathologie, Centre Hospitalier Régional Universitaire, Hôpital Morvan, Brest, France (P.P.; P.M.); Génétique Médicale, Centre Hospitalier Intercommunal, Poissy, Saint-Germain-En-Laye, France (J.R.); Department of Human Genetics and Pathology, Hadassah University Hospital, Jerusalem (V.M.; K.M.); Services d’Anatomie Pathologique et Génétique Médicale, Hôpital Hôtel Dieu, Clermont-Ferrand, France (A.M.-B.; C.F.); Service d’Anatomopathologie, Centre Hospitalier Intercommunal de Créteil, Créteil, France (M.S.); and Section of Ophthalmology and Neurosciences, Leeds Institute of Molecular Medicine, St. James’s University Hospital, Leeds, United Kingdom (C.A.J.)
1,
Pascale Marcorelles
Pascale Marcorelles
1From the INSERM U781 (L.B.; S. Sivanandamoorthy; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.) and U574 (S. Saunier; R.S.; M.-C.G.), Université René Descartes (L.B.; C.E.; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), Département de Génétique (S.A.; J.M.; C.O.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), and Service Maternité (Y.D.), Hôpital Necker-Enfants Malades, Service de Génétique et d’Embryologie Médicales, Hôpital Armand Trousseau (M.G.), Service d’Anatomie Pathologique, Hôpital Bichat (M.-C.D.), Service de Biologie du Développement, Hôpital Robert Debré (F.M.), and Service d’Anatomopathologie, Hôpital Saint-Vincent de Paul (J.T.), Assistance Publique–Hôpitaux de Paris, Paris; INSERM U535 and Université Paris Sud, Villejuif, France (M.C.-B.; E.G.); West Midlands Regional Genetics, Birmingham Women’s Hospital, Birmingham, United Kingdom (E.R.; F.M.); Service de Génétique, Centre Hospitalo–Universitaire Hôpital Bretonneau, Tours, France (A.T.; C.M.); Département de Pédiatrie et Génétique Médicale et Laboratoire d’Anatomopathologie, Centre Hospitalier Régional Universitaire, Hôpital Morvan, Brest, France (P.P.; P.M.); Génétique Médicale, Centre Hospitalier Intercommunal, Poissy, Saint-Germain-En-Laye, France (J.R.); Department of Human Genetics and Pathology, Hadassah University Hospital, Jerusalem (V.M.; K.M.); Services d’Anatomie Pathologique et Génétique Médicale, Hôpital Hôtel Dieu, Clermont-Ferrand, France (A.M.-B.; C.F.); Service d’Anatomopathologie, Centre Hospitalier Intercommunal de Créteil, Créteil, France (M.S.); and Section of Ophthalmology and Neurosciences, Leeds Institute of Molecular Medicine, St. James’s University Hospital, Leeds, United Kingdom (C.A.J.)
1,
Marie-Christine Dauge
Marie-Christine Dauge
1From the INSERM U781 (L.B.; S. Sivanandamoorthy; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.) and U574 (S. Saunier; R.S.; M.-C.G.), Université René Descartes (L.B.; C.E.; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), Département de Génétique (S.A.; J.M.; C.O.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), and Service Maternité (Y.D.), Hôpital Necker-Enfants Malades, Service de Génétique et d’Embryologie Médicales, Hôpital Armand Trousseau (M.G.), Service d’Anatomie Pathologique, Hôpital Bichat (M.-C.D.), Service de Biologie du Développement, Hôpital Robert Debré (F.M.), and Service d’Anatomopathologie, Hôpital Saint-Vincent de Paul (J.T.), Assistance Publique–Hôpitaux de Paris, Paris; INSERM U535 and Université Paris Sud, Villejuif, France (M.C.-B.; E.G.); West Midlands Regional Genetics, Birmingham Women’s Hospital, Birmingham, United Kingdom (E.R.; F.M.); Service de Génétique, Centre Hospitalo–Universitaire Hôpital Bretonneau, Tours, France (A.T.; C.M.); Département de Pédiatrie et Génétique Médicale et Laboratoire d’Anatomopathologie, Centre Hospitalier Régional Universitaire, Hôpital Morvan, Brest, France (P.P.; P.M.); Génétique Médicale, Centre Hospitalier Intercommunal, Poissy, Saint-Germain-En-Laye, France (J.R.); Department of Human Genetics and Pathology, Hadassah University Hospital, Jerusalem (V.M.; K.M.); Services d’Anatomie Pathologique et Génétique Médicale, Hôpital Hôtel Dieu, Clermont-Ferrand, France (A.M.-B.; C.F.); Service d’Anatomopathologie, Centre Hospitalier Intercommunal de Créteil, Créteil, France (M.S.); and Section of Ophthalmology and Neurosciences, Leeds Institute of Molecular Medicine, St. James’s University Hospital, Leeds, United Kingdom (C.A.J.)
