Abstract
The enteric nervous system (ENS) forms from migrating neural crest-derived precursors that differentiate into neurons and glia, aggregate into ganglion cell clusters, and extend neuronal processes to form a complex interacting network that controls many aspects of intestinal function. Bone morphogenetic proteins (BMPs) have diverse roles in development and influence the differentiation, proliferation and survival of ENS precursors. We hypothesized that BMP signaling might also be important for the ENS precursor migration, ganglion cell aggregation, and neurite fasciculation necessary to form the enteric nervous system. We now demonstrate that BMP signaling restricts murine ENS precursors to the outer bowel wall during migration. In addition, blocking BMP signaling causes faster colonization of the murine colon, reduces ganglion cell aggregation, and reduces neurite fasciculation. BMP signaling also influences patterns of neurite extension within the developing bowel wall. These effects on ENS precursor migration and neurite fasciculation appear to be mediated at least in part by increased polysialic acid addition to neural cell adhesion molecule (Ncam1) in response to BMP. Removing PSA enzymatically reverses the BMP effects on ENS precursor migration and neurite fasciculation. These studies demonstrate several novel roles for BMP signaling and highlight new functions for sialyltransferases in the developing ENS.
Keywords: bone morphogenetic protein, enteric nervous system, polysialic acid, migration, neurite fasciculation, neural cell adhesion molecule, noggin
Introduction
The enteric nervous system (ENS) is a highly organized collection of ganglia that develops from vagal and sacral neural crest cells through a complex process of migration, proliferation, and differentiation (Gariepy, 2004; Gershon, 1997; Newgreen and Young, 2002a; Newgreen and Young, 2002b; Parisi and Kapur, 2000). Failure of these neural crest cell-derived ENS precursors (NCC) to colonize the distal bowel causes Hirschsprung disease, which occurs in 1:5000 human infants. More subtle problems with ENS development also cause abnormal intestinal motility resulting in intestinal pseudo-obstruction syndromes (Kapur, 2001). When the ENS forms correctly, two distinct layers of enteric neurons are established in the region of the myenteric and the submucosal plexus. Within these regions there are many different enteric neuron subtypes with distinct patterns of axon and dendrite extension, neurotransmitter expression, electrophysiology and function (Furness, 2000). These ENS precursors form small clusters of ganglion cells in both the submucosal and myenteric plexus. Especially within the myenteric plexus, these ganglion cell clusters are connected by fasciculated nerve fiber bundles. While molecular mechanisms controlling some aspects of ENS pattern formation are known, mechanisms influencing ENS precursor migration and neurite fasciculation are incompletely understood. For several reasons, we hypothesized that bone morphogenetic proteins (BMPs) would regulate migration and neurite fasciculation within the developing ENS.
BMPs control many aspects of development including differentiation of neural ectoderm and establishment of the neural crest (Chen et al., 2004; Kishigami and Mishina, 2005). These proteins affect a wide variety of developing cells by binding to their receptors and activating Smad proteins to alter gene transcription. The influence of BMP activity, however, varies depending on both BMP concentration and the specific cell type affected. For this reason, localized BMP activity is required for normal development and BMP antagonists limit BMP actions to a well defined regions of the embryo (Canalis et al., 2003; Warren et al., 2003; Wijgerde et al., 2005).
Several recent studies have demonstrated that BMP signaling influences the developing ENS. First, PCR studies demonstrated that BMP-2, BMP-4, BMPR-IA (BMP receptor subunit), BMPR-IB, and BMPR-II, and the BMP antagonists, noggin, gremlin, chordin, and follistatin are all expressed in both ENS precursors and intestinal non-neural crest-derived cells at embryonic day 12 (E12) in rat when neurons first appear in the colon (Chalazonitis et al., 2004). Furthermore, BMP2 and BMP4 have concentration-dependent effects on enteric neural crest cell survival and differentiation in culture (Chalazonitis et al., 2004; Pisano et al., 2000). In addition, noggin expression from the neuron specific enolase promoter in vivo causes an increase in enteric neuron number, but a reduction in the percentage of cells expressing Trk C (Chalazonitis et al., 2004). These observations may result from BMP induced neuronal differentiation of enteric neural crest stem cells (Bixby et al., 2002) or effects on more differentiated ENS precursors (Chalazonitis et al., 2004; Pisano et al., 2000).
BMP expression in developing chick, mouse, rat and human is strongest in the gut mesenchyme adjacent to the epithelium (Bitgood and McMahon, 1995; Bixby et al., 2002; Brewer et al., 2005; Goldstein et al., 2005; Ramalho-Santos et al., 2000; Roberts et al., 1995) and is induced by epithelial expression of sonic hedgehog (Shh) (Bixby et al., 2002; Roberts et al., 1995; Sukegawa et al., 2000). Both BMP4 and Shh are important for concentric patterning of the bowel wall as well as for ENS development. For example, disrupting BMP4 signaling in the chick or mouse with noggin or blocking Shh signaling with cyclopamine or by gene deletion causes abnormal development of the gut wall and ectopic enteric neurons located close to the gut epithelium (Chalazonitis et al., 2004; De Santa Barbara et al., 2005; Fu et al., 2004; Goldstein et al., 2005; Ramalho-Santos et al., 2000; Sukegawa et al., 2000). In addition, a recent study in chick that parallels our current evaluation demonstrated that noggin treatment of gut explants delays ENS precursor migration into the distal bowel, reduces neural crest derived cell migration from gut explants toward sources of GDNF, and reduces the size of ganglia within the myenteric plexus (Goldstein et al., 2005).
These studies demonstrate that BMP signaling critically influences ENS precursor development in vitro and in vivo. The current studies demonstrate several novel effects of BMP signaling on the developing mouse ENS. We specifically demonstrate that BMPs reduce the rate of ENS precursor migration into the mouse colon, encourage ganglion cell aggregation, enhance neurite fasciculation, control radial migration within the bowel wall and influence the orientation of nerve fibers within the developing myenteric plexus. Remarkably, we found some similarities to the effects recently described using noggin to block BMP signaling in the chick gut (Goldstein et al., 2005), but also some striking differences. Most surprising is the observation that noggin increases migration of neural crest derived precursors into the distal bowel in the mouse, but prevents migration of neural crest derived cells into the distal bowel in the chick.
To understand the molecular mechanisms through which BMP may alter ENS precursor migration and neurite fasciculation, we pursued the hypothesis that these BMP effects might be mediated by polysialic acid (PSA) addition to neural cell adhesion molecule (Ncam1). PSA is an unusual linear homopolymer of alpha-2, 8-linked neuraminic acid that is added to the fifth immunoglobulin domain of Ncam1 (Cunningham et al., 1983; Finne et al., 1983) and influences both homophilic and heterophilic cell-cell interaction (Acheson et al., 1991; Durbec and Cremer, 2001; Nguyen et al., 2003). In vertebrates, Ncam1 is the only confirmed PSA modified protein (Acheson et al., 1991; Kiss and Rougon, 1997; Nelson et al., 1995) and this modification has been previously hypothesized to be important for ENS ganglion cell aggregation in response to BMP (Chalazonitis et al., 2004; Faure et al., 2003). To test the hypothesis that PSA addition to Ncam1 also influences ENS precursor migration and neurite fasciculation, we treated developing gut explants with endo-N-acylneuraminidase (endo-N), an enzyme that specifically removes PSA from Ncam1. These studies confirm that PSA levels increase in neurites after BMP treatment and that PSA removal increases ENS precursor migration and decreases neurite fasciculation. Together these observations demonstrate that BMP signaling influences neural crest precursor migration, proliferation, and neuronal fiber extension suggesting that mutations in BMPs, BMP antagonists, BMP receptors or adhesion molecules like PSA-Ncam1 may influence Hirschsprung’s disease penetrance.
Materials and methods
Mice
All studies used CF-1 mice from Charles River. Use and care of animals was approved by the Washington University animal care committee.
Dissociated gut culture
E12.5 CF-1 mouse stomach, small bowel and colon were dissociated with collagenase (0.2 mg/mL) and dispase (0.2 mg/mL) to yield a single cell suspension. Cells were filtered two times through a 40 μm cell strainer (BD Falcon) followed by plating at 8000 cells/well on fibronectin (250 μg/ml, Invitrogen) coated 4-well Permanox Sonic Seal chamber slides (NUNC). Cells were grown in DMEM plus 10% chick embryo extract for 48 hours as previously described (Fu et al., 2004) with BMP4 (60 ng/mL, R & D Systems), noggin (200 ng/mL, R & D Systems) or no added factor. After fixation (4% paraformaldehyde (PFA), 30 minutes, 25°C), immunocytochemistry was performed with antibodies to neuron specific class III β-tubulin (TuJ1) or polysialic acid (PSA) (# 735, a generous gift from Dr. Rita Gerardy-Schahn, (Frosch et al., 1985; Hayrinen et al., 1995)). Pixel intensity measurements for PSA immunohistochemistry were made on neurites with less than 10 μm diameter (n = 30 neurites for each condition) using Photoshop 8.0. Background pixel intensity was subtracted from the measured values. Digital images for this analysis were obtained using an Olympus BX60 microscope and AxioCam (Zeiss) camera with AxioVision 3.1 software. All images were obtained using identical exposure times.
Whole gut explant cultures
Gut cultures from E11.5 mouse embryos were prepared as described (Fu et al., 2004). Briefly, the entire gastrointestinal tract was dissected from the embryo, transferred to a 4 well-dish with a 2.5% agarose gel bed and pinned to the gel with 4-0 stainless steel filaments (Ethicon). Explants were cultured in 500 μL of organ culture medium (DMEM, 10% fetal calf serum (FCS), 1% penicillin/streptomycin) containing BMP2 (60 ng/mL, R&D Systems, Minneapolis, MN), BMP4 (60 ng/mL, R&D Systems), noggin (200 ng/mL, R&D Systems), anti-BMP4 blocking antibody (3 μg/mL, R&D Systems), or no added factor. The cultures were maintained for 48 hours at 37 °C in a 5% CO2 incubator before fixation in 4% PFA (30 minutes, 25°C) and processing for whole-mount immunohistochemistry. The extent of migration into the distal bowel was measured for 12 explants after treatment with BMP2 or BMP4, and for 20 explants grown in control medium, with noggin or with anti-BMP4 blocking antibody. Orientation of neurites within the bowel was determined by measuring the angle between the neurite and the long axis of the bowel at approximately 300 μm proximal to the leading edge of the wave front of migrating cells. Angle measurements were performed for 10 fibers/explant and 6 explants for each culture condition.
Modified suspension culture
Gut explants from E11.5 mouse embryos containing the stomach and small bowel were cut in the mid-small bowel as described (Fu et al., 2004) and the distal end was placed onto a filter membrane (0.45 μm; black gridded HABG; Millipore). The proximal end was secured to an agarose gel bed with a 4-0 stainless steel line. Explants were maintained in culture with BMP2 (60 ng/mL), BMP4 (60 ng/mL), noggin (200 ng/mL), or anti-BMP4 blocking antibody (3 μg/mL) with or without added glial cell line-derived neurotrophic factor (GDNF, 100 ng/mL, PeproTech Inc) in organ culture medium. After 48 hours, cultured explants together with the filter membrane were removed from the gel bed and fixed with 4% PFA (30 minutes, 25°C) before immunohistochemistry.
