Abstract
One of the notable features of Alzheimer's disease (AD) is the overabundance of beta-amyloid peptides in brain fluids, leading to the formation and deposition of insoluble amyloid plaques. Previous work in this lab demonstrates that the normal choroid plexus, a primary component of the blood-cerebrospinal fluid barrier, has the capacity to remove beta-amyloid from the cerebrospinal fluid, potentially preventing the formation of beta-amyloid plaques. The purpose of this work was to determine whether the choroid plexus and/or the brain capillaries, a primary component of the blood-brain barrier, possessed the capacity to produce or degrade beta-amyloid peptides. Using quantitative real-time RT-PCR, immunodetection and enzyme activity assays, we demonstrated the presence in brain barriers of several key enzymes involved in beta-amyloid production, namely, amyloid precursor protein and beta-secretase, and in beta-amyloid metabolism and alternate processing, such as insulin degrading enzyme, endothelin-converting enzyme-1, neprilysin and alpha-secretase. Furthermore, beta-amyloid presence, in the absence of its application in culture media, was detected in an immortalized choroidal epithelial cell line, known as Z310 cells. The ability of the choroid plexus to produce and degrade beta-amyloid, in addition to its transport function, suggests a vital role of this tissue in maintaining beta-amyloid homeostasis. Disruption of this homeostasis due to aging, injury or toxicant exposure may contribute to accumulation of beta-amyloid peptides in the brain fluids, leading to AD.
Keywords: Alzheimer's disease, brain barriers, choroid plexus, beta-amyloid, beta-secretase, neprilysin
1. Introduction
The accumulation and aggregation of beta-amyloid peptides into insoluble plaques are thought to be crucial steps in the etiology of Alzheimer's disease (AD). The amyloid cascade hypothesis suggests that stopping or slowing formation of the Aβ plaques would delay the onset of the disease symptoms. Beta-amyloid (Aβ) is found in the extracellular fluids of the brain, including the cerebrospinal fluid (CSF) supporting the brain and circulating in brain ventricles, and the interstitial fluid surrounding the neurons and glial cells in brain lobes (Price et al., 1998; Selkoe, 2001; Seubert et al., 1992; Vigo-Pelfrey et al., 1993). It is unclear, however, whether the accumulated Aβ is produced inside the brain or imported across brain barriers from other tissues.
Brain barriers contribute to the etiology of AD mainly in three aspects: (i) the aging of cerebral vascular structure in the overall aging process of the brain, (ii) as the site of transport of extracerebral Aβ into the brain, and (iii) the ability to prevent Aβ aggregation by removing it (Zheng, 2001). The blood-brain barrier (BBB) separates blood from the interstitial fluid surrounding the neurons and neuroglia, and the blood-CSF barrier (BCB) separates the blood and CSF in and around the brain. Several studies have shown the presence of Aβ and its transport across the BBB (Deane et al., 2003; Mackic et al., 2002; Pluta et al., 2000; Poduslo et al., 1999; Shibata et al., 2000; Zlokovic, 2004) and BCB (Crossgrove et al., 2005; Monro et al., 2002; Sasaki et al., 1997; Serot et al., 2003; Serot et al., 2001). The choroid plexus, a major component of the BCB, has been demonstrated to be immunoreactive to antibodies against Aβ and its precursor protein, APP (Sasaki et al., 1997), but the origin of the Aβ peptide has not been definitively established. Our recent finding suggests that the choroid plexus may remove Aβ from the CSF (Crossgrove et al., 2005). It is also possible that the choroid plexus may metabolize Aβ sequestered from the CSF, as it exhibits high levels of metabolic enzymes effective in the detoxification of endogenous compounds, drugs and other xenobiotics (Ghersi-Egea et al., 1994; Lee et al., 2001; Morse et al., 1998; Philbert et al., 1995).
The objective of this work was to determine whether the choroid plexus produces and/or degrades Aβ peptides. We investigated the presence of several key enzymes involved in Aβ production and metabolism, including amyloid precursor protein (APP), α- and β-secretase, insulin degrading enzyme (IDE), endothelin-converting enzyme-1 (ECE), and neprilysin. We also examined the presence of Aβ in an immortalized cell line of choroid plexus epithelia, known as Z310 cells, established in this lab as a model for the BCB (Zheng and Zhao, 2002). Taken together with the body of knowledge concerning β-amyloid homeostasis, we sought to identify the relative roles of the BCB and the BBB in Aβ production and metabolism.
2. Results
By real time RT-PCR analysis, the choroid plexus, the brain capillaries and surrounding parenchyma, and Z310 cells expressed genes capable of producing and metabolizing Aβ peptides (Fig. 1 and 2). APP and β-secretase contribute to the production of Aβ peptides, while the remaining genes function to degrade the peptides (ECE-1, IDE and neprilysin) or form an alternate APP product (α-secretase). The brain capillaries in the striatum tended to have relatively higher expression of Aβ producing and metabolizing genes than the choroid plexus (Fig. 2). This was not due to a difference in GAPDH expression in the choroid plexus compared to the striatal brain capillaries (28.3 ± 1.4 ng vs. 27.3 ± 1.8 ng, respectively; mean ± SEM; p = 0.67). In fact, there were no statistically significant differences in GAPDH expression among any of the regions tested by one-way ANOVA with post hoc comparisons using Bonferroni's test for multiple comparisons. Furthermore, the brain capillaries from the striatum had significantly greater expression of these genes than the surrounding brain parenchyma and greater expression than the capillary endothelia of the other tested regions. Compared to the immortalized choroidal epithelial cell line, the freshly isolated choroid plexus usually had a greater expression of the selected genes.
Figure 1.
