Abstract
OX40 (CD134) is a potent costimulatory molecule found on the surface of activated CD4+ and CD8+ T cells. Immunotherapy with OX40 agonists administered in vivo has demonstrated efficacy in several murine tumor models. A phase I clinical trial is currently underway in patients with advanced cancer using a mouse anti-CD134 monoclonal antibody. Therapy with this antibody will likely be limited to one cycle because patients develop neutralizing human anti-mouse antibody (HAMA). Therefore, we developed a humanized OX40 agonist that links the extracellular domain of human OX40L to the Fc domain of human IgG1 via a trimerizing isoleucine zipper domain (ILZ). Physical characterization by velocity sedimentation revealed that this novel construct, hFcILZOX40L, was assembled into hexamers in which the Fc domains formed three disulfide-bonded dimers and the ILZ-OX40L domains formed two trimers. Trimerization of the ILZ domain was necessary to achieve appropriate assembly. In vitro biologic activity of the hFcILZOX40L hexamer was equivalent to the activity of agonist antibodies in plate-bound assays and was superior when the agonists were tested as soluble agents. Our ultimate goal is to use this recombinant molecule in a future clinical trial and we feel that the OX40L hexamer will have equivalent or superior agonist activity in vivo when compared to an anti-OX40 antibody.
Keywords: OX40L, costimulation, immunotherapy, recombinant, structure
Introduction
Members of the TNF superfamily and their receptors play critical roles in T-cell immunity. These include initiation, expansion, differentiation and down-regulation of immune responses (see Watts (2005), for review). Typically, T-cell priming is initiated by the engagement of the T-cell receptor (TCR) and peptide/MHC complex accompanied by costimulation via CD28/B7.1. Reciprocal interactions between the APC and T cell can lead to additional costimulation late during priming that can substantially modulate the overall T-cell response. The TNF super family receptor, OX40, provides one such late priming costimulatory signal (Mallett et al., 1990; Paterson et al., 1987). OX40 expression peaks 24–48 hours after TCR engagement and then diminishes over the following three days (Gramaglia et al., 1998), while OX40L is expressed on APCs during a similar time frame. The expression of both OX40 receptor and OX40L depends on early events such as the strength of TCR engagement, CD28 signaling, and CD40 ligation (Croft, 2003; Weinberg, 2002).
Maximizing signaling via OX40 using exogenous agonists has profound effects on the T-cell response to both proteins and tumor cells following immunization (Croft, 2003; Sugamura et al., 2004). Analysis of CD4+ T-cell responses following in vivo OX40 engagement showed an increase in antigen-specific T-cell expansion, enhanced cytokine production and an increase in the generation and stability of a memory T cells (Evans et al., 2001; Huddleston et al., 2006; Kaleeba et al., 1998; Weinberg et al., 1998). In mouse tumor models, OX40 engagement, with an agonist antibody or soluble ligand in the absence of immunization, produced antitumor effects against breast and colon cancers, melanoma, sarcoma and glioma (Kjaergaard et al., 2001; Kjaergaard et al., 2000; Morris et al., 2001; Pan et al., 2002; Weinberg et al., 2000). The detection of OX40+ T cells in a variety of human solid tumors by immunohistochemistry (Ladanyi et al., 2004; Petty et al., 2002; Ramstad et al., 2000) suggests that activated tumor-infiltrating T cells could serve as targets for activating tumor immunotherapy. In animal models for cancer and autoimmune disease, these OX40+ T cells were sorted and shown to recognize tumor or auto antigens respectively (Weinberg, 2002). Strategies to exploit the OX40-OX40L system include agonists such as anti-OX40 monoclonal antibodies or recombinant soluble OX40L, and antagonists, which include monoclonal antibodies to OX40L or OX40:Ig (al-Shamkhani et al., 1996; Ali et al., 2004; Weinberg et al., 1999). We have initiated a phase I clinical trial using a mouse anti-human OX40 monoclonal antibody in patients with advanced cancer. As expected, human anti-mouse antibody responses (HAMA) developed within these patients and limited application of anti-OX40 therapy to one cycle. While a single cycle of therapy was sufficient in mouse tumor models, it is likely that repeated cycles of OX40 agonist therapy would be needed to treat patients most effectively. Therefore we developed a recombinant soluble human OX40L protein, which upon binding its target could potentially be a more potent agonist than the antibody and/or may also have a shorter half-life in vivo compared to humanized antibody. The shorter half-life might be beneficial because it would limit the agonist signal to a narrower window and the human origin of the construct would permit multiple cycles of therapy.
Ligands in the TNF family are typically homotrimeric type II transmembrane proteins that contain a small cytoplasmic N-terminal domain, a transmembrane domain, a stalk region and a C-terminal receptor-binding domain that contains the signature TNF homology sequence and the trimerizing interactions (Bodmer et al., 2002). When CD40L (Haswell et al., 2001; Morris et al., 1999; Shiraishi et al., 2004), FASL (Holler et al., 2003; Shiraishi et al., 2004), TRAIL (Rabu et al., 2005; Walczak et al., 1999; Wu et al., 2001), and 4-1BBL (Rabu et al., 2005) were expressed as soluble recombinant molecules, their biological activity was enhanced by inclusion, individually or in some cases in combination, of (a) the stalk region, (b) an additional trimerizing domain, and (c) an oligomerization domain that linked trimers together. A modified version of the alpha helical coiled coil domain of the yeast transcription factor, GCN4, has been used as a trimerization domain in this context. When isoleucine residues occupy the a and d positions of the heptad repeat in coiled-coil alpha helical sequences (Isoleucine zipper, ILZ), trimer formation with high thermal stability, Tm >100°C, is strongly preferred (Harbury et al., 1993). Linking together two or more trimers can be achieved by crosslinking after biosynthesis (Rabu et al., 2005) or by incorporating a fusion partner like the Fc domain of IgG. The Fc:FasL fusion protein, which has a flexible linker between these two domains, was shown to assemble into a hexamer that contained two FasL trimers linked to three Fc dimers (Holler et al., 2003) (figure 1B). This arrangement provided adjacent FasL trimers that proved essential for FasL activity. The Fc domain fusion partners also enhance protein expression, provide stability/longevity in the circulation, and offer a convenient tool for purification (Lo et al., 1998). In an effort to optimize the structure and function of a recombinant OX40L molecule for therapeutic use, the complete extracellular domain of human OX40L was joined to the Fc domain of IgG1 via an ILZ domain. This is the first description of a TNF-family member Ig fusion protein joined via a trimerization domain which was produced efficiently by a eukaryotic cell line, formed a hexameric structure, and exhibited potent biologic activity.
Figure 1.