Gut slice cultures
E11.5 midgut (300–400 μm long) from a region 500 μm proximal to the cecum were placed onto filter membranes (0.45 μm; black gridded HABG; Millipore) and then covered one minute later with 500 μl of organ culture medium. BMP2 (60 ng/mL), BMP4 (60 ng/mL), noggin (200 ng/mL) or anti-BMP4 blocking antibody (3 μg/mL) were applied to the medium immediately after the culture was established and explants were maintained in culture for 48 hours before fixation with 4% PFA (30 minutes, 25 °C).
Collagen gel culture
Collagen gel was prepared by dissolving 4 mg collagen (Roche) in 1 mL 0.2% acetic acid. This solution was placed on ice before adding 0.8 M NaHCO3 (40 μL), 200 mM NaOH to bring pH to approximately 7.8 (about 30 μL), and 4 mL of DMEM/10% FCS. Final solution contained collagen (750 μg/mL), FCS (7.5%), NaHCO3 (6 mM) and DMEM (1x). For some experiments, GDNF was added directly to the gel (100 ng/mL). For these studies, 300–400 μm long midgut explants from E11.5 mouse embryo were placed into 100 μL of collagen gel and cultures were maintained in a 5% CO2, 37°C incubator for 48 hours before fixation with 4% PFA (60 minutes, 25 °C). For co-culture experiments, noggin producing or control CHO cells (generously provided by Dr. Richard Harland; University of California) were placed within the gel at a distance of 400 μm from the gut segment on the anti-mesenteric border of the gut. To create small clumps of CHO or CHO-Noggin cells for these studies, cells were cultured in round bottom cell culture plates for 2 hours before transfer to the collagen gel.
Quantitative real time reverse transcriptase polymerase chain reaction (qRT-PCR)
The following primers were designed to generate short amplicons (50–100 bp, Tm about 60 °C), synthesized by Integrated DNA Technologies Inc (IDT, Coralville, IA), and used to perform qRT-PCR: Ncam1: forward primer gtctgtcaccctggtgtgtg and reverse primer atcctttgtccagctcatgg, Cdh2 primers: forward primer agtttctgcaccaggtttgg and reverse primer catacgtcccaggctttgat and L1cam; forward primer caccctgaggcattacacct and reverse primer tgccagtgcagtagcagact. qRT-PCR was performed in triplicate for each cDNA with SYBR green PCR Master mix (Applied Biosystems, Foster City, CA) and the iCycler iQ (Bio-Rad, Hercules, CA). Control reactions were performed omitting reverse transcriptase from the cDNA synthesis. For each gene, qRT-PCR was performed with RNA from E11.5 gut explants that had been maintained in control medium for 48 hours with or without BMP2, BMP4 and noggin as described above (n = 3 under each condition). The RNA content of samples was normalized based on GAPDH (glyceraldehyde-3-phosphate dehydrogenase) amplification. The threshold cycle (CT value) at which a significant increase in PCR product is first detected, was recorded. ΔCT = CT of gene of interest minus CT of GAPDH.
Endo-N treatment for neurite fasciculation studies
300–500μm thick E12.5 mouse mid-gut slices from the region 100 to 250 μm distal to the pylorus were cultured on fibronectin (250 μg/mL) coated dishes in organ culture medium as described (Fu et al., 2004). The slices were maintained with or without endo-N (200 ng/mL, a generous gift from Dr. Rita Gerardy-Schahn (Gerardy-Schahn et al., 1995) ) at 37°C for 2 hours before adding BMP4 (60ng/mL), noggin (200ng/mL), or no added factor. Cultures were then maintained for 48 hours (37°C, 5 % CO2) before fixation (4 % PFA, 1 hour, 25°C) and immunohistochemical analysis (see below) with TuJ1 and anti-PSA antibody. Neurite fiber bundle thickness was measured 100 μm from the explant’s edge. Fiber bundles were classified into three groups based on diameter (small (less than 5 μm), medium (5–10 μm), and large (more than 10 μm)). 10 explants were analyzed for each culture condition.
Immunohistochemistry
After fixation tissues or slides were washed three times in TBST (100 mM Tris, 150 mM NaCl, 0.2% Triton X-100), and then blocked with 4% donkey serum in TBST (1 hour, 25°C) before incubation with primary antibody (4°C, overnight) in TBST. Primary antibodies (Tuj1 (rabbit, Covance, 1:100), anti-Ret (Goat; Neuromics, 1:100), anti-smooth muscle actin (SMA) (Mouse; Dako, 1: 200), anti-PSA (mouse, # 735, 1:100) were visualized using donkey anti-goat Alexa 594; (1:200, Molecular Probes) and donkey anti-rabbit Alexa 488 (1:100, Molecular Probes) secondary antibodies after washing 3 times with TBST. Hoechst 33342 (1 μg/mL, Molecular Probes Inc., Eugene, OR) was used for nuclear staining.
In situ hybridization
Wild type CF-1 mouse gut was fixed with cold 4 % PFA overnight at 4°C and then frozen in OCT before sectioning at 14 μm thickness. Slides were warmed to 25°C, baked 15 minutes at 50°C and then fixed again in 4% PFA for 20 min at 25°C. After washing twice in diethylpyrocarbonate treated phosphate buffered saline (PBS-DEPC, 10mM) for 5 minutes, tissues were digested in Proteinase K (25 μg/mL) for 14–19 minutes in (50 mM Tris pH 7.5, 5 mM EDTA, DEPC treated water). Slides were then washed again in PBS-DEPC (2 × 5 minutes), incubated in 4 % PFA for (15 minutes, 25°C), and rinsed in DEPC treated water. Tissues were then blocked with 0.2 % acetic anhydride/0.1 M triethanolamine (10 minutes, 25°C), washed in PBS-DEPC (5 minutes, 25°C), and pre-hybridized for 3 hr at 65°C in pre-hybridization solution (50% formamide, 5x SSC, 1 mg/ml Yeast tRNA, 100 μg/ml Heparin, 1x Denhardt’s Solution, 0.1% Tween 20 (Sigma P-1379), 0.1% CHAPS (Sigma C-3023), 5mM EDTA pH 8.0). Riboprobes (2 ng/mL final concentration) were then added to fresh pre-hybridization solution, slides were covered with coverslips and tissues were hybridized overnight at 65°C in humidified chamber. Following hybridization, tissues were washed in 1x SSC/50% formamide at 65°C (3 × 30minutes), then twice in PBT (10 mM PBS with 0.1% Triton X-100 and 2 mg/mL BSA) for 20 min at 25°C, and then blocked with PBT/20% NSS (normal sheep serum) for 1hr at 25°C. Hybridized probe was detected after incubation (overnight, 4°C) with an anti-digoxigenin antibody conjugated to alkaline phosphatase (Roche, 1:2000) in fresh blocking solution (PBT/20% NSS). Slides were then washed in PBT (3 × 30 min, 25°C) followed by washing once in alkaline phosphatase (AP) buffer (100 mM Tris pH 9.5, 50 mM MgCl2, 100 mM NaCl, 0.1% TWEEN 20) with levamisole (5 mM, DakoCytomation) for 5 min and once in AP buffer without levamisole. Finally slides were incubated in AP buffer with 3.5 μL/mL BCIP (0.35% final concentration) and 1.5 μL/mL NBT (0.15% final concentration) for 1–3 days in dark at 4°C, or until desired stain was attained.
Protein immunoblot for PSA and Ncam1
Ten E11.5 CF-1 mouse small bowel, stomach and colon pieces were maintained in organ culture medium with or without endo-N (200 ng/mL, 2 hours, 37°C). E11.5 brain was used as a positive control. Explants and brain tissue were lysed in 200 μl of (100 mM Tris, 0.5% Triton X-100, pH 7.6) plus protease inhibitor cocktail (Complete Mini, Roche, Penzberg, Germany) and centrifuged (15,000 x g, 10 minutes) before analyzing the supernatant. 60 μg of protein/sample was separated by electrophoresis before transfer to Hybond nitrocellulose membranes (Amersham Buckinghamshire, UK). Equal loading was confirmed by Ponceau S staining. Membranes were blocked by 5 % nonfat dry milk in (100 mM Tris-HCl, 150 mM NaCl, 0.1% Tween-20, pH 7.8) for 1 hour before incubation with anti-PSA (735) antibody (1:1000, 4 °C overnight), anti-Ncam1 antibody (1:1000, 4°C overnight, Sigma, St. Louis, MO) or anti-GAPDH antibody (1:10,000, 4 °C overnight, RDI, Flanders, NJ). HRP-conjugated secondary antibody (2 hours, 25 °C), enhanced chemiluminescence reagent, and Hyperfilm (Amersham) were used to visualized immunoreactive protein.
Statistical analysis
Data were analyzed by t-test. Data are presented as the mean +/− standard error of the mean (SEM).
Results
Noggin and BMP4 are expressed in concentric rings in the bowel wall
Noggin, BMP2, BMP4 and BMP receptor mRNA signals have been previously identified in the developing rat bowel by RT-PCR (Bixby et al., 2002; Chalazonitis et al., 2004) and BMP4 has been localized in the chick (Bixby et al., 2002; Goldstein et al., 2005; Sukegawa et al., 2000) and mouse (Bitgood and McMahon, 1995) bowel by in situ hybridization. To more completely define the expression of BMP4 and noggin relative to the NCC that form the ENS, we performed in situ hybridization on mouse small bowel at E10.5 shortly after NCC first enter the small bowel as well as at E11.5 and E14.5 (Figure 1). These studies confirm and extend previous observations demonstrating that BMP4 is produced in mesenchymal cells adjacent to the intestinal epithelium (Figure 1A–C). Interestingly, noggin is produced by a thin ring of cells that surrounds the BMP producing cells at each age (figure 1D–F). To better define the relationship of noggin and BMP4 expressing cells to the developing ENS, we performed immunohistochemical staining at E14.5 (Figure 1G–J) on a section adjacent to the in situ hybridization (Figures 1C, 1F) using antibodies to Ret (a marker for ENS precursors and enteric neurons, Figure 1G), neuron specific class III β-tubulin (identified with TuJ1 antibody, Figure 1H) and smooth muscle actin (SMA, Figure 1I). A schematic prepared by overlaying images 1C, 1F, 1G, and 1I demonstrates that noggin expression overlaps with a small subset of SMA+ cells (Figure 1K). Furthermore, noggin producing cells are directly adjacent to the Ret expressing NCC and clearly surround the BMP4 producing cells. This expression pattern suggests that BMP and noggin might not only influence neural crest differentiation, but could also influence cell migration and plexus formation within the ENS.
Figure 1. Noggin and BMP4 are expressed in the developing mouse gut.