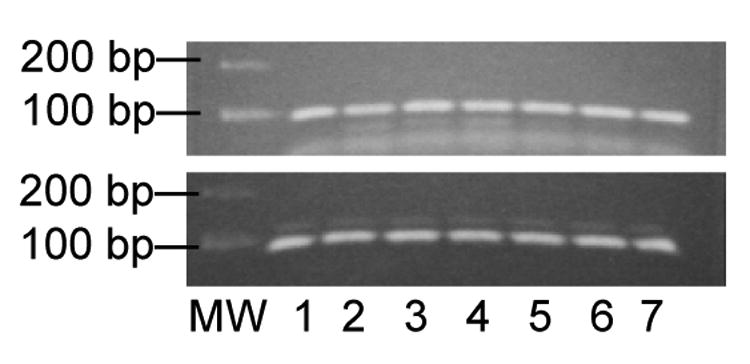
Expression of APP mRNA in choroid plexus tissues and Z310 cells by real-time RT-PCR. Following PCR, the double-stranded products were separated by gel electrophoresis and visualized with ethidium bromide. Upper: APP produced a double stranded product of 86 base pairs (bp). Lower: GAPDH produced a double stranded product of 82 bp. Lanes 1–5 used cDNA generated from Z310 cells. Lanes 6 and 7 are choroid plexus cDNAs.
Figure 2.
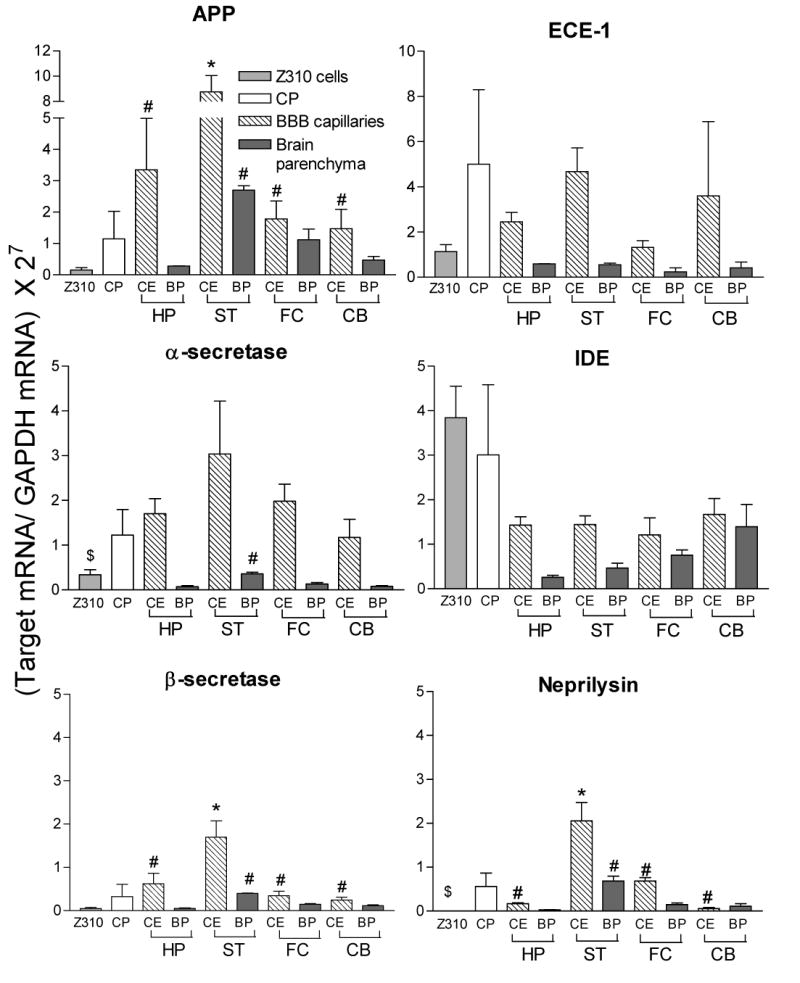
Gene expression of six Aβ-producing and -metabolizing enzymes in the choroid plexus, brain capillaries that comprise the BBB, and the surrounding brain parenchyma. Values are mean ± SEM (n=3). Significant differences are noted by: *different from choroid plexus by one-way ANOVA with Newman-Keuls post hoc test, $different from choroid plexus by t-test, #different from capillary endothelium of the striatum. Abbreviations: APP: amyloid precursor protein; ECE-1 endothelin converting enzyme-1; IDE: insulin degrading enzyme; HP: hippocampus; ST: striatum; FC: frontal cortex; CB: cerebellum; CE: capillary endothelium; BP: brain parenchyma. Note the differences in the values of the y-axes.
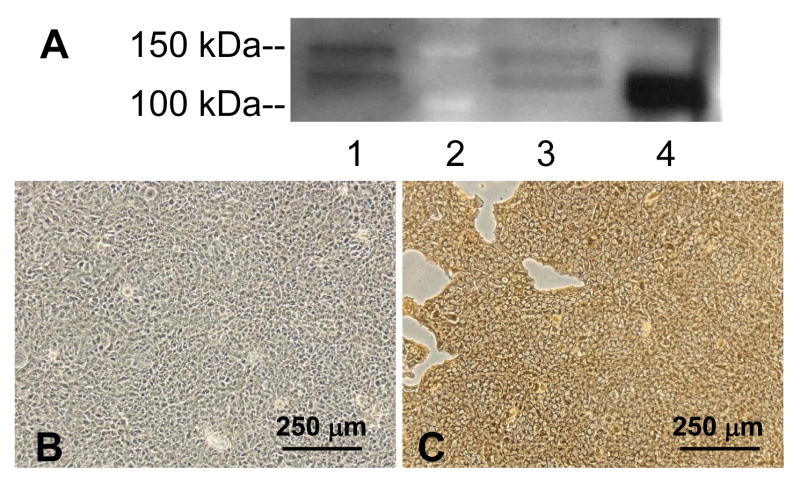
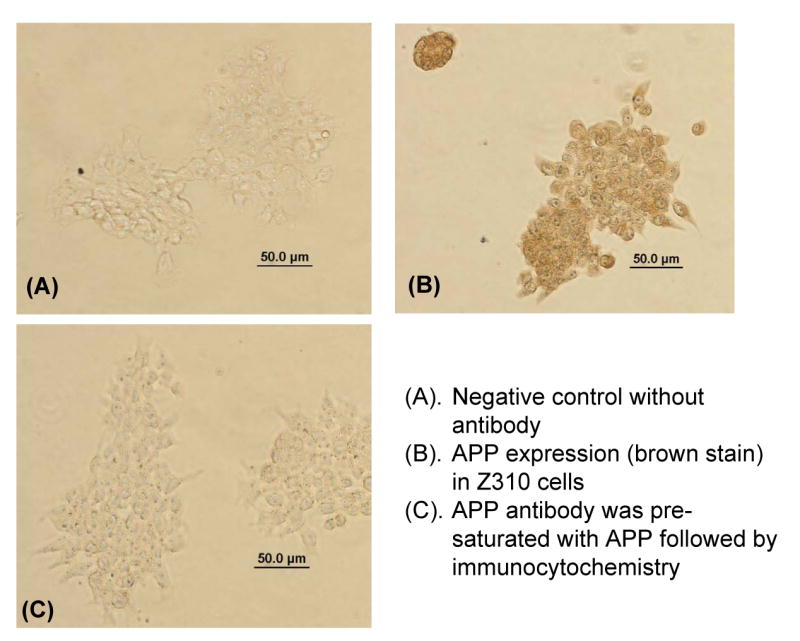
The choroid plexus clearly produces APP protein as demonstrated by Western blot analysis and immunohistochemical staining (Fig. 3). The band in both choroid plexus tissue and choroidal Z310 cells (Fig. 3A) corresponds to the band in the positive control, the whole brain homogenate. The upper band (about 150 kDa) in Fig. 3A is a non-specific band that was uniquely eliminated when the primary antibody was pre-exposed to APP (data not shown).