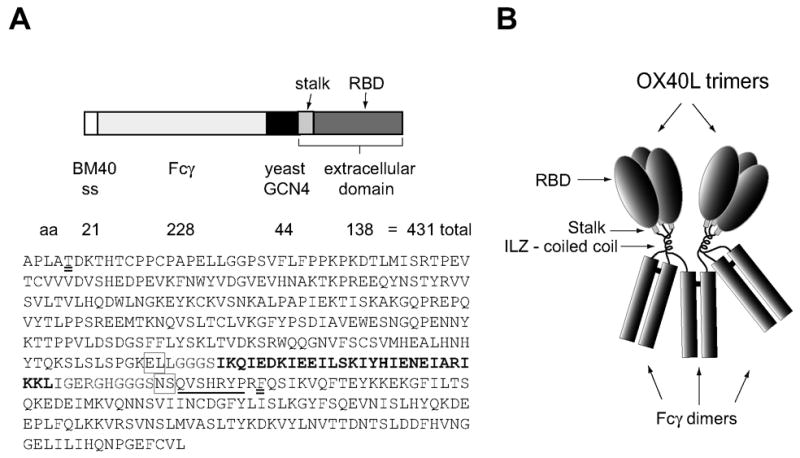
Schematic representation of recombinant human-Fcγ:human-OX40L fusion protein. A. The expression plasmid cloned into pCEP4 composed of a signal sequence from BM40 basement membrane protein, the hinge and Fc domain of human IgG1, the coiled coil trimerization domain derived from yeast GCN4 (Isoleucine zipper-ILZ) and the complete extracellular domain of human OX40L, including the short stalk region (see methods). The amino acid sequence of the secreted protein is also shown. The initial APLA is BM40 peptide sequence proximal to the signal peptide cleavage site. The two amino acids that were changed in making the construct are double underlined. The ILZ sequence is in bold type and the stalk region of OX40L is underlined. Pairs of amino acids introduced via restriction sites are boxed. B. Model of the folded fusion protein hFcILZOX40L based on the hexameric structure of Fc:FasL suggested by Holler et al. (2003). The Fc domains form three disulfide-bonded dimers and the ILZOX40L domains form two noncovalently associated trimers.
Methods
Construction of the FcILZOX40L expression plasmid
The Fc domain from human IgG1 was obtained by PCR amplification of plasmid pMT-Fc provided by Dr. Hu. This domain (accession #BC041037) begins in the hinge region at Cys251 that has been mutated to Thr (see figure 1). The 5′ primer contained a NheI restriction site and an extra base to preserve reading frame. The 3′ primer contained a SacI restriction site. The ILZ domain from yeast GCN4 was obtained from pCMV-Flag1 TriZP (EcoRI-Baff(Q136) provided b Dr. Hu. The ILZ domain was amplified by PCR using primers that contained SacI (5′) and EcoRI (3′) restriction sites. The hOX40L extracellular domain encoding amino acids 51–183 was obtained by PCR-amplification of pJOX obtained from Celtic Pharma (Hamilton, Bermuda) using primers containing EcoRI (5′) and XhoI (3″) restriction sites. The 5′-primer contained also contained a mutation (GAATTC to GATTTC) to alter an intrinsic EcoRI site. This converted the ninth amino acid of the hOX40L extracellular domain from I to F (see figure 1A). The 3′ primer contained a silent mutation to alter another intrinsic EcoRI site. The cDNAs encoding these three domains were cloned into a derivative of pCEP4 (Invitrogen, Carlsbad, CA), pCEPD4–7 that contained the signal sequence (SS) of the basement membrane protein BM40 (Mayer et al., 1993). The final expression plasmid contained SS-(NheI)-hFc-(SacI)-ILZ-(EcoRI)-hOX40L-(XhoI). Restriction enzymes and Quick Ligase T4 ligase were obtained from New England Biolabs (Ipswich MA) and competent DH5α bacteria were obtained from Invitrogen (Carlsbad CA). PCR amplifications utilized pfu taq polymerase (Stratagene, Cedar Creek TX).
Expression and purification
For expression, 293 HEK cells were transfected using Lipofectamine (Invitrogen, Carlsbad CA) and selected with hygromycin (Invitrogen, Carlsbad CA), 200 μg/ml and cultured in Dulbecco’s modified eagles medium (DMEM, Cambrex, Rockland ME) with 10% fetal bovine serum (Cambrex, Rockland ME) and penicillin/streptomycin (Invitrogen, Carlsbad CA). For maximum expression, transfected 293 cells were cultured in a CellMax hollow fiber bioreactor apparatus (Spectrum, Rancho Dominguez, CA) as above except that fungizone (Cambrex) was included in the medium and the FBS was stripped of bovine IgG by passage over a protein-G column (GE Healthcare, Fairfield CT). Culture media recovered from the bioreactor was loaded onto a protein G column, the column washed, and finally eluted with either 0.1M-glycine buffer, pH 2.7 or with Actisep (Sterogene, Carlsbad, CA) at neutral pH according to manufacturer’s instructions. Fractions were pooled, dialyzed and quantified by the Bicinchoninic Acid (BCA) method (Sigma, St Louis, MO). To measure rates of HFcILZOX40L synthesis, transfected cell line, D1, was plated in 24 well plates and cultured to confluency. The medium (1.0 ml) was replaced and recovered after 24 hours and the cells counted. The concentration of hFcILZOX40L was calculated by ELISA using the purified and quantified hFcILZOX40L as the standard.
Size exclusion chromatography (SEC)
Preparations of hFcILZOX40L and hFcOX40L were chromatographed on a tandem, Tricorn, 10 × 300cm, Superdex G-200 column (GE Healthcare, Fairfield CT) in Dulbecco’s phosphate buffered saline (Cambrex, Rockland ME) at 0.5 ml/min and the elution was monitored at A220. For preparative SEC, FcILZOX40L was chromatographed on one HR16 –Superose 6 column (GE Healthcare, Fairfield CT) in the same buffer at 0.3 ml/min and monitored at A280. 1 ml fractions were collected. Chromatography was performed using the AKTA basic system (GE Healthcare, Fairfield CT)
Proliferation assay
Preparation of cells: 10e8 frozen peripheral blood mononuclear cells (PBMCs) were thawed and fractionated on magnetic beads. Using the autoMACS system and the human CD4 isolation kit from Miltenyi (Auburn, CA), CD4+ T cells were purified by negative selection to >90%. The cells were cultured in RPMI (Cambrex, Rockland ME) with 10% FBS at 1 to 2 million cells/ml in the presence of 10 IU/ml IL2 after activation with 1μg/ml phytohemagglutinin (PHA) (Sigma, St. Louis, MO). On the fourth day of culture the cells were washed with RPMI and utilized in the proliferation assay. Plate-bound assay: 96-well plates (E&K Scientific, Santa Clara CA) were coated with goat anti-mouse, Fcγ-specific, and goat anti-human Fcγ-specific (Jackson Immunoresearch, West Grove, PA) both at 2μg/ml in PBS at 4°C, over night. The plates were blocked with 1% bovine serum albumin (Fisher Scientific, Pittsburg, PA) in PBS, incubated with anti-CD3 (OKT3, Oregon Health Sciences University) at 2 ng/ml in 1% BSA and finally incubated with serial 2-fold dilutions of FcIlzOX40L. Each incubation was conducted at 37°C for 90 min and followed by three washes with PBS. CD4+ T cells were diluted to 250,000 cells/ml and 200 μl of cell suspension was added to each well (50,000 cells/well). After 48 hours, 1μCi 3H-thymidine (MP Biomedicals, Solon, OH) was added and the cells were harvested 16 hours later. Soluble assay: 96-well plates were coated with anti-CD3 2μg/ml overnight at 4°C, washed with PBS, and then blocked with 1% BSA for 90min at 37°C. Serial 2-fold dilution of anti-hOX40 antibody (L106, Becton Dickenson or 9B12 (Weinberg et al., 2006)) or FcIlzOX40L in RPMI (100μL volumes) was followed by the addition of 50,000 activated T cells (see above) in 100μL for a final volume of 200 μL. Cells were incubated, labeled and harvested and counted as described for the plate-bound assay. Student’s T test was performed using GraphPad Prism version 4.0 for Macintosh, GraphPad Software, San Diego California USA, www.graphpad.com.