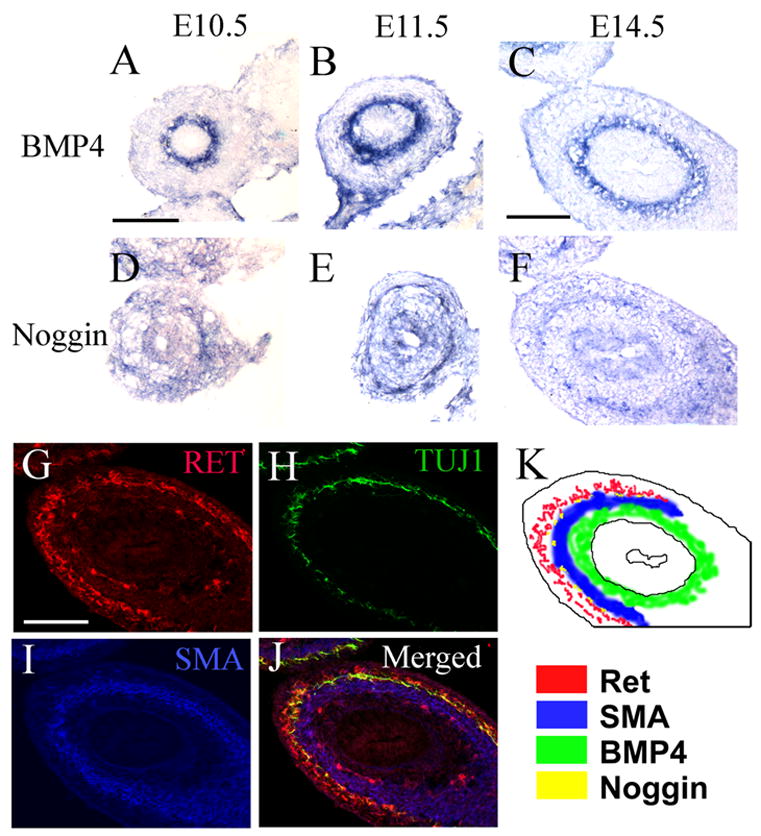
(A–F) In situ hybridization of E10.5, E11.5 and E14.5 mouse midgut for BMP4 (A–C) and noggin (D–F). (G–J) Immunohistochemistry for (G) Ret, (H) TuJ1 and (I) smooth muscle actin (SMA) was performed on a section adjacent to images (C and F). (J) Shows merged immunohistochemistry for Ret, TuJ1, and SMA. (K) The localization of Ret, SMA, BMP4 and noggin expression was recorded based on the location of in situ hybridization and immunohistochemical signals in Figures C, F, G, and I. Scale bar = 200 μm.
BMP4 reduces neural crest precursor migration from gut explants in response to GDNF
BMP effects on NCC migration were first evaluated by examining Ret expressing cells that migrate from the distal end of E11.5 mouse mid-gut explants cultured for 48 hours with the cut end attached to a filter membrane (see modified suspension culture schematic in Figure 2G). Explants were maintained with GDNF (Figure 2A, 2A′, 2A″), GDNF plus noggin (Figure 2B, 2B′, 2B″), GDNF plus anti-BMP4 blocking antibody (Figure 2C, C′, C″), GDNF plus BMP2 (Figure 2D, 2D′, 2D″), or GDNF plus BMP4 (Figure 2E, 2E′, 2E″). NCC that migrated out of the explant and neuronal fibers were visualized by immunohistochemistry with Ret and TuJ1 antibodies respectively. Nuclear staining with Hoechst 33342 confirmed the location of Ret+ cell bodies that had migrated from the explant (Figure 2I–P). Both BMP2 and BMP4 reduced the distance traveled by Ret+ cells from the edge of the explant (Figure 2 F; P < 0.001, n = 12). In contrast, treatment of explants with noggin or anti-BMP4 blocking antibody increased the distance traveled by these cells (Figure 2F; P < 0.001, n = 12). Control experiments demonstrated almost no migration of NCC from the explant if GDNF was omitted from the culture medium (Supplemental Figure 1) suggesting that BMP signaling influences GDNF induced NCC migration.
Figure 2. BMP and Noggin influence neural crest-derived migration and neurite patterning in E11.5 mouse midgut.
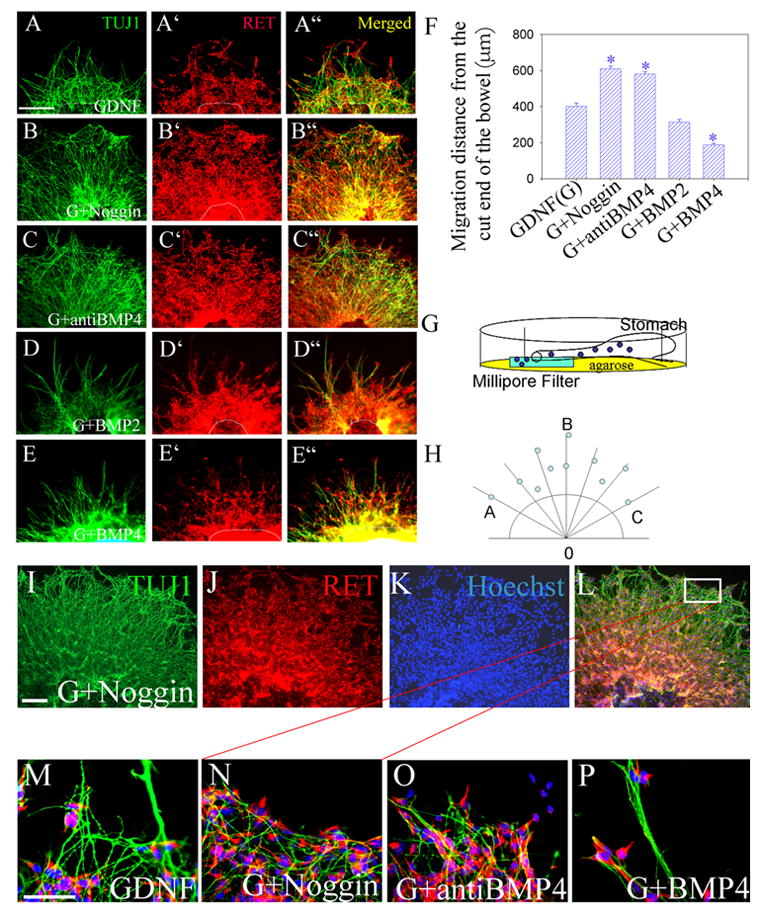
Gut explants were grown in culture with the cut end of the midgut placed onto filter paper to allow outgrowth (see diagram G). Migration and neurite extension from the midgut was examined after 48 hours in culture with GDNF (A, A′, A″), GDNF plus noggin (B, B′, B″), GDNF plus anti-BMP4 blocking antibody (C, C′, C″), GDNF plus BMP2 (D, D′, D″), or GDNF plus BMP4 (E, E′, E″). Immunohistochemistry was performed for TuJ1 (A–E) or Ret (A′–E′). Merged images (A″ – E″). (H) The schematic shows how the extent of migration from the edge of the explant was measured. Seven radiating lines were drawn in standard positions extending from the edge of the explant. The position of the most distant Ret+ cell along these lines was recorded. (F) Mean distance that Ret+ cells migrated from the edge of the explant confirms increased migration with GDNF plus noggin or GDNF plus anti-BMP4 blocking antibody, and reduced migration with GDNF plus BMP4 (n = 12 explants) from 6 separate experiments. Scale bar = 100 μm. (I–L) To confirm the location of Ret+ cells that migrated from the explants, cell nuclei were visualized using Hoechst 33342 dye. Low magnification images of an explant grown in GDNF plus noggin are shown after immunohistochemistry for TuJ1 (I), Ret (J) and Hoechst 33342 (K) with an additional merged image (L). The white box in (L) marks the region shown in (N). (M–P) Show higher magnification images of cells and neurites at the edge of the outgrowth from explants after growth in GDNF (M), GDNF plus noggin (N), GDNF plus BMP4 blocking antibody (O) or GDNF plus BMP4 (P). These images demonstrate cell bodies at the tips of the furthest neurites and highlight BMP4 induced neurite fasciculation (P). * P < 0.001. Scale bar (A–E) = 100 μm. Scale bar (I–L) = 50 μm. Scale bar (M–P) = 25 μm.
Noggin promotes migration of ENS precursors into collagen gel in the presence of GDNF
Because our murine results seemed to contradict recent studies of the effects of noggin on NCC migration from chick gut explants (Goldstein et al., 2005), we decided to replicate their migration experiments using a modified collagen gel assay system with embryonic murine gut. For these studies, E11.5 mid small bowel segments were cultured in collagen gel in the presence or absence of GDNF. Noggin secreting or control CHO cells were also placed in the collagen gel near the explants. When GDNF was included in the collagen gel, there was extensive growth of neuronal fibers and migration of NCC out of the gut into collagen gel (Figure 3 A, B). This was confirmed by TUJ1 immunohistochemistry (Supplemental Figure 2). After 48 hours the distance that NCC migrated either toward or away from the CHO cells was determined (Figure 3 D). These analyses demonstrated a consistent statistically significant increase in the distance that cells migrated toward the noggin secreting CHO cells (proximal to distal ratio: 1.5 +/− 0.26, P = 0.013, n = 6). In contrast, with control CHO cells, NCC migrated symmetrically from the explant into collagen gel (Figure 3D, P = 0.39, n = 6). These data suggest that noggin either increases the rate of GDNF-induced NCC migration or that noggin acts as a chemoattractant for ENS precursors. To determine whether noggin alone was adequate to support NCC migration and neurite extension, GDNF was omitted from the collagen gel. In the absence of GDNF, very few cells migrated from the explant and few fibers were extended into the collagen gel (Figure 3C, 3D, n = 6). Thus, in contrast to the studies in chick, noggin appears to enhance GDNF induced NCC migration, but does not adequately support NCC migration or neurite extension in the absence of GDNF.
Figure 3. Neural crest cells migrate faster toward noggin producing cells.
