INTRODUCTION
The 9+2 microtubule arrangement is the most conserved axonemal configuration, suggesting that this organization has been retained under evolutionary pressure for crucial functions. The primary role of the nine outer doublet microtubules is well established: the associated dynein motors power the microtubule sliding that ultimately results in flagellar bending [Satir, 1968; Brokaw, 1972; Shingyoji et al., 1977]. In contrast, the role of the two central pair of microtubules (CP) and the radial spokes (RS) that project towards the CP is far less definitive. The CP/RS system appears to play an important role in motility of 9+2 axonemes since mutant organelles defective in either of these structural complexes are paralyzed [Witman et al., 1978; Sturgess and Chao, 1982; Neugebauer et al., 1990]. However, these mutant axonemes can be experimentally induced to beat using modified reactivation conditions [Frey et al., 1997; Wakabayashi et al., 1997; Yagi and Kamiya, 2000]. In addition, motility of naturally occurring central pairless cilia and flagella has been reported [Baccetti et al., 1979; Prensier et al., 1980; Nonaka et al., 1998]. Most notably, recent studies have demonstrated that the 9+0 nodal cilia of developing embryos are motile, and this motility plays a critical role in establishing left-right asymmetry [Nonaka et al., 1998; Marszalek et al., 1999; Nonaka et al., 2002].
These observations provide compelling evidence that the central apparatus and radial spokes are not absolutely required for oscillatory beating. What, then, is their function in cilia and flagella that possess the 9+2 arrangement of microtubules? Recent structural, biochemical, and functional studies have provided important new insights into these seemingly contradictory phenomena. Here, we review these findings and propose a model in which the central apparatus and radial spokes serve as mechano-chemical sensors to control motility in 9+2 cilia and flagella.
FUNCTIONAL ANALYSIS OF THE CENTRAL APPARATUS / RADIAL SPOKE SYSTEM
Much of our current understanding of CP/RS function is derived from genetic, structural, and functional studies. As illustrated in Figure 1, the radial spokes are attached to each outer doublet microtubule; they project toward and interact with the projections of the central apparatus. Mutants defective in the central apparatus or radial spokes are found in many organisms and have paralyzed cilia and flagella [for example, Witman et al., 1978; Afzelius and Eliasson, 1979; Afzelius, 1985]. In Chlamydomonas, extragenic suppressor mutations have been identified that restore beating to these paralyzed mutants without restoring the missing structures [Huang et al., 1982]. Importantly, the flagella from the suppressed mutant cells produce only symmetric waveforms [Brokaw et al., 1982], indicating that the radial spokes and central apparatus may be involved in converting simple symmetric bends into the asymmetric waveforms required for forward swimming. The suppressor mutations were later found to be mutations in outer [Huang et al., 1982; Porter et al., 1994] or inner [Porter et al., 1992] dynein arm components, or in polypeptides comprising a dynein regulatory complex [Huang et al., 1982; Piperno et al., 1992, 1994]. These observations provide strong evidence that the central apparatus ultimately regulates dynein-driven microtubule sliding in a pathway that includes the radial spokes and dynein regulatory complex. Presumably, the paralysis caused by radial spoke and central pair defects reveals a control system that inhibits flagellar movement when these components are missing; the suppressor mutations restore motility by bypassing the inhibited state.
Fig. 1.
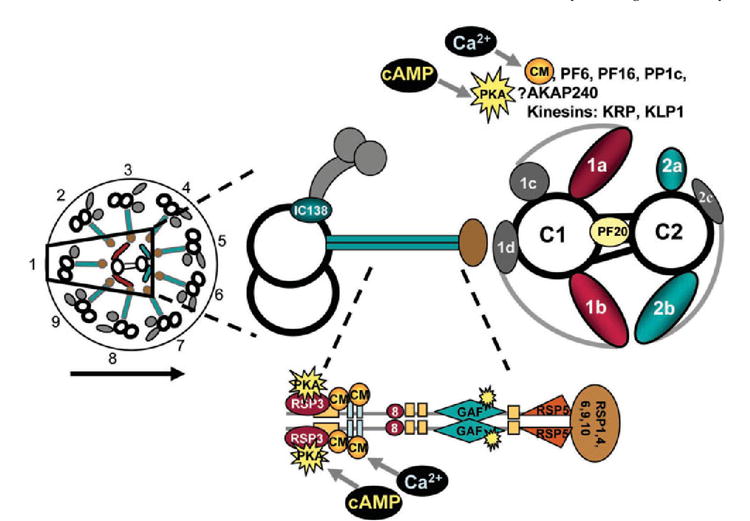
A transverse view of a Chlamydomonas flagellum as viewed looking proximal to distal; the outer doublet microtubules are numbered according to Hoops and Witman [1983]. The direction of the effective stroke is indicated by the arrow. Only doublet number one lacks an outer dynein arm. A single doublet microtubule (doublet one) with associated radial spoke, as well as the entire central apparatus, is enlarged. The radial spoke diagram is further enlarged to include details of the possible location of particular radial spoke components. RSP2 serves as the primary stalk component and consists of GAF domains as well as calmodulin (CM) binding domains [Yang et al., 2002]. The central apparatus diagram is adapted from Mitchell and Sale [1999]. While PF6, PF16, PP1c, AKAP240, KRP, and KLP1 have been tentatively localized to specific central tubules (see Table I), it is unknown whether they localize to specific projections. Therefore, they are listed above the central apparatus diagram. For references, see Table I.
Omoto et al. [1996] have proposed that the inhibited state may be due to ATP interaction with the regulatory P-loops of the dynein heavy chains. By using ATP analogs or low concentrations of ATP (<50 μM ATP), axonemes isolated from radial spoke or central apparatus defective mutants could be reactivated to produce modest waveforms at very low beat frequency (<5 Hz) [Omoto et al., 1996]. These results suggest that ATP plays an inhibitory role, and that the function of the CP/RS system may be to release the ATP inhibition in a controlled manner. In similar studies using 200 μM ATP, various salts (MgSO4 in particular), and organic compounds, Yagi and Kamiya [2000] were able to reactivate central pairless and radial spokeless mutant axonemes with increased beat frequencies (~20 Hz) and larger amplitude waveforms [Yagi and Kamiya, 2000]. These results demonstrate that the central pair/radial spokes are not required for high frequency oscillatory beating and are consistent with the observation that some naturally occurring 9+0 cilia and flagella, such as nodal cilia and 9+0 eel sperm, can beat with frequencies of ~20 and 90 Hz, respectively. Wakabayashi et al. [1997] and Frey et al. [1997] have demonstrated that isolated axonemes from central pairless and radial spokeless mutants reactivated at low ATP concentration also undergo modest waveform conversion in response to changes in calcium concentration. Therefore, the central apparatus and radial spokes are not an absolute requirement for calcium-induced waveform conversion.