Figure 3.
APP protein is expressed in the choroid plexus tissue and the choroidal epithelial cell line. A) Representative Western blot for APP presence in Z310 cells (lane 1), choroid plexus (lane 3) and homogenized whole brain (lane 4; positive control). Lane 2 is the molecular weight markers for 100 and 150 kDa. Bands are present at 110 kDa. Results are not quantitative. B) Negative control in which the primary antibody against APP was excluded from the immunocytochemistry in Z310 cells. C) APP expression (brown stain) in Z310 cells. B and C are representative of three immunocytochemistry experiments.
Immunocytochemical staining further confirmed the presence of APP in Z310 cells (Fig. 3C). Negative controls, including a lack of primary antibody (Fig.3 B) and the addition of antibody that was pre-exposed to APP (data not shown), revealed no specific binding to the cells, suggesting that the APP staining was not an artifact. Subsequently, the presence of Aβ peptides was detected in Z310 cells (Fig. 4A&B) as well as in the choroid plexus tissue (Fig. 4C&D). Within the choroid plexus tissue, Aβ appears to be present both intracellularly and along the plasma membrane. It should be noted that the culture medium of Z310 cells did not contain exogenous Aβ peptides. Furthermore, any bovine Aβ that may be present from the serum would not be expected to react with the 6E10 antibody against Aβ, since the predicted bovine sequence of Aβ has at least eight amino acid substitutions within the 17-amino acid immunogenic fragment.
Figure 4.
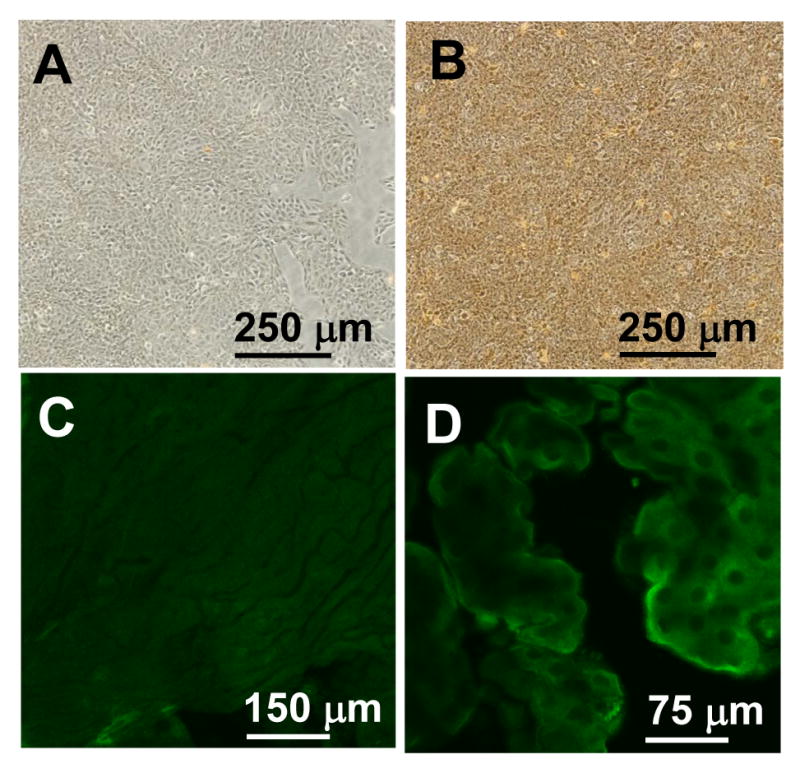
The choroid plexus produces Aβ from APP. Left, negative controls excluding primary antibody. Right, expression of immunoreactive Aβ peptide in tissues and cells. A, B) Aβ expression (brown stain) in Z310 cells by immunocytochemistry. C, D) Green fluorescence corresponds to Aβ peptide in choroid plexus tissue. Note the differences in magnification.
The level of neprilysin mRNA in our experiment (Fig. 2) appeared to be lower than that reported in the literature (Matsas et al., 1986). This may be due to an inefficient primer. Nonetheless, our immunohistochemical data confirmed the presence of neprilysin in the choroid plexus tissue (Fig. 5). As a positive control, the kidney also produced a significant signal in the glomeruli (data not shown). Z310 cells did not exhibit any significant immunoreactivity to the neprilysin antibody (data not shown), suggesting that the epithelial cell line lost neprilysin expression following immortalization.
Figure 5.
