Abstract
The ability of Pelobacter carbinolicus to oxidize electron donors with electron transfer to the anodes of microbial fuel cells was evaluated because microorganisms closely related to Pelobacter species are generally abundant on the anodes of microbial fuel cells harvesting electricity from aquatic sediments. P. carbinolicus could not produce current in a microbial fuel cell with electron donors which support Fe(III) oxide reduction by this organism. Current was produced using a coculture of P. carbinolicus and Geobacter sulfurreducens with ethanol as the fuel. Ethanol consumption was associated with the transitory accumulation of acetate and hydrogen. G. sulfurreducens alone could not metabolize ethanol, suggesting that P. carbinolicus grew in the fuel cell by converting ethanol to hydrogen and acetate, which G. sulfurreducens oxidized with electron transfer to the anode. Up to 83% of the electrons available in ethanol were recovered as electricity and in the metabolic intermediate acetate. Hydrogen consumption by G. sulfurreducens was important for ethanol metabolism by P. carbinolicus. Confocal microscopy and analysis of 16S rRNA genes revealed that half of the cells growing on the anode surface were P. carbinolicus, but there was a nearly equal number of planktonic cells of P. carbinolicus. In contrast, G. sulfurreducens was primarily attached to the anode. P. carbinolicus represents the first Fe(III) oxide-reducing microorganism found to be unable to produce current in a microbial fuel cell, providing the first suggestion that the mechanisms for extracellular electron transfer to Fe(III) oxides and fuel cell anodes may be different.
It has been generally regarded that microorganisms which have the ability to use Fe(III) oxides as an electron acceptor are also able to transfer electrons to the anodes of microbial fuel cells without the requirement for an exogenous electron shuttle mediator (21, 35). Anodes and Fe(III) oxides both represent insoluble, extracellular electron acceptors, and it is reasonable to assume that the mechanisms for electron transfer to Fe(III) oxides and electrodes might be similar, because anodes are not natural electron acceptors (21). For example, Geobacter sulfurreducens produces current from the oxidation of acetate or hydrogen (3), and several outer-surface proteins known to be important in Fe(III) oxide reduction also appear to play a role in electricity production. Deletion of the gene for the outer-membrane c-type cytochrome OmcS specifically inhibits growth on Fe(III) oxides but not on soluble electron acceptors (29), and the OmcS-deficient strain is also severely inhibited in current production (14). The electrically conductive pili of G. sulfurreducens are also required for Fe(III) oxide reduction (31) as well as for maximum power production in fuel cells (32). Similar mechanisms for electron transfer to Fe(III) oxides and the anodes of microbial fuel cells would greatly facilitate the study of mechanisms for electricity production because studies with Fe(III) oxides as electron acceptors are often more technically tractable than studies with fuel cells.
Although a substantial number of Fe(III) oxide-reducing microorganisms, such as Aeromonas (30), Desulfobulbus (12), Desulfuromonas (2), Geobacter (2, 3), Geopsychrobacter (17), Geothrix (4), Rhodoferax (6), and Shewanella (18; M. Lanthier, unpublished data) species, have been evaluated for the potential for current production, Pelobacter species have not. Pelobacter species are of interest, in part, because they are in the family Geobacteraceae, and Geobacteraceae are often specifically enriched on the surfaces of anodes harvesting electricity from aquatic sediments (2, 13, 36). Geobacter species are the most abundant Geobacteraceae on anodes of sediment fuel cells harvesting electricity from freshwater sediments, and Desulfuromonas species are the predominant members of this family on anodes from marine sediment fuel cells. However, microorganisms with 16S rRNA gene sequences most closely related to known Pelobacter species can account for ca. 20% of the Geobacteraceae sequences on both freshwater and marine anodes (13).
Like Geobacter and Desulfuromonas species, Pelobacter species are capable of Fe(III) reduction (11, 20, 26), but unlike these other members of the Geobacteraceae, which completely oxidize acetate and other organic compounds, Pelobacter species incompletely oxidize organic substrates and are typically cultured under fermentative conditions. For example, Pelobacter carbinolicus, which is the Pelobacter species that can be cultured most readily on Fe(III) oxides, ferments substrates, such as 2,3-butanediol, acetoin, and ethylene glycol, to ethanol and acetate (33) and incompletely oxidizes ethanol to acetate with Fe(III) or S° serving as the electron acceptor (26). P. carbinolicus and P. acetylenicus can also ferment ethanol to acetate with the production of hydrogen when grown with a hydrogen-consuming partner that maintains hydrogen concentrations low enough for this reaction to be thermodynamically favorable (33, 34).
In order to evaluate the potential for Pelobacter species to contribute to current production in sediment microbial fuel cells, current production in P. carbinolicus was investigated. The results of the investigation suggest that although this organism readily reduces Fe(III) oxides, it cannot effectively transfer electrons to the anode of a microbial fuel cell.
MATERIALS AND METHODS
Organisms, media, and growth conditions.