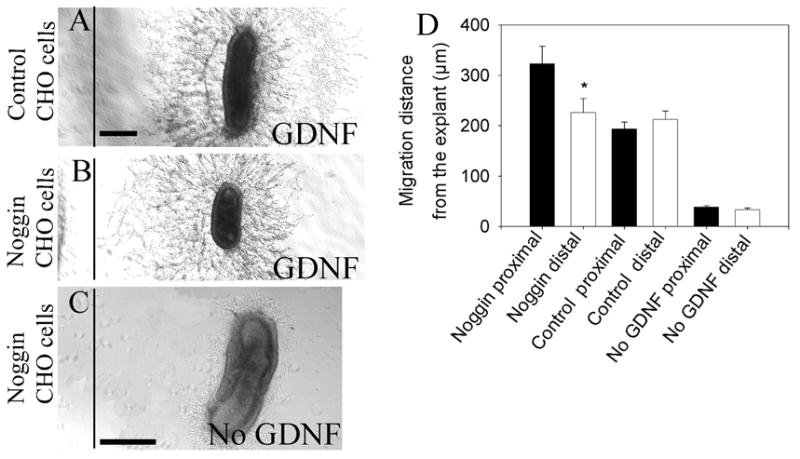
300–400 μm of E11.5 mouse midgut was cultured in collagen gel with (A, B) or without (C) GDNF (200 ng/mL). (A) Control or (B) noggin secreting CHO cells were placed 400 μm away from the anti-mesenteric border of the explant edge and cultures were maintained for 48 hours. (D) The distance that neural crest derived cells migrated toward (proximal) or away (distal) from the CHO cells was measured. With control CHO cells, proximal and distal migration distances were equal. In contrast, cells migrated a greater distance on the side of the gut with noggin producing CHO cells. (C) Migration out of the gut in response to noggin alone (i.e., without GDNF) was minimal. Scale bar = 100 μm
Noggin and BMP signaling influence ENS precursor migration into the colon in vitro
To test more directly whether modifying BMP signaling influenced NCC migration into the colon, the entire E11.5 mouse bowel from the stomach to the end of the colon was maintained in organ culture for 48 hours. At the start of the culture the NCC migration wave front had reached the ileo-cecal junction. Explants treated with BMP2, BMP4, noggin, anti-BMP4 blocking antibody or no added factors were processed for Ret and TuJ1 immunohistochemistry. Hindgut length and the length of the hindgut containing NCC were determined (see landmarks in Figure 4F). Similar results were obtained with either Ret or TuJ1 immunohistochemistry and hindgut length was similar under all conditions tested (Supplemental Figure 3). Under control conditions, NCC colonized the rostral three quarters of the hindgut (74 +/− 1.2 %, n = 20, Figure 4A). BMP2 and BMP4 treatment significantly reduced hindgut colonization by NCC (BMP4: 51 +/− 2.9 %, BMP2: 49 +/− 5 %, Figure 4D, 4E; P<0.001, n = 12). In contrast, noggin treatment increased the colon colonization by NCC (84.3 +/− 1.5 % colon colonization, n = 20, Figure 4B, P<0.001). Similar results were obtained using BMP4 blocking antibody (80 +/− 1.5 %, n = 20, Figure 4C, P = 0.006). Overall these data demonstrate that in contrast to the developing chick ENS (Goldstein et al., 2005), noggin enhances NCC migration and increases colonization of the distal colon in the mouse while BMP signaling reduces NCC migration in several different assay systems.
Figure 4. Noggin enhances neural crest migration into the hindgut.
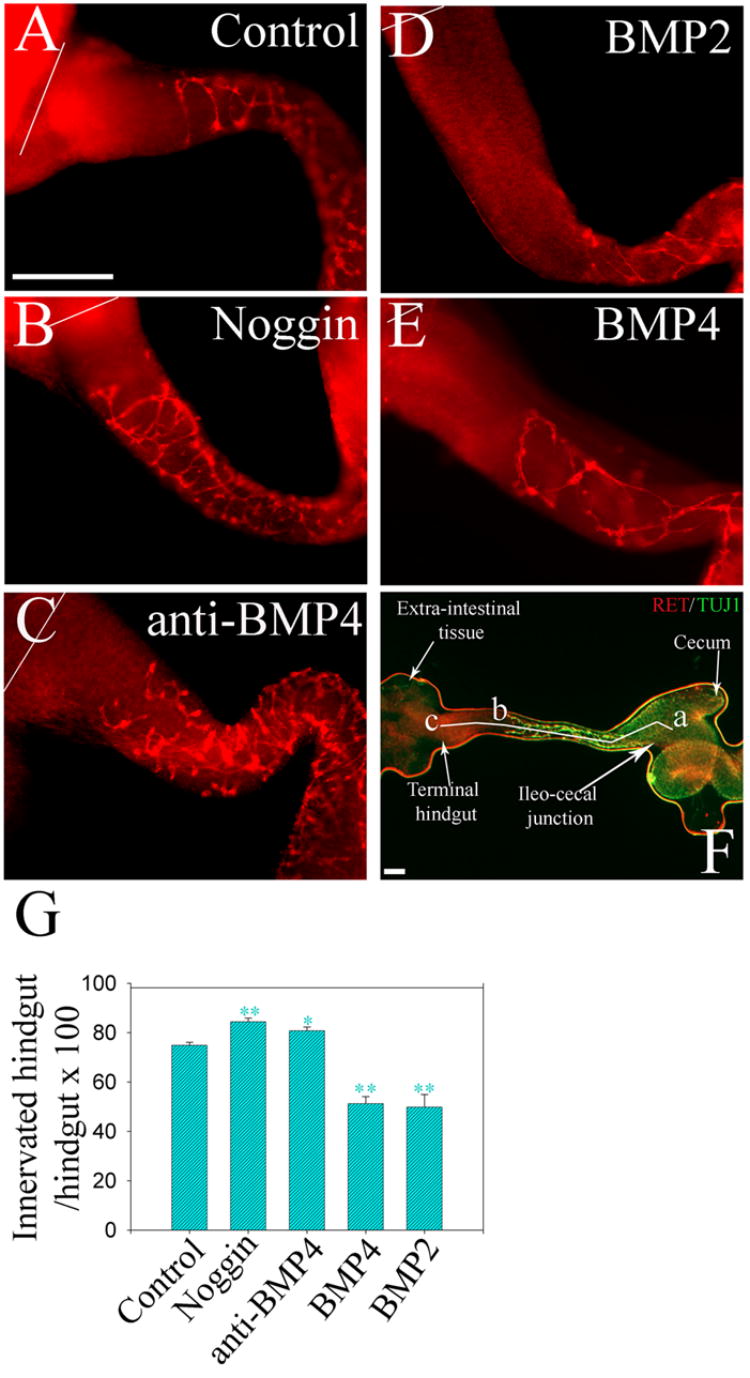
E11.5 mouse gut explants including the esophagus, stomach, small bowel and colon were cultured for 48 hours (A) under control conditions, or (B) with noggin, (C) anti-BMP4 blocking antibody, (D) BMP2, or (E) BMP4. Whole mount immunohistochemistry for Ret demonstrated NCC within the bowel. White lines indicate the end of the colon. (F) Shows landmarks for measurements: segment “a–b” = the region of colon colonized by NCC; segment “a–c” = the whole length of the hindgut. “a” is the ileo-cecal junction; “b” is the position of the most distal Ret expressing cell at the migration wave front. “c” is the terminal hindgut. Also seen is the extra-intestinal tissue we used to secure the explants to the gel bed. (G) Quantitative analysis demonstrated that noggin or anti-BMP4 blocking antibody treatment increased the percentage of the colon innervated, while BMP treatment reduced the extent of colon innervation. Scale bar = 100 μm. * P<0.001; **:P<0.01.
BMP and Noggin influence neurite fasciculation and neuronal fiber orientation in the developing ENS
BMPs have been previously reported to influence rat enteric ganglion cell aggregation in culture (Chalazonitis et al., 2004) and neurons/ganglion in the developing chick (Goldstein et al., 2005). During our studies of murine NCC migration, we also observed striking effects of noggin and BMP signaling on neurite fasciculation and fiber orientation both within the bowel wall and growing from the edge of gut explants. For example, neurites extending onto Millipore filter membrane from E 11.5 mid-gut slices were markedly fasciculated when BMP4 was included in the culture medium and typically extend straight out from the edge of the explant (Figures 5D). Similar neurite fasciculation was observed in response to BMP treatment using the modified suspension culture (Figure 2D, 2P). In contrast, neurites extending from similar explants grown in noggin or BMP4 blocking antibody were minimally fasciculated and generally extended a short distance before turning back toward the explant (Figures 2N, 2O, 5B, 5C). These data suggested that BMP or noggin might influence neurite pathfinding in response to factors produced by the gut.
Figure 5. Neuronal fibers growing out of gut explants are highly fasciculated after BMP treatment.
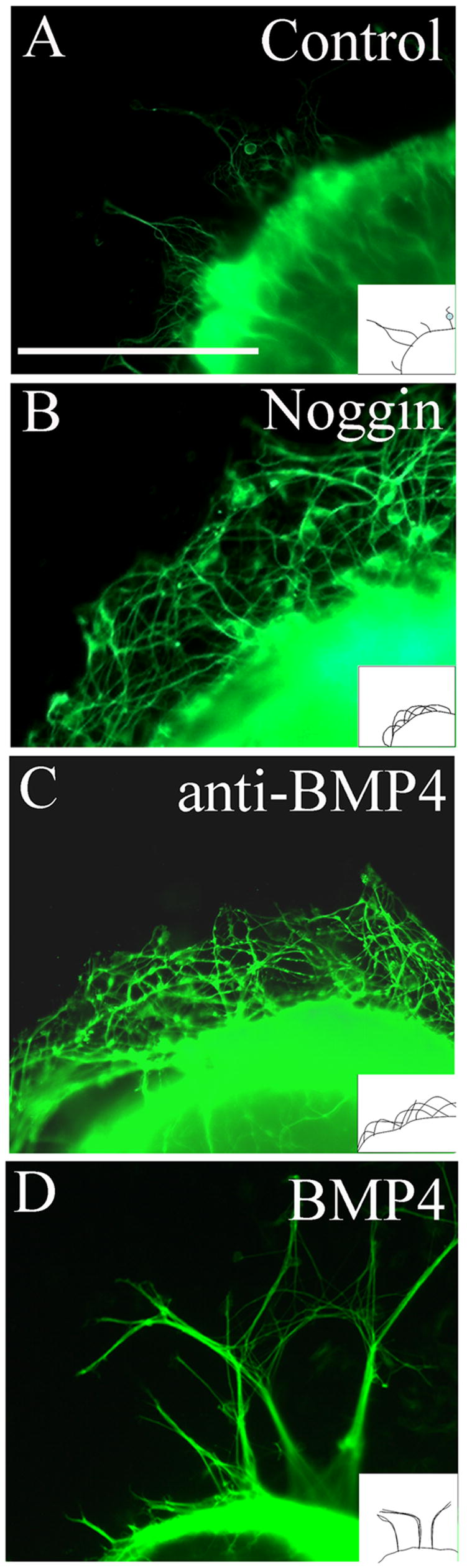
300–400 μm long E11.5 mouse midgut slices were cultured for 48 hours on Millipore filters covered with culture medium containing (A) organ culture medium alone (DMEM plus 10% FCS) or with added (B) noggin, (C) anti-BMP4 blocking antibody or (D) BMP4. Immunohistochemistry for TuJ1 revealed neuronal fibers extending from the gut explant onto filter paper. BMP treatment resulted in many fasciculated fibers. Control culture medium resulted in only a few neuronal fibers extending from the explant. Noggin or anti-BMP4 blocking antibody treatment resulted in many fibers extended from the explant. These fibers were not fasciculated and frequently curved back toward the explant. Diagrams show a schematic of the observed fiber patterns. Scale bar = 100 μm.
Corresponding to the alterations in neurite orientation observed in slice cultures, there was a significant effect on the orientation of neuronal fibers within the bowel wall after BMP treatment of whole gut explants in culture. Specifically, cultures maintained in BMP containing medium typically had fibers that extended parallel to the long axis of the bowel (Figure 6C, 6D) while fibers in the bowel wall of explants grown in control media or in the presence of noggin typically were more perpendicular to the long axis of the bowel (Figure 6A, 6B). These observations were substantiated by measuring the angle between neuronal fibers and a line parallel to the long axis of the bowel within 300 μm of the wave front of migrating NCC (Figure 6N, 6O). The angles measured were similar in control and noggin treated gut explants (Control 64° +/− 7, Noggin 71° +/− 5, P = 0.3), but clearly different after BMP treatment (BMP2 38° +/− 4, P = 0.0015 versus control; BMP4 25° +/− 4, P < 0.0001 versus control, n = 6 explants grown under each condition).