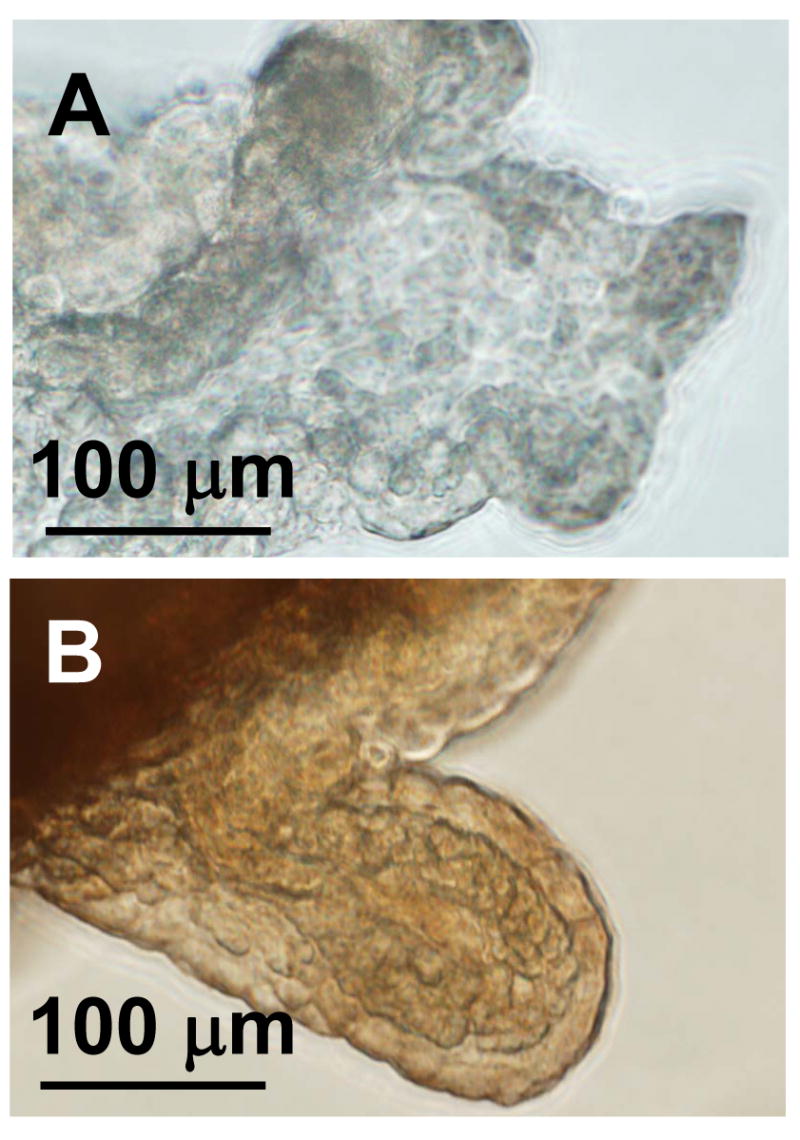
Immunohistochemistry of neprilysin in the choroid plexus. Neprilysin expression is evident in choroid plexus tissue (B) compared to a control tissue, in which the primary antibody was excluded (A). These images are representative of at least three trials.
The enzymatic assay of β-secretase activity revealed a detectable enzyme activity in all tested samples with an activity order of choroid plexus > BBB > Z310 cells (Fig. 6). The activity is consistent with the design of the assay, with 5–20% of the substrate being cleaved. The β-secretase activity in the choroid plexus, the structural basis of BCB, was 38% higher than that in brain capillary endothelia, which comprise the BBB.
Figure 6.
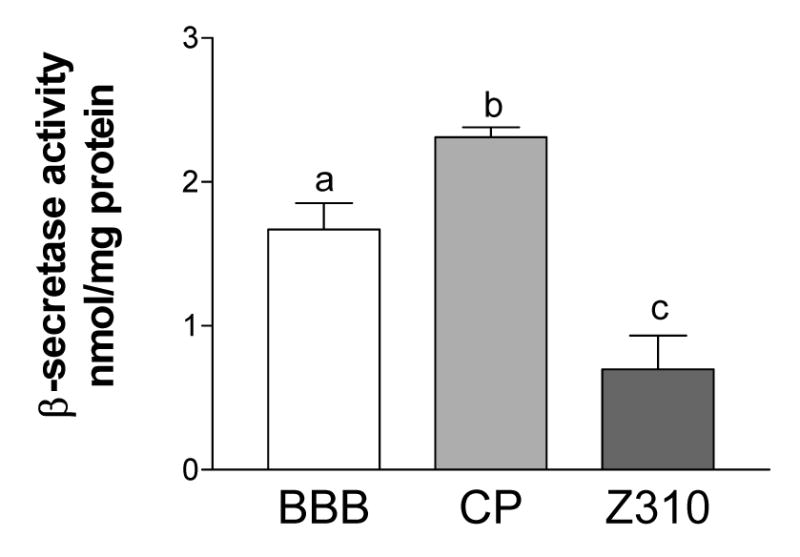
β-Secretase activity in the choroid plexus and the blood-brain barrier. The choroid plexus has more β-secretase activity (nmol product formed/ mg total protein) than either the brain capillaries (BBB; from whole brain) or Z310 cells. Values shown are mean ± SEM (n=3). Each sample is significantly different from all others.
3. Discussion
The brain barrier system, i.e., BBB and BCB, protects the central nervous system against the imbalanced buildup of neuroactive materials such as toxic chemicals, endogenous metabolites, and macromolecules (Zheng et al., 2003). The dynamic homeostasis of Aβ in brain extracellular fluid compartments, namely interstitial fluid and CSF, is presumed to be similarly maintained by both the BCB and BBB. Our previous study has established that the choroid plexus is capable of capturing Aβ molecules in the CSF with a relatively large capacity (Crossgrove et al., 2005). This study was designed to address whether the choroid plexus possesses the ability to produce Aβ, whether there is an enzymatic system in the tissue to degrade the captured Aβ, and what the relative role of the BCB is in comparison to BBB on these aspects.
Our data clearly demonstrate that both the BBB and the BCB are capable of producing Aβ peptides. Consistent with previous reports of APP expression in the choroid plexus and BBB (Kalaria et al., 1996; Sasaki et al., 1997), the mRNA expression of APP and β-secretase were confirmed by these protein and activity assays. While we did not directly examine γ-secretase activity or the presence of any of its four subunits (presenilin-1 or -2, nicastrin, Aph-1, and presenilin enhancer-2) in the choroid plexus, the presence of a correctly-cleaved APP product (Aβ) in the cultured choroidal cell line, in the absence of its addition in the cell culture medium, implies that both β- and γ-secretase are present and active in the choroid plexus. Other studies have confirmed the existence of γ-secretase mRNA and protein complex (determined by immunoprecipitation with presenilin) in whole brain (Hebert et al., 2004), but not specifically at either barrier.