For maintenance, P. carbinolicus (DSMZ 2380) was cultured at 30°C under strict anaerobic conditions in medium containing NaCl (20.0 g/liter), MgCl2·6H2O (3.0 g/liter), NaHCO3 (2.5 g/liter), NH4Cl (0.25 g/liter), KH2PO4 (0.2 g/liter), KCl (0.5 g/liter), and CaCl2·2H2O (0.15 g/liter). Vitamins and trace minerals (23), acetoin (10 mM) and Na2S (1.7 mM) were added from stock solutions (26). These cultures were also used to inoculate the fuel cells. The medium in the fuel cells was freshwater medium (24) containing NaHCO3 (2.5 g/liter), NH4Cl (0.25 g/liter), NaH2PO4 (0.6 g/liter), and KCl (0.1 g/liter). Vitamins and trace minerals (23) were added from stock solutions. The medium was additionally amended (5%, vol/vol) with a salt stock solution (containing NaCl [180 g/liter], MgCl2·6H2O [54 g/liter], and CaCl2·2H2O [2.7 g/liter]) to establish a salt concentration at which both P. carbinolicus and G. sulfurreducens thrived.
Geobacter sulfurreducens strain PCA (ATCC 51573) was maintained in anoxic pressure tubes with acetate-fumarate (NBAF) medium containing 10 mM acetate and 40 mM fumarate, as described previously (7). Before inoculation in fuel cells, the bacteria were slowly adapted to the high salt concentration by sequentially transferring them into NBAF medium with increasing contents (1, 2, 3, 4, and 5% [vol/vol]) of the salt stock solution described above.
Fuel cells.
Dual-chamber fuel cells (H type) were assembled and operated as described previously (3), with the exceptions that 5% (vol/vol) of the salt stock solution described above was added, the fuel cells had a liquid volume of 200 ml and a headspace of 100 ml, and each chamber was equipped with a glass screw thread aperture on top, which was sealed with a rubber stopper and screw cap. Ethanol (5 mM) or acetate (10 mM) served as the electron donor and carbon source in the anode chamber. The electrodes were connected via a 560Ω resistor over which the voltage was recorded hourly.
When fuel cells were switched to potentiostat mode, the anode became the working electrode, and the setup was adjusted as described previously (3). Briefly, while the chambers were sparged with N2-CO2 (80:20), the medium in both chambers was replaced and the Tris-buffered medium in the cathode chamber was exchanged with bicarbonate-buffered FW medium. An Ag/AgCl reference electrode was placed in the working electrode chamber, and the working electrode was poised at +300 mV with a potentiostat. The counter electrode chamber was continuously sparged with N2-CO2, while sparging of the working electrode was stopped after adding ethanol (5 mM), to avoid further losses of ethanol by evaporation.
Analytical techniques.
Concentrations of ethanol and acetate were determined with high-pressure liquid chromatography using an LC-10ATVP high-pressure liquid chromatograph (Shimadzu, Kyoto, Japan) equipped with an Aminex HPX-87H column (300 by 7.8 mm; Bio-Rad, Hercules, CA), with 8 mM H2SO4 eluent. Acetate was detected with an SPD-10AVP UV detector set at 210 nm. Ethanol was quantified with an RID-10A refractive index detector (Shimadzu, Kyoto, Japan).
Hydrogen partial pressures were measured using an RGD2 reduction gas analyzer (Trace Analytical, Menlo Park, CA) as described previously (25).
The protein content in fuel cell medium and on electrodes was determined using the bicinchoninic assay (K. P. Nevin, H. Richter, S. F. Covalla, J. P. Johnson, T. L. Woodard, H. Jia, M. Zhang, and D. R. Lovley, submitted for publication).
Calculations.
Electron recovery from ethanol metabolism was calculated by the following formulas: moles of electrons recovered as electricity = amps produced × s/96,500 columbs per mole of electron, moles of electrons available from ethanol metabolism = (moles/liter of ethanol consumed × 12) − (moles/liter of acetate remaining × 8) − (moles/liter × hydrogen remaining × 2) × liters in anode chamber, and electron recovery = moles of electrons recovered as electricity/moles of electrons available from ethanol metabolism. The concentration of dissolved hydrogen was calculated from hydrogen partial pressure (22, 38) in the headspace of fuel cells.
16S rRNA gene clone libraries.
The proportion of P. carbinolicus in the biofilm on anodes or in the fuel cell medium of cocultures was determined from the abundance of 16S rRNA genes. DNA was extracted from the biofilm on the anodes as described previously (13) or from an aliquot (2 ml) of the anode chamber medium with the Bio101 soil kit (Bio Systems, Carlsbad, CA). The DNA was purified, and 16S rRNA gene fragments were amplified with the primer 8 forward and 519 reverse (13). The PCR conditions were as described previously (16). PCR products were purified, and clone libraries were constructed with a TOPO TA cloning kit, version K2 (Invitrogen, Carlsbad, CA). 16S rRNA gene fragments were sequenced, and the proportion of P. carbinolicus sequences was determined as described previously (16).
Fluorescent in situ hybridization.
Biofilms growing on graphite electrodes were fixed for 1 h in paraformaldehyde-phosphate-buffered saline (PBS) (19) in plastic bags. Electrodes were then washed in PBS for 5 min and stored at −20°C in PBS buffer-50% ethanol until hybridization. For hybridization, samples were dehydrated in a graded series of 50, 80, and 95% ethanol solutions for 5 min each. Rectangular sections were drawn on one side of the electrode with a hydrophobic pen (Super PAP pen liquid blocker; Ted Pella, Inc., Redding, CA). Each section was for hybridization with a different set of probes. Electrodes were exposed for 20 min to 1 to 2 ml of an acetylation solution (19) and then washed gently with deionized water.
