Abstract
Enteric pathogens in animal waste that is not properly processed can contaminate the environment and food. The persistence of pathogens in animal waste depends upon the waste treatment technology, but little is known about persistence of porcine viruses. Our objectives were to characterize the porcine enteric viruses (porcine noroviruses [PoNoVs], porcine sapoviruses [PoSaVs], rotavirus A [RV-A], RV-B, and RV-C) in fresh feces or manure and to evaluate the effects of different candidate environmentally superior technologies (ESTs) for animal waste treatment on the detection of these viruses. Untreated manure and samples collected at different stages during and after treatment were obtained from swine farms that used conventional waste management (CWM) and five different candidate ESTs. The RNA from porcine enteric viruses was detected by reverse transcription-PCR and/or seminested PCR; PoSaV and RV-A were also detected by enzyme-linked immunosorbent assay. Cell culture immunofluorescence (CCIF) and experimental inoculation of gnotobiotic (Gn) pigs were used to determine RV-A/C infectivity in posttreatment samples. The PoSaV and RV-A were detected in pretreatment samples from each farm, whereas PoNoV and RV-C were detected in pretreatment feces from three of five and four of five farms using the candidate ESTs, respectively. After treatment, PoSaV RNA was detected only in the samples from the farm using CWM and not from the farms using the candidate ESTs. RV-A and RV-C RNAs were detected in four of five and three of four candidate ESTs, respectively, after treatment, but infectious particles were not detected by CCIF, nor were clinical signs or seroconversion detected in inoculated Gn pigs. These results indicate that only RV-A/C RNA, but no viral infectivity, was detected after treatment. Our findings address a public health concern regarding environmental quality surrounding swine production units.
Increased animal production and a decline in the numbers of producers in the United States have led to megascale livestock operations (36). The confinement of animals on relatively small land areas has resulted in the generation, accumulation, and need for disposal of large amounts of animal wastes worldwide (6, 32). The causative agents of many infectious diseases are excreted by the fecal route by animals with acute and chronic infections but also sometimes from clinically healthy animals. In all types of livestock housing, waste material on site produces odors and contains antimicrobials, nutrients, organic matter, and pathogens. Storage and treatment of this waste before land application are typically done in wastewater lagoons. However, lagoon management presents significant concerns (18). With a greater opportunity for horizontal spread of infectious agents among closely confined animals, manure contains pathogens that can be transmitted to other animals or to farm workers or to the public via contaminated meat products or water sources (12).
Environmentally superior technologies (ESTs) for animal waste treatment have been defined as those that are not only technically, economically, and operationally feasible but also are able to eliminate the direct discharge of animal waste to surface and groundwater and to reduce the levels of odors, ammonium, pathogens, nutrients, and heavy metals. At least 18 candidate ESTs have been developed for treatment of animal wastes to reduce their impact on the environment, the food supply, and public health (20). Several studies to determine the presence, persistence, and rates of reduction of bacterial pathogens in the newly developed treatments have been performed (6, 17, 39; X. Li, J. B. Payne, F. B. O. Santos, C. M. Williams, and B. W. Sheldon, presented at the 2005 Animal Waste Management Symposium, Raleigh, NC, 5 to 7 October 2005; C. A. Likirdopulos, O. D. Simmons, G. P. Ko, and M. D. Sobsey, presented at the 2005 Animal Waste Management Symposium, Raleigh, NC, 5 to 7 October 2005; O. D. Simmons, C. A. Likirdopulos, L. Worley-Davis, C. M. Williams, and M. D. Sobsey, presented at the 2005 Animal Waste Management Symposium, Raleigh, NC, 5 to 7 October 2005; P. W. Westerman and J. Arogo, presented at the North Carolina Animal Waste Management Workshop, Raleigh, NC, 16 to 17 October 2005). However, little is known about the presence and persistence of viruses in swine waste. In 2001, the USEPA and USDA identified several infectious disease agents associated with manure, and the research needs to better document their presence and fate through different treatments (31). Among the most common causes of viral infectious diarrhea in pigs, porcine enteric caliciviruses (porcine noroviruses [PoNoVs] and porcine sapoviruses [PoSaVs]) and rotaviruses (RVs; groups A, B, and C) are of particular concern because of their environmental stability, resistance to disinfectants, and prolonged infectivity in feces.