Sedimentation velocity measurements
Sedimentation analysis was performed at the National Analytical Ultracentrifugation Facility at the University of Connecticut. Samples were equilibrated by dialysis into Dulbecco’s PBS (Mg++ and Ca++ -free) buffer (pH 7.0). Extinction coefficient, molecular mass, and partial specific volume of the protein portion of hFcILZOX40L were determined using the Sednterp program (Laue, 1992). Sedimentation velocity analytical ultracentrifugation was performed with a Beckman Coulter XL-I instrument at 20°C and 44,000 RPM. Interference data was collected using a scan interval of 40 seconds. Data were first analyzed with the program DcDt+ (Philo, 2000, 2006). A rough estimate of the carbohydrate content of the sample was made by performing a separate synthetic boundary run. Data were collected using the absorbance optical system at 280 nm, and the interference optical system. The excess interference signal over that calculated for the protein contribution, as measured by the A280 signal, can be accounted for by the presence of approximately 15% by weight of carbohydrate. Assuming 15% carbohydrate content, a value for the partial specific volume of the glycoprotein was estimated to be 0.72 ml/gm, using an average value for the carbohydrate contribution of 0.63 ml/gm (Shire, 1994). The molecular mass and sedimentation coefficient of the predominant species was determined using the program Sedphat Version 4.3 (Schuck, 2003). Data obtained at the three concentrations were globally fit using a hybrid local continuous and global discrete model where the major peak was fit as a single discrete species and the material at higher and lower S was accounted for by two continuous distributions.
Rotary Shadowing and Electron Microscopy. Rotary shadowing and electron microscopy was performed as previously described (Morris et al., 1986).
Results
The cDNA construct used to express the soluble form of human OX40L which contained the complete extracellular domain of OX40 ligand fused to the N-terminus of the Fcγ domain of human IgG1 via an isoleucine zipper (ILZ) trimerization domain is depicted in figure 1A. The construct and protein will be referred to as hFcILZOX40L. The amino acid sequence encoded by this construct includes two additional amino acids encoded by each of the two restriction sites used to link the domains together and contains four consensus N-linked glycosylation sites (NXS/T) within the hOX40L sequence. This cDNA was cloned into a derivative of the expression vector pCEP4 that contained the signal sequence of the basement membrane protein, BM40 (Mayer et al., 1993). hFcILZOX40L was expressed in human embryonic kidney 293 cells at a rate of 2 ug/106 cells/24 hours when cultured in plates indicating suitability for large-scale production. For characterization of hFcILZOX40L, transfected cell lines were cultured in a hollow fiber bioreactor system and the conditioned medium fractionated by protein-G affinity chromatography. Elution of hFcILZOX40L from protein-G at pH 2.8 resulted in the formation of micro-aggregates of protein (see figure 5c). We found that functional hFcILZOX40L was obtained when the protein was eluted from protein G at neutral pH (see methods). The resulting recombinant OX40L fusion protein was expected to assemble into the structure pictured in figure 1B, which was based on the previously described structure of the Fc:FasL fusion protein (Holler et al., 2003). Six polypeptide chains would be expected to form three disulfide-bonded dimers through the naturally dimeric Fc domains and two trimers via the ILZ and OX40L domains. In one of the Fc dimer pairs, the ILZ and OX40L domains of the two polypeptide chains would contribute to two different OX40L trimers.
Figure 5.
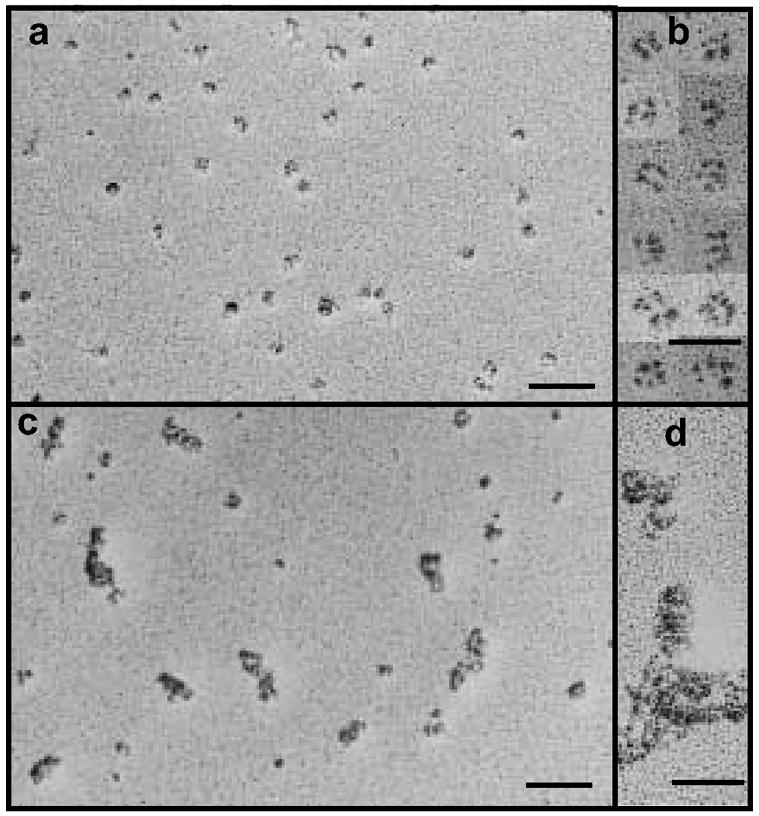
Analysis of the structure of hFcILZOX40L by rotary shadowing and electron microscopy. (a, b) hFcILZOX40L eluted form protein-g at neutral pH; (c, d) hfcILZOX40L eluted from protein-G at acidic pH. The scale bars for the fields in a and c are 100 nm and for the selected images in b and d, 50 nm.
Secreted hFcILZOX40L isolated from bioreactor supernatant by protein G chromatography was characterized by SDS polyacrylamide gel electrophoresis. When stained with coomassie blue (fig 2A), the gel (under reducing conditions) showed a predominant band corresponding to a polypeptide chain of approximately 55 kDa, instead of the 46.8 kDa predicted from the amino acid sequence. This discrepancy and the diffuseness of the band suggested that glycosylation had occurred at one or more of the consensus sites. The presence of other bands indicates that further purification, by size exclusion chromatography for example (see fig 3), would be necessary. A diffuse band of approximately 90–100kDa was observed under non-reducing conditions and was consistent with a disulfide-bonded dimer, the highest order covalent structure possible. An unidentified band of approximately 50 kDa was also present. The gels were subjected to immunoblotting with antibodies to either human IgG or to human OX40L to confirm the presence of these domain in the molecule (fig. 2B). Under reducing conditions, the anti-human IgG antibody identified the broad 55kDa band seen in the coomassie stained gel; this band was only very weakly stained with the anti-human OX40L antibody. However, under non-reducing conditions, both antibodies detected the 90–100kDa dimer bands suggesting that the OX40L antibody recognized an epitope that is dependent on the intrachain disulfide bond present in human OX40L. An unidentified faster migrating band (Mr approximately 50 kDa) was also detected by anti-IgG but not by anti-OX40L. This could be the same band observed after staining with coomassie blue and suggests the presence of a truncated form of the hFcILZOX40L protein or an unrelated contaminant that binds anti-human IgG antibody.
Figure 2.