Cilia and flagella with 9+2 axonemes generally have large amplitude, planar waveforms during at least one phase, if not the entire, beat cycle. It is possible that the CP/RS system contributes to the production of planar waveforms. The 9+0 axonemal variants generally beat with a low amplitude, 3-dimensional, helical waveform [Okada et al., 1999]. The CP/RS system may be required to establish the more powerful planar effective stroke as the intrinsic waveform for 9+2 axonemes beating under physiological conditions. This type of waveform would be particularly useful for cells encountering high workload, such as viscous mucous. For example, in mammals the helical waveform of the 9+0 nodal cilia may be crucial to establish a morphogen gradient or directed flow in early development [see, for example, Nonaka et al., 2002], whereas the 9+2 respiratory cilia beat with high-frequency, asymmetric, planar waveforms to sweep mucous and debris from the lungs. Therefore, the type of axoneme assembled appears to be tailored to the unique function of the particular cell type.
STRUCTURAL ANALYSIS OF CENTRAL PAIR AND RADIAL SPOKE FUNCTION
Although cilia and flagella isolated from diverse species differ in the number, length, and spacing of the central pair projections, the two tubules, C1 and C2, are structurally distinguishable (Fig. 1) [reviewed in Smith and Lefebvre, 1997b]. For example, in Chlamydomonas flagella the two prominent projections on the C1 microtubule, 1A and 1B, are longer (18 nm) than the two prominent projections on the C2 microtubule (8 nm), termed 2A and 2B [Adams et al., 1981; Dutcher et al., 1984; Goodenough and Heuser, 1985]. In addition, less prominent projections on both the C1 and C2 microtubules, termed the 1C, 1D, and 2C projections, have been resolved [Mitchell and Sale, 1999]. The projections on C2 repeat every 16 nm [Witman et al., 1978], whereas the C1 microtubule appears to have both 16- and 32-nm repeating structures [Witman et al., 1978; Goodenough and Heuser, 1985]. Mitchell [2003b] has recently identified 32-nm repeating structures on the C1 microtubule, which include the 1C and 1D projections and the central sheath between 1A and 1C [Mitchell, 2003b]. Based on the inherent structural asymmetry of the central apparatus, one prediction is that the C1 and C2 projections differ in their interaction with the radial spoke heads.
The radial spokes consist of a thin stalk attached to the A-tubule of the doublet microtubules and a bulbous head projecting toward the central apparatus [reviewed in Curry and Rosenbaum, 1993]. They repeat in pairs (Chlamydomonas) or triplet groups (Tetrahymena) every 96 nm and maintain a right-handed helix along the length of the axoneme [Warner, 1970; Warner and Satir, 1974; Goodenough and Heuser, 1985]. The anchoring position of the radial spoke stalk on the A-tubule of the doublet microtubules suggests that the radial spokes may ultimately provide a structural linkage between the central apparatus and the dynein arms. It is not yet known if there are direct interactions between the radials spokes and dynein arm components. However, structural analyses indicate that the base of the spokes may be in a position to interact with subsets of inner dynein arms or components of the dynein regulatory complex (DRC) [Piperno et al., 1990, 1992; Kagami and Kamiya, 1992; Gardner et al., 1994].
Interactions of the radials pokes heads with central apparatus structures are thought to occur in a transient fashion. In studies of Elliptio gill cilia, Warner and Satir [1974] observed that as the axoneme bends, the radial spokes are not extensible and undergo cycles of detachment and re-attachment with the central pair projections. These contacts cause the radial spokes to tilt relative to the longitudinal axis of the axoneme as the microtubules slide. Structural studies of Paramecia cilia revealed that the central apparatus rotates within the nine doublet microtubules; it rotates once per beat and with a slight twist that has the same period as that of the propagating bend [Omoto and Kung, 1979]. Rotation of the central pair has also been observed for flagella of other species including Chlamydomonas [reviewed in Omoto et al., 1999]. It is not known whether interactions of the central pair projections with the radial spoke heads during rotation induce tilt in the spokes relative to the circumferential axis of the axoneme. If this is the case, then the radial spokes would potentially experience distortion in two directions: longitudinally as the microtubules slide, and circumferentially as the central apparatus rotates.
One model is that as the central apparatus rotates, the projections make periodic contacts with the radial spokes, which in turn transduce a signal to the dynein arms [reviewed in Omoto et al., 1999]. The rotation and twist of the central apparatus may provide positional regulatory cues both circumferentially and along the length of the axoneme. It is unknown whether central apparatus rotation is an active or passive process. The association of kinesins with the central microtubules suggests enzymes may be involved in actively rotating the central apparatus [Bernstein et al., 1994; Fox et al., 1994; Johnson et al., 1994]; however, motor activity has not yet been demonstrated for these central apparatus associated kinesins. On the other hand, the inherent twist of the central apparatus [Kamiya, 1982; Shingyoji et al., 1991] may allow for the passive rotation of the central pair as the axoneme bends. Regardless of the mechanism of central apparatus rotation, one prediction of this model is that the asymmetry of the central apparatus coordinates dynein activity on specific subsets of doublet microtubules to regulate the size and shape of the bend, consistent with a switch point hypothesis for alternating bends and control of bending symmetry [Satir, 1985].
Recent studies in Chlamydomonas indicate that the position of the two central microtubules may specify dynein activity on specific doublets. Wargo and Smith [2003] have analyzed the orientation of the central apparatus in flagellar axonemes isolated from Chlamydomonas cells using an in vitro microtubule sliding assay. Their results indicate that the orientation of the C1 central microtubule correlates with the position of active sliding for subsets of doublet microtubules. Mitchell has rapidly fixed Chlamydomonas cells with beating flagella to examine the orientation of the central apparatus relative to flagellar bends [Mitchell, 2003a]. During forward swimming, the central apparatus is parallel to the bend plane throughout the principal bend of both the effective and recovery stroke. The C1 microtubule always faces the outer edge of the curve. Based on the doublet numbering system of Hoops and Witman [1983], these observations indicate that during the principal bend of the effective stroke, dynein arms on doublets two-four are active and central tubule C1 is adjacent to doublet one (see Fig. 1); during the principal bend of the recovery stroke, central tubule C1 is also adjacent to doublet one, yet, dynein arms on doublets six-eight are active. These studies provide additional support for a model in which central pair orientation is linked to dynein activity on specific doublets. However, in this case the specific orientation of C1 does not always correlate with dynein activity as noted above for isolated axonemes induced to slide in vitro. Based on the combined results of Wargo and Smith [2003] and Mitchell [2003a], the sliding assay appears to capture the equivalent of one phase of flagellar beating, the principal bend of the effective stroke.
The central apparatus does not rotate in all cilia and flagella [Tamm and Tamm, 1981]. However, the absence of rotation does not rule out the possibility that the central apparatus plays a role in specifying dynein activity on specific doublets. For example, in mussel gill cilia and echinoderm sperm the central apparatus maintains a specific orientation relative to the position of active sliding [Sale, 1986; Mohri et al., 1987; Satir and Matsuoka, 1989; Holwill and Satir, 1994]. In these cell types, specific interactions of the radial spokes with the central apparatus may positively or negatively control dynein activity by a switching mechanism that does not involve central apparatus rotation. This notion is supported by the recent studies of isolated sea urchin sperm axonemes using a modified microtubule sliding assay [Yoshimura and Shingyoji, 1999; Nakano et al., 2003]. It is possible that rotation provides additional regulatory mechanisms that are primarily founded in the asymmetry of the central apparatus and that provide axonemes with versatility and an array of waveforms [for example, see Gray, 1928].