The main contributor to food-borne illness is Norovirus (a genus in the Caliciviridae family), which is estimated to cause 82% of the illnesses caused by known pathogens (34). PoNoV was detected in Japan (33) and Europe (37) and recently in the United States (40). PoNoV was detected in cecal contents of healthy pigs in Japan and in fecal samples from 3- to 9-month-old healthy pigs in The Netherlands. In the United States, PoNoV was detected in fecal samples but only from finisher pigs. Most positive samples were from healthy animals, suggesting that, as previously observed for cases of human norovirus infection, asymptomatic shedding of norovirus occurs among adults, thereby contributing to virus persistence in the field (43).
Sapovirus (another genus in the Caliciviridae family) is also associated with gastroenteritis and plays an important role in outbreaks of infantile and elderly gastroenteritis (16). The PoSaV has emerged as an important pathogen associated with diarrhea and subclinical infections among pigs. The PoSaVs were first discovered in the United States in 1980 (29). Since then, PoSaVs have been detected in fecal samples from diarrheic or normal nursing and postweaning pigs and also from normal finisher pigs (43). However, pigs in all age groups are seropositive for PoSaV antibodies, indicative of high exposure rates. These findings suggest that PoSaV may be a major cause of diarrhea, but subclinical PoSaV infections may also occur. Moreover, identification of genetically closely related caliciviruses in humans and animals, as well as studies of the seroprevalence and antigenic cross-reactivity between them, suggests possible interspecies or zoonotic transmission (10, 41).
RVs are the leading cause of acute viral gastroenteritis in the young of both avian and mammalian species, including pigs and humans. Group A, B, and C RVs infect pigs (30, 46), and both RV-A and non-group A RV can be detected in the same herd. RV-A infection accounts for 53% of the cases of diarrhea in preweaning swine and 44% of the cases of diarrhea in postweaning swine, whereas 89% of all cases of RV diarrhea in commercial pig operations have been attributed to RV-A (15, 44). RV-B and RV-C have been identified in humans and pigs, but at lower percentages (4.6 to 18.2% and 10.8 to 22.7%, respectively) (13, 21). In the United States, RV-C was identified in fecal samples collected from finishing pigs with diarrhea by immunoelectron microscopy, cell culture immunofluorescence (CCIF), and reverse transcription-PCR (RT-PCR) (23). Furthermore, the finding of a low prevalence of antibodies to RV-C in humans and a higher prevalence of these antibodies in pigs has led to the suggestion that RV-C could be a zoonotic infection (15). Likewise, for RV-A, pig-like serotypes were detected in children, and human serotypes have been shown to infect pigs (11, 30), confirming their interspecies transmission. RV is highly stable, and it can survive in the feces as long as 7 to 9 months at room temperature (18 to 20°C) (45). Farm expansion, all-in all-out production, the presence of an RV strain that was not previously circulating, and high population density are considered the major risk factors that contribute to an outbreak (2, 7).
The objectives of this study were to characterize the occurrence of PoNoV, PoSaV, RV-A, RV-B, and RV-C in fresh excreta and swine manure under defined animal waste treatment systems and to evaluate the effects of five different candidate ESTs for swine waste management on the detection of these viral pathogens.
MATERIALS AND METHODS
ESTs.