1,
Joëlle Roume
Joëlle Roume
1From the INSERM U781 (L.B.; S. Sivanandamoorthy; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.) and U574 (S. Saunier; R.S.; M.-C.G.), Université René Descartes (L.B.; C.E.; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), Département de Génétique (S.A.; J.M.; C.O.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), and Service Maternité (Y.D.), Hôpital Necker-Enfants Malades, Service de Génétique et d’Embryologie Médicales, Hôpital Armand Trousseau (M.G.), Service d’Anatomie Pathologique, Hôpital Bichat (M.-C.D.), Service de Biologie du Développement, Hôpital Robert Debré (F.M.), and Service d’Anatomopathologie, Hôpital Saint-Vincent de Paul (J.T.), Assistance Publique–Hôpitaux de Paris, Paris; INSERM U535 and Université Paris Sud, Villejuif, France (M.C.-B.; E.G.); West Midlands Regional Genetics, Birmingham Women’s Hospital, Birmingham, United Kingdom (E.R.; F.M.); Service de Génétique, Centre Hospitalo–Universitaire Hôpital Bretonneau, Tours, France (A.T.; C.M.); Département de Pédiatrie et Génétique Médicale et Laboratoire d’Anatomopathologie, Centre Hospitalier Régional Universitaire, Hôpital Morvan, Brest, France (P.P.; P.M.); Génétique Médicale, Centre Hospitalier Intercommunal, Poissy, Saint-Germain-En-Laye, France (J.R.); Department of Human Genetics and Pathology, Hadassah University Hospital, Jerusalem (V.M.; K.M.); Services d’Anatomie Pathologique et Génétique Médicale, Hôpital Hôtel Dieu, Clermont-Ferrand, France (A.M.-B.; C.F.); Service d’Anatomopathologie, Centre Hospitalier Intercommunal de Créteil, Créteil, France (M.S.); and Section of Ophthalmology and Neurosciences, Leeds Institute of Molecular Medicine, St. James’s University Hospital, Leeds, United Kingdom (C.A.J.)
1,
Martine Le Merrer
Martine Le Merrer
1From the INSERM U781 (L.B.; S. Sivanandamoorthy; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.) and U574 (S. Saunier; R.S.; M.-C.G.), Université René Descartes (L.B.; C.E.; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), Département de Génétique (S.A.; J.M.; C.O.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), and Service Maternité (Y.D.), Hôpital Necker-Enfants Malades, Service de Génétique et d’Embryologie Médicales, Hôpital Armand Trousseau (M.G.), Service d’Anatomie Pathologique, Hôpital Bichat (M.-C.D.), Service de Biologie du Développement, Hôpital Robert Debré (F.M.), and Service d’Anatomopathologie, Hôpital Saint-Vincent de Paul (J.T.), Assistance Publique–Hôpitaux de Paris, Paris; INSERM U535 and Université Paris Sud, Villejuif, France (M.C.-B.; E.G.); West Midlands Regional Genetics, Birmingham Women’s Hospital, Birmingham, United Kingdom (E.R.; F.M.); Service de Génétique, Centre Hospitalo–Universitaire Hôpital Bretonneau, Tours, France (A.T.; C.M.); Département de Pédiatrie et Génétique Médicale et Laboratoire d’Anatomopathologie, Centre Hospitalier Régional Universitaire, Hôpital Morvan, Brest, France (P.P.; P.M.); Génétique Médicale, Centre Hospitalier Intercommunal, Poissy, Saint-Germain-En-Laye, France (J.R.); Department of Human Genetics and Pathology, Hadassah University Hospital, Jerusalem (V.M.; K.M.); Services d’Anatomie Pathologique et Génétique Médicale, Hôpital Hôtel Dieu, Clermont-Ferrand, France (A.M.-B.; C.F.); Service d’Anatomopathologie, Centre Hospitalier Intercommunal de Créteil, Créteil, France (M.S.); and Section of Ophthalmology and Neurosciences, Leeds Institute of Molecular Medicine, St. James’s University Hospital, Leeds, United Kingdom (C.A.J.)
1,
Vardiella Meiner
Vardiella Meiner
1From the INSERM U781 (L.B.; S. Sivanandamoorthy; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.) and U574 (S. Saunier; R.S.; M.-C.G.), Université René Descartes (L.B.; C.E.; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), Département de Génétique (S.A.; J.M.; C.O.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), and Service Maternité (Y.D.), Hôpital Necker-Enfants Malades, Service de Génétique et d’Embryologie Médicales, Hôpital Armand Trousseau (M.G.), Service d’Anatomie Pathologique, Hôpital Bichat (M.-C.D.), Service de Biologie du Développement, Hôpital Robert Debré (F.M.), and Service d’Anatomopathologie, Hôpital Saint-Vincent de Paul (J.T.), Assistance Publique–Hôpitaux de Paris, Paris; INSERM U535 and Université Paris Sud, Villejuif, France (M.C.-B.; E.G.); West Midlands Regional Genetics, Birmingham Women’s Hospital, Birmingham, United Kingdom (E.R.; F.M.); Service de Génétique, Centre Hospitalo–Universitaire Hôpital Bretonneau, Tours, France (A.T.; C.M.); Département de Pédiatrie et Génétique Médicale et Laboratoire d’Anatomopathologie, Centre Hospitalier Régional Universitaire, Hôpital Morvan, Brest, France (P.P.; P.M.); Génétique Médicale, Centre Hospitalier Intercommunal, Poissy, Saint-Germain-En-Laye, France (J.R.); Department of Human Genetics and Pathology, Hadassah University Hospital, Jerusalem (V.M.; K.M.); Services d’Anatomie Pathologique et Génétique Médicale, Hôpital Hôtel Dieu, Clermont-Ferrand, France (A.M.-B.; C.F.); Service d’Anatomopathologie, Centre Hospitalier Intercommunal de Créteil, Créteil, France (M.S.); and Section of Ophthalmology and Neurosciences, Leeds Institute of Molecular Medicine, St. James’s University Hospital, Leeds, United Kingdom (C.A.J.)