This study also provides evidence that the BBB and BCB are capable of metabolizing Aβ by IDE, ECE and/or neprilysin, the enzymes known to degrade Aβ (Eckman and Eckman, 2005). This is consistent with previous reports in the literature of IDE in cultures of human brain endothelial cells (Lynch et al., 2006), ECE in human brain cerebral vessels (Davenport and Kuc, 2000; Vatter et al., 2002) and in rat choroid plexus (Sluck et al., 1999), and neprilysin protein expression in choroid plexus (Matsas et al., 1986) and its activity in bovine brain microvasculature (Brownson et al., 1994). Although we were unable to determine the protein level of IDE due to the difficulty in obtaining rat-specific antibody, our real-time RT-PCR data indicated that the gene was being transcribed in the choroid plexus at a rate marginally greater than in the BBB (p=0.06). The presence of α-secretase in the BBB and BCB, which processes APP into a non-amyloid product, was also confirmed by PCR in this study.
The presence of these enzymes in both the BBB and the BCB raises several interesting questions for further investigation. First, in which direction(s) do the brain barrier systems secrete the produced Aβ and to what extent does this contribute to the overall Aβ homeostasis? The finding that brain capillary endothelia possess a significantly higher level of APP and β-secretase than brain parenchyma is remarkable. Aβ production has been commonly viewed as a process uniquely related to the functions or malfunction of neurons and neuroglia. Our data provide evidence of an additional source of brain Aβ that is derived from brain barrier cells. As the function of brain barriers deteriorates with aging and is affected by exposure to other damaging substances in blood circulation (Serot et al., 2001; Shah and Mooradian, 1997; Zheng et al., 1996), there is sound reasoning to postulate that the damaged brain barriers may become the primary source of Aβ in the brain, which in turn facilitates the course and progression of AD. Clearly, an in-depth study is warranted to explore the contribution of brain barriers in the production of brain Aβ.
Secondly, which barrier plays a more important role in Aβ production and processing? The BBB expression of these genes was remarkably region-specific. For example, only the striatal BBB expressed significantly more APP, β-secretase and neprilysin than the choroid plexus. Interestingly, these same genes were also expressed significantly less in the neurons and neuroglia comprising the striatal parenchyma than in the striatal BBB. The striatal BBB also expressed more APP, β-secretase and neprilysin than the capillary endothelia of the other tested regions. There were no differences between or among the capillary endothelia and brain parenchyma of any other tested region, nor did they differ from the choroid plexus. Because the striatum had more expression than the BCB for both production (APP and β-secretase) and metabolism (neprilysin) enzymes, it is difficult to determine whether the striatal BBB increases or decreases the net Aβ amount in the normal brain and how that contribution may compare to the Aβ production by the BCB. That the choroid plexus has marginally more IDE than the combined BBB is important due to a recent finding that IDE inhibitors had a greater effect on Aβ degradation than either ECE or neprilysin inhibitors in a BBB cell model (Lynch et al., 2006). Since Aβ permeability at the choroid plexus is about 10-fold greater than that of the BBB in either flux direction (Crossgrove et al., 2005; Strazielle et al., 2000), and the choroid plexus surface area has been estimated to be about half that of the BBB (Keep and Jones, 1990), it is possible that the choroid plexus sequesters five times more Aβ than the BBB. Thus, even a marginal increase in IDE expression at the BCB versus the BBB may contribute to considerably more Aβ degradation by this barrier. It is therefore temping to presume that the BBB of the striatum may play a larger role in the volume of Aβ produced than other tested regions, whereas the BCB may have a larger role in Aβ degradation.
Finally, what is the role of transthyretin (TTR), an Aβ-binding protein produced in the brain exclusively by the choroid plexus, to the etiology of AD? First identified as a thyroxine-binding protein in blood and CSF, TTR has been implicated in Aβ transport in the CSF and also functions to bind Aβ and prevent amyloid aggregation and/or deposition in the CSF (Lignelid et al., 1997; Schreiber et al., 1990; Schreiber et al., 2001; Schwarzman et al., 1994). The level of TTR in the CSF decreases with aging and by exposure to the toxic metal lead (Zheng et al., 2001; Zheng et al., 1996). In AD patients, TTR levels in the CSF are reportedly significantly reduced compared to age-matched controls (Merched et al., 1998). In a genetically engineered animal model, over-expression of TTR molecules appears to protect against the progression of AD pathology (Stein et al., 2004). It would be interesting to determine how the production and degradation of Aβ in the choroid plexus may relate to its binding to TTR and/or TTR production.
In summary, the BCB and the BBB possess the definite capacity to produce and degrade Aβ peptides, with the BBB having higher expression of proteins associated with Aβ production. Taken together with our earlier studies on Aβ uptake and transport, this study suggests that the BCB plays a major and complex role in removing Aβ from the CSF by sequestration and metabolism in the choroid plexus under normal, healthy conditions. It is possible that any damage to the BCB or BBB due to illness, injury or toxicant exposure could alter the Aβ homeostasis and may contribute to the etiology of AD.
4. Experimental Procedure
4.1 Materials
Materials were purchased from the following sources: primary antibodies against APP from Sigma (St. Louis, MO), against Aβ (clone 6E10) from Signet (Dedham, MA), against neprilysin from Vision BioSystems (Norwell, MA); secondary antibodies labeled with horseradish peroxidase from GE Healthcare (Piscataway, NJ) or labeled with Alexa Fluor 488™ from Invitrogen (Carlsbad, CA); and custom-produced real-time RT-PCR primers from Sigma-Genosys (Woodlands, TX). Unless otherwise noted, all other chemicals were obtained from Sigma.
The immortalized choroidal epithelial cell line, known as Z310 cells, was maintained in Dulbecco's modified essential medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and 40 μg/mL gentamycin at 37°C as described previously (Zheng and Zhao, 2002). Fresh choroid plexus and kidney tissue were isolated from male Sprague-Dawley rats that were anesthetized by ketamine and xylazine mixture (75 and 10 mg/kg) prior to tissue collection and euthanasia. All animal experiments were conducted with the approval of the Purdue University Animal Care and Use Committee.