The following conventional (lagoon) operation and five different candidate ESTs were evaluated: (i) conventional waste management (CWM), (ii) the aerobic upflow biofiltration system (AUFBS), (iii) solids separation/constructed-wetland system (CWS), (iv) the solids separation/nitrification-denitrification/soluble-phosphorus-removal solids-processing system (SSS; Super Soil Systems), (v) in-ground ambient-temperature anaerobic digester and greenhouse system (ATAnD), and (vi) high-rise hog building (HRHB). The CWM, AUFBS, CWS, SSS, and ATAnD were tested in North Carolina as part of the agreement between the Attorney General of North Carolina and Smithfield Foods, Premium Standard Farms, and Frontline Farmers to identify alternative technologies to replace anaerobic lagoons (C. M. Williams, presented at the International Symposium Addressing Animal Production and Environmental Issues, Raleigh, NC, 2001; C. M. Williams, presented at the 2005 Animal Waste Management Symposium, Raleigh, NC, 5 to 7 October 2005). The HRHB was developed as an alternative method for treatment of animal waste to produce composted solids without liquids (22). The system was originally built in Ohio and has been evaluated by researchers at the Ohio Agricultural Research and Development Center.
Site characteristics.
Seven swine production sites were selected for this study. Each site consisted of a medium (750 to 2,499 pigs weighing over 55 pounds) or large (2,500 or more pigs weighing over 55 pounds) concentrated animal feeding operation according to the U.S. Environmental Protection Agency definitions (9), and the sites were similar to each other in terms of production phase (with the exception of ATAnD technology). When it was possible, technologies were evaluated at two different time points during a year. Site characteristics are summarized in Table 1.
TABLE 1.
Site characteristics
| EST | No. of sites | Type of farm | No. of houses | Total no. of pigs | Testing mo.e |
|---|---|---|---|---|---|
| CWM | 2 | Finishing | 4/8b | 5,000/7,000 | February or May |
| AUFBS | 1 | Finishing | 5 | 4,000 | April and June |
| CWS | 1 | Finishing | 4 | 3,500 | November |
| SSS | 1 | Finishing | 6 | 4,400 | April and May |
| ATAnD | 1 | Farrow to weaninga | 6c | 7,000d | August and November |
| HRHB | 1 | Finishing | 1 | 1,000 | June to September and October to February |
This type of farm houses sows and newborns up to 3 weeks old.
Four houses in one farm and eight houses in the other farm.
The six houses include two farrowing and four gestation houses.
This total includes 4,000 sows and 3,000 weaning pigs.
Technologies were tested twice per year, in the same or different farms, except for CWS.
Samples.
Fresh swine fecal specimens were aseptically collected from 10 different spots along a zig-zag path through an animal pen or waste pit. To evaluate the effects of these technologies, fresh excreta, as well as samples of manure and litter, pre- and posttreatment samples, and samples taken at different steps during treatment were collected from each swine waste management system. Samples were collected at different treatment points according to the technology (Table 2), frozen, and shipped overnight to our lab.
TABLE 2.
Samples and points of collection for each system
| Sample sources from indicated point of collection in waste treatment technologies
| |||||
|---|---|---|---|---|---|
| CWM | AUFBS | CWS | SSS | ATAnD | HRHB |
| Fecesa | Fecesa | Fecesa | Fecesa | Fecesa,c | Fecesa |
| House effluent | House effluent | House effluent | House effluent | House effluentc | Bedding |
| Lagoonb | Separated solids | Inner effluent | Homogenizer tank | Digester | Initial compost |
| Separated liquids | Outer effluent | Separated solids | Biofilter no. 1 | Final compostb | |
| Equalization tank | Solid for separation | Separated liquids | Storage pond | ||
| Biofilter effluent | Inner effluent | Pre-phosphorus removal (liquid) | Biofilter no. 2 | ||
| Biofilter backwash | Outer effluent | Post-phosphorus removal (liquid)b | Greenhouse samplesb | ||
| Lagoon 1 (liquids)b | Storage pondb | Bagged final product (solid)b | Tomato mediab | ||
| Lagoon 2 (biosolids)b | |||||
| Lagoon 3 (barn waste)b,d | |||||
Pretreatment samples.
Posttreatment samples.
Includes samples from four gestation houses and two farrowing houses.
Lagoon 3 is not connected to the treatment technology (bypass) and it receives manure from another five houses with 4,000 pigs (total).
Sample preparation and nucleic acid extraction.