1,
Karen Meir
Karen Meir
1From the INSERM U781 (L.B.; S. Sivanandamoorthy; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.) and U574 (S. Saunier; R.S.; M.-C.G.), Université René Descartes (L.B.; C.E.; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), Département de Génétique (S.A.; J.M.; C.O.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), and Service Maternité (Y.D.), Hôpital Necker-Enfants Malades, Service de Génétique et d’Embryologie Médicales, Hôpital Armand Trousseau (M.G.), Service d’Anatomie Pathologique, Hôpital Bichat (M.-C.D.), Service de Biologie du Développement, Hôpital Robert Debré (F.M.), and Service d’Anatomopathologie, Hôpital Saint-Vincent de Paul (J.T.), Assistance Publique–Hôpitaux de Paris, Paris; INSERM U535 and Université Paris Sud, Villejuif, France (M.C.-B.; E.G.); West Midlands Regional Genetics, Birmingham Women’s Hospital, Birmingham, United Kingdom (E.R.; F.M.); Service de Génétique, Centre Hospitalo–Universitaire Hôpital Bretonneau, Tours, France (A.T.; C.M.); Département de Pédiatrie et Génétique Médicale et Laboratoire d’Anatomopathologie, Centre Hospitalier Régional Universitaire, Hôpital Morvan, Brest, France (P.P.; P.M.); Génétique Médicale, Centre Hospitalier Intercommunal, Poissy, Saint-Germain-En-Laye, France (J.R.); Department of Human Genetics and Pathology, Hadassah University Hospital, Jerusalem (V.M.; K.M.); Services d’Anatomie Pathologique et Génétique Médicale, Hôpital Hôtel Dieu, Clermont-Ferrand, France (A.M.-B.; C.F.); Service d’Anatomopathologie, Centre Hospitalier Intercommunal de Créteil, Créteil, France (M.S.); and Section of Ophthalmology and Neurosciences, Leeds Institute of Molecular Medicine, St. James’s University Hospital, Leeds, United Kingdom (C.A.J.)
1,
Françoise Menez
Françoise Menez
1From the INSERM U781 (L.B.; S. Sivanandamoorthy; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.) and U574 (S. Saunier; R.S.; M.-C.G.), Université René Descartes (L.B.; C.E.; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), Département de Génétique (S.A.; J.M.; C.O.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), and Service Maternité (Y.D.), Hôpital Necker-Enfants Malades, Service de Génétique et d’Embryologie Médicales, Hôpital Armand Trousseau (M.G.), Service d’Anatomie Pathologique, Hôpital Bichat (M.-C.D.), Service de Biologie du Développement, Hôpital Robert Debré (F.M.), and Service d’Anatomopathologie, Hôpital Saint-Vincent de Paul (J.T.), Assistance Publique–Hôpitaux de Paris, Paris; INSERM U535 and Université Paris Sud, Villejuif, France (M.C.-B.; E.G.); West Midlands Regional Genetics, Birmingham Women’s Hospital, Birmingham, United Kingdom (E.R.; F.M.); Service de Génétique, Centre Hospitalo–Universitaire Hôpital Bretonneau, Tours, France (A.T.; C.M.); Département de Pédiatrie et Génétique Médicale et Laboratoire d’Anatomopathologie, Centre Hospitalier Régional Universitaire, Hôpital Morvan, Brest, France (P.P.; P.M.); Génétique Médicale, Centre Hospitalier Intercommunal, Poissy, Saint-Germain-En-Laye, France (J.R.); Department of Human Genetics and Pathology, Hadassah University Hospital, Jerusalem (V.M.; K.M.); Services d’Anatomie Pathologique et Génétique Médicale, Hôpital Hôtel Dieu, Clermont-Ferrand, France (A.M.-B.; C.F.); Service d’Anatomopathologie, Centre Hospitalier Intercommunal de Créteil, Créteil, France (M.S.); and Section of Ophthalmology and Neurosciences, Leeds Institute of Molecular Medicine, St. James’s University Hospital, Leeds, United Kingdom (C.A.J.)
1,
Anne-Marie Beaufrère
Anne-Marie Beaufrère
1From the INSERM U781 (L.B.; S. Sivanandamoorthy; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.) and U574 (S. Saunier; R.S.; M.-C.G.), Université René Descartes (L.B.; C.E.; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), Département de Génétique (S.A.; J.M.; C.O.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), and Service Maternité (Y.D.), Hôpital Necker-Enfants Malades, Service de Génétique et d’Embryologie Médicales, Hôpital Armand Trousseau (M.G.), Service d’Anatomie Pathologique, Hôpital Bichat (M.-C.D.), Service de Biologie du Développement, Hôpital Robert Debré (F.M.), and Service d’Anatomopathologie, Hôpital Saint-Vincent de Paul (J.T.), Assistance Publique–Hôpitaux de Paris, Paris; INSERM U535 and Université Paris Sud, Villejuif, France (M.C.-B.; E.G.); West Midlands Regional Genetics, Birmingham Women’s Hospital, Birmingham, United Kingdom (E.R.; F.M.); Service de Génétique, Centre Hospitalo–Universitaire Hôpital Bretonneau, Tours, France (A.T.; C.M.); Département de Pédiatrie et Génétique Médicale et Laboratoire d’Anatomopathologie, Centre Hospitalier Régional Universitaire, Hôpital Morvan, Brest, France (P.P.; P.M.); Génétique Médicale, Centre Hospitalier Intercommunal, Poissy, Saint-Germain-En-Laye, France (J.R.); Department of Human Genetics and Pathology, Hadassah University Hospital, Jerusalem (V.M.; K.M.); Services d’Anatomie Pathologique et Génétique Médicale, Hôpital Hôtel Dieu, Clermont-Ferrand, France (A.M.-B.; C.F.); Service d’Anatomopathologie, Centre Hospitalier Intercommunal de Créteil, Créteil, France (M.S.); and Section of Ophthalmology and Neurosciences, Leeds Institute of Molecular Medicine, St. James’s University Hospital, Leeds, United Kingdom (C.A.J.)