4.2 Real-time, reverse-transcriptase PCR
mRNA levels of Aβ processing genes were determined by real-time RT-PCR as described previously (Li et al., 2005). Choroid plexus and selected brain regions were extracted from health adult rat brains. Brain tissue was homogenized on ice and separated by centrifugation (5400 × g, 15 min, 4°C) in 18% dextran into the capillary fraction (pellet) and the brain parenchyma fraction (supernatant). Total RNA was extracted from each sample by TRIzol (Invitrogen, Carlsbad, CA) and purified on RNeasy columns (Qiagen, Palo Alto, CA). One microgram of purified RNA was converted to cDNA by MuLV reverse transcriptase with oligo dT primers and stored at −80°C until use. Forward and reverse primers for each gene of interest and a housekeeping gene, GAPDH, were designed with Primer Express 2.0 software. The primer sequences (forward; reverse, both in the 5′ to 3′ direction) are: APP (ccttaccggtgcctagttggt; gtccatccgctcctggtgta), α-secretase (ggagagttggccccacagtt; cccctgggattggagttaaga), β-secretase (gatccccctggaccacatct; gctctggccacaggtaccat), IDE (ggttggagagttcccctctca; aggccgcgcttgaattc), ECE-1 (cacccacagcatcacctaaca; ctcacacacaggtcacaagagctt) neprilysin (aggccctttacgggactacat; gcctccccacagcattctc), GADPH (cctggagaaacctgccaagtat; agcccaggatgccctttagt). Real-time PCR was completed with the ABsolute QPCR SYBR green mix kit (ABgene, Rochester, NY) in the Mx3000P real-time RT-PCR system (Stratagene, LaJolla, CA). The amplification process was 15 min at 95°C followed by 40 cycles of 30 sec at 95°C, 1 min at 55°C and 30 sec at 72°C. Values were expressed in cycle threshold time (Ct) and were normalized to the Ct times for housekeeping gene, GAPDH. Primer specificity was confirmed by a single peak in a dissociation curve or by a single lane following gel electrophoresis of the primer products (Fig. 1).
4.3 Western blot analysis
Total protein extracted from choroid plexus, whole brain homogenate and Z310 cells was separated by gel electrophoresis on a 4–20% Tris-HCl linear gradient gel (Bio-Rad, Hercules, CA) and transferred to a PVDF membrane. Membranes were blocked with 1% milk powder and immunoblotted with a 1:200 dilution of rabbit anti-APP antibody, followed by incubation with goat anti-rabbit secondary antibody conjugated to horseradish peroxidase. The protein bands were visualized by an ECL system (GE Healthcare, Piscataway, NJ).
4.4 Immunohistochemistry
Confluent Z310 cells grown in Lab Tek II 8-well chamber slides, freshly isolated choroid plexus or kidney tissue was fixed in paraformaldehye (0.3%) and glutaraldehyde (0.25%) in phosphate-buffered saline (PBS) for 10 minutes at room temperature. Tissues were permeabilized by incubation in 0.5% Triton-X100 in PBS for 30 min. Non-specific antibody binding was blocked by incubation with 1% bovine serum albumin in PBS. Primary antibody was added for overnight incubation at 4°C (anti-neprilysin, 1:80; anti-β-amyloid 6E10, 1:100, anti-APP, 1:200). After washing the cells and tissues 5X in PBS, secondary antibody conjugated to horseradish peroxidase was added for at least one hour incubation at 37°C. Samples were washed 5 times in PBS and protein visualized by 3,3′-diaminobenzidine staining.
4.5 Confocal microscopy
Freshly isolated choroid plexus tissue was fixed, permeabilized and blocked as above. Tissues were incubated in primary antibody (at above concentrations) for 1 hour at 37°C, washed and incubated with a conjugated secondary antibody conjugated to Alexa Fluor 488™ for at least one hour at 37°C. Tissues were mounted and dried before visualization with a BioRad MRC 1024 confocal microscope, Purdue University Cytology Lab.
4.6 Determination of β-secretase activity
β-Secretase activity was determined in freshly isolated protein fractions by an assay kit (Sigma, St. Louis, MO), according to the manufacturer's instruction and utilizing the fluorescence resonance energy transfer technique. Briefly, proteins from cells or tissues were incubated for 2 hours at 37°C with a substrate whose fluorescence signal is increased following substrate cleavage by β-secretase. Enzyme activity was then determined from a standard curve and was normalized to total protein concentration of the sample.
4.7 Data Analysis
All values are expressed as the mean ± SEM. Gene expression was compared between the choroid plexus and Z310 cell line by student's t-test. For the regional tissue analysis of gene expression (Fig. 2), choroid plexus and brain regional data were analyzed by one-way ANOVA. Post hoc examination using Newman-Keuls multiple comparison test.compared gene expression (i) in the choroid plexus versus each regional capillary fraction, (ii) in each regional capillary fraction versus its corresponding brain parenchyma fraction and (iii) in each capillary fraction versus the other capillary fractions. In the cases where no differences were noted between the choroid plexus and each capillary fraction, data from the capillary fractions were combined and tested against the choroid plexus fraction by student's t-test. For the β-secretase activity assay, the data were analyzed by ANOVA using Newman-Keuls comparison of the three groups as the post hoc test.
Appendix-1. Specificity Study of APP Antibody (supplement to Fig. 3).
Acknowledgments
This study was supported in part by NIH/National Institute of Environmental Health Sciences grants ES08164 and ES013118, and Purdue Research Foundation.