Hybridization solution (100 to 200 μl) (19), which contained 25 ng of fluorescent probe per microliter and helper oligonucleotides (Table 1) to increase the fluorescence signal (10), if required, was added to each rectangular section on the electrode. Autofluorescence controls were prepared without fluorescent probes, and nonspecific probes (37) were applied to rule out nonspecific hybridization. All fluorescent probes were coupled to Cy3, but another aliquot of GEO2 was also available with Cy5. Hybridization was carried out in a hybridization oven (Shake n Bake, model 136400; Boekel Scientific) for 3 h at 46°C. The humidity chamber was filled with towels soaked with 50 ml of hybridization buffer. Then electrodes were washed once for 20 min at 48°C in washing buffer (19), rinsed with deionized water, counterstained in the dark with 100 to 200 μl of 25 μM YOYO-1 (Molecular Probes, Eugene, OR) per section for 30 min, and rinsed for 5 min with deionized water. Electrodes were mounted with an antifade kit (ProLong; Molecular Probes).
TABLE 1.
Fluorescent probes used in this studya
| Common probe name | Generic name | Target(s) | Sequence (5′-3′) | Position no. | Reference(s) |
|---|---|---|---|---|---|
| NON338 | NA | None | ACTCCTACGGGAGGCAGC | NA | 37 |
| EUB338-I | S-D-Bact-0338-a-A-18 | Most Bacteria | GCTGCCTCCCGTAGGAGT | 338-355 | 1, 9 |
| EUB338-II | S-*-BactP-0338-a-A-18 | Planctomycetales | GCAGCCACCCGTAGGTGT | 338-355 | |
| EUB338-III | S-*-BactV-0338-a-A-18 | Verrucomicrobiales | GCTGCCACCCGTAGGTGT | 338-355 | |
| DELTA495A | S-C-dProt-0495-a-A-18 | Most Deltaproteobacteria | AGTTAGCCGGTGCTTCCT | 495-512 | 27 |
| DELTA495B | S-*-dProt-0495-b-A-18 | Some Deltaproteobacteria | AGTTAGCCGGCGCTTCCT | 495-512 | |
| DELTA495C | S-*-dProt-0495-c-A-18 | Some Deltaproteobacteria | AATTAGCCGGTGCTTCCT | 495-512 | |
| GEO3-A | S-G-Geob-0818-a-A-21 | Geobacter cluster | CCGCAACACCTAGTACTCATC | 818-838 | This study |
| GEO3-B | S-G-Geob-0818-b-A-21 | CCGCAACACCTAGTTCTCATC | 818-838 | ||
| GEO3-C | S-G-Geob-0818-c-A-21 | CCGCAACACCTGGTTCTCATC | 818-838 | ||
| DMONAS-A | S-G-Dmona-0433-a-A-21 | Desulfuromonas cluster | TTTCTTCCCCTCTGACAGAGC | 433-453 | This study |
| DMONAS-B | S-G-Dmona-0433-b-A-21 | TTTCTTCCCTCTTGACAGAGC | 433-453 | ||
| DMONAS-C | S-G-Dmona-0433-c-A-21 | TTTCTTTCCTCCTGACAGAGC | 433-453 | ||
| DMONAS-D | S-G-Dmona-0433-d-A-21 | GTTCTTCCCCTCTGACAGAGC | 433-453 | ||
| PCARB1 | S-S-Pcarb-0455-a-A-18 | P. carbinolicus | GCCTATTCGACCACGATA | 455-470 | This study |
| GEO2 | S-S-Gsulf-0207-a-A-19 | G. sulfurreducens | GAAGACAGGAGGCCCGAAA | 207-225 | This study |
| HGEO2-1 | S-S-Gsuh1-0114-(PCA)-a-A-22 | Helper probes for GEO2 | GTCCCCCCCTTTTCCCGCAAGA | 114-135 | This study |
| HGEO2-2 | S-S-Gsuh2-0226-a-A-22 | G. sulfurreducens | CTAATGGTACGCGGACTCATCC | 226-243 | |
| HGEO3-3 | S-G-Geoh3-0798-a-A-20 | Helper probes for GEO3 | GTTTACGGCGGGTACTACC | 798-817 | This study |
| HGEO3-4 | S-G-Geoh4-0839-a-A-18 | CACTGCAGGGGTCAATAC | 839-856 |
NA, not available.
Samples were examined on a Zeiss Axiovert LSM 510 Meta confocal system equipped with a 63× Zeiss Plan-Apochromat oil immersion objective (numerical aperture of 1.4) and a Meta detector (Carl Zeiss MicroImaging, Inc., Thornwood, NY). The confocal microscope was equipped with an Argon laser (lines at 458, 477, 488, and 514 nm; 25 mW total) and two HeNe lasers (lines at 543 nm, 1 mW, and 633 nm, 5 mW), a krypton-argon dual laser (488 and 568 nm) and a diode laser (638 nm). Representative three-dimensional scans of biofilm sections on the electrodes were taken and displayed as orthoviews. Images were averaged by Kalman filtration with eight running scans per image (28). The acquisition software was LSM 510 Meta, version 3.2 SP2.
RESULTS AND DISCUSSION
Current with P. carbinolicus alone and in culture with G. sulfurreducens.