1,
Christine Francannet
Christine Francannet
1From the INSERM U781 (L.B.; S. Sivanandamoorthy; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.) and U574 (S. Saunier; R.S.; M.-C.G.), Université René Descartes (L.B.; C.E.; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), Département de Génétique (S.A.; J.M.; C.O.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), and Service Maternité (Y.D.), Hôpital Necker-Enfants Malades, Service de Génétique et d’Embryologie Médicales, Hôpital Armand Trousseau (M.G.), Service d’Anatomie Pathologique, Hôpital Bichat (M.-C.D.), Service de Biologie du Développement, Hôpital Robert Debré (F.M.), and Service d’Anatomopathologie, Hôpital Saint-Vincent de Paul (J.T.), Assistance Publique–Hôpitaux de Paris, Paris; INSERM U535 and Université Paris Sud, Villejuif, France (M.C.-B.; E.G.); West Midlands Regional Genetics, Birmingham Women’s Hospital, Birmingham, United Kingdom (E.R.; F.M.); Service de Génétique, Centre Hospitalo–Universitaire Hôpital Bretonneau, Tours, France (A.T.; C.M.); Département de Pédiatrie et Génétique Médicale et Laboratoire d’Anatomopathologie, Centre Hospitalier Régional Universitaire, Hôpital Morvan, Brest, France (P.P.; P.M.); Génétique Médicale, Centre Hospitalier Intercommunal, Poissy, Saint-Germain-En-Laye, France (J.R.); Department of Human Genetics and Pathology, Hadassah University Hospital, Jerusalem (V.M.; K.M.); Services d’Anatomie Pathologique et Génétique Médicale, Hôpital Hôtel Dieu, Clermont-Ferrand, France (A.M.-B.; C.F.); Service d’Anatomopathologie, Centre Hospitalier Intercommunal de Créteil, Créteil, France (M.S.); and Section of Ophthalmology and Neurosciences, Leeds Institute of Molecular Medicine, St. James’s University Hospital, Leeds, United Kingdom (C.A.J.)
1,
Julia Tantau
Julia Tantau
1From the INSERM U781 (L.B.; S. Sivanandamoorthy; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.) and U574 (S. Saunier; R.S.; M.-C.G.), Université René Descartes (L.B.; C.E.; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), Département de Génétique (S.A.; J.M.; C.O.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), and Service Maternité (Y.D.), Hôpital Necker-Enfants Malades, Service de Génétique et d’Embryologie Médicales, Hôpital Armand Trousseau (M.G.), Service d’Anatomie Pathologique, Hôpital Bichat (M.-C.D.), Service de Biologie du Développement, Hôpital Robert Debré (F.M.), and Service d’Anatomopathologie, Hôpital Saint-Vincent de Paul (J.T.), Assistance Publique–Hôpitaux de Paris, Paris; INSERM U535 and Université Paris Sud, Villejuif, France (M.C.-B.; E.G.); West Midlands Regional Genetics, Birmingham Women’s Hospital, Birmingham, United Kingdom (E.R.; F.M.); Service de Génétique, Centre Hospitalo–Universitaire Hôpital Bretonneau, Tours, France (A.T.; C.M.); Département de Pédiatrie et Génétique Médicale et Laboratoire d’Anatomopathologie, Centre Hospitalier Régional Universitaire, Hôpital Morvan, Brest, France (P.P.; P.M.); Génétique Médicale, Centre Hospitalier Intercommunal, Poissy, Saint-Germain-En-Laye, France (J.R.); Department of Human Genetics and Pathology, Hadassah University Hospital, Jerusalem (V.M.; K.M.); Services d’Anatomie Pathologique et Génétique Médicale, Hôpital Hôtel Dieu, Clermont-Ferrand, France (A.M.-B.; C.F.); Service d’Anatomopathologie, Centre Hospitalier Intercommunal de Créteil, Créteil, France (M.S.); and Section of Ophthalmology and Neurosciences, Leeds Institute of Molecular Medicine, St. James’s University Hospital, Leeds, United Kingdom (C.A.J.)