From these combined studies, we can draw several conclusions independent of central apparatus rotation. First, the orientation and asymmetry of the central apparatus most likely correlates with dynein activity on specific doublet microtubules. Second, the central pair projections make contact with the radial spoke heads in cycles of detachment and re-attachment. And finally, these contacts induce distortions in the radial spokes that are manifest as significant tilt of the spoke stalk. The key question is, what is the relationship of radial spoke distortion to changes in dynein activity?
One approach to addressing this question has been the application of mathematical models to simulate beating sperm flagella [for example, see Lindemann, 1994; Brokaw, 2002; Cibert, 2003]. Lindemann [1994] has proposed a “geometric clutch” model for flagellar beating in which dynein cross-bridge formation on specific doublets is limited by inter-doublet spacing. Once dynein arms induce sliding between doublets, a transverse force is generated that is sufficiently strong to exceed the force developed by the dynein arms and results in the detachment of the dynein cross-bridges. At this switch-point, dynein arms on the opposite side of the axoneme are now in a position to form cross-bridges. In a recent publication, Lindemann proposes that the radial spokes and central apparatus play a role in redistributing this transverse force during the switching event, and that movable attachments between the radial spokes and central pair projections are required for mediating the transmission of this force [Lindemann, 2003]. Testing the prediction that strain in the radial spoke stalk locally modulates dynein activity will most likely require the development of a new in vitro assay combined with micromanipulation technology [for example, see Shingyoji et al., 1998].
CONSERVED SIGNALING PROTEINS LOCATED IN THE CENTRAL PAIR AND RADIAL SPOKE STRUCTURES
While mechanical interactions between the central apparatus and radial spokes may provide an intrinsic mechanism for control of ciliary and flagellar beating, substantial evidence has emerged to indicate that cells utilize chemical signals to alter ciliary and flagellar motility [reviewed in Porter and Sale, 2000]. A combination of physiological, pharmacological, and biochemical analyses of isolated wild-type and mutant axonemes from Chlamydomonas and Tetrahymena as well as axonemes isolated from sperm tails of several species has been used to elucidate these signal transduction pathways.
Chemical signaling involves the interaction of second messengers, such as cyclic nucleotide monophosphates and calcium [reviewed in Brokaw, 1987; Tash, 1989; Satir, 1995, 1999] with conserved protein receptors and enzymes anchored to the axoneme, some of which are components of the radial spokes and central apparatus (Table I). Motility changes mediated by these second messengers include inducing quiescence in beating flagella, activating motility, increasing beat frequency, reversing the direction of the effective stroke, or changing waveform. The effect of second messengers is mediated at least in part through second-messenger-dependent kinases and phosphatases, such as cAMP-dependent protein kinase A (PKA), cGMP-dependent protein kinase (PKG), calmodulin dependent kinase, and calcineurin (PP2B) [Tash and Means, 1983; Tash et al., 1988; Chaudhry et al., 1995; San Agustin et al., 1998; Smith, 2002a]. It is possible that some second messengers affect motility by additional mechanisms that are independent of phosphorylation. For example, several subunits of dynein motors are EF-hand proteins that may bind calcium directly, altering dynein activity [Piperno et al., 1992; King and Patel-King, 1995; Yanagisawa and Kamiya, 2001; Casey et al., 2003; Guerra et al., 2003]. Chemical signaling also includes axonemal enzymes that are not directly sensitive to second messengers including casein kinase 1 (CK1), PP1, and PP2A, all of which appear to be involved in the control of dynein-driven motility [Walczak and Nelson, 1993; Habermacher and Sale, 1996; Yang et al., 2000; Yang and Sale, 2000; Smith, 2002b].
TABLE I.
Central Apparatus and Radial Spoke-Associated Polypeptides
| Protein | Notes | References |
|---|---|---|
| Central apparatus-associated polypeptides | ||
| PF6 | 238-kDa alanine/proline rich protein; pf6 flagella lack the 1a projection on the C1 microtubule and display only twitching motion | [Dutcher et al., 1984; Rupp et al., 2001] |
| PF16 | 57-kDa armadillo repeat containing protein that localizes to the c1 microtubule; pf16 flagella lack the C1 microtubule and are paralyzed. PF16 may correspond to CP14 | [Dutcher et al., 1984; Smith and Lefebvre, 1996] |
| PF20 | 63-kDa WD-repeat containing protein that localizes to the inter-microtubule bridge connecting C1 and C2; pf20 flagella lack the entire central apparatus and are paralyzed | [Adams et al., 1981; Smith and Lefebvre, 1997a] |
| PP1c | Localized primarily, but not exclusively, to the C1 microtubule | [Yang et al., 2001] |
| KLP1 | 83-kDa kinesin-like protein that localizes to the C2 microtubule | [Bernstein et al., 1994] |
| KRP | 110-kDa kinesin related protein associated with the central apparatus, most likely the C1 microtubule | [Fox et al., 1994; Johnson et al., 1994; Mitchell and Sale, 1999] |
| AKAP240 | 240-kDa A-kinase anchoring protein; most likely associated with C2 | [Gaillard et al., 2001] |
| Calmodulin | Most likely associated with C1, sediments as a complex at 10S | [Yang et al., 2001; Dymek et al., 2002] |
| Radial spoke-associated polypeptides | ||
| RSP2 | PF24 gene product, GAF, and calmodulin binding domain; pf24 mutants lack radial spoke heads and have paralyzed flagella | [Huang et al., 1981; Piperno et al., 1981; Yang et al., 2002] |
| RSP3 | PF14 gene product, 97-kDa A-kinase anchoring protein most likely localized to the base of the spokes; pf14 mutants lack radial spokes and have paralyzed flagella | [Huang et al., 1981; Piperno et al., 1981; Diener et al., 1993; Gaillard et al., 2001] |
| RSP4 | PF1 gene product, 49.8-kDa proline rich polypeptide similar to RSP6, pf1 mutants lack spoke heads and have paralyzed flagella | [Huang et al., 1981; Piperno et al., 1981; Curry et al., 1992] |
| RSP6 | PF26 gene product, 48.8-kDa proline rich polypeptide similar to RSP4, pf26 mutants lack spoke heads and have paralyzed flagella | [Huang et al., 1981; Piperno et al., 1981; Curry et al., 1992] |
| LC8 | RSP22, originally identified as light chain of outer dynein arm, also present in radial spoke stalk | [Piperno and Luck, 1979; Yang et al., 2001] |
| Calmodulin | RSP20, component of the radial spoke stalk | [Yang et al., 2001] |
| LRR37 | Leucine-rich repeat protein, most likely localizes to radial spoke head | [Padma et al., 2003] |
The use of available Chlamydomonas mutants and pharmacological reagents in conjunction with the micro-tubule sliding assay has proven to be an extremely powerful approach to reveal that the CP/RS control system is involved in integrating chemical signals. Chlamydomonas mutants with defects in the assembly of either the radial spoke or central apparatus have paralyzed flagella. However, axonemes isolated from these mutants undergo dynein-driven microtubule sliding in vitro [Witman et al., 1978], albeit at significantly reduced velocity compared to wild-type axonemes [Smith and Sale, 1992, 1994; Habermacher and Sale, 1997; Smith, 2002b]. By reconstituting dynein arms isolated from wild-type axonemes onto extracted spokeless axonemes, wild-type dynein activity was restored [Smith and Sale, 1992]; these results led to the prediction that the radial spokes, and possibly the central apparatus, controlled dynein motors by a stable post-translational modification [Smith and Sale, 1992].