Figure 6. BMP signaling alters the patterns of neurite extension, NCC migration and NCC clustering within the bowel wall.
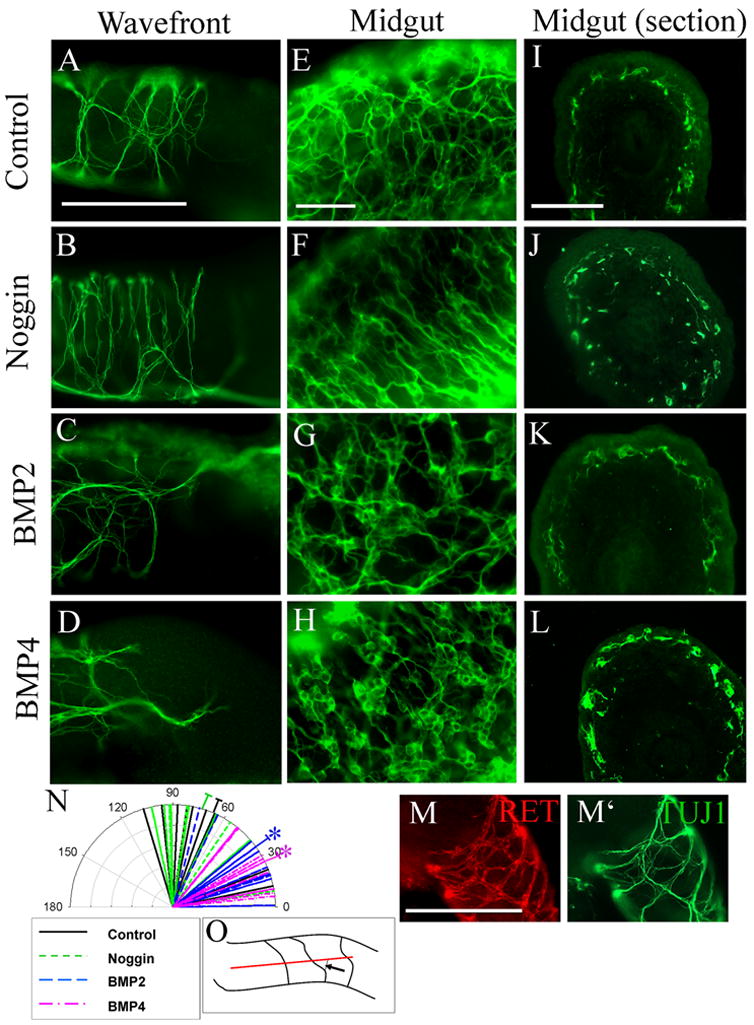
(A–D) TuJ1 immunohistochemistry demonstrated neuronal fiber patterns in E11.5 whole mouse gut explants maintained in culture for 48 hours (A) under control conditions or in the presence of (B) noggin, (C) BMP2, (D) or BMP4. (N) Quantitative analysis of the angle between neuronal fibers and the long axis of the bowel demonstrated a striking difference in fiber patterns in control or noggin treated gut explants and BMP treated explants. Longer lines represent the mean angles measured under each condition. Shorter lines represent individual angle measurements. N = 6 explants under each condition from a total of 4 separate experiments; *P = 0.001 for BMP2 or BMP4 versus control or noggin. (O) Schematic of how the angles were measured. The black arrow indicates the angle measured. (E–H) Treatment of gut explants with BMP2 or BMP4 increased ganglion cell clustering (G, H) compared to control (E). Noggin reduced ganglion cell aggregation (F). (I–L) Cross sections demonstrate that noggin treatment also induced ectopic NCC near the intestinal mucosa (J), but this did not occur under control conditions (I) or after BMP treatment (K, L). RET immunoreactive cells (M) were closely adherent to TUJ1 immunoreactive nerve fibers (M′) at the hindgut migration wavefront under all conditions. This explant was grown in culture under control conditions. Scale bar = 100 μm.
BMP signaling influences ganglion cell aggregation and restricts NCC migration to a region in the outer bowel wall
Similar to observed BMP-induced ganglion cell aggregation in dispersed cell culture (Chalazonitis et al., 2004), we observed marked aggregation of ganglion cells within the wall of the developing murine bowel after 48 hours in media with BMP2 or BMP4 (Figure 6G, 6H). In contrast, while ganglion cells were easily visible in the gut segments grown in control (Figure 6E) or noggin containing media (Figure 6F), large cell clusters were more difficult to find. In addition, when the gut was cultured in media containing noggin, ganglion cells appeared to be in more than one layer of the bowel wall whereas gut segments maintained in control or BMP containing medium had a defined layer of NCC in the outer bowel wall. To confirm this impression, sections through the midgut were obtained after 2 days in culture with noggin, BMP2, BMP4, or control media (Figure 6I–6L). Immunohistochemistry for Ret confirmed that NCC were typically in a very restricted region of the bowel wall in gut explants maintained in BMP2 (n = 12/14), BMP4 (n = 12/14) or control (n = 10/10) media (Figure 6I, 6K, 6L). In contrast, NCC in noggin treated explants (n = 10/14) were found throughout the bowel wall including in the region near the mucosa (Figure 6J). Thus, BMP signaling encourages the clustering of NCC into ganglia and restricts radial migration of NCC within the bowel wall.
BMP4 enhances the expression of PSA on NCCs and their neuronal fibers
BMP signaling clearly affects NCC aggregation and migration, neurite fasciculation, and patterns of neurite extension within the bowel wall. All of these effects suggest that BMP signaling modulates the adhesion of ENS precursors to each other and their environment. To more precisely define the molecular mechanisms by which BMPs influence ENS morphogenesis, we first used qRT-PCR to determine whether BMP or noggin treatment influenced expression of adhesion molecules known to be present within the developing ENS at E11.5. Analysis of mRNA levels for Ncam1, L1cam, and Cdh2 (N-cadherin), however, demonstrated no significant difference in gene expression for these molecules under the conditions tested (Table 1).
Table 1. Quantitative measurement of mRNA levels for Ncam1, L1cam, and Cdh2.
Explants were maintained in culture for 48 hours under control conditions or with noggin or BMP4. Levels of mRNA for Ncam1, L1cam and Cdh2 were determined by quantitative real time reverse transcriptase-PCR compared to the level of GAPDH in the same sample. Data represent the difference in crossing threshold between the gene of interest and GAPHD (ΔCT). The levels of Ncam1, L1cam and Cdh2 were not affected by the treatment conditions tested. N = 3 samples under each condition. P > 0.05 versus control explants for all comparisons.
| Gene Name | Control | Noggin | BMP2 | BMP4 |
|---|---|---|---|---|
| Ncam1 | 8.00 ±0.20 | 7.80 ±0.15 | 7.80 ±0.08 | 8.33 ±0.22 |
| L1cam | 7.76 ±0.17 | 7.93 ±0.38 | 7.86 ±0.03 | 7.98 ±0.16 |
| Cdh2 (N-cadherin) | 7.34 ±0.22 | 6.82 ±0.37 | 7.34 ±0.24 | 6.72 ±0.31 |
As an alternative explanation, we pursued the hypothesis that increased polysialic acid (PSA) addition to Ncam1 on NCC would influence neurite fasciculation and NCC migration as it has been previously suggested to influence NCC aggregation (Chalazonitis et al., 2004; Faure et al., 2003). To verify that BMP signaling altered PSA addition to Ncam1, we first used protein immunoblot analysis of E11.5 gut explants that had been maintained in culture for 48 hours with BMP or noggin, but we were unable to detect altered PSA levels in these tissues (data not shown). We hypothesized that PSA-Ncam1 expression might not be restricted to NCC. To determine which cells within the gut express PSA, cross sections from E12.5 mouse mid-gut were stained with a PSA specific antibody (#735) (Hayrinen et al., 1995). These analyses demonstrated PSA on both mesenchymal cells and NCCs, with stronger expression in the NCCs (Figure 7D, 7E). Because PSA expression in gut mesenchyme might make it difficult to detect changes in PSA-Ncam1 within the NCC, dissociated E12.5 embryonic gut cultures were maintained for 48 hours with either BMP2, BMP4 or noggin before immunohistochemical analysis with a PSA specific antibody. Neither BMP4 nor noggin treatment altered the proportion of TuJ1+ NCC expressing PSA (Figure 7G), but BMP4 treatment did increase the intensity of PSA immunoreactivity on neurites (Figure 7A′, 7B′, 7C′). This impression was confirmed by quantitative image analysis (Figure 7F). (Pixel intensity for PSA on neurites: Control 22 +/− 1, noggin 15 +/− 1, BMP4 41 +/− 3; Control versus BMP4: P<0.001, Control versus noggin: P < 0.001). These data suggested the possibility that PSA addition to Ncam1 might mediate the effects of BMP signaling on NCC migration or neurite fasciculation.
Figure 7. BMP increases PSA addition to Ncam1.
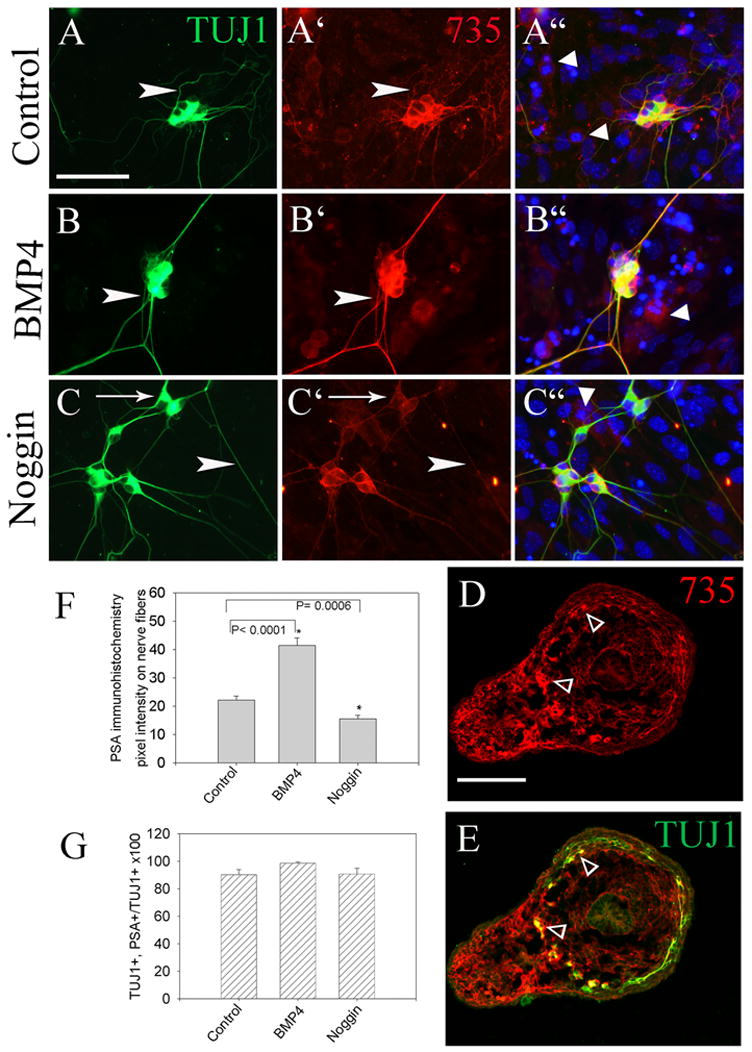
(A–E) PSA-Ncam1 immunofluorescence detected with the 735 antibody was compared to TuJ1 immunoreactivity in developing enteric neurons after dispersed cell culture. BMP4 treatment significantly increased 735 reactivity on neurites, while noggin reduced 735 immunoreactivity. (A–C) Arrowheads show similar sized TuJ1 reactive nerve fibers. (A′–C′) Arrowheads show the same fibers with PSA antibody staining. (C) Arrow shows a TUJ1 immunoreactive cell body with minimal PSA immunoreactivity after growth in GDNF plus noggin. (A”–C”) Merged images including Hoechst 33342 staining confirm that PSA immunoreactivity is most intense on NCC, but can also be detected in some non-NCC in these cultures (arrowheads). (D, E) Cross sections of the bowel further demonstrate PSA immunoreactivity is most intense on NCC (opened small arrow heads), but is present at lower levels in other cells in the bowel wall and mesentery. (F) Quantitative analysis of pixel intensity for PSA on neurites in cultured cells demonstrated that BMP4 increased PSA immunoreactivity on neurites, while noggin treatment reduced PSA immunoreactivity. Even after growth in noggin, however, most TuJ1+ cell bodies had some detectable PSA immunoreactivity (G). (A–C) Scale bar = 25 μm. (D, E) Scale bar = 100 μm.