1,
Martine Sinico
Martine Sinico
1From the INSERM U781 (L.B.; S. Sivanandamoorthy; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.) and U574 (S. Saunier; R.S.; M.-C.G.), Université René Descartes (L.B.; C.E.; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), Département de Génétique (S.A.; J.M.; C.O.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), and Service Maternité (Y.D.), Hôpital Necker-Enfants Malades, Service de Génétique et d’Embryologie Médicales, Hôpital Armand Trousseau (M.G.), Service d’Anatomie Pathologique, Hôpital Bichat (M.-C.D.), Service de Biologie du Développement, Hôpital Robert Debré (F.M.), and Service d’Anatomopathologie, Hôpital Saint-Vincent de Paul (J.T.), Assistance Publique–Hôpitaux de Paris, Paris; INSERM U535 and Université Paris Sud, Villejuif, France (M.C.-B.; E.G.); West Midlands Regional Genetics, Birmingham Women’s Hospital, Birmingham, United Kingdom (E.R.; F.M.); Service de Génétique, Centre Hospitalo–Universitaire Hôpital Bretonneau, Tours, France (A.T.; C.M.); Département de Pédiatrie et Génétique Médicale et Laboratoire d’Anatomopathologie, Centre Hospitalier Régional Universitaire, Hôpital Morvan, Brest, France (P.P.; P.M.); Génétique Médicale, Centre Hospitalier Intercommunal, Poissy, Saint-Germain-En-Laye, France (J.R.); Department of Human Genetics and Pathology, Hadassah University Hospital, Jerusalem (V.M.; K.M.); Services d’Anatomie Pathologique et Génétique Médicale, Hôpital Hôtel Dieu, Clermont-Ferrand, France (A.M.-B.; C.F.); Service d’Anatomopathologie, Centre Hospitalier Intercommunal de Créteil, Créteil, France (M.S.); and Section of Ophthalmology and Neurosciences, Leeds Institute of Molecular Medicine, St. James’s University Hospital, Leeds, United Kingdom (C.A.J.)
1,
Yves Dumez
Yves Dumez
1From the INSERM U781 (L.B.; S. Sivanandamoorthy; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.) and U574 (S. Saunier; R.S.; M.-C.G.), Université René Descartes (L.B.; C.E.; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), Département de Génétique (S.A.; J.M.; C.O.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), and Service Maternité (Y.D.), Hôpital Necker-Enfants Malades, Service de Génétique et d’Embryologie Médicales, Hôpital Armand Trousseau (M.G.), Service d’Anatomie Pathologique, Hôpital Bichat (M.-C.D.), Service de Biologie du Développement, Hôpital Robert Debré (F.M.), and Service d’Anatomopathologie, Hôpital Saint-Vincent de Paul (J.T.), Assistance Publique–Hôpitaux de Paris, Paris; INSERM U535 and Université Paris Sud, Villejuif, France (M.C.-B.; E.G.); West Midlands Regional Genetics, Birmingham Women’s Hospital, Birmingham, United Kingdom (E.R.; F.M.); Service de Génétique, Centre Hospitalo–Universitaire Hôpital Bretonneau, Tours, France (A.T.; C.M.); Département de Pédiatrie et Génétique Médicale et Laboratoire d’Anatomopathologie, Centre Hospitalier Régional Universitaire, Hôpital Morvan, Brest, France (P.P.; P.M.); Génétique Médicale, Centre Hospitalier Intercommunal, Poissy, Saint-Germain-En-Laye, France (J.R.); Department of Human Genetics and Pathology, Hadassah University Hospital, Jerusalem (V.M.; K.M.); Services d’Anatomie Pathologique et Génétique Médicale, Hôpital Hôtel Dieu, Clermont-Ferrand, France (A.M.-B.; C.F.); Service d’Anatomopathologie, Centre Hospitalier Intercommunal de Créteil, Créteil, France (M.S.); and Section of Ophthalmology and Neurosciences, Leeds Institute of Molecular Medicine, St. James’s University Hospital, Leeds, United Kingdom (C.A.J.)
1,
Fiona MacDonald
Fiona MacDonald
1From the INSERM U781 (L.B.; S. Sivanandamoorthy; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.) and U574 (S. Saunier; R.S.; M.-C.G.), Université René Descartes (L.B.; C.E.; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), Département de Génétique (S.A.; J.M.; C.O.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), and Service Maternité (Y.D.), Hôpital Necker-Enfants Malades, Service de Génétique et d’Embryologie Médicales, Hôpital Armand Trousseau (M.G.), Service d’Anatomie Pathologique, Hôpital Bichat (M.-C.D.), Service de Biologie du Développement, Hôpital Robert Debré (F.M.), and Service d’Anatomopathologie, Hôpital Saint-Vincent de Paul (J.T.), Assistance Publique–Hôpitaux de Paris, Paris; INSERM U535 and Université Paris Sud, Villejuif, France (M.C.-B.; E.G.); West Midlands Regional Genetics, Birmingham Women’s Hospital, Birmingham, United Kingdom (E.R.; F.M.); Service de Génétique, Centre Hospitalo–Universitaire Hôpital Bretonneau, Tours, France (A.T.; C.M.); Département de Pédiatrie et Génétique Médicale et Laboratoire d’Anatomopathologie, Centre Hospitalier Régional Universitaire, Hôpital Morvan, Brest, France (P.P.; P.M.); Génétique Médicale, Centre Hospitalier Intercommunal, Poissy, Saint-Germain-En-Laye, France (J.R.); Department of Human Genetics and Pathology, Hadassah University Hospital, Jerusalem (V.M.; K.M.); Services d’Anatomie Pathologique et Génétique Médicale, Hôpital Hôtel Dieu, Clermont-Ferrand, France (A.M.-B.; C.F.); Service d’Anatomopathologie, Centre Hospitalier Intercommunal de Créteil, Créteil, France (M.S.); and Section of Ophthalmology and Neurosciences, Leeds Institute of Molecular Medicine, St. James’s University Hospital, Leeds, United Kingdom (C.A.J.)