Consistent with this hypothesis, PKA inhibitors restored dynein activity of radial spoke defective axonemes to wild-type levels [Howard et al., 1994]. And furthermore, cAMP, which stimulates PKA activity, inhibits reactivation of wild-type Chlamydomonas axonemes [Hasegawa et al., 1987]. These experiments indicate that PKA is a component of the axoneme and plays a role in regulating dynein activity. Predictably, when the radial spokes are defective, PKA activity is not regulated and leads to inhibition of flagellar dynein activity. The radial spokes may be involved in control of cAMP-stimulated PKA activity. Of possible relevance, radial spoke protein three has been shown to be an A-kinase anchoring protein, AKAP [Gaillard et al., 2001].
In contrast, PKA inhibitors do not restore wild-type sliding velocities to central apparatus defective mutants [Smith, 2002b], suggesting that either the PKA affected by the inhibitor or its substrate is missing in central pairless axonemes. Alternatively, the presence of the radial spokes may be sufficient to inhibit PKA in central pairless axonemes. The reduced microtubule sliding velocity in central pair mutants may be due to mechanism independent of PKA activity. However, a 240-kD central apparatus component is a candidate AKAP [Gaillard et al., 2001]. Therefore, both the radial spokes and central apparatus may serve as scaffolds for the localization of PKA (see Fig. 1), although their roles in regulating motility may differ.
It has also been shown that CK1 is anchored to the axoneme. Like PKA, CK1 is inappropriately activated in mutants with either radial spoke or central apparatus defects, resulting in the inhibition of dynein activity; inhibitors of CK1 restore wild-type dynein activity to both radial spokeless and central pairless axonemes [Yang and Sale, 2000; Smith, 2002b]. Interestingly, the effect of both PKA and CK1 inhibitors requires the activity of phosphatases. In the presence of PP1 and/or PP2A inhibitors, PKA and CK1 inhibitors failed to restore dynein activity to either radial spokeless or central pairless axonemes [Habermacher and Sale, 1996; Yang and Sale, 2000; Smith, 2002b]. Analyses of axonemes isolated from mutant Chlamydomonas strains indicate that PP1 is primarily, but not exclusively, localized to the central apparatus [Yang et al., 2000]. Both the mechanism for anchoring PP1 to the axoneme and the potential substrates of PP1 are unknown.
As noted above, calcium is also an important second messenger involved in regulating ciliary and flagellar bending. It has long been suspected that calmodulin is involved in calcium signaling [reviewed in Otter, 1989]. Biochemical analyses of axonemal components have recently revealed that calmodulin is a structural component of both the radial spokes and central apparatus [Yang et al., 2001; Dymek et al., 2002]. In addition, using the microtubule sliding assay, Smith [2002a] discovered that wild-type dynein activity is restored to central apparatus defective mutants, but not radial spokeless mutants, in the presence of high calcium. The application of either calmodulin inhibitors or calmodulin-dependent kinase inhibitors blocked the calcium-induced increase in dynein activity in central pairless axonemes [Smith, 2002a]. These results indicate that calcium regulation of flagellar motility in Chlamydomonas involves the regulation of dynein-driven microtubule sliding, that calmodulin and calmodulin-dependent kinase may mediate the calcium signal, and that the central apparatus and radial spokes are among the key components of the calcium signaling pathway [Smith, 2002a]. Similar microtubule sliding assays and reactivation studies using sea urchin sperm axonemes have also implicated the central apparatus in calcium-induced modulation of motility [Bannai et al., 2000; Nakano et al., 2003].
The discovery of multiple axonemal kinases and phosphatases implies that the function of specific axonemal components must be regulated by phosphorylation. While it has been known for some time that many axonemal components are phosphoproteins [Piperno and Luck, 1976], we are only beginning to identify potential substrates for particular kinases and phosphatases. For example, in Paramecia, Hamasaki and co-workers have identified one dynein light chain that is phosphorylated in a cAMP-dependent manner, and this phosphorylation correlated with increased swimming velocity [Hamasaki et al., 1991]. And, in Tetrahymena phosphorylation of a dynein light chain resulted in an approximately 70% increase in in vitro microtubule translocation velocity compared with its unphosphorylated counterpart [Christensen et al., 2001].
In Chlamydomonas, the activity of inner dynein arm subform I1, located proximal to spoke 1 [Piperno et al., 1990], appears to be regulated by the control system. Inhibitors of PKA and CK1 had no effect on dynein activity in axonemes from double mutants with radial spoke defects and lacking I1 [Habermacher and Sale, 1997; Yang and Sale, 2000]. In contrast, for double mutants lacking I1 and the central apparatus, microtubule sliding velocities are restored to nearly wild-type velocity in the absence of any kinase inhibitors [Smith, 2002b]. And, mutations that affect I1 assembly can suppress paralysis in mutants lacking the C1 microtubule of the central apparatus [Porter et al., 1992]. These observations reveal a distinct role for the I1 dynein subtype and provide a functional link between the central apparatus, radial spokes, and inner dynein arm I1.
It was subsequently demonstrated that phosphorylation of IC138, an intermediate chain subunit of I1, is correlated with the inhibition of dynein activity [Habermacher and Sale, 1997; Yang and Sale, 2000]. The role of IC138 phosphorylation in modulating motility has also been suggested by the phenotype of mutants lacking subunits of I1 [Perrone et al., 2000] or with hyper-phosphorylated IC138 [King and Dutcher, 1997]. In both cases, these mutants are defective for phototactic behavior. Therefore, the phosphorylation state of I1 appears to be important for light-induced motility changes, a process that is dependent on changes in intraflagellar calcium concentration [Hyams and Borisy, 1978; Bessen et al., 1980]. It is not yet known if the phosphorylation state of I1 is modulated in response to calcium, or if the phosphorylation state of I1 is specific to particular doublet microtubules. Nevertheless, the functional assays described above suggest that regulation of I1 phosphorylation includes the integration of second messenger signals using the network of enzymes associated with the central apparatus and radial spokes.
CONCLUSIONS AND FUTURE DIRECTIONS
Diverse evidence indicates that the CP/RS system is not required for oscillatory beating of the axoneme. Rather, the outer doublet microtubules and associated dynein arms appear to be sufficient for the initiation and propagation of bends. Thus, the question remains, what is the role of the central apparatus and radial spokes? The simplest model is that the CP/RS system performs as a signal transducer for controlling the size and shape of the bend and for modifying motility in response to specific signals.