Samples were diluted 1:10 in phosphate-buffered saline (PBS; pH 7.4), homogenized, and centrifuged for 30 min at 1,500 × g at 4°C. The supernatants were aspirated, aliquoted, and stored at −20°C until analysis. Samples were tested by enzyme-linked immunosorbent assay (ELISA), RT-PCR, and/or seminested PCR.
Nucleic acids were extracted with TRIzol (Invitrogen) according to the manufacturer's directions and the RNA was resuspended in 50 μl of diethyl pyrocarbonate (DEPC)-treated water. The presence of natural PCR inhibitors was tested by RT-PCR using a competitive internal control (IC) and primers PEC-65 and PEC-66, which are specific for this IC as described below (42).
Detection of PCR and RT-PCR inhibitors in fecal samples.
One microliter of a competitive IC was added to each RNA sample, and RT-PCR with primers PEC-65/PEC-66, specific for this IC, was performed as follows: 42°C for 60 min and 94°C for 3 min, followed by 35 cycles of 94°C for 30 s, 50°C for 60 s, and 72°C for 60 s and a final extension of 10 min at 72°C. Amplified products were analyzed by electrophoresis using 2% agarose gel staining with ethidium bromide and visualized under UV. If inhibition was detected, the RNA template was diluted 1:10 to overcome it and tested again.
RT-PCR for animal enteric calicivirus and seminested PCR for RV detection.
For the detection of animal enteric caliciviruses, RT-PCR was performed with primers PEC-66 (5′-GACTACAGCAAGTGGGATTCC-3′) and PEC-65 (5′-ATACACACAATCATCCCCGTA-3′) for PoSaVs as previously described (19), and RT-PCR was performed with primers PNV7 (5′-AGGTGGTGGCCGAGGAYC-3′) and PNV8 (5′-TCGCCATAGAAGTARAAG-3′) for PoNoVs as previously described by Wang et al. (42).
For RV-A, RT-PCR was performed with primers sBeg-9 (5′-GGCTTTAAAAGAGAGAATTTCCGTCTGG-3′) and End-9 (5′-GGTCACATCATACAATTCTAATCTAAG-3′) as previously described (14). The RT-PCR products were diluted with DEPC-treated water (1:10) and a seminested PCR was performed with primers GA75M (5′-TAGGTATTGAATATACCACAA-3′) and End-9 for 30 cycles at 94°C for 1 min, 52°C for 2 min, and 72°C for 1 min and a final incubation at 72°C for 10 min. For RV-B, RT-PCR was performed with primers 9B3 (5′-CAGTAACTCTATCCTTTTACC-3′) and 9B4 (5′-CGTATCGCAAATACAATCCG-3′) as previously described (4). For RV-C, RT-PCR was performed with primers C1 (5′-CTCGATGCTACTACAGAATCA-3′) and C4 (5′-AGCCACATAGTTCACATTTCATCC-3′). The RT-PCR products were diluted 1:10 in DEPC-treated water and seminested PCR was performed with primers C1 and C3 (5′-GGGATCATCCACGTCATGCG-3′) as previously described (15). Amplified products were analyzed by electrophoresis using 2% agarose gels staining with ethidium bromide and visualized under UV light.
Detection of RV-A and PoSaV by antigen ELISA.
Separate antigen capture ELISAs were performed to detect RV-A and PoSaV as previously described (1, 19). Briefly, for RV-A, half of the 96-well plates were coated with a gnotobiotic (Gn) pig hyperimmune anti-bovine RV-A serum (positive coating) or Gn pig RV-A antibody-negative serum (negative coating) and incubated overnight at 4°C. After that, plates were washed with PBS-0.05% Tween and blocked with PBS-0.05% Tween-2% nonfat dry milk for 2 h at 37°C. After being washed, each sample was added in duplicate to antibody-positive serum- and antibody-negative serum-coated wells. Positive and negative controls were added to each plate (1). Plates were incubated for 1 h at 37°C. Hyperimmune guinea pig anti-bovine RV-A serum was added as a secondary antibody. After incubation, anti-guinea pig immunoglobulin G (IgG; heavy plus light chains)-horseradish peroxidase (HRP)-labeled antibody (KPL, Inc., MD) was added to each well and incubated for 1 h at 37°C. Plates were developed by using the ABTS two-component microwell peroxidase substrate kit (KPL, Inc., MD). Absorbance values at 450 nm were read with an ELISA reader (Spectra Max 340PC; Molecular Devices, CA).