1,
Arnold Munnich
Arnold Munnich
1From the INSERM U781 (L.B.; S. Sivanandamoorthy; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.) and U574 (S. Saunier; R.S.; M.-C.G.), Université René Descartes (L.B.; C.E.; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), Département de Génétique (S.A.; J.M.; C.O.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), and Service Maternité (Y.D.), Hôpital Necker-Enfants Malades, Service de Génétique et d’Embryologie Médicales, Hôpital Armand Trousseau (M.G.), Service d’Anatomie Pathologique, Hôpital Bichat (M.-C.D.), Service de Biologie du Développement, Hôpital Robert Debré (F.M.), and Service d’Anatomopathologie, Hôpital Saint-Vincent de Paul (J.T.), Assistance Publique–Hôpitaux de Paris, Paris; INSERM U535 and Université Paris Sud, Villejuif, France (M.C.-B.; E.G.); West Midlands Regional Genetics, Birmingham Women’s Hospital, Birmingham, United Kingdom (E.R.; F.M.); Service de Génétique, Centre Hospitalo–Universitaire Hôpital Bretonneau, Tours, France (A.T.; C.M.); Département de Pédiatrie et Génétique Médicale et Laboratoire d’Anatomopathologie, Centre Hospitalier Régional Universitaire, Hôpital Morvan, Brest, France (P.P.; P.M.); Génétique Médicale, Centre Hospitalier Intercommunal, Poissy, Saint-Germain-En-Laye, France (J.R.); Department of Human Genetics and Pathology, Hadassah University Hospital, Jerusalem (V.M.; K.M.); Services d’Anatomie Pathologique et Génétique Médicale, Hôpital Hôtel Dieu, Clermont-Ferrand, France (A.M.-B.; C.F.); Service d’Anatomopathologie, Centre Hospitalier Intercommunal de Créteil, Créteil, France (M.S.); and Section of Ophthalmology and Neurosciences, Leeds Institute of Molecular Medicine, St. James’s University Hospital, Leeds, United Kingdom (C.A.J.)
1,
Stanislas Lyonnet
Stanislas Lyonnet
1From the INSERM U781 (L.B.; S. Sivanandamoorthy; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.) and U574 (S. Saunier; R.S.; M.-C.G.), Université René Descartes (L.B.; C.E.; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), Département de Génétique (S.A.; J.M.; C.O.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), and Service Maternité (Y.D.), Hôpital Necker-Enfants Malades, Service de Génétique et d’Embryologie Médicales, Hôpital Armand Trousseau (M.G.), Service d’Anatomie Pathologique, Hôpital Bichat (M.-C.D.), Service de Biologie du Développement, Hôpital Robert Debré (F.M.), and Service d’Anatomopathologie, Hôpital Saint-Vincent de Paul (J.T.), Assistance Publique–Hôpitaux de Paris, Paris; INSERM U535 and Université Paris Sud, Villejuif, France (M.C.-B.; E.G.); West Midlands Regional Genetics, Birmingham Women’s Hospital, Birmingham, United Kingdom (E.R.; F.M.); Service de Génétique, Centre Hospitalo–Universitaire Hôpital Bretonneau, Tours, France (A.T.; C.M.); Département de Pédiatrie et Génétique Médicale et Laboratoire d’Anatomopathologie, Centre Hospitalier Régional Universitaire, Hôpital Morvan, Brest, France (P.P.; P.M.); Génétique Médicale, Centre Hospitalier Intercommunal, Poissy, Saint-Germain-En-Laye, France (J.R.); Department of Human Genetics and Pathology, Hadassah University Hospital, Jerusalem (V.M.; K.M.); Services d’Anatomie Pathologique et Génétique Médicale, Hôpital Hôtel Dieu, Clermont-Ferrand, France (A.M.-B.; C.F.); Service d’Anatomopathologie, Centre Hospitalier Intercommunal de Créteil, Créteil, France (M.S.); and Section of Ophthalmology and Neurosciences, Leeds Institute of Molecular Medicine, St. James’s University Hospital, Leeds, United Kingdom (C.A.J.)
1,
Marie-Claire Gubler
Marie-Claire Gubler
1From the INSERM U781 (L.B.; S. Sivanandamoorthy; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.) and U574 (S. Saunier; R.S.; M.-C.G.), Université René Descartes (L.B.; C.E.; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), Département de Génétique (S.A.; J.M.; C.O.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), and Service Maternité (Y.D.), Hôpital Necker-Enfants Malades, Service de Génétique et d’Embryologie Médicales, Hôpital Armand Trousseau (M.G.), Service d’Anatomie Pathologique, Hôpital Bichat (M.-C.D.), Service de Biologie du Développement, Hôpital Robert Debré (F.M.), and Service d’Anatomopathologie, Hôpital Saint-Vincent de Paul (J.T.), Assistance Publique–Hôpitaux de Paris, Paris; INSERM U535 and Université Paris Sud, Villejuif, France (M.C.-B.; E.G.); West Midlands Regional Genetics, Birmingham Women’s Hospital, Birmingham, United Kingdom (E.R.; F.M.); Service de Génétique, Centre Hospitalo–Universitaire Hôpital Bretonneau, Tours, France (A.T.; C.M.); Département de Pédiatrie et Génétique Médicale et Laboratoire d’Anatomopathologie, Centre Hospitalier Régional Universitaire, Hôpital Morvan, Brest, France (P.P.; P.M.); Génétique Médicale, Centre Hospitalier Intercommunal, Poissy, Saint-Germain-En-Laye, France (J.R.); Department of Human Genetics and Pathology, Hadassah University Hospital, Jerusalem (V.M.; K.M.); Services d’Anatomie Pathologique et Génétique Médicale, Hôpital Hôtel Dieu, Clermont-Ferrand, France (A.M.-B.; C.F.); Service d’Anatomopathologie, Centre Hospitalier Intercommunal de Créteil, Créteil, France (M.S.); and Section of Ophthalmology and Neurosciences, Leeds Institute of Molecular Medicine, St. James’s University Hospital, Leeds, United Kingdom (C.A.J.)