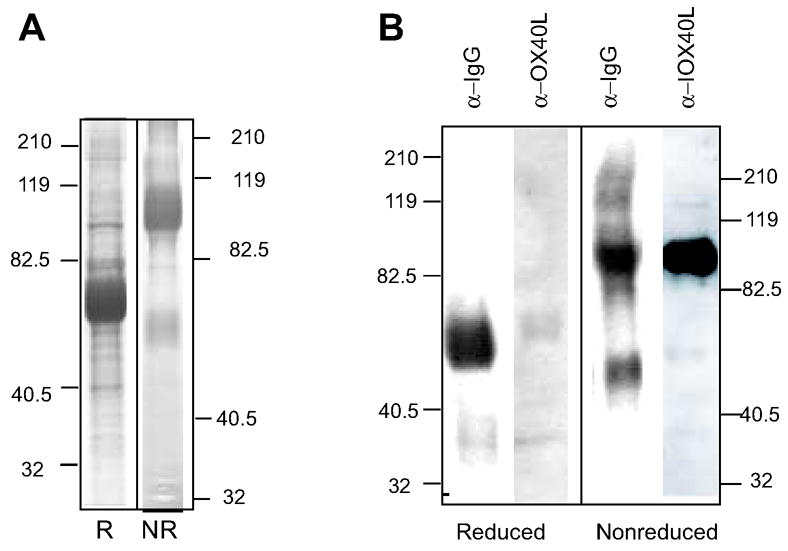
Analysis of hFcILXOX40L by SDS PAGE. hFCcILZOX40L was fractionated by chromatography on Protein-G sepharose and analyzed by SDS PAGE. A. The reduced (R) and non-reduced (NR) samples were run on separate gels and stained with Coomassie Blue. B. After SDS PAGE of reduced and non-reduced samples, hFCILZOX40L was analyzed by immunoblotting using antibodies to human IgG (α-IgG) and to human OX40L (α-OX40L) (see methods). Detection of the OX40L domain by α-OX40L after reduction of the internal disulfide bond in this domain was greatly diminished.
Figure 3.
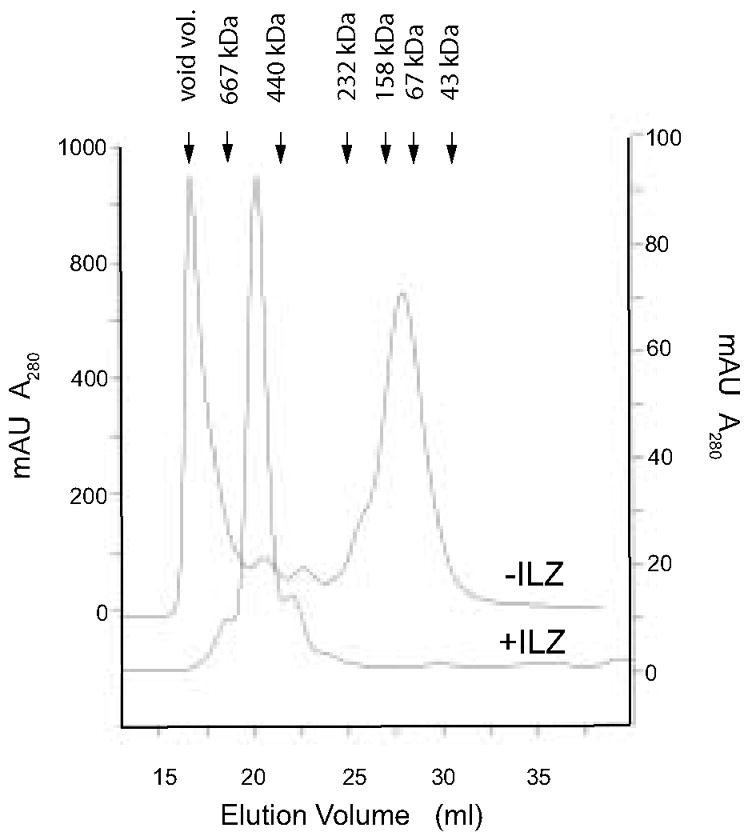
Analysis of hFcILZOX40L by molecular sieve chromatography on Superdex G200. After fractionation by protein G chromatography, an aliquot of the fusion protein was applied to Superdex G200 in PBS. hFcOX40L that lacked the ILZ was chromatographed in the same way and its elution profile overlaid. The position of molecular weight standards is indicated. The molecular weight of an hFCILZOX40L hexamer calculated from the amino acid sequence alone is 240kDa, however it elutes at approximately 540kDa.
To investigate the native structure of the recombinant protein, hFcILZOX40L was analyzed by size exclusion chromatography (SEC) (fig 3). A single symmetrical major peak eluting at 540 kDa relative to globular standards was observed. The small leading and trailing peaks likely correspond to the contaminants observed by SDS PAGE in figure 2A. From the mobility observed by SDS PAGE, we expected a mass of approximately 330kDa for the hexameric hFcILZOX40L. This suggested that either some higher order structure, such as two hexamers, was obtained, or that the hexamer was asymmetric and behaved as a larger molecule. Also depicted in figure 3 is the SEC analysis of a preparation of Fc:OX40L produced without an ILZ domain. This material eluted either as aggregates in the void volume or in a broad peak between 67 and 158 kDa that suggested formation of dimers rather than hexamers. A small peak eluted at the same position as hFcILZOX40L and was recovered from chromatography as a purified fraction of the Fc:OX40L total preparation.
In order to exclude asymmetry as a factor responsible for the unexpected molecular weight and to confirm a hexameric assembly, hFcILZOX40L was purified by SEC and analyzed by velocity sedimentation (figure 4). The apparent sedimentation coefficient distribution function g(s*) versus s* shows the frequency of encountering a specific sedimenting species, g(s*), as a function of the sedimentation velocity as indicated by the sedimentation coefficient, s*. Sedimentation is strongly affected by the reversible interaction between sedimenting molecules and such interaction is concentration dependent. The fact that the normalized g(s*) plots for the three different concentrations can be superimposed and that there was no concentration-dependent shift in the peak position was strong evidence that, over the concentration range studied (0.15 mg/ml to 1.18 mg/ml), there were no reversible reactions occurring. The diffusion coefficient was also measured and allowed shape-independent calculation of molecular weight from sedimentation analysis. A composition of 15% carbohydrate for hFcILZOX40L was estimated by sedimentation and using this value (see methods), the fitted value for the molecular weight of the main species (74 ± 2 %) was 336 kDa [324 kDa, 347 kDa, 95% confidence limits]. This is consistent with a hexameric assembly of 55kDa protomers.
Figure 4.
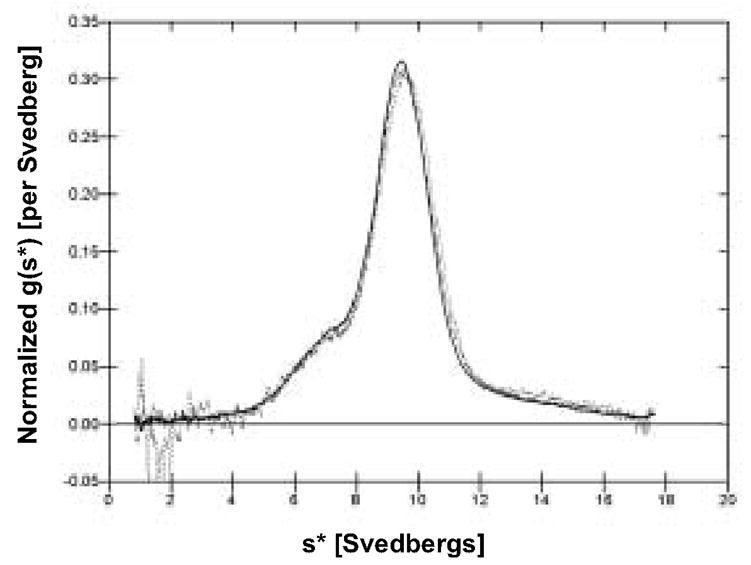
Sedimentation analysis of hFcILZOX40L. Sedimentation was performed at three concentrations, 1.18 mg/ml (solid line), 0.46 mg/ml (dashed line) and 0.15 mg/ml (dotted line). The overlaid plots of the frequency distribution of molecules with different sedimentation coefficients [normalized g(s*) versus s*] are presented. Superimposition of sedimentation profiles at all three concentrations indicates a non-interacting system and validates the determination of the sedimentation coefficient and the molecular weight. Time-derivative analysis (Stafford, 1992) was performed on the sedimentation velocity concentration profiles to obtain g(s*) profiles using the program, DcDt+, Version 2.07 (Philo, 2006).