Based on the experimental results cited above, we propose that the CP/RS network of structures operates as both a mechanical and chemical transducer. Mechanical input includes interactions between the radial spokes and central apparatus. Chemical input includes binding of second messengers, changes in enzymatic activity of anchored kinases and phosphatases, and localized changes in phosphorylation of axonemal proteins in the CP/RS control system. In each case, output involves changes in the physical and chemical properties of the radial spokes on subsets of doublet microtubules for local control of dynein activity. Localized interactions of the radial spokes and central apparatus are predicted to differentially alter dynein activity on one side of the axoneme relative to the propagating bend [Mitchell, 2003a; Wargo and Smith, 2003]; this axonemal axis is defined by the inherent asymmetry of the central apparatus.
Modulation of microtubule sliding by localized control of dynein activity may involve the localized phosphorylation of key regulatory proteins including subunits of the dynein arms [Hamasaki et al., 1991; Habermacher and Sale, 1997; King and Dutcher, 1997; Yang and Sale, 2000; Christensen et al., 2001] and possibly components of the DRC. These modifications are not likely to operate on the time scale of a single beat cycle to modulate the inherent oscillatory behavior of beating cilia and flagella. Rather, additional beat parameters, such as waveform and stroke direction, most likely result from specific modifications that occur on a slower time course and affect particular axonemal components, including the dynein arms and CP/RS control system. The continued molecular dissection of the CP/RS control system is essential for elucidating the physical and biochemical properties of the radial spokes and central apparatus; Table I represents only a small subset of the polypeptides associated with the central apparatus and radial spoke structures. This information will provide a substantial foundation for generating and testing hypotheses using molecular biological approaches and functional assays.
References
- 1.Adams GM, Huang B, Piperno G, Luck DJ. Central-pair micro-tubular complex of Chlamydomonas flagella: polypeptide composition as revealed by analysis of mutants. J Cell Biol. 1981;91:69–76. doi: 10.1083/jcb.91.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afzelius BA. The immotile-cilia syndrome: a microtubule-associated defect. CRC Crit Rev Biochem. 1985;19:63–87. doi: 10.3109/10409238509086788. [DOI] [PubMed] [Google Scholar]
- 3.Afzelius BA, Eliasson R. Flagellar mutants in man: on the heterogeneity of the immotile-cilia syndrome. J Ultrastruct Res. 1979;69:43–52. doi: 10.1016/s0022-5320(79)80041-6. [DOI] [PubMed] [Google Scholar]
- 4.Baccetti B, Burrini AG, Dallai R, Pallini V. The dynein electrophoretic bands in axonemes naturally lacking the inner or the outer arm. J Cell Biol. 1979;80:334–340. doi: 10.1083/jcb.80.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bannai H, Yoshimura M, Takahashi K, Shingyoji C. Calcium regulation of microtubule sliding in reactivated sea urchin sperm flagella. J Cell Sci. 2000;113:831–839. doi: 10.1242/jcs.113.5.831. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein M, Beech PL, Katz SG, Rosenbaum JL. A new kinesin-like protein (Klp1) localized to a single microtubule of the Chlamydomonas flagellum. J Cell Biol. 1994;125:1313–1326. doi: 10.1083/jcb.125.6.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bessen M, Fay RB, Witman GB. Calcium control of waveform in isolated flagellar axonemes of Chlamydomonas. J Cell Biol. 1980;86:446–455. doi: 10.1083/jcb.86.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brokaw CJ. Flagellar movement: a sliding filament model. Science. 1972;178:455–462. doi: 10.1126/science.178.4060.455. [DOI] [PubMed] [Google Scholar]
- 9.Brokaw CJ. Regulation of sperm flagellar motility by calcium and cAMP-dependent phosphorylation. J Cell Biochem. 1987;35:175–184. doi: 10.1002/jcb.240350302. [DOI] [PubMed] [Google Scholar]
- 10.Brokaw CJ. Computer simulation of flagellar movement VIII: Coordination of dynein by local curvature control can generate helical bending waves. Cell Motil Cytoskeleton. 2002;53:103–124. doi: 10.1002/cm.10067. [DOI] [PubMed] [Google Scholar]
- 11.Brokaw CJ, Luck DJ, Huang B. Analysis of the movement of Chlamydomonas flagella:“ the function of the radial-spoke system is revealed by comparison of wild-type and mutant flagella. J Cell Biol. 1982;92:722–732. doi: 10.1083/jcb.92.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casey DM, Yagi T, Kamiya R, Witman GB. DC3, the smallest subunit of the Chlamydomonas flagellar outer dynein arm-docking complex, is a redox-sensitive calcium-binding protein. J Biol Chem. 2003 doi: 10.1074/jbc.M303064200. in press. [DOI] [PubMed] [Google Scholar]
- 13.Chaudhry PS, Creagh S, Yu N, Brokaw CJ. Multiple protein kinase activities required for activation of sperm flagellar motility. Cell Motil Cytoskeleton. 1995;32:65–79. doi: 10.1002/cm.970320108. [DOI] [PubMed] [Google Scholar]
- 14.Christensen ST, Guerra C, Wada Y, Valentin T, Angeletti RH, Satir P, Hamasaki T. A regulatory light chain of ciliary outer arm dynein in Tetrahymena thermophila. J Biol Chem. 2001;276:20048–20054. doi: 10.1074/jbc.M008412200. [DOI] [PubMed] [Google Scholar]
- 15.Cibert C. Entropy and information in flagellar axoneme cybernetics: A radial spokes integrative function. Cell Motil Cytoskeleton. 2003;54:296–316. doi: 10.1002/cm.10100. [DOI] [PubMed] [Google Scholar]
- 16.Curry AM, Rosenbaum JL. Flagellar radial spoke: a model molecular genetic system for studying organelle assembly. Cell Motil Cytoskeleton. 1993;24:224–232. doi: 10.1002/cm.970240403. [DOI] [PubMed] [Google Scholar]
- 17.Curry AM, Williams BD, Rosenbaum JL. Sequence analysis reveals homology between two proteins of the flagellar radial spoke. Mol Cell Biol. 1992;12:3967–3977. doi: 10.1128/mcb.12.9.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diener DR, Ang LH, Rosenbaum JL. Assembly of flagellar radial spoke proteins in Chlamydomonas: identification of the axoneme binding domain of radial spoke protein 3. J Cell Biol. 1993;123:183–190. doi: 10.1083/jcb.123.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dutcher SK, Huang B, Luck DJ. Genetic dissection of the central pair microtubules of the flagella of Chlamydomonas reinhardtii. J Cell Biol. 1984;98:229–236. doi: 10.1083/jcb.98.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dymek E, Wargo M, Smith EF. Characterization of calmodulin and calmodulin binding proteins associated with the flagellar central apparatus. Mol Biol Cell. 2002;13:328a. [Google Scholar]