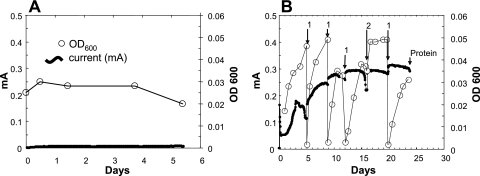
P. carbinolicus did not produce current in fuel cells operated either in true fuel cell mode (Fig. 1) or when the anode was artificially poised at +300 mV with a potentiostat (data not shown). If acetoin, which supports fermentative growth, was provided, the cells grew via fermentation in the fuel cell chamber. There was no growth when ethanol was the potential electron donor (Fig. 1A). The loss of ethanol over time in fuel cells inoculated with P. carbinolicus was similar to the evaporative loss of ethanol due to the N2-CO2 sparging in uninoculated fuel cells (Fig. 2).
FIG. 1.
Fuel cells with 5 mM ethanol, inoculated with P. carbinolicus (A) or a coculture of G. sulfurreducens and P. carbinolicus (B). In the coculture, the anode chamber medium was replaced several times and supplemented with 5 mM ethanol (1) or ethanol was added without medium replacement (2). OD600, optical density at 600 nm.
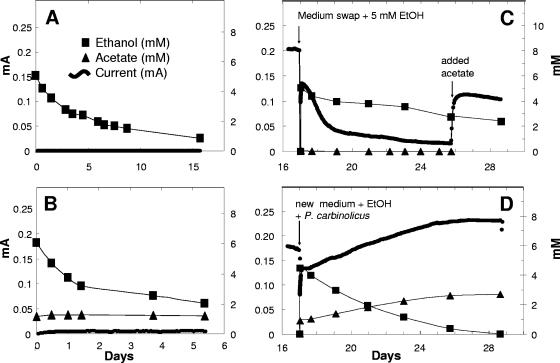
FIG. 2.
Comparison of current generation, ethanol consumption, and acetate production in fuel cells with sterile control plus ethanol (A), P. carbinolicus plus ethanol (B), G. sulfurreducens grown with acetate until day 16 and then fed with ethanol (EtOH) (C), and a coculture of P. carbinolicus and G. sulfurreducens fed with ethanol (D). In panels C and D, G. sulfurreducens was grown with acetate in the fuel cell until current production was stable and then media were exchanged (acetate was omitted, and ethanol or ethanol and P. carbinolicus were added).
However, current was produced when Geobacter sulfurreducens was inoculated into an ethanol-amended anode chamber along with P. carbinolicus (Fig. 1B). Current production was associated with an increase in culture density. When the current began to decline, the medium in the anode chamber was replaced. This replacement resulted in increased current production and continued growth of planktonic cells (Fig. 1B). With continued medium replacements, the current production increased to a maximum of ca. 0.3 mA, which is comparable to the power output previously observed for other microorganisms incubated in similar fuel cells (2, 3; data not shown).
Fuel cells inoculated with just G. sulfurreducens did not produce current with ethanol as the electron donor (data not shown). In order to further evaluate whether ethanol supported electricity production by G. sulfurreducens, a fuel cell was established with just G. sulfurreducens and acetate as the electron donor. As expected from previous studies (3) G. sulfurreducens produced current under these conditions (Fig. 2C).
However, when the medium was switched to one with ethanol as the electron donor, current production rapidly declined. Current resumed when acetate was reintroduced (Fig. 2C). These results are consistent with a previous report that G. sulfurreducens does not use ethanol as an electron donor for the reduction of Fe(III) (5).
Ethanol metabolism and current production in the coculture fuel cells were associated with the production of acetate (Fig. 2D). Acetate accumulation was less than ethanol removal (Fig. 2D), suggesting that G. sulfurreducens was oxidizing some of the acetate with electron transfer to the anode. Acetate production from ethanol requires that four electrons be transferred to an electron acceptor. When P. carbinolicus lacks electron acceptors, such as Fe(III) or S° (26), it can transfer electrons to protons, producing hydrogen as long as hydrogen concentrations are maintained at sufficiently low levels (33, 34). The fact that ethanol was not significantly metabolized in fuel cells containing just P. carbinolicus suggests that G. sulfurreducens was required to consume hydrogen as well as acetate to make ethanol metabolism thermodynamically favorable.
Significance of hydrogen consumption by G. sulfurreducens.
In order to determine the significance of hydrogen consumption by G. sulfurreducens in the coculture fuel cells, wild-type G. sulfurreducens was replaced with a strain in which the gene hybL was deleted. This gene encodes the large subunit of the uptake hydrogenase, HyB. The hybL-deficient strain is capable of metabolizing acetate, but does not grow with hydrogen as an electron donor (8). In fuel cells, it produced current from acetate, but not hydrogen (data not shown). In order to establish P. carbinolicus with the hydrogenase-deficient mutant in a fuel cell coculture, it was necessary to vigorously sparge the anode chamber with N2-CO2 to strip hydrogen from the system. Once the coculture was established, the media in both chambers were exchanged as described in Materials and Methods, ethanol (5 mM) was added, and the anode was poised at +300 mV. Sparging was stopped 1 day after this ethanol addition. Controls were treated similarly, but grown with wild-type G. sulfurreducens.