The fitted value for the corrected sedimentation coefficient was 9.86 Svedbergs [9.84 S, 9.88 S, 95% confidence limits]. There was 16 ± 2 % of the sample sedimenting slower and 10 ± 2 % of the sample sedimenting faster than the main species. The faster sedimenting material is likely small aggregates arising from ultrafiltration used to concentrate the sample from SEC prior to sedimentation. The nature of the slower sedimenting species is unknown but could be dimers or trimers (instead of hexamers) that were unresolved by SEC.
Further evidence that hFcILZOX40L assembled into the expected hexamer was obtained by rotary shadowing and electron microscopy (figure 5). At lower magnification (fig. 5a) the field showed a number of individual molecules with apparent substructure and little evidence of aggregation. When selected molecules were observed at higher magnification (fig 5b), a subunit-like organization was evident. At the resolution obtained, we were unable to delineate five distinct domains, but the pattern was consistent with the molecular architecture predicted in figure 1B (Holler et al., 2003). As mentioned previously, exposure to acidic pH disrupted the native structure of hFcILZOX40L. This was visualized by examination of the rotary shadowed images of hFcILZOX40L eluted from protein-G columns at pH 2.8 (fig. 5c,d)., in which most of the protein was found in aggregates.
The ability of FcILZOX40L to costimulate the proliferation of human CD4+ T cells that had received sub-mitogenic T-cell receptor stimulation with low levels of anti-CD3 antibody was assessed. In the assay utilized for figure 6, both the anti-CD3 and the OX40 agonist, anti-OX40 antibody or recombinant OX40L, were plate-bound by means of anti-mouse or anti-human IgG capture antibodies. Purified CD4+ T-cells were initially stimulated with PHA for three days so figures 6 and 7 depict proliferation of CD4+ effector T cells after OX40 costimulation. FcILZOX40L increased CD4+ T-cell proliferation, as measured by 3H-thymidine incorporation, 8-fold compared to anti-CD3 alone (figure 6A). FcOX40L, lacking the ILZ domain, showed no activity in this assay. However, the small fraction of hexameric FcOX40L that was purified from this preparation (purified FcOX40L, see figure 3) had the same effective concentration (EC50) as hFcILZOX40L. Costimulation of proliferation by hFcILZOX40L was very similar to that achieved by the OX40 agonist antibodies L106 and 9B12 (figure 6B); the latter is currently in a phase I clinical trial in cancer patients. The effective concentrations (EC50) of the different OX40 agonists were similar, between 3 – 8.0 nM, and results from several experiments indicated that the EC50 of plate-bound hFcILZOX40L, 9B12 and L106 were the same.
Figure 6.
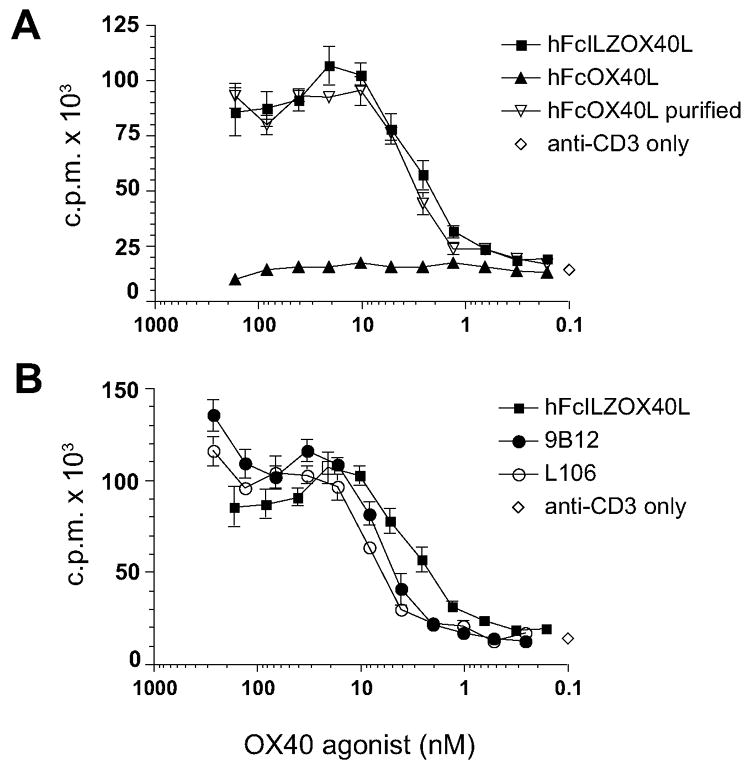
Plate-bound costimulation assay. The biological activity of hFcILZOX40Lwas measured by its ability to promote effector CD4+ T-cell division, as measured by 3H-thymidine incorporation, at suboptimal concentrations of anti-CD3. A. The costimulatory activity of hFcILZOX40L was compared to FcOX40L, lacking the ILZ, and the purified fraction of FcOX40L that co-elutes with hFcILZOX40L by SEC (see fig 3). B. Costimulation of proliferation by FcILZOX40L was compared to the OX40 agonist mouse monoclonal antibodies, L106 and 9B12. The curve for hFcILZOX40L is the same in both A and B. Anti-mouse and/or anti-human capture antibodies were used to anchor anti-CD3, L106, FcOX40L and hFcILZOX40L to the plate and the graphs show 2-fold serial dilutions of the OX40 agonists.
Figure 7.
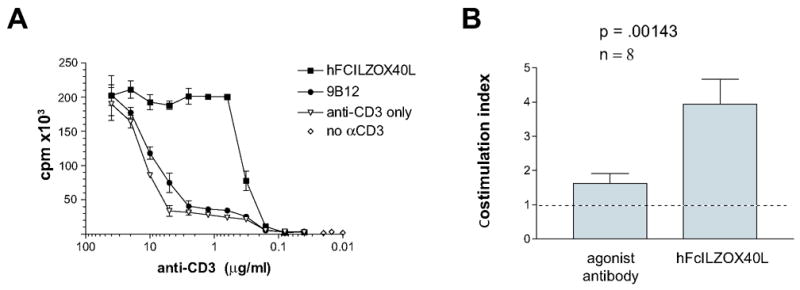
Soluble costimulation assay. The ability of soluble preparations of hFcILZOX40L and the agonist antibody, 9B12, to enhance CD4+ effector T-cell proliferation was measured by incorporation of 3H-thymidine as a function of plate-bound anti-CD3 concentration. A. The concentration of anti-CD3 used to coat the plate is indicated on the abscissa. The final concentration of hFcXILOX40L and 9B12 added to the cells was 35.7 nM and 66.7 nM respectively. These concentrations exceeded the amount needed to provide maximal costimulation in this assay. B. The maximum costimulation was determined from eight experiments and the costimulation provided by agonist antibody or hFcILZOX40L normalized to anti-CD3 alone is presented as a stimulation index. The range for hFCILZOX40L was 2.21 to 8.33 and for agonist antibody 1.08 to 3.63. The results for the L106 and 9B12 agonist antibodies were combined.