- 21.Fox LA, Sawin KE, Sale WS. Kinesin-related proteins in eukaryotic flagella. J Cell Sci. 1994;107:1545–1550. doi: 10.1242/jcs.107.6.1545. [DOI] [PubMed] [Google Scholar]
- 22.Frey E, Brokaw CJ, Omoto CK. Reactivation at low ATP distinguishes among classes of paralyzed flagella mutants. Cell Motil Cytoskeleton. 1997;38:91–99. doi: 10.1002/(SICI)1097-0169(1997)38:1<91::AID-CM8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 23.Gaillard AR, Diener DR, Rosenbaum JL, Sale WS. Flagellar radial spoke protein 3 is an A-kinase anchoring protein (AKAP) J Cell Biol. 2001;153:443–448. doi: 10.1083/jcb.153.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gardner LC, O’Toole E, Perrone CA, Giddings T, Porter ME. Components of a “dynein regulatory complex” are located at the junction between the radial spokes and the dynein arms in Chlamydomonas flagella. J Cell Biol. 1994;127:1311–1325. doi: 10.1083/jcb.127.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodenough UW, Heuser JE. Substructure of inner dynein arms, radial spokes, and the central pair/projection complex of cilia and flagella. J Cell Biol. 1985;100:2008–2018. doi: 10.1083/jcb.100.6.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gray J. Ciliary movement. Cambridge, UK: Cambridge University Press; 1928. [Google Scholar]
- 27.Guerra C, Wada Y, Leick V, Bell A, Satir P. Cloning, localization, and axonemal function of tetrahymena centrin. Mol Biol Cell. 2003;14:251–261. doi: 10.1091/mbc.E02-05-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Habermacher G, Sale WS. Regulation of flagellar dynein by an axonemal type-1 phosphatase in Chlamydomonas. J Cell Sci. 1996;109:1899–1907. doi: 10.1242/jcs.109.7.1899. [DOI] [PubMed] [Google Scholar]
- 29.Habermacher G, Sale WS. Regulation of flagellar dynein by phosphorylation of a 138-kD inner arm dynein intermediate chain. J Cell Biol. 1997;136:167–176. doi: 10.1083/jcb.136.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamasaki T, Barkalow K, Richmond J, Satir P. cAMP-stimulated phosphorylation of an axonemal polypeptide that copurifies with the 22S dynein arm regulates microtubule translocation velocity and swimming speed in Paramecium. Proc Natl Acad Sci USA. 1991;88:7918–7922. doi: 10.1073/pnas.88.18.7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hasegawa E, Hayashi H, Asakura S, Kamiya R. Stimulation of in vitro motility of Chlamydomonas axonemes by inhibition of cAMP-dependent phosphorylation. Cell Motil Cytoskeleton. 1987;8:302–311. doi: 10.1002/cm.970080403. [DOI] [PubMed] [Google Scholar]
- 32.Holwill ME, Satir P. Physical model of axonemal splitting. Cell Motil Cytoskeleton. 1994;27:287–298. doi: 10.1002/cm.970270402. [DOI] [PubMed] [Google Scholar]
- 33.Hoops HJ, Witman GB. Outer doublet heterogeneity reveals structural polarity related to beat direction in Chlamydomonas flagella. J Cell Biol. 1983;97:902–908. doi: 10.1083/jcb.97.3.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Howard DR, Habermacher G, Glass DB, Smith EF, Sale WS. Regulation of Chlamydomonas flagellar dynein by an axonemal protein kinase. J Cell Biol. 1994;127:1683–1692. doi: 10.1083/jcb.127.6.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang B, Piperno G, Ramanis Z, Luck DJ. Radial spokes of Chlamydomonas flagella: genetic analysis of assembly and function. J Cell Biol. 1981;88:80–88. doi: 10.1083/jcb.88.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang B, Ramanis Z, Luck DJ. Suppressor mutations in Chlamydomonas reveal a regulatory mechanism for Flagellar function. Cell. 1982;28:115–124. doi: 10.1016/0092-8674(82)90381-6. [DOI] [PubMed] [Google Scholar]
- 37.Hyams JS, Borisy GG. Isolated flagellar apparatus of Chlamydomonas: characterization of forward swimming and alteration of waveform and reversal of motion by calcium ions in vitro. J Cell Sci. 1978;33:235–253. doi: 10.1242/jcs.33.1.235. [DOI] [PubMed] [Google Scholar]
- 38.Johnson KA, Haas MA, Rosenbaum JL. Localization of a kinesin-related protein to the central pair apparatus of the Chlamydomonas reinhardtii flagellum. J Cell Sci. 1994;107:1551–1556. doi: 10.1242/jcs.107.6.1551. [DOI] [PubMed] [Google Scholar]
- 39.Kagami O, Kamiya R. Translocation and rotation of microtubules caused by multiple species of Chlamydomonas inner-arm dynein. J Cell Sci. 1992;103:653–664. [Google Scholar]
- 40.Kamiya R. Extrusion and rotation of the central-pair microtubules in detergent-treated flagella. Cell Motil. 1982;1:169–173. doi: 10.1002/cm.970020732. [DOI] [PubMed] [Google Scholar]
- 41.King SJ, Dutcher SK. Phosphoregulation of an inner dynein arm complex in Chlamydomonas reinhardtii is altered in phototactic mutant strains. J Cell Biol. 1997;136:177–191. doi: 10.1083/jcb.136.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.King SM, Patel-King RS. Identification of a Ca(2+)-binding light chain within Chlamydomonas outer arm dynein. J Cell Sci. 1995;108:3757–3764. doi: 10.1242/jcs.108.12.3757. [DOI] [PubMed] [Google Scholar]
- 43.Lindemann CB. A model of flagellar and ciliary functioning which uses the forces transverse to the axoneme as the regulator of dynein activation. Cell Motil Cytoskeleton. 1994;29:141–154. doi: 10.1002/cm.970290206. [DOI] [PubMed] [Google Scholar]
- 44.Lindemann CB. Structural-functional relationships of the dynein, spokes, and central-pair projections predicted from an analysis of the forces acting within a flagellum. Biophys J. 2003;84:4115–4126. doi: 10.1016/S0006-3495(03)75136-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marszalek JR, Ruiz-Lozano P, Roberts E, Chien KR, Goldstein LS. Situs inversus and embryonic ciliary morphogenesis defects in mouse mutants lacking the KIF3A subunit of kinesin-II. Proc Natl Acad Sci USA. 1999;96:5043–5048. doi: 10.1073/pnas.96.9.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitchell DR. Orientation of the central pair complex during flagellar bend formation in Chlamydomonas. Cell Motil Cytoskeleton. 2003a;56:120–129. doi: 10.1002/cm.10142. [DOI] [PubMed] [Google Scholar]
- 47.Mitchell DR. Reconstruction of the projection periodicity and surface architecture of the flagellar central pair complex. Cell Motil Cytoskeleton. 2003b;55:188–199. doi: 10.1002/cm.10121. [DOI] [PubMed] [Google Scholar]
- 48.Mitchell DR, Sale WS. Characterization of a Chlamydomonas insertional mutant that disrupts flagellar central pair microtubule-associated structures. J Cell Biol. 1999;144:293–304. doi: 10.1083/jcb.144.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mohri H, Mohri T, Okuno M. Topographical relationship between the axonemal arrangement and the bend direction in starfish sperm flagella. Cell Motil Cytoskeleton. 1987;8:76–84. doi: 10.1002/cm.970080111. [DOI] [PubMed] [Google Scholar]