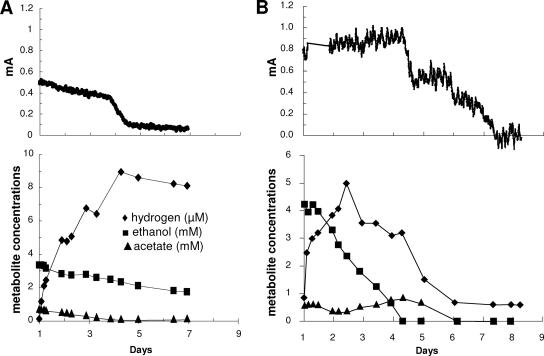
The nonsparged coculture with the hydrogenase-deficient G. sulfurreducens mutant initially produced current with the consumption of the remaining acetate that had been produced from the metabolism of ethanol during the sparging phase, when hydrogen was able to escape the fuel cell (Fig. 3A). The ethanol concentration declined at a rate which was an order of magnitude less than that in the coculture with wild-type G. sulfurreducens, and hydrogen accumulated. Once the acetate was consumed, current declined to very low levels and hydrogen concentrations stabilized, indicating that even though P. carbinolicus can oxidize hydrogen with the reduction of Fe(III) (26), it did not significantly oxidize hydrogen with electron transfer to the fuel cell anode. In contrast, ethanol was rapidly degraded in the coculture with wild-type G. sulfurreducens (Fig. 3B). Hydrogen initially accumulated, but to lower levels than that in the coculture with the hydrogenase-deficient mutant, and in the presence of wild-type cells, the hydrogen levels subsequently declined. These results suggest that hydrogen uptake by G. sulfurreducens plays an important role in promoting the ethanol metabolism of P. carbinolicus and, thus, current production by the coculture.
FIG. 3.
Current generation and metabolite concentrations in cocultures of P. carbinolicus and a G. sulfurreducens strain with the uptake hydrogenase gene deleted (A) or wild-type G. sulfurreducens (B). The anode was poised at +300 mV with a potentiostat. Cultures were initiated with vigorous sparging in order to remove hydrogen produced as outlined in the text. Time courses are shown after the sparging was stopped on day 1.
Electron recovery.
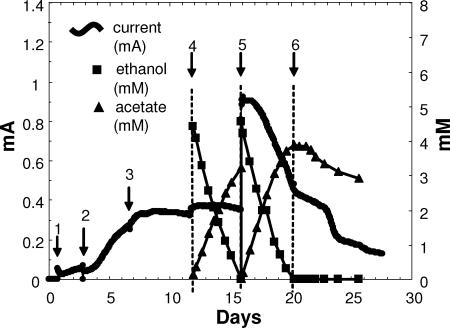
The stoichiometry of ethanol consumption and recovery of electrons as current with wild-type G. sulfurreducens and P. carbinolicus were determined in fuel cell mode because this mode most closely represents the conversion of fuels to current for practical applications. However, when oxygen was used as the oxidant for the cathode, the anode chamber had to be bubbled with N2/CO2 in order to remove oxygen diffusing into the anode chamber. As noted above, this caused evaporative losses of ethanol, resulting in low values of electron recovery (37 to 49%). To alleviate the need for sparging the anode chamber, 50 mM K3Fe(CN)6 was added as the oxidant in the cathode chamber and the fuel cells were placed in an anaerobic glove bag to prevent oxygen diffusion into the system (Fig. 4). Under these conditions, 74 to 83% of the electrons that were present in the ethanol consumed were recovered as current and in the accumulated acetate.
FIG. 4.
Current and concentrations of ethanol and acetate in a coculture fuel cell in an anaerobic glove bag. First the fuel cell was set up to sparge the cathode (air) and anode (N2-CO2). It was then inoculated with G. sulfurreducens plus 10 mM acetate (arrow 1). After initial growth, the anode medium was exchanged (P. carbinolicus and 5 mM ethanol were added) (arrow 2). Medium plus ethanol was replaced two more times until stable current was achieved (arrows 3 and 4). Ethanol, acetate, and current were measured in “sparging mode” until ethanol was completely consumed (arrows 4 and 5). Then oxygen was sparged out of the cathode chamber with N2-CO2, while the cathode medium was supplemented with K3Fe(III)(CN)6. Sparging of both chambers was stopped, and the fuel cell was put into an anaerobic glove bag (arrow 5). Electron recovery was then determined without evaporative loss of metabolites (arrows 5 and 6).
Biomass distribution.
The measurable optical density in the P. carbinolicus-G. sulfurreducens fuel cells (Fig. 1B) contrasts with that of previously described G. sulfurreducens fuel cells in which the anode chamber typically exhibits little or no turbidity upon repeated exchanges of the medium (3). The amounts of planktonic protein, 1.00 to 1.44 mg, and protein on the anode, 1.03 to 1.25 mg, were comparable. An analysis of 16S rRNA gene clone libraries constructed from planktonic cells demonstrated that 98% of the planktonic cells were P. carbinolicus. In contrast, 16S rRNA gene clone libraries constructed from cells scraped from the anode surface indicated that P. carbinolicus and G. sulfurreducens each accounted for 48 to 52% of the cells on the anode. The fact that ca. two-thirds of the P. carbinolicus biomass was not associated with the anode is consistent with the concept that P. carbinolicus is important for ethanol metabolism, but not for current production, and that G. sulfurreducens associated with the anode is responsible for electron transfer to the anode. The average current generated per milligram of cell protein attached to the anode surface in the coculture fuel cells was 0.27 mA/mg. This value is low compared to the 0.34 to 1.93 mA/mg protein from G. sulfurreducens pure cultures (3). This can be attributed to the inability of P. carbinolicus to participate in current production.