Abbreviations
- AD
Alzheimer's disease
- APP
amyloid precursor protein
- Aβ
beta-amyloid
- BBB
blood-brain barrier
- BCB
blood-cerebrospinal fluid barrier
- CSF
cerebrospinal fluid
- ECE
endothelin converting enzyme-1
- IDE
insulin degrading enzyme
- PBS
phosphate buffered saline
- RT-PCR
reverse-transcriptase polymerase chain reaction
- TTR
transthyretin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brownson EA, Abbruscato TJ, Gillespie TJ, Hruby VJ, Davis TP. Effect of peptidases at the blood brain barrier on the permeability of enkephalin. J Pharmacol Exp Ther. 1994;270:675–80. [PubMed] [Google Scholar]
- Crossgrove JS, Li GJ, Zheng W. The choroid plexus removes beta-amyloid from brain cerebrospinal fluid. Exp Biol Med (Maywood) 2005;230:771–6. doi: 10.1177/153537020523001011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport AP, Kuc RE. Cellular expression of isoforms of endothelin-converting enzyme-1 (ECE-1c, ECE-1b and ECE-1a) and endothelin-converting enzyme-2. J Cardiovasc Pharmacol. 2000;36:S12–4. doi: 10.1097/00005344-200036051-00006. [DOI] [PubMed] [Google Scholar]
- Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L, Lin C, Yu J, Zhu H, Ghiso J, Frangione B, Stern A, Schmidt AM, Armstrong DL, Arnold B, Liliensiek B, Nawroth P, Hofman F, Kindy M, Stern D, Zlokovic B. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 2003;9:907–13. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- Eckman EA, Eckman CB. Abeta-degrading enzymes: modulators of Alzheimer's disease pathogenesis and targets for therapeutic intervention. Biochem Soc Trans. 2005;33:1101–5. doi: 10.1042/BST20051101. [DOI] [PubMed] [Google Scholar]
- Ghersi-Egea JF, Leninger-Muller B, Suleman G, Siest G, Minn A. Localization of drug-metabolizing enzyme activities to blood-brain interfaces and circumventricular organs. J Neurochem. 1994;62:1089–96. doi: 10.1046/j.1471-4159.1994.62031089.x. [DOI] [PubMed] [Google Scholar]
- Hebert SS, Serneels L, Dejaegere T, Horre K, Dabrowski M, Baert V, Annaert W, Hartmann D, De Strooper B. Coordinated and widespread expression of gamma-secretase in vivo: evidence for size and molecular heterogeneity. Neurobiol Dis. 2004;17:260–72. doi: 10.1016/j.nbd.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Premkumar DR, Pax AB, Cohen DL, Lieberburg I. Production and increased detection of amyloid beta protein and amyloidogenic fragments in brain microvessels, meningeal vessels and choroid plexus in Alzheimer's disease. Brain Res Mol Brain Res. 1996;35:58–68. doi: 10.1016/0169-328x(95)00180-z. [DOI] [PubMed] [Google Scholar]
- Keep RF, Jones HC. A morphometric study on the development of the lateral ventricle choroid plexus, choroid plexus capillaries and ventricular ependyma in the rat. Brain Res Dev Brain Res. 1990;56:47–53. doi: 10.1016/0165-3806(90)90163-s. [DOI] [PubMed] [Google Scholar]
- Lee G, Dallas S, Hong M, Bendayan R. Drug transporters in the central nervous system: brain barriers and brain parenchyma considerations. Pharmacol Rev. 2001;53:569–96. [PubMed] [Google Scholar]
- Li GJ, Zhao Q, Zheng W. Alteration at translational but not transcriptional level of transferrin receptor expression following manganese exposure at the blood-CSF barrier in vitro. Toxicol Appl Pharmacol. 2005;205:188–200. doi: 10.1016/j.taap.2004.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lignelid H, Collins VP, Jacobsson B. Cystatin C and transthyretin expression in normal and neoplastic tissues of the human brain and pituitary. Acta Neuropathol (Berl) 1997;93:494–500. doi: 10.1007/s004010050644. [DOI] [PubMed] [Google Scholar]
- Lynch JA, George AM, Eisenhauer PB, Conn K, Gao W, Carreras I, Wells JM, McKee A, Ullman MD, Fine RE. Insulin degrading enzyme is localized predominantly at the cell surface of polarized and unpolarized human cerebrovascular endothelial cell cultures. J Neurosci Res. 2006;83:1262–70. doi: 10.1002/jnr.20809. [DOI] [PubMed] [Google Scholar]
- Mackic JB, Bading J, Ghiso J, Walker L, Wisniewski T, Frangione B, Zlokovic BV. Circulating amyloid-beta peptide crosses the blood-brain barrier in aged monkeys and contributes to Alzheimer's disease lesions. Vascul Pharmacol. 2002;38:303–13. doi: 10.1016/s1537-1891(02)00198-2. [DOI] [PubMed] [Google Scholar]
- Matsas R, Kenny AJ, Turner AJ. An immunohistochemical study of endopeptidase-24.11 (“enkephalinase”) in the pig nervous system. Neuroscience. 1986;18:991–1012. doi: 10.1016/0306-4522(86)90113-2. [DOI] [PubMed] [Google Scholar]
- Merched A, Serot JM, Visvikis S, Aguillon D, Faure G, Siest G. Apolipoprotein E, transthyretin and actin in the CSF of Alzheimer's patients: relation with the senile plaques and cytoskeleton biochemistry. FEBS Lett. 1998;425:225–8. doi: 10.1016/s0014-5793(98)00234-8. [DOI] [PubMed] [Google Scholar]
- Monro OR, Mackic JB, Yamada S, Segal MB, Ghiso J, Maurer C, Calero M, Frangione B, Zlokovic BV. Substitution at codon 22 reduces clearance of Alzheimer's amyloid-beta peptide from the cerebrospinal fluid and prevents its transport from the central nervous system into blood. Neurobiol Aging. 2002;23:405–12. doi: 10.1016/s0197-4580(01)00317-7. [DOI] [PubMed] [Google Scholar]
- Morse DC, Stein AP, Thomas PE, Lowndes HE. Distribution and induction of cytochrome P450 1A1 and 1A2 in rat brain. Toxicol Appl Pharmacol. 1998;152:232–9. doi: 10.1006/taap.1998.8477. [DOI] [PubMed] [Google Scholar]
- Philbert MA, Beiswanger CM, Manson MM, Green JA, Novak RF, Primiano T, Reuhl KR, Lowndes HE. Glutathione S-transferases and gamma-glutamyl transpeptidase in the rat nervous systems: a basis for differential susceptibility to neurotoxicants. Neurotoxicology. 1995;16:349–62. [PubMed] [Google Scholar]