- 50.Nakano I, Kobayashi T, Yoshimura M, Shingyoji C. Central-pair-linked regulation of microtubule sliding by calcium in flagellar axonemes. J Cell Sci. 2003;116:1627–1636. doi: 10.1242/jcs.00336. [DOI] [PubMed] [Google Scholar]
- 51.Neugebauer DC, Neuwinger J, Jockenhovel F, Nieschlag E. ’9 + 0’ axoneme in spermatozoa and some nasal cilia of a patient with totally immotile spermatozoa associated with thickened sheath and short midpiece. Hum Reprod. 1990;5:981–986. doi: 10.1093/oxfordjournals.humrep.a137232. [DOI] [PubMed] [Google Scholar]
- 52.Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–837. doi: 10.1016/s0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- 53.Nonaka S, Shiratori H, Saijoh Y, Hamada H. Determination of left-right patterning of the mouse embryo by artificial nodal flow. Nature. 2002;418:96–99. doi: 10.1038/nature00849. [DOI] [PubMed] [Google Scholar]
- 54.Okada Y, Nonaka S, Tanaka Y, Saijoh Y, Hamada H, Hirokawa N. Abnormal nodal flow precedes situs inversus in iv and inv mice. Mol Cell. 1999;4:459–468. doi: 10.1016/s1097-2765(00)80197-5. [DOI] [PubMed] [Google Scholar]
- 55.Omoto CK, Kung C. The pair of central tubules rotates during ciliary beat in Paramecium. Nature. 1979;279:532–534. doi: 10.1038/279532a0. [DOI] [PubMed] [Google Scholar]
- 56.Omoto CK, Yagi T, Kurimoto E, Kamiya R. Ability of paralyzed flagella mutants of Chlamydomonas to move. Cell Motil Cytoskeleton. 1996;33:88–94. doi: 10.1002/(SICI)1097-0169(1996)33:2<88::AID-CM2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 57.Omoto CK, Gibbons IR, Kamiya R, Shingyoji C, Takahashi K, Witman GB. Rotation of the central pair microtubules in eukaryotic flagella. Mol Biol Cell. 1999;10:1–4. doi: 10.1091/mbc.10.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Otter T. Cell Movement. Vol. 1. New York: Alan R. Liss; 1989. Calmodulin and the control of flagellar movement; pp. 281–298. [Google Scholar]
- 59.Padma P, Satouh Y, Wakabayashi K, Hozumi A, Ushimaru Y, Kamiya R, Inaba K. Identification of a novel leucine-rich repeat protein as a component of flagellar radial spoke in the Ascidian Ciona intestinalis. Mol Biol Cell. 2003;14:774–785. doi: 10.1091/mbc.02-06-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perrone CA, Myster SH, Bower R, O’Toole ET, Porter ME. Insights into the structural organization of the I1 inner arm dynein from a domain analysis of the 1beta dynein heavy chain. Mol Biol Cell. 2000;11:2297–2313. doi: 10.1091/mbc.11.7.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Piperno G, Luck DJ. Phosphorylation of axonemal proteins in Chlamydomonas reinhardtii. J Biol Chem. 1976;251:2161–2167. [PubMed] [Google Scholar]
- 62.Piperno G, Luck DJ. Axonemal adenosine triphosphatases from flagella of Chlamydomonas reinhardtii. Purification of two dyneins. J Biol Chem. 1979;254:3084–3090. [PubMed] [Google Scholar]
- 63.Piperno G, Huang B, Ramanis Z, Luck DJ. Radial spokes of Chlamydomonas flagella: polypeptide composition and phosphorylation of stalk components. J Cell Biol. 1981;88:73–79. doi: 10.1083/jcb.88.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Piperno G, Ramanis Z, Smith EF, Sale WS. Three distinct inner dynein arms in Chlamydomonas flagella: molecular composition and location in the axoneme. J Cell Biol. 1990;110:379–389. doi: 10.1083/jcb.110.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Piperno G, Mead K, Shestak W. The inner dynein arms I2 interact with a “dynein regulatory complex” in Chlamydomonas flagella. J Cell Biol. 1992;118:1455–1463. doi: 10.1083/jcb.118.6.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Piperno G, Mead K, LeDizet M, Moscatelli A. Mutations in the “dynein regulatory complex” alter the ATP-insensitive binding sites for inner arm dyneins in Chlamydomonas axonemes. J Cell Biol. 1994;125:1109–1117. doi: 10.1083/jcb.125.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Porter ME, Sale WS. The 9 + 2 axoneme anchors multiple inner arm dyneins and a network of kinases and phosphatases that control motility. J Cell Biol. 2000;151:F37–42. doi: 10.1083/jcb.151.5.f37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Porter ME, Power J, Dutcher SK. Extragenic suppressors of paralyzed flagellar mutations in Chlamydomonas reinhardtii identify loci that alter the inner dynein arms. J Cell Biol. 1992;118:1163–1176. doi: 10.1083/jcb.118.5.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Porter ME, Knott JA, Gardner LC, Mitchell DR, Dutcher SK. Mutations in the SUP-PF-1 locus of Chlamydomonas reinhardtii identify a regulatory domain in the beta-dynein heavy chain. J Cell Biol. 1994;126:1495–1507. doi: 10.1083/jcb.126.6.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Prensier G, Vivier E, Goldstein S, Schrevel J. Motile flagellum with a “3 + 0” ultrastructure. Science. 1980;207:1493–1494. doi: 10.1126/science.7189065. [DOI] [PubMed] [Google Scholar]
- 71.Rupp G, O’Toole E, Porter ME. The Chlamydomonas PF6 locus encodes a large alanine/proline-rich polypeptide that is required for assembly of a central pair projection and regulates flagellar motility. Mol Biol Cell. 2001;12:739–751. doi: 10.1091/mbc.12.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sale WS. The axonemal axis and Ca2+-induced asymmetry of active microtubule sliding in sea urchin sperm tails. J Cell Biol. 1986;102:2042–2052. doi: 10.1083/jcb.102.6.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.San Agustin J, Leszyk J, Nuwaysir L, Witman G. The catalytic subunit of the cAMP-dependent protein kinase of ovine sperm flagella has a unique amino-terminal sequence. J Biol Chem. 1998;273:24874–24883. doi: 10.1074/jbc.273.38.24874. [DOI] [PubMed] [Google Scholar]
- 74.Satir P. Studies on cilia. 3. Further studies on the cilium tip and a “sliding filament” model of ciliary motility. J Cell Biol. 1968;39:77–94. doi: 10.1083/jcb.39.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Satir P. Switching mechanisms in the control of ciliary motility. New York: Alan R. Liss, Inc.; 1985. [Google Scholar]
- 76.Satir P. Landmarks in cilia research from Leeuwenhoek to us. Cell Motil Cytoskeleton. 1995;32:90–94. doi: 10.1002/cm.970320203. [DOI] [PubMed] [Google Scholar]
- 77.Satir P. The cilium as a biological nanomachine. Faseb J. 1999;13(Suppl 2):S235–237. doi: 10.1096/fasebj.13.9002.s235. [DOI] [PubMed] [Google Scholar]
- 78.Satir P, Matsuoka T. Splitting the ciliary axoneme: implications for a “switch-point” model of dynein arm activity in ciliary motion. Cell Motil Cytoskeleton. 1989;14:345–358. doi: 10.1002/cm.970140305. [DOI] [PubMed] [Google Scholar]