A similar procedure was followed for PoSaV (19). The 96-well plates were coated with hyperimmune guinea pig anti-PoSaV Cowden strain virus-like particle serum (positive coating) or preimmunization guinea pig antibody-negative serum and incubated overnight at 4°C. Plates were washed and blocked, and samples and controls were added and incubated after each reagent was added as described above. Hyperimmune pig antiserum to PoSaV Cowden strain was added as a secondary antibody, followed by mouse monoclonal antibody to pig IgG conjugated to biotin. Streptavidin-HRP (Roche) was added, and plates were developed with the TMB two-component microwell peroxidase substrate kit (KPL, Inc., MD). Their absorbance values at 650 nm were read with an ELISA reader.
Detection of RV-A and RV-C infectious viral particles by CCIF in pre- and posttreatment samples.
A representative group of pretreatment samples and all posttreatment samples that were positive by seminested PCR for RV-A and/or RV-C were analyzed by a CCIF assay to detect infectious viral particles, as previously described (1, 35). Supernatants were diluted 1:10 in serum-free minimum essential medium and serially diluted 10-fold thereafter. Confluent monolayers of MA104 cells were inoculated with 100 μl of each dilution in duplicate. Fifty microliters of trypsin (2 μg/ml) was added to each well, and plates were centrifuged at 1,200 × g for 1 h at room temperature. After that, plates were incubated in a 5% CO2 atmosphere at 37°C for 18 h. After incubation, monolayers were fixed with 80% acetone for 10 min and incubated overnight at 4°C with fluorescein isothiocyanate-conjugated hyperimmune Gn pig anti-RV-C serum or fluorescein isothiocyanate-conjugated hyperimmune anti-bovine RV-A serum. Fluorescing cells were visualized by using a fluorescent microscope.
Sequence analysis.
RT-PCR and seminested PCR products were purified from agarose gels by use of the Geneclean kit as described by the manufacturer (QIAGEN, MD). The DNA was sequenced directly by using BigDye Terminator Cycle chemistry and a 3700 DNA analyzer (Applied Biosystems, Foster, CA). Sequence data were aligned by using the Lasergene software package (DNASTAR Inc., Madison, WI) and compared with published sequences by using the Basic Local Alignment Search Tool (BLAST) and Clustal methods.
Challenge of Gn pigs with posttreatment samples.
To assess infectivity of posttreatment samples positive by seminested PCR but negative by CCIF, hysterectomy-derived, colostrum-deprived 5-day-old Gn pigs were orally inoculated. Pigs were surgically derived and maintained in sterile isolator units (25). Selected posttreatment samples had the highest seminested PCR titers (based on endpoint titration) for RV-A or RV-C but were negative by CCIF for RV-A or RV-C, respectively. Samples were diluted 1:10 in minimum essential medium, homogenized, and centrifuged, and the recovered supernatants were filtered (with a 0.2-μm-pore-size filter). Briefly, two Gn pigs were first orally inoculated with 5 ml of sample H-125 (positive for RV-C by seminested PCR but negative by CCIF). Clinical parameters, including diarrhea and fecal scores (0, normal; 1, pasty; 2, semiliquid; 3, liquid), were recorded. Fecal shedding of viral RNA was assayed by seminested PCR from postinoculation days (PID) 0 to 5. At 7 PID and in the absence of clinical signs, both pigs were orally inoculated with 5 ml of sample H-134 (positive for RV-A by seminested PCR but negative by CCIF). Clinical signs were recorded, whereas fecal shedding (viral RNA or virus) was assayed by seminested PCR (RV-A and RV-C) and ELISA (RV-A) from PID 7 to 12. Serum samples were collected at 0, 14, 21, and 31 PID and stored at −20°C until tested for antibodies to RV-A and RV-C by ELISA. Large and small intestine contents were collected after euthanasia at PID 31.