1,
Emmanuelle Génin
Emmanuelle Génin
1From the INSERM U781 (L.B.; S. Sivanandamoorthy; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.) and U574 (S. Saunier; R.S.; M.-C.G.), Université René Descartes (L.B.; C.E.; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), Département de Génétique (S.A.; J.M.; C.O.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), and Service Maternité (Y.D.), Hôpital Necker-Enfants Malades, Service de Génétique et d’Embryologie Médicales, Hôpital Armand Trousseau (M.G.), Service d’Anatomie Pathologique, Hôpital Bichat (M.-C.D.), Service de Biologie du Développement, Hôpital Robert Debré (F.M.), and Service d’Anatomopathologie, Hôpital Saint-Vincent de Paul (J.T.), Assistance Publique–Hôpitaux de Paris, Paris; INSERM U535 and Université Paris Sud, Villejuif, France (M.C.-B.; E.G.); West Midlands Regional Genetics, Birmingham Women’s Hospital, Birmingham, United Kingdom (E.R.; F.M.); Service de Génétique, Centre Hospitalo–Universitaire Hôpital Bretonneau, Tours, France (A.T.; C.M.); Département de Pédiatrie et Génétique Médicale et Laboratoire d’Anatomopathologie, Centre Hospitalier Régional Universitaire, Hôpital Morvan, Brest, France (P.P.; P.M.); Génétique Médicale, Centre Hospitalier Intercommunal, Poissy, Saint-Germain-En-Laye, France (J.R.); Department of Human Genetics and Pathology, Hadassah University Hospital, Jerusalem (V.M.; K.M.); Services d’Anatomie Pathologique et Génétique Médicale, Hôpital Hôtel Dieu, Clermont-Ferrand, France (A.M.-B.; C.F.); Service d’Anatomopathologie, Centre Hospitalier Intercommunal de Créteil, Créteil, France (M.S.); and Section of Ophthalmology and Neurosciences, Leeds Institute of Molecular Medicine, St. James’s University Hospital, Leeds, United Kingdom (C.A.J.)
1,
Colin A Johnson
Colin A Johnson
1From the INSERM U781 (L.B.; S. Sivanandamoorthy; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.) and U574 (S. Saunier; R.S.; M.-C.G.), Université René Descartes (L.B.; C.E.; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), Département de Génétique (S.A.; J.M.; C.O.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), and Service Maternité (Y.D.), Hôpital Necker-Enfants Malades, Service de Génétique et d’Embryologie Médicales, Hôpital Armand Trousseau (M.G.), Service d’Anatomie Pathologique, Hôpital Bichat (M.-C.D.), Service de Biologie du Développement, Hôpital Robert Debré (F.M.), and Service d’Anatomopathologie, Hôpital Saint-Vincent de Paul (J.T.), Assistance Publique–Hôpitaux de Paris, Paris; INSERM U535 and Université Paris Sud, Villejuif, France (M.C.-B.; E.G.); West Midlands Regional Genetics, Birmingham Women’s Hospital, Birmingham, United Kingdom (E.R.; F.M.); Service de Génétique, Centre Hospitalo–Universitaire Hôpital Bretonneau, Tours, France (A.T.; C.M.); Département de Pédiatrie et Génétique Médicale et Laboratoire d’Anatomopathologie, Centre Hospitalier Régional Universitaire, Hôpital Morvan, Brest, France (P.P.; P.M.); Génétique Médicale, Centre Hospitalier Intercommunal, Poissy, Saint-Germain-En-Laye, France (J.R.); Department of Human Genetics and Pathology, Hadassah University Hospital, Jerusalem (V.M.; K.M.); Services d’Anatomie Pathologique et Génétique Médicale, Hôpital Hôtel Dieu, Clermont-Ferrand, France (A.M.-B.; C.F.); Service d’Anatomopathologie, Centre Hospitalier Intercommunal de Créteil, Créteil, France (M.S.); and Section of Ophthalmology and Neurosciences, Leeds Institute of Molecular Medicine, St. James’s University Hospital, Leeds, United Kingdom (C.A.J.)