We also assessed in vitro costimulation of T-cell proliferation when the OX40 agonists were added in solution rather than being bound to the plate. This made the assay more stringent (i.e. more difficult to demonstrate costimulatory activity) and thus may reveal differences between the OX40 agonists. PHA-activated CD4+ T cells were exposed to decreasing amounts of plate bound anti-CD3 and a constant amount of soluble OX40 agonist. Under these conditions hFcILZOX40L showed a seven-to 10-fold stimulation index compared to anti-CD3 alone, over the range of 0.5 – 2.0 ug/ml anti-CD3 (figure 7A). The agonist antibody, 9B12, showed almost no costimulatory activity in this experiment. Proliferation by soluble OX40 agonists compared to CD3 alone was assessed in eight separate experiments (figure 7B) and hFcILZOX40L showed significantly greater costimulatory activity compared to the OX40 agonist antibody.
Discussion
The antitumor effects following OX40 engagement in mouse tumor models (Kjaergaard et al., 2001; Kjaergaard et al., 2000; Morris et al., 2001; Pan et al., 2002; Weinberg et al., 2000) and the safety of anti-OX40 antibodies in primates (Weinberg et al., 2006) make a compelling case for pursuing this approach in patients with cancer. A phase I clinical trial in patients with advanced cancer is currently underway at our institution. The anti-OX40 is a mouse anti-human monoclonal antibody (9B12) and the development of HAMA limits therapy to one round of treatment in the first two weeks. While this scheme is effective in mouse tumor models, it is not likely to be adequate to treat patients with cancer where several treatments may be needed. To overcome this limitation, we designed and produced a recombinant human Ig:OX40L fusion protein. Direct linkage of the extracellular domain of OX40L to the Fcγ domain was not effective as the final protein product due to aggregation (fig 3) and lack of costimulatory activity (fig. 6). The lack of activity could have been due to instability within the receptor-binding domain as was observed for CD40L, FasL, TRAIL and 4-1BBL (Morris et al., 1999; Rabu et al., 2005; Shiraishi et al., 2004; Wu et al., 2001). The stalk region enhanced the stability and function of 4-1BBL (Rabu et al., 2005) and CD40L (Morris et al., 1999); however, the OX40L stalk region has only seven amino acids (Compaan and Hymowitz, 2006) and despite its inclusion in the original construct, the Ig:OX40L fusion protein failed.
One approach that has led to TNF-ligand stability is the addition of a trimerization domain in the form of an isoleucine zipper (ILZ) from yeast GCN4. When ILZ was added to CD40L, the EC50 for B-cell proliferation was lowered by two orders of magnitude compared to the receptor-binding domain alone and one order of magnitude lower than the receptor binding domain plus stalk region (Morris et al., 1999). In this case, the stabilization was attributed to maintenance of the protomer structure by anchoring the N-terminus. TRAIL and FasL also exhibited enhanced activity after inclusion of an ILZ domain, which reinforced the trimeric interaction of the receptor-binding domain (Shiraishi et al., 2004; Walczak et al., 1999; Wu et al., 2001). For hFcILZOX40L, inclusion of the ILZ domain may help to stabilize the OX40L trimer as well, although no direct comparison was made to extracellular OX40L domain alone. The mouse Ig:OX40L seems to function without an additional trimerizing domain (Ali et al., 2004; Morris et al., 2001), but the mouse and human sequences are quite different. Compared to the human OX40L extracellular domain, the mouse has only about 40% sequence identity, contains an additional non-homologous 13 amino acids at the C-terminus and has 1.5 times the trimer interface surface area (Compaan and Hymowitz, 2006). Hence the ILZ may be necessary to contribute to the stability of hFcILZOX40L. Since biosynthesis of human Ig:OX40L without the ILZ resulted in aggregates as well as lower molecular weight forms, it is also possible that the ILZ domain is required to provide a productive path for the assembly of protomers into hexamers.
In a previous report, the fusion protein, Fc:FasL, was prepared by linking the TNF homology domain to the Fc domain of IgG via a flexible linker. The authors concluded that this design resulted in the formation of a molecular hexamer with five distinct domains: the Fc domains assembled into three dimers stabilized by interchain disulfide bonds and the C-terminal TNF domains assembled into two noncovalent trimers (Holler et al., 2003). Using the same strategy, with the isoleucine zipper serving as the linker region, the recombinant soluble OX40L molecule described here appears to follow this assembly mechanism effectively. This was confirmed by the determination of a molecular weight for the native molecule of 336 kDa by sedimentation analysis, a mass that was consistent with a hexamer of 55 kDa protomers. The molecular weight of 55 kDa for the protomer included 15–17% contributed by glycosylation that was determined indirectly by SDS PAGE (48.6 kDa mass calculated from the sequence) and by optical methods (the difference between interference optics and A280). The glycosylation is likely N-linked involving N152 and perhaps one of the other three potential glycosylation sites in the OX40L sequence, N90, N114, N157 (Compaan and Hymowitz, 2006). As predicted from the model, a disulfide bonded dimer (SDS PAGE, figure 2) is the largest covalently linked subunit structure after denaturation. Finally, the images of hFcILZOX40L obtained by rotary shadowing and electron microscopy (fig. 5) were suggestive of linked globular domains and were very similar to the images obtained for the Fc:FasL molecule (Holler et al., 2003).
One possible limitation on the applicability of hFcILZOX40L in clinical trials is the yeast origin of the GCN4 ILZ domain that may induce neutralizing immunity in patients. The immunogenicity of this GCN4 ILZ domain has already been investigated in clinical trials of ILZ-CD40L, where only two of thirty-one patients developed antibodies to ILZ-CD40L (Vonderheide et al., 2001). Whether the ILZ itself or junctional domains contributed to the immunogenicity was not determined. We cannot predict the immunogenicity of ILZ in the context of human FcILZOX40L since this construct presents unique junctional domains. However based on the experience with ILZ-CD40L, neutralizing immunity may not interfere with repetitive therapy for most prospective patients.
The assembly of six or more TNF-family protomers [(TNF3)n, n≥2] within one signaling molecule appears to have advantages with regard to function. At least two associated trimers (n=2) of FASL and 4-1BBL are required to achieve the most effective signal transduction through their respective receptors (Holler et al., 2003; Rabu et al., 2005) and lung surfactant CD40L fusion protein that links four CD40L trimers (n=4) is significantly more effective than a single stabilized CD40L trimer (n=1) (Haswell et al., 2001). Interestingly, lung surfactant protein also provides an endogenous coiled-coil trimerization domain adjacent to CD40L, although the size and complexity (dodecamer) may promote rapid clearance and therefore limit therapeutic capability. As discussed by Holler et al., (2003), the assembling of two or more trimeric ligands would be expected to generate and bring into close proximity multiple trimeric receptors. Such an arrangement may better resemble the two interacting cell surfaces present in vivo and indicates an advantage for close association of neighboring receptor complexes. In this study, hFcILZOX40L showed potent costimulatory activity in vitro not only when plate-bound (fig 6) where clustering of ligand is achieved by capture antibody, but also when added in solution to purified T cells (fig 7) where clustering of adjacent trimeric receptors is dependent on the structure of the soluble ligand.