PSA addition to Ncam1 is required for BMP induced nerve fiber fasciculation
To determine whether PSA addition affects NCC neurite fasciculation, slices of gut from E12.5 mid small bowel were cultured on fibronectin coated dishes. Explants were treated with endo-N for 2 hours before BMP4 or noggin addition to remove PSA and then maintained in culture for 48 hours to allow neurite outgrowth from the explant. Protein immunoblot analysis confirmed that endo-N treatment effectively removed PSA from Ncam1 and resulted in a shift in the migration of Ncam1 on SDS-PAGE gels, but did not cause loss of Ncam1 (Figure 8E). As previously observed, BMP4 caused significant neurite fasciculation (Figure 8C, C′). In contrast, endo-N treatment blocked neurite fasciculation even when explants were cultured with BMP4 (Figure 8D, D′). Because fibers in bundles were often closely adherent it was difficult to directly count neurites/fiber bundle, but fasciculation in response to BMP treatment was observed in several different assay systems (Figure 2P, 5D and 8C, Supplemental Figure 4). Quantitative analysis of neurite fasciculation was therefore performed by measuring fiber bundle diameter (Figure 8F). These analyses confirmed that BMP4 dramatically increased the percentage of both large and medium sized fiber bundles while decreasing the percentage of small fiber bundles. In contrast, although endo-N treatment alone did not affect neurite fasciculation, endo-N did dramatically reduce BMP4 induced neurite fasciculation (Figure 8B, C, D). Quantitative analysis also confirmed that endo-N treatment did not alter the number of fibers or fiber bundles extending from gut explants (Figure 8G). BMP4 treatment, however, reduced the total number of fiber bundles extending from explants consistent with an effect on fasciculation. This effect was partially reversed by endo-N. Together these studies confirm that BMP4 increases PSA addition to Ncam1 on neurites of cultured NCC and that this PSA addition is important for BMP4 induced effects on neurite fasciculation.
Figure 8. PSA removal with endo-N prevents BMP induced neurite fasciculation.
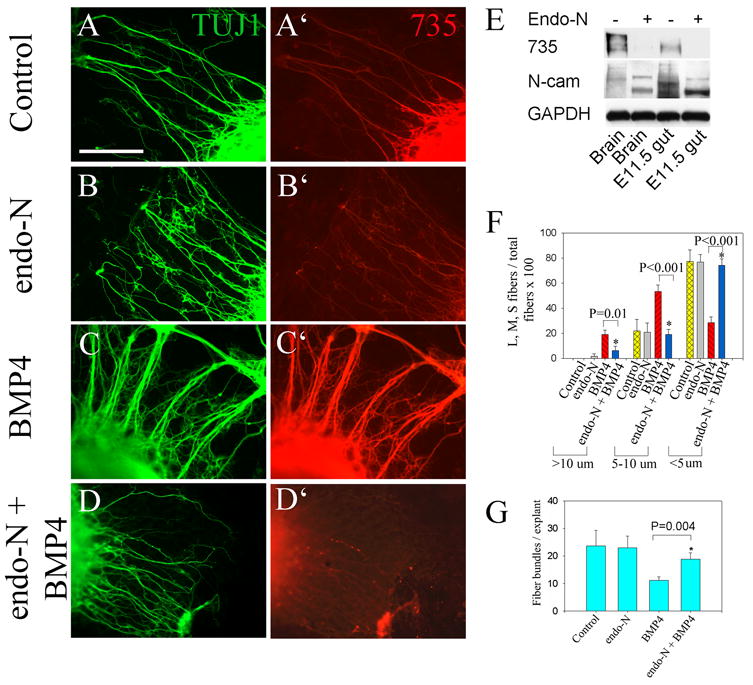
(A–D) E12.5 gut slice explants were grown for 48 hours under control conditions (A, A′), with endo-N (B, B′), BMP4 (C, C′) or BMP4 plus endo-N (D, D′) on fibronectin coated dishes. Immunohistochemistry with TuJ1 and 735 antibodies demonstrate that PSA removal influences neurite fasciculation. (E) Removal of PSA from Ncam1 was confirmed by protein immunoblot using antibodies to PSA (735) or Ncam1. The PSA antibody recognizes only a single broad band on the SDS-PAGE gels. Endo-N treatment results in a change in migration pattern for Ncam1 protein and a marked reduction in PSA immunoreactivity. As a loading control, the blot was probed with an antibody to GAPDH. Reduced PSA immunoreactivity is also observed after endo-N treatment in images B and D (compared to A and C). Although some fluorescent fibers are still visible after endo-N treatment in these images, these immunofluorescence signals are comparable in intensity to the fluorescence seen with no primary antibody (see Supplemental Figure 5). (F) Quantitative analysis of fiber bundle thickness confirms that BMP4 induces neurite fasciculation and that this fasciculation can be reversed by endo-N treatment. (G) The number of fiber bundles/explant is not affected by endo-N, but is reduced by BMP4 because of fasciculation. The BMP4 induced neurite fasciculation, is partially reversed by endo-N. Scale bar = 100 μm.
PSA addition to Ncam1 reduced NCC migration into the distal bowel
To determine whether PSA addition to Ncam1 influences NCC migration, E11.5 gut explants were treated with endo-N for 2 hours and then maintained in suspension culture for 48 hours with or without BMP4 (Figure 9). Quantitative analysis of NCC migration confirmed that endo-N treatment did not affect the extent of colon innervation under control conditions, but reversed the effect of added BMP4 on colon innervation (Figure 9E). These data suggest that the BMP4 induced reduction in NCC migration into the distal bowel is at least partially mediated by increased PSA addition to NCAM.
Figure 9. PSA removal with endo-N prevents BMP induced delays in NCC migration through the colon.
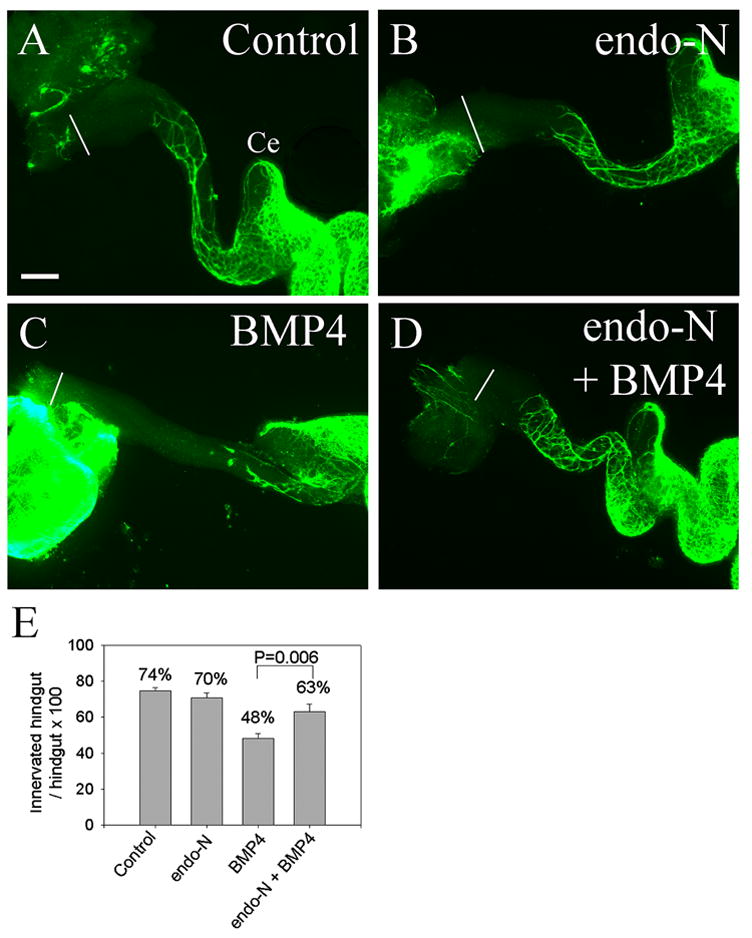
(A–D) E11.5 gut explants were maintained in culture for 48 hours (A) under control conditions, or with (B) endo-N, (C) BMP4, or (D) BMP4 plus endo-N. Endo-N did not affect NCC migration under control conditions, but prevented the delay in NCC migration induced by BMP4. White lines indicate the end of the colon. Ce = cecum. (E) Quantitative analysis of colon innervation (n = 10 explants for each treatment). Scale bar = 100 μm. Landmarks for these measurements are in Figure 4F.
Discussion
Several recent papers have highlighted important effects of BMP signaling on ENS morphogenesis (Bixby et al., 2002; Chalazonitis et al., 2004; De Santa Barbara et al., 2005; Goldstein et al., 2005; Pisano et al., 2000). Our new studies add to this literature by demonstrating effects of BMP and noggin on murine ENS precursor migration, neurite fasciculation, ganglion cell aggregation, and neurite orientation within the bowel wall. We specifically demonstrate that: 1. BMP4 and noggin are expressed in the bowel wall during the entire period of NCC migration through the small bowel and colon. 2. Blocking BMP signaling with noggin or BMP4 blocking antibody increases the rate of murine NCC migration into the colon, while excess BMP reduces the rate of NCC migration into the colon. 3. Blocking BMP signaling with noggin allows NCC to migrate toward the gut epithelium resulting in ectopic enteric neurons. 4. BMP signaling promotes both ganglion cell aggregation and neurite fasciculation. 5. BMP increases PSA addition to Ncam1 on NCC. 6. Removing PSA from Ncam1 reverses the effect of BMP on both NCC migration and neurite fasciculation. These results complement previously published studies on the effect of BMP signaling on NCC proliferation and differentiation. They also in some ways resemble the recently reported effects of BMP signaling on developing chick enteric NCC, but differ from the results in chick in some surprising ways.