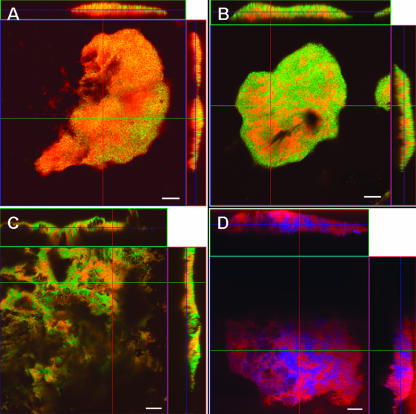
In order to further evaluate the association of P. carbinolicus and G. sulfurreducens, the biofilm on the anode surface was examined with confocal laser scanning microscopy and fluorescent in situ hybridization. There was no autofluorescence when no probe was added to the hybridization buffer, or with a nonspecific probe (NON338), which does not hybridize with the rRNA. All cells in the biofilm on the anode hybridized with a set of probes targeting all Bacteria (EUB338-I, -II, and -III) (data not shown) or all Deltaproteobacteria (DELTA495A, -B, and -C) (Fig. 5A). Probes targeting the Geobacter cluster (15) of the Geobacteraceae (GEO3A, -B, and -C) (data not shown), which includes G. sulfurreducens, or the Desulfuromonas cluster, which includes P. carbinolicus (15) (DMONAS-A, -B, -C, and -D) (Fig. 5B), revealed that both organisms colonized the anode in near-equal numbers, in accordance with the results from the clone libraries. This result was further confirmed with species-specific probes targeting G. sulfurreducens (GEO2) or P. carbinolicus (PCARB1) (Fig. 5C and D). Although there appeared to be species-specific clusters of cells within the biofilm, neither organism appeared to have a preference for growth near the anode surface or the outer surface of the biofilm.
FIG. 5.
Top views (large squares) and orthogonal side views (top and side rectangles) of P. carbinolicus and G. sulfurreducens biofilm growing on an anode and hybridized with probes targeting Deltaproteobacteria (probe DELTA495, red) (A), Desulfuromonas cluster (probe DMONAS, red) (B), G. sulfurreducens (probe GEO2, red) (C), and P. carbinolicus (probe PCARB1, red) and G. sulfurreducens (GEO2, blue) (D). All biofilm sections are shown with YOYO-1 counterstain (green), except in panel D. Helper probes HGEO2-1 and HGEO2-2 were used in panels C and D. Bar = 10 μm.
Implications.
These results demonstrate that P. carbinolicus has little, if any, capacity for electron transfer to electrodes. This result is surprising, because P. carbinolicus readily grows with insoluble Fe(III) oxides as the electron acceptor (26), and, typically, microorganisms capable of dissimilatory Fe(III) oxide reduction are also able to use anodes as an electron acceptor (21, 35). The lack of current production by P. carbinolicus was not due to improper culture conditions in the fuel cell, because P. carbinolicus grew readily during fermentation of acetoin or ethanol in coculture with G. sulfurreducens in the same system. The current produced from the coculture resulted from G. sulfurreducens oxidizing acetate and hydrogen not removed by sparging, with the anode serving as the electron acceptor. Both acetate and hydrogen oxidation can serve as electron donors for current production by G. sulfurreducens (3).
An apparent difference in extracellular electron transfer strategies in P. carbinolicus and G. sulfurreducens is that P. carbinolicus contains far fewer c-type cytochromes than G. sulfurreducens does (11). Most notably, P. carbinolicus lacks the outer-membrane cytochromes that are thought to serve as an electrical contact between the cell and the anode in G. sulfurreducens cells closely associated with the anode surface (14). P. carbinolicus does contain genes for pili, which in G. sulfurreducens are considered to be electrically conductive (31) and provide long-range electron transfer through the biofilm on fuel cell anodes (32). Reverse transcriptase PCR and gel electrophoresis demonstrated that in P. carbinolicus, the putative pilA gene (Pcar_2144, VIMSS 586177) coding for the pilin subunit of type IV pili is expressed during growth on the electrode in coculture with G. sulfurreducens (unpublished results). However, whether the pili in G. sulfurreducens are sufficient for electron transfer to anodes in the absence of outer-membrane c-type cytochromes has not been determined. Attempts to determine whether the pili of P. carbinolicus are conductive have been inconclusive due to technical difficulties in obtaining sufficient pili for evaluation. Therefore, it is not clear whether the inability of P. carbinolicus to generate current is due to the lack of required c-type cytochromes or due to pili which are nonconductive.
Although the possibility for current production in other Pelobacter species has yet to be determined, the difficulties in growing the other available pure cultures on Fe(III) oxides suggest that it is unlikely that any of these strains are able to directly generate current as a pure culture in a microbial fuel cell. Although Pelobacter species clearly colonize the anodes of sediment fuel cells (13), it seems likely that their primary role in current production is indirect, converting organic substrates to acetate and hydrogen, which Geobacter or Desulfuromonas species can oxidize with electron transfer to the anode.