In the context of immunotherapy, exploration of the efficacy of hFcILZOX40L rather than developing a humanized anti-OX40 antibody can be justified for the following reasons. hFcILZOX40L appears to be a more potent OX40 agonist than murine anti-hOX40 antibody in vitro (fig. 7) and there is no reason to expect better agonistic activity from a humanized antibody. In addition to activated CD4+ T cells, OX40 signaling also occurs in activated OX40+ CD8+T cells (Bansal-Pakala et al., 2004) T regulatory cells (Ito et al., 2006; Valzasina et al., 2005), and neutrophils. Because it better mimics in vivo ligation of OX40, hFcILZOX40L may impart both more potent and qualitatively different signaling than agonist antibody (murine or humanized) in these different target cells as has been discussed for CD40L vs. anti-CD40 (Fanslow et al., 1994). While the Fc domain of hFcILZOX40L may enhance circulatory half-life, we expect that its half-life would be significantly shorter than that of a humanized antibody. This may be advantageous for a T-cell agonist activity where excessive and prolonged exposure could have a tolerizing effect or conversely cause excessive agonist activity leading to unwanted inflammation and toxicity. We think that ultimately hFcILZOX40L protein could become a powerful tool for cancer immunotherapy and also could be used as a vaccine adjuvant to help clear harmful pathogens.
Acknowledgments
The authors wish to thank Drs, Jeffery Lary and James Cole at the National Analytical Ultracentrifugation Facility, University of Connecticut, for the velocity sedimentation analysis. The authors also wish to thank Doug Keene at the Shriners Hospital for Children, Portland OR, for the analysis of recombinant hFcILZOX40L by rotary shadowing and electron microscopy. This work was funded by grant #R01CA109563 from the National Institutes of Health and by the Providence Portland Medical Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- al-Shamkhani A, Birkeland ML, Puklavec M, Brown MH, James W, Barclay AN. OX40 is differentially expressed on activated rat and mouse T cells and is the sole receptor for the OX40 ligand. Eur J Immunol. 1996;26:1695–1699. doi: 10.1002/eji.1830260805. [DOI] [PubMed] [Google Scholar]
- Ali SA, Ahmad M, Lynam J, McLean CS, Entwisle C, Loudon P, Choolun E, McArdle SE, Li G, Mian S, Rees RC. Anti-tumour therapeutic efficacy of OX40L in murine tumour model. Vaccine. 2004;22:3585–3594. doi: 10.1016/j.vaccine.2004.03.041. [DOI] [PubMed] [Google Scholar]
- Bansal-Pakala P, Halteman BS, Cheng MH, Croft M. Costimulation of CD8 T cell responses by OX40. J, Immunol. 2004;172:4821–4825. doi: 10.4049/jimmunol.172.8.4821. [DOI] [PubMed] [Google Scholar]
- Bodmer JL, Schneider P, Tschopp J. The molecular architecture of the TNF superfamily. Trends in biochemical sciences. 2002;27:19–26. doi: 10.1016/s0968-0004(01)01995-8. [DOI] [PubMed] [Google Scholar]
- Compaan DM, Hymowitz SG. The crystal structure of the costimulatory OX40-OX40L complex. Structure. 2006;14:1321–1330. doi: 10.1016/j.str.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3:609–620. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- Evans DE, Prell RA, Thalhofer CJ, Hurwitz AA, Weinberg AD. Engagement of OX40 enhances antigen-specific CD4(+) T cell mobilization/memory development and humoral immunity: comparison of alphaOX-40 with alphaCTLA-4. J Immunol. 2001;167:6804–6811. doi: 10.4049/jimmunol.167.12.6804. [DOI] [PubMed] [Google Scholar]
- Fanslow WC, Srinivasan S, Paxton R, Gibson MG, Spriggs MK, Armitage RJ. Structural characteristics of CD40 ligand that determine biological function. Semin Immunol. 1994;6:267–278. doi: 10.1006/smim.1994.1035. [DOI] [PubMed] [Google Scholar]
- Gramaglia I, Weinberg AD, Lemon M, Croft M. Ox-40 ligand: a potent costimulatory molecule for sustaining primary CD4 T cell responses. J Immunol. 1998;161:6510–6517. [PubMed] [Google Scholar]
- Harbury PB, Zhang T, Kim PS, Alber T. A switch between two-, three-, and four-stranded coiled coils in GCN4 leucine zipper mutants. Science. 1993;262:1401–1407. doi: 10.1126/science.8248779. [DOI] [PubMed] [Google Scholar]
- Haswell LE, Glennie MJ, Al-Shamkhani A. Analysis of the oligomeric requirement for signaling by CD40 using soluble multimeric forms of its ligand, CD154. Eur J Immunol. 2001;31:3094–3100. doi: 10.1002/1521-4141(2001010)31:10<3094::aid-immu3094>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Holler N, Tardivel A, Kovacsovics-Bankowski M, Hertig S, Gaide O, Martinon F, Tinel A, Deperthes D, Calderara S, Schulthess T, et al. Two adjacent trimeric Fas ligands are required for Fas signaling and formation of a death-inducing signaling complex. Mol Cell Biol. 2003;23:1428–1440. doi: 10.1128/MCB.23.4.1428-1440.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huddleston CA, Weinberg AD, Parker DC. OX40 (CD134) engagement drives differentiation of CD4(+) T cells to effector cells. Eur J Immunol. 2006;36:1093–1103. doi: 10.1002/eji.200535637. [DOI] [PubMed] [Google Scholar]
- Ito T, Wang YH, Duramad O, Hanabuchi S, Perng OA, Gilliet M, Qin FX, Liu YJ. OX40 ligand shuts down IL-10-producing regulatory T cells. Proc Natl Acad Sci U S A. 2006;103:13138–13143. doi: 10.1073/pnas.0603107103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaleeba JA, Offner H, Vandenbark AA, Lublinski A, Weinberg AD. The OX-40 receptor provides a potent co-stimulatory signal capable of inducing encephalitogenicity in myelin-specific CD4+ T cells. Int Immunol. 1998;10:453–461. doi: 10.1093/intimm/10.4.453. [DOI] [PubMed] [Google Scholar]
- Kjaergaard J, Peng L, Cohen PA, Drazba JA, Weinberg AD, Shu S. Augmentation versus inhibition: effects of conjunctional OX-40 receptor monoclonal antibody and IL-2 treatment on adoptive immunotherapy of advanced tumor. J Immunol. 2001;167:6669–6677. doi: 10.4049/jimmunol.167.11.6669. [DOI] [PubMed] [Google Scholar]
- Kjaergaard J, Tanaka J, Kim JA, Rothchild K, Weinberg A, Shu S. Therapeutic efficacy of OX-40 receptor antibody depends on tumor immunogenicity and anatomic site of tumor growth. Cancer Res. 2000;60:5514–5521. [PubMed] [Google Scholar]
- Ladanyi A, Somlai B, Gilde K, Fejos Z, Gaudi I, Timar J. T-cell activation marker expression on tumor-infiltrating lymphocytes as prognostic factor in cutaneous malignant melanoma. Clin Cancer Res. 2004;10:521–530. doi: 10.1158/1078-0432.ccr-1161-03. [DOI] [PubMed] [Google Scholar]
- Laue TM, Shah BD, Ridgeway TM, Pelletier SL. Computer-Aided Interpretation of Analytical Sedimentation Data For Proteins. In: Harding S, Rowe A, editors. Analytical Ultracentrifugation in Biochemistry and Polymer Science. Royal Society of Chemistry; London: 1992. pp. 90–125. [Google Scholar]