- 79.Shingyoji C, Murakami A, Takahashi K. Local reactivation of Triton-extracted flagella by iontophoretic application of ATP. Nature. 1977;265:269–270. doi: 10.1038/265269a0. [DOI] [PubMed] [Google Scholar]
- 80.Shingyoji C, Katada J, Takahashi K, Gibbons IR. Rotating the plane of imposed vibration can rotate the plane of flagellar beating in sea-urchin sperm without twisting the axoneme. J Cell Sci. 1991;98:175–181. doi: 10.1242/jcs.98.2.175. [DOI] [PubMed] [Google Scholar]
- 81.Shingyoji C, Higuchi H, Yoshimura M, Katayama E, Yanagida T. Dynein arms are oscillating force generators. Nature. 1998;393:711–714. doi: 10.1038/31520. [DOI] [PubMed] [Google Scholar]
- 82.Smith EF. Regulation of flagellar dynein by calcium and a role for an axonemal calmodulin and calmodulin-dependent kinase. Mol Biol Cell. 2002a;13:3303–3313. doi: 10.1091/mbc.E02-04-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Smith EF. Regulation of flagellar dynein by the axonemal central apparatus. Cell Motil Cytoskeleton. 2002b;52:33–42. doi: 10.1002/cm.10031. [DOI] [PubMed] [Google Scholar]
- 84.Smith EF, Lefebvre PA. PF16 encodes a protein with armadillo repeats and localizes to a single microtubule of the central apparatus in Chlamydomonas flagella. J Cell Biol. 1996;132:359–370. doi: 10.1083/jcb.132.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith EF, Lefebvre PA. PF20 gene product contains WD repeats and localizes to the intermicrotubule bridges in Chlamydomonas flagella. Mol Biol Cell. 1997a;8:455–467. doi: 10.1091/mbc.8.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smith EF, Lefebvre PA. The role of central apparatus components in flagellar motility and microtubule assembly. Cell Motil Cytoskeleton. 1997b;38:1–8. doi: 10.1002/(SICI)1097-0169(1997)38:1<1::AID-CM1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 87.Smith EF, Sale WS. Regulation of dynein-driven microtubule sliding by the radial spokes in flagella. Science. 1992;257:1557–1559. doi: 10.1126/science.1387971. [DOI] [PubMed] [Google Scholar]
- 88.Smith EF, Sale WS. Mechanisms of flagellar movement: functional interactions between dynein arms and radial spoke-central apparatus complex. In: Hyams JS, Lloyd C, editors. Microtubules. New York: Wiley Liss Inc.; 1994. pp. 381–392. [Google Scholar]
- 89.Sturgess JM, Chao J. Ultrastructural features of a human genetic defect of cilia. Prog Clin Biol Res. 1982;80:7–12. doi: 10.1002/cm.970020704. [DOI] [PubMed] [Google Scholar]
- 90.Tamm SL, Tamm S. Ciliary reversal without rotation of axonemal structures in Ctenophore comb plates. J Cell Biol. 1981;89:495–509. doi: 10.1083/jcb.89.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tash JS. Protein phosphorylation: the second messenger signal transducer of flagellar motility. Cell Motil Cytoskeleton. 1989;14:332–339. doi: 10.1002/cm.970140303. [DOI] [PubMed] [Google Scholar]
- 92.Tash JS, Krinks M, Patel J, Means RL, Klee CB, Means AR. Identification, characterization, and functional correlation of calmodulin-dependent protein phosphatase in sperm. J Cell Biol. 1988;106:1625–1633. doi: 10.1083/jcb.106.5.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tash JS, Means AR. Cyclic adenosine 3′,5′ monophosphate, calcium and protein phosphorylation in flagellar motility. Biol Reprod. 1983;28:75–104. doi: 10.1095/biolreprod28.1.75. [DOI] [PubMed] [Google Scholar]
- 94.Wakabayashi K, Yagi T, Kamiya R. Ca2+-dependent waveform conversion in the flagellar axoneme of Chlamydomonas mutants lacking the central-pair/radial spoke system. Cell Motil Cytoskeleton. 1997;38:22–28. doi: 10.1002/(SICI)1097-0169(1997)38:1<22::AID-CM3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 95.Walczak CE, Nelson DL. In vitro phosphorylation of ciliary dyneins by protein kinases from Paramecium. J Cell Sci. 1993;106:1369–1376. doi: 10.1242/jcs.106.4.1369. [DOI] [PubMed] [Google Scholar]
- 96.Wargo MJ, Smith EF. Asymmetry of the central apparatus defines the location of active microtubule sliding in Chlamydomonas flagella. Proc Natl Acad Sci USA. 2003;100:137–142. doi: 10.1073/pnas.0135800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Warner FD. New observations on flagellar fine structure. The relationship between matrix structure and the microtubule component of the axoneme. J Cell Biol. 1970;47:159–182. doi: 10.1083/jcb.47.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Warner FD, Satir P. The structural basis of ciliary bend formation. Radial spoke positional changes accompanying microtubule sliding. J Cell Biol. 1974;63:35–63. doi: 10.1083/jcb.63.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Witman GB, Plummer J, Sander G. Chlamydomonas flagellar mutants lacking radial spokes and central tubules. Structure, composition, and function of specific axonemal components. J Cell Biol. 1978;76:729–747. doi: 10.1083/jcb.76.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yagi T, Kamiya R. Vigorous beating of Chlamydomonas axonemes lacking central pair/radial spoke structures in the presence of salts and organic compounds. Cell Motil Cytoskeleton. 2000;46:190–199. doi: 10.1002/1097-0169(200007)46:3<190::AID-CM4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 101.Yanagisawa HA, Kamiya R. Association between actin and light chains in Chlamydomonas flagellar inner-arm dyneins. Biochem Biophys Res Commun. 2001;288:443–447. doi: 10.1006/bbrc.2001.5776. [DOI] [PubMed] [Google Scholar]
- 102.Yang P, Sale WS. Casein kinase I is anchored on axonemal doublet microtubules and regulates flagellar dynein phosphorylation and activity. J Biol Chem. 2000;275:18905–18912. doi: 10.1074/jbc.M002134200. [DOI] [PubMed] [Google Scholar]
- 103.Yang P, Fox L, Colbran RJ, Sale WS. Protein phosphatases PP1 and PP2A are located in distinct positions in the Chlamydomonas flagellar axoneme. J Cell Sci. 2000;113:91–102. doi: 10.1242/jcs.113.1.91. [DOI] [PubMed] [Google Scholar]
- 104.Yang P, Diener DR, Rosenbaum JL, Sale WS. Localization of calmodulin and dynein light chain LC8 in flagellar radial spokes. J Cell Biol. 2001;153:1315–1326. doi: 10.1083/jcb.153.6.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang C, Bhatti S, Yang P. Initiation Codon Mutation of RSP2 in Chlamydomonas pf24 mutant leads to reduced expression and defective assembly of radial spokes. Mol Biol Cell. 2002;13:327a. [Google Scholar]
- 106.Yoshimura M, Shingyoji C. Effects of the central pair apparatus on microtubule sliding velocity in sea urchin sperm flagella. Cell Struct Funct. 1999;24:43–54. doi: 10.1247/csf.24.43. [DOI] [PubMed] [Google Scholar]