Acknowledgments
This research was supported by the Office of Science (BER), U.S. Department of Energy, cooperative agreement number DE-FC02-02ER63446, and the Office of Naval Research, award number N00014-06-1-0802. Confocal laser scanning microscopy was supported by National Science Foundation grant BBS8714235 to the University of Massachusetts—Amherst Microscopy Facility.
Footnotes
Published ahead of print on 15 June 2007.
REFERENCES
- 1.Amann, R. I., B. J. Binder, R. J. Olson, S. W. Chisholm, R. Devereux, and D. A. Stahl. 1990. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl. Environ. Microbiol. 56:1919-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bond, D. R., D. E. Holmes, L. M. Tender, and D. R. Lovley. 2002. Electrode-reducing microorganisms that harvest energy from marine sediments. Science 295:483-485. [DOI] [PubMed] [Google Scholar]
- 3.Bond, D. R., and D. R. Lovley. 2003. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl. Environ. Microbiol. 69:1548-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bond, D. R., and D. R. Lovley. 2005. Evidence for involvement of an electron shuttle in electricity generation by Geothrix fermentans. Appl. Environ. Microbiol. 71:2186-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caccavo, F., Jr., D. J. Lonergan, D. R. Lovley, M. Davis, J. F. Stolz, and M. J. McInerney. 1994. Geobacter sulfurreducens sp. nov., a hydrogen- and acetate-oxidizing dissimilatory metal-reducing microorganism. Appl. Environ. Microbiol. 60:3752-3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaudhuri, S. K., and D. R. Lovley. 2003. Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat. Biotechnol. 21:1229-1232. [DOI] [PubMed] [Google Scholar]
- 7.Coppi, M. V., C. Leang, S. J. Sandler, and D. R. Lovley. 2001. Development of a genetic system for Geobacter sulfurreducens. Appl. Environ. Microbiol. 67:3180-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coppi, M. V., R. A. O'Neil, and D. R. Lovley. 2004. Identification of an uptake hydrogenase required for hydrogen-dependent reduction of Fe(III) and other electron acceptors by Geobacter sulfurreducens. J. Bacteriol. 186:3022-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daims, H., A. Bruhl, R. Amann, K. H. Schleifer, and M. Wagner. 1999. The domain-specific probe EUB338 is insufficient for the detection of all bacteria: development and evaluation of a more comprehensive probe set. Syst. Appl. Microbiol. 22:434-444. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs, B. M., F. O. Glockner, J. Wulf, and R. Amann. 2000. Unlabeled helper oligonucleotides increase the in situ accessibility to 16S rRNA of fluorescently labeled oligonucleotide probes. Appl. Environ. Microbiol. 66:3603-3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haveman, S. A., D. E. Holmes, Y. H. Ding, J. E. Ward, R. J. Didonato, Jr., and D. R. Lovley. 2006. c-Type cytochromes in Pelobacter carbinolicus. Appl. Environ. Microbiol. 72:6980-6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holmes, D. E., D. R. Bond, and D. R. Lovley. 2004. Electron transfer by Desulfobulbus propionicus to Fe(III) and graphite electrodes. Appl. Environ. Microbiol. 70:1234-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmes, D. E., D. R. Bond, R. A. O'Neil, C. E. Reimers, L. R. Tender, and D. R. Lovley. 2004. Microbial communities associated with electrodes harvesting electricity from a variety of aquatic sediments. Microb. Ecol. 48:178-190. [DOI] [PubMed] [Google Scholar]
- 14.Holmes, D. E., S. K. Chaudhuri, K. P. Nevin, T. Mehta, B. A. Methe, A. Liu, J. E. Ward, T. L. Woodard, J. Webster, and D. R. Lovley. 2006. Microarray and genetic analysis of electron transfer to electrodes in Geobacter sulfurreducens. Environ. Microbiol. 8:1805-1815. [DOI] [PubMed] [Google Scholar]
- 15.Holmes, D. E., K. P. Nevin, and D. R. Lovley. 2004. Comparison of 16S rRNA, nifD, recA, gyrB, rpoB and fusA genes within the family Geobacteraceae fam. nov. Int. J. Syst. Evol. Microbiol. 54:1591-1599. [DOI] [PubMed] [Google Scholar]
- 16.Holmes, D. E., K. P. Nevin, R. A. O'Neil, J. E. Ward, L. A. Adams, T. L. Woodard, H. A. Vrionis, and D. R. Lovley. 2005. Potential for quantifying expression of the Geobacteraceae citrate synthase gene to assess the activity of Geobacteraceae in the subsurface and on current-harvesting electrodes. Appl. Environ. Microbiol. 71:6870-6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmes, D. E., J. S. Nicoll, D. R. Bond, and D. R. Lovley. 2004. Potential role of a novel psychrotolerant member of the family Geobacteraceae, Geopsychrobacter electrodiphilus gen. nov., sp. nov., in electricity production by a marine sediment fuel cell. Appl. Environ. Microbiol. 70:6023-6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim, H. J., H. S. Park, M. S. Hyun, I. S. Chang, M. Kim, and B. H. Kim. 2002. A mediator-less microbial fuel cell using a metal reducing bacterium, Shewanella putrefaciens. Enzyme Microb. Technol. 30:145-152. [Google Scholar]