- Lo KM, Sudo Y, Chen J, Li Y, Lan Y, Kong SM, Chen L, An Q, Gillies SD. High level expression and secretion of Fc-X fusion proteins in mammalian cells. Protein Eng. 1998;11:495–500. doi: 10.1093/protein/11.6.495. [DOI] [PubMed] [Google Scholar]
- Mallett S, Fossum S, Barclay AN. Characterization of the MRC OX40 antigen of activated CD4 positive T lymphocytes--a molecule related to nerve growth factor receptor. Embo J. 1990;9:1063–1068. doi: 10.1002/j.1460-2075.1990.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer U, Nischt R, Poschl E, Mann K, Fukuda K, Gerl M, Yamada Y, Timpl R. A single EGF-like motif of laminin is responsible for high affinity nidogen binding. Embo J. 1993;12:1879–1885. doi: 10.1002/j.1460-2075.1993.tb05836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A, Vetto JT, Ramstad T, Funatake CJ, Choolun E, Entwisle C, Weinberg AD. Induction of anti-mammary cancer immunity by engaging the OX-40 receptor in vivo. Breast Cancer Res, Treat. 2001;67:71–80. doi: 10.1023/a:1010649303056. [DOI] [PubMed] [Google Scholar]
- Morris AE, Remmele RL, Jr, Klinke R, Macduff BM, Fanslow WC, Armitage RJ. Incorporation of an isoleucine zipper motif enhances the biological activity of soluble CD40L (CD154) J Biol Chem. 1999;274:418–423. doi: 10.1074/jbc.274.1.418. [DOI] [PubMed] [Google Scholar]
- Morris NP, Keene DR, Glanville RW, Bentz H, Burgeson RE. The tissue form of type VII collagen is an antiparallel dimer. J Biol Chem. 1986;261:5638–5644. [PubMed] [Google Scholar]
- Pan PY, Zang Y, Weber K, Meseck ML, Chen SH. OX40 ligation enhances primary and memory cytotoxic T lymphocyte responses in an immunotherapy for hepatic colon metastases. Mol Ther. 2002;6:528–536. doi: 10.1006/mthe.2002.0699. [DOI] [PubMed] [Google Scholar]
- Paterson DJ, Jefferies WA, Green JR, Brandon MR, Corthesy P, Puklavec M, Williams AF. Antigens of activated rat T lymphocytes including a molecule of 50,000 Mr detected only on CD4 positive T blasts. Mol Immunol. 1987;24:1281–1290. doi: 10.1016/0161-5890(87)90122-2. [DOI] [PubMed] [Google Scholar]
- Petty JK, He K, Corless CL, Vetto JT, Weinberg AD. Survival in human colorectal cancer correlates with expression of the T-cell costimulatory molecule OX-40 (CD134) Am J Surg. 2002;183:512–518. doi: 10.1016/s0002-9610(02)00831-0. [DOI] [PubMed] [Google Scholar]
- Philo JS. A method for directly fitting the time derivative of sedimentation velocity data and an alternative algorithm for calculating sedimentation coefficient distribution functions. Anal Biochem. 2000;279:151–163. doi: 10.1006/abio.2000.4480. [DOI] [PubMed] [Google Scholar]
- Philo JS. Improved methods for fitting sedimentation coefficient distributions derived by time-derivative techniques. Anal Biochem. 2006;354:238–246. doi: 10.1016/j.ab.2006.04.053. [DOI] [PubMed] [Google Scholar]
- Rabu C, Quemener A, Jacques Y, Echasserieau K, Vusio P, Lang F. Production of recombinant human trimeric CD137L (4-1BBL). Cross-linking is essential to its T cell co-stimulation activity. J Biol Chem. 2005;280:41472–41481. doi: 10.1074/jbc.M506881200. [DOI] [PubMed] [Google Scholar]
- Ramstad T, Lawnicki L, Vetto J, Weinberg A. Immunohistochemical analysis of primary breast tumors and tumor-draining lymph nodes by means of the T-cell costimulatory molecule OX-40. Am J Surg. 2000;179:400–406. doi: 10.1016/s0002-9610(00)00361-5. [DOI] [PubMed] [Google Scholar]
- Schuck P. On the analysis of protein self-association by sedimentation velocity analytical ultracentrifugation. Anal Biochem. 2003;320:104–124. doi: 10.1016/s0003-2697(03)00289-6. [DOI] [PubMed] [Google Scholar]
- Shiraishi T, Suzuyama K, Okamoto H, Mineta T, Tabuchi K, Nakayama K, Shimizu Y, Tohma J, Ogihara T, Naba H, et al. Increased cytotoxicity of soluble Fas ligand by fusing isoleucine zipper motif. Biochem Biophys Res Commun. 2004;322:197–202. doi: 10.1016/j.bbrc.2004.07.098. [DOI] [PubMed] [Google Scholar]
- Shire S. In: Modern Analytical Ultracentrifugation. Schuster TM, Laue TM, editors. Birkhauser; Boston: 1994. pp. 261–297. [Google Scholar]
- Stafford WF., 3rd Boundary analysis in sedimentation transport experiments: a procedure for obtaining sedimentation coefficient distributions using the time derivative of the concentration profile. Anal Biochem. 1992;203:295–301. doi: 10.1016/0003-2697(92)90316-y. [DOI] [PubMed] [Google Scholar]
- Sugamura K, Ishii N, Weinberg AD. Therapeutic targeting of the effector T-cell co-stimulatory molecule OX40. Nat Rev Immunol. 2004;4:420–431. doi: 10.1038/nri1371. [DOI] [PubMed] [Google Scholar]
- Valzasina B, Guiducci C, Dislich H, Killeen N, Weinberg AD, Colombo MP. Triggering of OX40 (CD134) on CD4(+)CD25+ T cells blocks their inhibitory activity: a novel regulatory role for OX40 and its comparison with GITR. Blood. 2005;105:2845–2851. doi: 10.1182/blood-2004-07-2959. [DOI] [PubMed] [Google Scholar]
- Vonderheide RH, Dutcher JP, Anderson JE, Eckhardt SG, Stephans KF, Razvillas B, Garl S, Butine MD, Perry VP, Armitage RJ, et al. Phase I study of recombinant human CD40 ligand in cancer patients. J Clin Oncol. 2001;19:3280–3287. doi: 10.1200/JCO.2001.19.13.3280. [DOI] [PubMed] [Google Scholar]
- Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- Weinberg AD. OX40: targeted immunotherapy - implications for tempering autoimmunity and enhancing vaccines. Trends in Immunology. 2002;23:102–109. doi: 10.1016/s1471-4906(01)02127-5. [DOI] [PubMed] [Google Scholar]
- Weinberg AD, Rivera MM, Prell R, Morris A, Ramstad T, Vetto JT, Urba WJ, Alvord G, Bunce C, Shields J. Engagement of the OX-40 receptor in vivo enhances antitumor immunity. J Immunol. 2000;164:2160–2169. doi: 10.4049/jimmunol.164.4.2160. [DOI] [PubMed] [Google Scholar]
- Weinberg AD, Thalhofer C, Morris N, Walker JM, Seiss D, Wong S, Axthelm MK, Picker LJ, Urba WJ. Anti-OX40 (CD134) administration to nonhuman primates: immunostimulatory effects and toxicokinetic study. J Immunother. 2006;29:575–585. doi: 10.1097/01.cji.0000211319.00031.fc. [DOI] [PubMed] [Google Scholar]
- Weinberg AD, Vella AT, Croft M. OX-40: life beyond the effector T cell stage. Semin Immunol. 1998;10:471–480. doi: 10.1006/smim.1998.0146. [DOI] [PubMed] [Google Scholar]
- Weinberg AD, Wegmann KW, Funatake C, Whitham RH. Blocking OX-40/OX-40 ligand interaction in vitro and in vivo leads to decreased T cell function and amelioration of experimental allergic encephalomyelitis. J Immunol. 1999;162:1818–1826. [PubMed] [Google Scholar]
- Wu X, He Y, Falo LD, Jr, Hui KM, Huang L. Regression of human mammary adenocarcinoma by systemic administration of a recombinant gene encoding the hFlex-TRAIL fusion protein. Mol Ther. 2001;3:368–374. doi: 10.1006/mthe.2001.0280. [DOI] [PubMed] [Google Scholar]