- Pluta R, Misicka A, Barcikowska M, Spisacka S, Lipkowski AW, Januszewski S. Possible reverse transport of beta-amyloid peptide across the blood-brain barrier. Acta Neurochir Suppl. 2000;76:73–7. doi: 10.1007/978-3-7091-6346-7_15. [DOI] [PubMed] [Google Scholar]
- Poduslo JF, Curran GL, Sanyal B, Selkoe DJ. Receptor-mediated transport of human amyloid beta-protein 1–40 and 1–42 at the blood-brain barrier. Neurobiol Dis. 1999;6:190–9. doi: 10.1006/nbdi.1999.0238. [DOI] [PubMed] [Google Scholar]
- Price DL, Sisodia SS, Borchelt DR. Genetic neurodegenerative diseases: the human illness and transgenic models. Science. 1998;282:1079–83. doi: 10.1126/science.282.5391.1079. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Iijima M, Yokoo H, Shoji M, Nakazato Y. Human choroid plexus is an uniquely involved area of the brain in amyloidosis: a histochemical, immunohistochemical and ultrastructural study. Brain Res. 1997;755:193–201. doi: 10.1016/s0006-8993(97)00097-8. [DOI] [PubMed] [Google Scholar]
- Schreiber G, Aldred AR, Jaworowski A, Nilsson C, Achen MG, Segal MB. Thyroxine transport from blood to brain via transthyretin synthesis in choroid plexus. Am J Physiol. 1990;258:R338–45. doi: 10.1152/ajpregu.1990.258.2.R338. [DOI] [PubMed] [Google Scholar]
- Schreiber G, Richardson SJ, Prapunpoj P. Structure and expression of the transthyretin gene in the choroid plexus: a model for the study of the mechanism of evolution. Microsc Res Tech. 2001;52:21–30. doi: 10.1002/1097-0029(20010101)52:1<21::AID-JEMT4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Schwarzman AL, Gregori L, Vitek MP, Lyubski S, Strittmatter WJ, Enghilde JJ, Bhasin R, Silverman J, Weisgraber KH, Coyle PK, Zagorski MJ, Talafous J, Eisenberg M, Saunders AM, Roses AD, Goldgaber D. Transthyretin sequesters amyloid beta protein and prevents amyloid formation. Proc Natl Acad Sci U S A. 1994;91:8368–72. doi: 10.1073/pnas.91.18.8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–66. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Serot JM, Bene MC, Faure GC. Choroid plexus, aging of the brain, and Alzheimer's disease. Front Biosci. 2003;8:s515–21. doi: 10.2741/1085. [DOI] [PubMed] [Google Scholar]
- Serot JM, Foliguet B, Bene MC, Faure GC. Choroid plexus and ageing in rats: a morphometric and ultrastructural study. Eur J Neurosci. 2001;14:794–8. doi: 10.1046/j.0953-816x.2001.01693.x. [DOI] [PubMed] [Google Scholar]
- Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, Davis D, Sinha S, Schlossmacher M, Whaley J, Swindlehurst C, et al. Isolation and quantification of soluble Alzheimer's beta-peptide from biological fluids. Nature. 1992;359:325–7. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- Shah GN, Mooradian AD. Age-related changes in the blood-brain barrier. Exp Gerontol. 1997;32:501–19. doi: 10.1016/s0531-5565(96)00158-1. [DOI] [PubMed] [Google Scholar]
- Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA, Strickland DK, Ghiso J, Zlokovic BV. Clearance of Alzheimer's amyloid-ss(1–40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest. 2000;106:1489–99. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluck JM, Lin RC, Katolik LI, Jeng AY, Lehmann JC. Endothelin converting enzyme-1-, endothelin-1-, and endothelin-3-like immunoreactivity in the rat brain. Neuroscience. 1999;91:1483–97. doi: 10.1016/s0306-4522(98)00692-7. [DOI] [PubMed] [Google Scholar]
- Stein TD, Anders NJ, DeCarli C, Chan SL, Mattson MP, Johnson JA. Neutralization of transthyretin reverses the neuroprotective effects of secreted amyloid precursor protein (APP) in APPSW mice resulting in tau phosphorylation and loss of hippocampal neurons: support for the amyloid hypothesis. J Neurosci. 2004;24:7707–17. doi: 10.1523/JNEUROSCI.2211-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strazielle N, Ghersi-Egea JF, Ghiso J, Dehouck MP, Frangione B, Patlak C, Fenstermacher J, Gorevic P. In Vitro Evidence that beta-Amyloid Peptide 1–40 Diffuses Across the Blood-Brain Barrier and Affects Its Permeability. J Neuropathol Exp Neurol. 2000;59:29–38. doi: 10.1093/jnen/59.1.29. [DOI] [PubMed] [Google Scholar]
- Vatter H, Mursch K, Zimmermann M, Zilliken P, Kolenda H, Seifert V, Schilling L. Endothelin-converting enzyme activity in human cerebral circulation. Neurosurgery. 2002;51:445–51. discussion 451–2. [PubMed] [Google Scholar]
- Vigo-Pelfrey C, Lee D, Keim P, Lieberburg I, Schenk DB. Characterization of beta-amyloid peptide from human cerebrospinal fluid. J Neurochem. 1993;61:1965–8. doi: 10.1111/j.1471-4159.1993.tb09841.x. [DOI] [PubMed] [Google Scholar]
- Zheng W. Neurotoxicology of the brain barrier system: new implications. J Toxicol Clin Toxicol. 2001;39:711–9. doi: 10.1081/clt-100108512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Aschner M, Ghersi-Egea JF. Brain barrier systems: a new frontier in metal neurotoxicological research. Toxicol Appl Pharmacol. 2003;192:1–11. doi: 10.1016/s0041-008x(03)00251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Lu YM, Lu GY, Zhao Q, Cheung O, Blaner WS. Transthyretin, thyroxine, and retinol-binding protein in human cerebrospinal fluid: effect of lead exposure. Toxicol Sci. 2001;61:107–14. doi: 10.1093/toxsci/61.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Shen H, Blaner WS, Zhao Q, Ren X, Graziano JH. Chronic lead exposure alters transthyretin concentration in rat cerebrospinal fluid: the role of the choroid plexus. Toxicol Appl Pharmacol. 1996;139:445–50. doi: 10.1006/taap.1996.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Zhao Q. The blood-CSF barrier in culture. Development of a primary culture and transepithelial transport model from choroidal epithelial cells. Methods Mol Biol. 2002;188:99–114. doi: 10.1385/1-59259-185-X:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. Clearing amyloid through the blood-brain barrier. J Neurochem. 2004;89:807–11. doi: 10.1111/j.1471-4159.2004.02385.x. [DOI] [PubMed] [Google Scholar]