- 19.Lanthier, M., P. Juteau, F. Lepine, R. Beaudet, and R. Villemur. 2005. Desulfitobacterium hafniense is present in a high proportion within the biofilms of a high-performance pentachlorophenol-degrading, methanogenic fixed-film reactor. Appl. Environ. Microbiol. 71:1058-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lonergan, D. J., H. L. Jenter, J. D. Coates, E. J. Phillips, T. M. Schmidt, and D. R. Lovley. 1996. Phylogenetic analysis of dissimilatory Fe(III)-reducing bacteria. J. Bacteriol. 178:2402-2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lovley, D. R. 2006. Bug juice: harvesting electricity with microorganisms. Nat. Rev. Microbiol. 4:497-508. [DOI] [PubMed] [Google Scholar]
- 22.Lovley, D. R., F. H. Chapelle, and J. C. Woodward. 1994. Use of dissolved H2 concentrations to determine distribution of microbially catalyzed redox reactions in anoxic groundwater. Environ. Sci. Technol. 28:1205-1210. [DOI] [PubMed] [Google Scholar]
- 23.Lovley, D. R., R. C. Greening, and J. G. Ferry. 1984. Rapidly growing rumen methanogenic organism that synthesizes coenzyme M and has a high affinity for formate. Appl. Environ. Microbiol. 48:81-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lovley, D. R., and E. J. Phillips. 1988. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl. Environ. Microbiol. 54:1472-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lovley, D. R., E. J. Phillips, and D. J. Lonergan. 1989. Hydrogen and formate oxidation coupled to dissimilatory reduction of iron or manganese by Alteromonas putrefaciens. Appl. Environ. Microbiol. 55:700-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lovley, D. R., E. J. Phillips, D. J. Lonergan, and P. K. Widman. 1995. Fe(III) and S° reduction by Pelobacter carbinolicus. Appl. Environ. Microbiol. 61:2132-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loy, A., A. Lehner, N. Lee, J. Adamczyk, H. Meier, J. Ernst, K. H. Schleifer, and M. Wagner. 2002. Oligonucleotide microarray for 16S rRNA gene-based detection of all recognized lineages of sulfate-reducing prokaryotes in the environment. Appl. Environ. Microbiol. 68:5064-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manz, W., K. Wendt-Potthoff, T. R. Neu, U. Szewzyk, and J. R. Lawrence. 1999. Phylogenetic composition, spatial structure, and dynamics of lotic bacterial biofilms investigated by fluorescent in situ hybridization and confocal laser scanning microscopy. Microb. Ecol. 37:225-237. [DOI] [PubMed] [Google Scholar]
- 29.Mehta, T., M. V. Coppi, S. E. Childers, and D. R. Lovley. 2005. Outer membrane c-type cytochromes required for Fe(III) and Mn(IV) oxide reduction in Geobacter sulfurreducens. Appl. Environ. Microbiol. 71:8634-8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pham, C. A., S. J. Jung, N. T. Phung, J. Lee, I. S. Chang, B. H. Kim, H. Yi, and J. Chun. 2003. A novel electrochemically active and Fe(III)-reducing bacterium phylogenetically related to Aeromonas hydrophila, isolated from a microbial fuel cell. FEMS Microbiol. Lett. 223:129-134. [DOI] [PubMed] [Google Scholar]
- 31.Reguera, G., K. D. McCarthy, T. Mehta, J. S. Nicoll, M. T. Tuominen, and D. R. Lovley. 2005. Extracellular electron transfer via microbial nanowires. Nature 435:1098-1101. [DOI] [PubMed] [Google Scholar]
- 32.Reguera, G., K. P. Nevin, J. S. Nicoll, S. F. Covalla, T. L. Woodard, and D. R. Lovley. 2006. Biofilm and nanowire production leads to increased current in Geobacter sulfurreducens fuel cells. Appl. Environ. Microbiol. 72:7345-7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schink, B. 1984. Fermentation of 2,3-butanediol by Pelobacter carbinolicus sp. nov., and Pelobacter propionicus sp. nov., and evidence for propionate formation from C2 compounds. Arch. Microbiol. 137:33-41. [Google Scholar]
- 34.Seitz, H. J., B. Schink, and R. Conrad. 1988. Thermodynamics of hydrogen metabolism in methanogenic cocultures degrading ethanol or lactate. FEMS Microbiol. Lett. 55:119-124. [Google Scholar]
- 35.Seop, C. I., H. Moon, O. Bretschger, J. K. Jang, H. I. Park, K. H. Nealson, and B. H. Kim. 2006. Electrochemically active bacteria (EAB) and mediator-less microbial fuel cells. J. Microbiol. Biotechnol. 16:163-177. [Google Scholar]
- 36.Tender, L. M., C. E. Reimers, H. A. Stecher III, D. E. Holmes, D. R. Bond, D. A. Lowy, K. Pilobello, S. J. Fertig, and D. R. Lovley. 2002. Harnessing microbially generated power on the seafloor. Nat. Biotechnol. 20:821-825. [DOI] [PubMed] [Google Scholar]
- 37.Wallner, G., R. Amann, and W. Beisker. 1993. Optimizing fluorescent in situ hybridization with rRNA-targeted oligonucleotide probes for flow cytometric identification of microorganisms. Cytometry 14:136-143. [DOI] [PubMed] [Google Scholar]
- 38.Wilhelm, E., R. Battino, and R. J. Wilcox. 1977. Low-pressure solubility of gases in liquid water. Chem. Rev. 77:219-262. [Google Scholar]