1,
Michel Vekemans
Michel Vekemans
1From the INSERM U781 (L.B.; S. Sivanandamoorthy; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.) and U574 (S. Saunier; R.S.; M.-C.G.), Université René Descartes (L.B.; C.E.; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), Département de Génétique (S.A.; J.M.; C.O.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), and Service Maternité (Y.D.), Hôpital Necker-Enfants Malades, Service de Génétique et d’Embryologie Médicales, Hôpital Armand Trousseau (M.G.), Service d’Anatomie Pathologique, Hôpital Bichat (M.-C.D.), Service de Biologie du Développement, Hôpital Robert Debré (F.M.), and Service d’Anatomopathologie, Hôpital Saint-Vincent de Paul (J.T.), Assistance Publique–Hôpitaux de Paris, Paris; INSERM U535 and Université Paris Sud, Villejuif, France (M.C.-B.; E.G.); West Midlands Regional Genetics, Birmingham Women’s Hospital, Birmingham, United Kingdom (E.R.; F.M.); Service de Génétique, Centre Hospitalo–Universitaire Hôpital Bretonneau, Tours, France (A.T.; C.M.); Département de Pédiatrie et Génétique Médicale et Laboratoire d’Anatomopathologie, Centre Hospitalier Régional Universitaire, Hôpital Morvan, Brest, France (P.P.; P.M.); Génétique Médicale, Centre Hospitalier Intercommunal, Poissy, Saint-Germain-En-Laye, France (J.R.); Department of Human Genetics and Pathology, Hadassah University Hospital, Jerusalem (V.M.; K.M.); Services d’Anatomie Pathologique et Génétique Médicale, Hôpital Hôtel Dieu, Clermont-Ferrand, France (A.M.-B.; C.F.); Service d’Anatomopathologie, Centre Hospitalier Intercommunal de Créteil, Créteil, France (M.S.); and Section of Ophthalmology and Neurosciences, Leeds Institute of Molecular Medicine, St. James’s University Hospital, Leeds, United Kingdom (C.A.J.)
1,
Férechté Encha-Razavi
Férechté Encha-Razavi
1From the INSERM U781 (L.B.; S. Sivanandamoorthy; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.) and U574 (S. Saunier; R.S.; M.-C.G.), Université René Descartes (L.B.; C.E.; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), Département de Génétique (S.A.; J.M.; C.O.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), and Service Maternité (Y.D.), Hôpital Necker-Enfants Malades, Service de Génétique et d’Embryologie Médicales, Hôpital Armand Trousseau (M.G.), Service d’Anatomie Pathologique, Hôpital Bichat (M.-C.D.), Service de Biologie du Développement, Hôpital Robert Debré (F.M.), and Service d’Anatomopathologie, Hôpital Saint-Vincent de Paul (J.T.), Assistance Publique–Hôpitaux de Paris, Paris; INSERM U535 and Université Paris Sud, Villejuif, France (M.C.-B.; E.G.); West Midlands Regional Genetics, Birmingham Women’s Hospital, Birmingham, United Kingdom (E.R.; F.M.); Service de Génétique, Centre Hospitalo–Universitaire Hôpital Bretonneau, Tours, France (A.T.; C.M.); Département de Pédiatrie et Génétique Médicale et Laboratoire d’Anatomopathologie, Centre Hospitalier Régional Universitaire, Hôpital Morvan, Brest, France (P.P.; P.M.); Génétique Médicale, Centre Hospitalier Intercommunal, Poissy, Saint-Germain-En-Laye, France (J.R.); Department of Human Genetics and Pathology, Hadassah University Hospital, Jerusalem (V.M.; K.M.); Services d’Anatomie Pathologique et Génétique Médicale, Hôpital Hôtel Dieu, Clermont-Ferrand, France (A.M.-B.; C.F.); Service d’Anatomopathologie, Centre Hospitalier Intercommunal de Créteil, Créteil, France (M.S.); and Section of Ophthalmology and Neurosciences, Leeds Institute of Molecular Medicine, St. James’s University Hospital, Leeds, United Kingdom (C.A.J.)
1,
Tania Attié-Bitach
Tania Attié-Bitach
1From the INSERM U781 (L.B.; S. Sivanandamoorthy; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.) and U574 (S. Saunier; R.S.; M.-C.G.), Université René Descartes (L.B.; C.E.; M.L.M.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), Département de Génétique (S.A.; J.M.; C.O.; A.M.; S.L.; M.V.; F.E.-R.; T.A.-B.), and Service Maternité (Y.D.), Hôpital Necker-Enfants Malades, Service de Génétique et d’Embryologie Médicales, Hôpital Armand Trousseau (M.G.), Service d’Anatomie Pathologique, Hôpital Bichat (M.-C.D.), Service de Biologie du Développement, Hôpital Robert Debré (F.M.), and Service d’Anatomopathologie, Hôpital Saint-Vincent de Paul (J.T.), Assistance Publique–Hôpitaux de Paris, Paris; INSERM U535 and Université Paris Sud, Villejuif, France (M.C.-B.; E.G.); West Midlands Regional Genetics, Birmingham Women’s Hospital, Birmingham, United Kingdom (E.R.; F.M.); Service de Génétique, Centre Hospitalo–Universitaire Hôpital Bretonneau, Tours, France (A.T.; C.M.); Département de Pédiatrie et Génétique Médicale et Laboratoire d’Anatomopathologie, Centre Hospitalier Régional Universitaire, Hôpital Morvan, Brest, France (P.P.; P.M.); Génétique Médicale, Centre Hospitalier Intercommunal, Poissy, Saint-Germain-En-Laye, France (J.R.); Department of Human Genetics and Pathology, Hadassah University Hospital, Jerusalem (V.M.; K.M.); Services d’Anatomie Pathologique et Génétique Médicale, Hôpital Hôtel Dieu, Clermont-Ferrand, France (A.M.-B.; C.F.); Service d’Anatomopathologie, Centre Hospitalier Intercommunal de Créteil, Créteil, France (M.S.); and Section of Ophthalmology and Neurosciences, Leeds Institute of Molecular Medicine, St. James’s University Hospital, Leeds, United Kingdom (C.A.J.)
1