Remarkably, noggin treatment was reported to delay NCC migration into the chick distal bowel, but enhanced NCC migration in our murine experiments. Because of these apparently contradictory results we evaluated the effect of noggin on murine NCC migration using several different systems. Noggin or BMP4 blocking antibody increased murine NCC migration into the distal bowel, or out of the bowel toward sources of GDNF in collagen gel, onto filter membranes or onto fibronectin coated plates. Consistent with these findings, BMP treatment reduced NCC migration in each of these experimental paradigms. While it is possible that these differences result from different delivery methods for noggin, or different concentrations of recombinant factor used, the similarity of our experimental approaches suggest that blocking BMP signaling has different effects on chick and mouse NCC migration at what appear to be comparable stages of development. This finding is consistent with the observation that BMP proteins have different effects on cell lineages at different stages of development and that these effects are both time and concentration dependent.
The observed differences in noggin effects on NCC migration between chick and mouse may also explain one difference in enteric nervous system development between these two species. In mouse small bowel and hindgut colonization by NCC occurs first in outer layers of the bowel wall in the region that will later become the myenteric plexus (McKeown et al., 2001). In chick, the region of the myenteric plexus is colonized first in the midgut (Allan and Newgreen, 1980), but in the hindgut, vagal NCC colonize the area of the submucosal plexus before colonizing the myenteric plexus region of the bowel (Burns and Le Douarin, 1998). Since BMP4 is produced adjacent to the intestinal epithelium, the reduced migration of murine ENS precursors in the presence of BMP could in part help keep mouse NCC out of the region of the submucosal plexus until later stages of development when they are attracted to the submucosal plexus by netrin/DCC signaling (Jiang et al., 2003). This would be consistent with our observation that noggin treatment of murine gut explants results in ectopic NCC located closer to the intestinal epithelium than normal. It is also consistent with the presence of similar ectopic neurons in Shh deficient mice since Shh is produced in the gut epithelium and induces BMP4 production in adjacent cells (Ramalho-Santos et al., 2000). The situation in chick, however, is more complex since reducing BMP4 expression in the chick duodenum by expressing Bapx1 or increasing BMP4 in the chick gizzard causes ectopic ganglia near the mucosa (De Santa Barbara et al., 2005). Together these data clearly demonstrate that the location and intensity of BMP signaling affect radial NCC migration. Because chick hindgut NCC migrate more slowly when BMP signaling is blocked by noggin, but mouse hindgut NCC migrate more quickly, BMP expression adjacent to the gut epithelium provides one potential explanation for the different hindgut migration pathways in these species. Of course, it may be more complicated since BMP signaling influences many aspects of gut development (De Santa Barbara et al., 2005), but the strikingly opposite effect of noggin on NCC migration in chick and mouse hindgut is consistent with the observed differences in migratory pathway. Furthermore, the effects on NCC migration out of the bowel wall toward sources of GDNF suggest that BMP signaling directly influences NCC migration in addition to any indirect effects via alterations in the gut mesenchyme. The observation that chick and mouse small bowel have similar NCC migratory pathways despite the differences in noggin effects on NCC migration in these species, however, suggests that additional factors critically influence radial migration of NCC.
In addition to effects on cell migration, BMP signaling promotes ganglion cell aggregation and neurite fasciculation. Our results demonstrating that BMP signaling promotes ganglion cell aggregation within the gut wall while noggin treatment prevents aggregation in organ culture, agree with the previously reported effects of BMP on the aggregation of isolated ENS precursors in vitro (Chalazonitis et al., 2004). The effect of BMP signaling on neurite fasciculation has not, to our knowledge, been previously reported in ENS precursors. In addition, we found striking effects of BMP signaling on the orientation of neurites within the gut wall. All of these effects of BMP signaling on ENS development suggest that BMP signaling alters cell adhesion.
To test the hypothesis that BMP signaling modulates cell adhesion, we used qRT-PCR to determine if the levels of adhesion molecules known to be expressed in the ENS change in response to BMP signaling. While we did not detect changes in the levels of the adhesion molecules tested, previous reports that BMP signaling induced PSA addition to Ncam1 (Chalazonitis et al., 2004; Faure et al., 2003) led us to test the hypothesis that Ncam1 polysialation might mediate the effects of BMP on neurite fasciculation and ENS precursor migration. Experiments using endo-N to remove PSA from Ncam1 demonstrated that both increased neurite fasciculation and reduced ENS precursor migration caused by BMP treatment were prevented by PSA removal.
PSA-Ncam1 has important functions in cell migration, axon growth, neurite fasciculation, and neuronal plasticity within the central nervous system (Durbec and Cremer, 2001; Franceschini et al., 2004; Yin et al., 1995). Because PSA addition to Ncam1 influences cell-cell and cell-substrate interactions, endo-N treatment has been reported to both increase and decrease neurite fasciculation, depending on the model system. Furthermore, PSA addition may alter the responsiveness of neural precursors to specific neurotrophic factors (Muller et al., 2000) or even to BMP4. Our data suggests that BMP induced neurite fasciculation in ENS precursors depends at least in part on increased PSA-Ncam1. This would imply that PSA addition either increases neurite-neurite interactions or decreases neurite-extracellular matrix interactions. Note, however, that we cannot determine from our studies whether the observed effects of Endo-N result from PSA removal from NCC or the surrounding mesenchymal cells. The effects of altered cell adhesion on NCC migration, also cannot be easily predicted from effects on cell aggregation or neurite fasciculation. NCC migrate in chains through the developing gut, but since migration requires interactions at the leading edge of the cell and release of interactions at the trailing edge of the cell, both increased and decreased cell-cell interaction could slow migration. Our data suggest that, at least in the mouse, increased PSA-Ncam1 in response to exogenous BMP slows migration of NCC through the colon.
These studies confirm and reinforce previous work in vitro and in vivo suggesting that BMP signaling is critical for many aspects of ENS development. We specifically demonstrate roles for endogenous BMP signaling in NCC migration both rostrocaudally along the bowel and radially toward the gut mucosa. We also demonstrate important and previously unappreciated roles for BMP signaling in neurite fasciculation and neurite orientation within the bowel. These studies suggest that altered BMP signaling could profoundly influence ENS development and intestinal function. They further suggest that regulating sialyltransferase activity could influence Hirschsprung disease penetrance.
Supplementary Material
Supplemental Figure 1. Very few neural crest cells migrate from the edge of the explant in the absence of GDNF in the medium. Cultures were prepared as described in Figure 2 except that GDNF was omitted from the culture medium. Under these conditions there is little migration of Ret+ cells from the explant and few fibers extend from the end of the explant onto the filter paper. Scale bar = 100 μm.
Supplemental Figure 2. Explants cultured in GDNF containing collagen gel extended many TuJ1 immunoreactive nerve fibers. Gut explant cultures were prepared as described for Figure 3 and then stained with TuJ1 antibody to demonstrate neurites extending from the explant into the collagen gel. Scale bar = 100 μm.
Supplemental Figure 3. Hindgut length. Hindgut length is the same after 48 hours of growth in control medium, or medium with added noggin, anti-BMP4 blocking antibody or BMP4.
Supplemental Figure 4. BMP4 induces fasciculation of neurites that grow from gut explants. E11.5 midgut explants were placed onto filter paper and cultured with GDNF, GDNF plus BMP4 or GDNF plus anti-BMP4 blocking antibody. Neurite fasciculation was consistently observed in explants grown in the presence of BMP4 (B), but not in explants grown in control media (A) or with anti-BMP4 blocking antibody (C). In these image, it is easy to see fibers joining to form fascicles after BMP4 treatment. This fasciculation was evaluated quantitatively by measuring neurite bundle diameter in Figure 8F.
Supplemental Figure 5. Endo-N treatment effectively eliminates PSA immunoreactivity. E12.5 gut slice explants were maintained in culture for 48 hours on fibronectin coated dishes either with (A) or without (B) endo-N treatment using conditions identical to those in Figure 8. (A, B) Immunohistochemistry for TuJ1. (A′) Shows the same explant as in (A), but with anti-PSA antibody (735) immunohistochemistry. Faint immunofluorescence on neurites is still visible, but this is comparable to the immunofluorescence observed using only the Alexa 594 secondary antibody (i.e., no primary antibody) (B′). These data demonstrate that endo-N treatment removes essentially all detectable PSA from neurites and that residual staining on neurites is attributable to secondary antibody staining.
Acknowledgments
We are grateful to Dr. Rita Gerardy-Schahn for generously sharing the PSA antibody (#735) and endo-N-acylneuraminidase (endo-N). We are also thankful to Dr. Richard Harland for graciously providing noggin secreting CHO cells. In addition we appreciate the technical guidance of Dr. Heather Young and Dr. Vincent Lui. R.O.H was supported by NIH/RO1 DK57038, NIH/RO1 DK6459201, the Digestive Disease Research Center Core (DDRCC) NIH/P30-DK52574 and a grant from the March of Dimes FY02-182 (R.O.H).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acheson A, Sunshine JL, Rutishauser U. NCAM polysialic acid can regulate both cell-cell and cell-substrate interactions. J Cell Biol. 1991;114:143–53. doi: 10.1083/jcb.114.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan IJ, Newgreen DF. The origin and differentiation of enteric neurons of the intestine of the fowl embryo. Am J Anat. 1980;157:137–54. doi: 10.1002/aja.1001570203. [DOI] [PubMed] [Google Scholar]
- Bitgood MJ, McMahon AP. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Developmental Biology. 1995;172:126–138. doi: 10.1006/dbio.1995.0010. [DOI] [PubMed] [Google Scholar]
- Bixby S, Kruger GM, Mosher JT, Joseph NM, Morrison SJ. Cell-intrinsic differences between stem cells from different regions of the peripheral nervous system regulate the generation of neural diversity. Neuron. 2002;35:643–56. doi: 10.1016/s0896-6273(02)00825-5. [DOI] [PubMed] [Google Scholar]
- Brewer KC, Mwizerva O, Goldstein AM. BMPRIA is a promising marker for evaluating ganglion cells in the enteric nervous system--a pilot study. Hum Pathol. 2005;36:1120–6. doi: 10.1016/j.humpath.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Burns AJ, Le Douarin NM. The sacral neural crest contributes neurons and glia to the post-umbilical gut: spatiotemporal analysis of the development of the enteric nervous system. Development. 1998;125:4335–4347. doi: 10.1242/dev.125.21.4335. [DOI] [PubMed] [Google Scholar]
- Canalis E, Economides AN, Gazzerro E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr Rev. 2003;24:218–35. doi: 10.1210/er.2002-0023. [DOI] [PubMed] [Google Scholar]
- Chalazonitis A, D’Autreaux F, Guha U, Pham TD, Faure C, Chen JJ, Roman D, Kan L, Rothman TP, Kessler JA, Gershon MD. Bone morphogenetic protein-2 and -4 limit the number of enteric neurons but promote development of a TrkC-expressing neurotrophin-3-dependent subset. J Neurosci. 2004;24:4266–82. doi: 10.1523/JNEUROSCI.3688-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–41. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- Cunningham BA, Hoffman S, Rutishauser U, Hemperly JJ, Edelman GM. Molecular topography of the neural cell adhesion molecule N-CAM: surface orientation and location of sialic acid-rich and binding regions. Proc Natl Acad Sci U S A. 1983;80:3116–20. doi: 10.1073/pnas.80.10.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santa Barbara P, Williams J, Goldstein AM, Doyle AM, Nielsen C, Winfield S, Faure S, Roberts DJ. Bone morphogenetic protein signaling pathway plays multiple roles during gastrointestinal tract development. Dev Dyn. 2005;234:312–22. doi: 10.1002/dvdy.20554. [DOI] [PubMed] [Google Scholar]
- Durbec P, Cremer H. Revisiting the function of PSA-NCAM in the nervous system. Mol Neurobiol. 2001;24:53–64. doi: 10.1385/MN:24:1-3:053. [DOI] [PubMed] [Google Scholar]
- Faure C, Rhéaume C, Chalazonitis A, Gershon MD. Expression of polysialylated neural cell adhesion molecule (PSA-NCAM) in the developing and mature rat enteric nervous system (ENS): relationship to neuronal differentiation and plasticity. Neurogastroenterol Motil. 2003;15:197. [Google Scholar]
- Finne J, Finne U, Deagostini-Bazin H, Goridis C. Occurrence of alpha 2-8 linked polysialosyl units in a neural cell adhesion molecule. Biochem Biophys Res Commun. 1983;112:482–7. doi: 10.1016/0006-291x(83)91490-0. [DOI] [PubMed] [Google Scholar]
- Franceschini I, Vitry S, Padilla F, Casanova P, Tham TN, Fukuda M, Rougon G, Durbec P, Dubois-Dalcq M. Migrating and myelinating potential of neural precursors engineered to overexpress PSA-NCAM. Mol Cell Neurosci. 2004;27:151–62. doi: 10.1016/j.mcn.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Frosch M, Gorgen I, Boulnois GJ, Timmis KN, Bitter-Suermann D. NZB mouse system for production of monoclonal antibodies to weak bacterial antigens: isolation of an IgG antibody to the polysaccharide capsules of Escherichia coli K1 and group B meningococci. Proc Natl Acad Sci U S A. 1985;82:1194–8. doi: 10.1073/pnas.82.4.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu M, Lui VC, Sham MH, Pachnis V, Tam PK. Sonic hedgehog regulates the proliferation, differentiation, and migration of enteric neural crest cells in gut. J Cell Biol. 2004;166:673–84. doi: 10.1083/jcb.200401077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness JB. Types of neurons in the enteric nervous system. J Autonomic Nervous System. 2000;81:87–96. doi: 10.1016/s0165-1838(00)00127-2. [DOI] [PubMed] [Google Scholar]
- Gariepy CE. Developmental disorders of the enteric nervous system: genetic and molecular bases. J Pediatr Gastroenterol Nutr. 2004;39:5–11. doi: 10.1097/00005176-200407000-00003. [DOI] [PubMed] [Google Scholar]
- Gerardy-Schahn R, Bethe A, Brennecke T, Muhlenhoff M, Eckhardt M, Ziesing S, Lottspeich F, Frosch M. Molecular cloning and functional expression of bacteriophage PK1E-encoded endoneuraminidase Endo NE. Mol Microbiol. 1995;16:441–50. doi: 10.1111/j.1365-2958.1995.tb02409.x. [DOI] [PubMed] [Google Scholar]
- Gershon M. Genes and lineages in the formation of the enteric nervous system. Current Opinion in Neurobiology. 1997;7:101–109. doi: 10.1016/s0959-4388(97)80127-4. [DOI] [PubMed] [Google Scholar]
- Goldstein AM, Brewer KC, Doyle AM, Nagy N, Roberts DJ. BMP signaling is necessary for neural crest cell migration and ganglion formation in the enteric nervous system. Mech Dev. 2005;122:821–33. doi: 10.1016/j.mod.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Hayrinen J, Jennings H, Raff HV, Rougon G, Hanai N, Gerardy-Schahn R, Finne J. Antibodies to polysialic acid and its N-propyl derivative: binding properties and interaction with human embryonal brain glycopeptides. J Infect Dis. 1995;171:1481–90. doi: 10.1093/infdis/171.6.1481. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Liu MT, Gershon MD. Netrins and DCC in the guidance of migrating neural crest-derived cells in the developing bowel and pancreas. Dev Biol. 2003;258:364–84. doi: 10.1016/s0012-1606(03)00136-2. [DOI] [PubMed] [Google Scholar]
- Kapur RP. Neuropathology of paediatric chronic intestinal pseudo-obstruction and related animal models. J Pathol. 2001;194:277–88. doi: 10.1002/path.885. [DOI] [PubMed] [Google Scholar]
- Kishigami S, Mishina Y. BMP signaling and early embryonic patterning. Cytokine Growth Factor Rev. 2005;16:265–78. doi: 10.1016/j.cytogfr.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Kiss JZ, Rougon G. Cell biology of polysialic acid. Curr Opin Neurobiol. 1997;7:640–6. doi: 10.1016/s0959-4388(97)80083-9. [DOI] [PubMed] [Google Scholar]
- McKeown SJ, Chow CW, Young HM. Development of the submucous plexus in the large intestine of the mouse. Cell Tissue Res. 2001;303:301–5. doi: 10.1007/s004410000303. [DOI] [PubMed] [Google Scholar]
- Muller D, Djebbara-Hannas Z, Jourdain P, Vutskits L, Durbec P, Rougon G, Kiss JZ. Brain-derived neurotrophic factor restores long-term potentiation in polysialic acid-neural cell adhesion molecule-deficient hippocampus. Proc Natl Acad Sci U S A. 2000;97:4315–20. doi: 10.1073/pnas.070022697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RW, Bates PA, Rutishauser U. Protein determinants for specific polysialylation of the neural cell adhesion molecule. J Biol Chem. 1995;270:17171–9. doi: 10.1074/jbc.270.29.17171. [DOI] [PubMed] [Google Scholar]
- Newgreen D, Young HM. Enteric nervous system: development and developmental disturbances--part 1. Pediatr Dev Pathol. 2002a;5:224–47. doi: 10.1007/s10024-001-0142-y. [DOI] [PubMed] [Google Scholar]
- Newgreen D, Young HM. Enteric nervous system: development and developmental disturbances--part 2. Pediatr Dev Pathol. 2002b;5:329–49. doi: 10.1007/s10024-001-0142-y. [DOI] [PubMed] [Google Scholar]
- Nguyen L, Rigo JM, Malgrange B, Moonen G, Belachew S. Untangling the functional potential of PSA-NCAM-expressing cells in CNS development and brain repair strategies. Curr Med Chem. 2003;10:2185–96. doi: 10.2174/0929867033456774. [DOI] [PubMed] [Google Scholar]
- Parisi MA, Kapur RP. Genetics of Hirschsprung disease. Curr Opin Pediatr. 2000;12:610–7. doi: 10.1097/00008480-200012000-00017. [DOI] [PubMed] [Google Scholar]
- Pisano JM, Colon-Hastings F, Birren SJ. Postmigratory enteric and sympathetic neural precursors share common, developmentally regulated, responses to BMP2. Developmental Biology. 2000;227:1–11. doi: 10.1006/dbio.2000.9876. [DOI] [PubMed] [Google Scholar]
- Ramalho-Santos M, Melton DA, McMahon AP. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development. 2000;127:2763–2772. doi: 10.1242/dev.127.12.2763. [DOI] [PubMed] [Google Scholar]
- Roberts DJ, Johnson RL, Burke AC, Nelson CE, Morgan BA, Tabin C. Sonic hedgehog is an endodermal signal inducing Bmp-4 and Hox genes during induction and regionalization of the chick hindgut. Development. 1995;121:3163–3174. doi: 10.1242/dev.121.10.3163. [DOI] [PubMed] [Google Scholar]
- Sukegawa A, Narita T, Kameda T, Saitoh K, Nohno T, Iba H, Yasugi S, Fukuda K. The concentric structure of the developing gut is regulated by Sonic hedgehog derived from endodermal epithelium. Development. 2000;127:1971–1980. doi: 10.1242/dev.127.9.1971. [DOI] [PubMed] [Google Scholar]
- Warren SM, Brunet LJ, Harland RM, Economides AN, Longaker MT. The BMP antagonist noggin regulates cranial suture fusion. Nature. 2003;422:625–9. doi: 10.1038/nature01545. [DOI] [PubMed] [Google Scholar]
- Wijgerde M, Karp S, McMahon J, McMahon AP. Noggin antagonism of BMP4 signaling controls development of the axial skeleton in the mouse. Dev Biol. 2005;286:149–57. doi: 10.1016/j.ydbio.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Yin X, Watanabe M, Rutishauser U. Effect of polysialic acid on the behavior of retinal ganglion cell axons during growth into the optic tract and tectum. Development. 1995;121:3439–46. doi: 10.1242/dev.121.10.3439. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Very few neural crest cells migrate from the edge of the explant in the absence of GDNF in the medium. Cultures were prepared as described in Figure 2 except that GDNF was omitted from the culture medium. Under these conditions there is little migration of Ret+ cells from the explant and few fibers extend from the end of the explant onto the filter paper. Scale bar = 100 μm.
Supplemental Figure 2. Explants cultured in GDNF containing collagen gel extended many TuJ1 immunoreactive nerve fibers. Gut explant cultures were prepared as described for Figure 3 and then stained with TuJ1 antibody to demonstrate neurites extending from the explant into the collagen gel. Scale bar = 100 μm.
Supplemental Figure 3. Hindgut length. Hindgut length is the same after 48 hours of growth in control medium, or medium with added noggin, anti-BMP4 blocking antibody or BMP4.
Supplemental Figure 4. BMP4 induces fasciculation of neurites that grow from gut explants. E11.5 midgut explants were placed onto filter paper and cultured with GDNF, GDNF plus BMP4 or GDNF plus anti-BMP4 blocking antibody. Neurite fasciculation was consistently observed in explants grown in the presence of BMP4 (B), but not in explants grown in control media (A) or with anti-BMP4 blocking antibody (C). In these image, it is easy to see fibers joining to form fascicles after BMP4 treatment. This fasciculation was evaluated quantitatively by measuring neurite bundle diameter in Figure 8F.
Supplemental Figure 5. Endo-N treatment effectively eliminates PSA immunoreactivity. E12.5 gut slice explants were maintained in culture for 48 hours on fibronectin coated dishes either with (A) or without (B) endo-N treatment using conditions identical to those in Figure 8. (A, B) Immunohistochemistry for TuJ1. (A′) Shows the same explant as in (A), but with anti-PSA antibody (735) immunohistochemistry. Faint immunofluorescence on neurites is still visible, but this is comparable to the immunofluorescence observed using only the Alexa 594 secondary antibody (i.e., no primary antibody) (B′). These data demonstrate that endo-N treatment removes essentially all detectable PSA from neurites and that residual staining on neurites is attributable to secondary antibody staining.