Detection of antibodies against RV-A and RV-C by ELISA.
For detection of anti-RV-A antibodies, 96-well plates were coated with guinea pig hyperimmune anti-bovine RV-A serum and incubated at 4°C overnight (26). The plates were blocked with PBS-0.05% Tween-2% nonfat dry milk for 2 h at 37°C. Reagents and samples were added in the following order: semipurified human RV-A Wa strain or mock cell culture supernatant, fourfold serial dilutions of serum, anti-swine IgG or IgM conjugated to biotin (KPL, Inc., MD), and streptavidin-HRP (Roche). Plates were developed by using the ABTS two-component microwell peroxidase substrate kit (KPL, Inc., MD), and absorbance values at 450 nm were read with an ELISA reader.
Similarly, for RV-C, plates were coated with semipurified swine RV-C strain L1049 or mock cell culture supernatants, incubated overnight at 4°C, and blocked with PBS-0.05% Tween-2% nonfat dry milk for 2 h at 37°C. Fourfold serial dilutions of serum or anti-swine IgG- or IgM-HRP antibody (KPL, Inc., MD) were sequentially added. Plates were developed by using the ABTS two-component microwell peroxidase substrate kit (KPL, Inc., MD), and absorbance values at 450 nm were read with an ELISA reader.
RESULTS
Occurrence of animal enteric viruses in fresh excreta.
Animal enteric caliciviruses and RVs were detected by ELISA and different RT-PCR and seminested PCR assays. PoSaVs were detected in 59/61 (97%) samples by RT-PCR, ELISA, or both techniques. Eight samples from different sites were sequenced, confirming the presence of PoSaV genogroup III. PoNoVs were detected in 12/61 (20%) samples by RT-PCR (Table 3). Sequence analysis of samples collected in different sites confirmed the presence of PoNoV genogroup II.
TABLE 3.
Detection of PoNoV and PoSaV in pre-, post-, and during treatment samples in different candidate ESTs
| Collecting time (no. of samples) | No. (%) of samples positive for animal enteric caliciviruses
|
||||
|---|---|---|---|---|---|
| PoNoV (RT-PCR) | PoSaV
|
||||
| RT-PCRa | ELISA | RT-PCRa and ELISAb | Total | ||
| Pretreatment (61) | 12 (20) | 19 (31) | 14 (23) | 26 (43) | 59 (97) |
| During treatment (75) | 0 (0) | 5 (7) | 18 (24) | 4 (5) | 27 (36) |
| Posttreatment (28) | 0 (0) | 2 (7) | 0 (0) | 0 (0) | 2 (7) |
| Total (164) | 12 (7) | 26 (16) | 32 (19) | 30 (18) | 88 (54) |
RT-PCR was performed with primers PEC-66/PEC-65.
Samples were positive by both ELISA and RT-PCR.
Samples were also tested for the presence of RV-A by ELISA and seminested PCR. Forty-one of 61 (67%) samples were positive for RV-A by seminested PCR or both ELISA and seminested PCR. None of the samples were positive for RV-B by RT-PCR. RV-C was detected in 27/61 (44%) samples by seminested PCR (Table 4).
TABLE 4.
Detection of RV-A, RV-B, and RV-C in pretreatment samples, posttreatment samples, and samples obtained during treatment in different candidate ESTsa
| Collecting time | RV-A
|
No. (%) of samples positive for RV-B by RT-PCR | RV-C
|
|||||
|---|---|---|---|---|---|---|---|---|
| No. (%) of samples positive
|
No. of samples positive by CCIF/ no. tested by CCIF (%)c | |||||||
| Seminested PCRb | ELISA | Seminested PCRb and ELISAd | Total | No. (%) of samples positive by seminested PCR | No. of samples positive by CCIF/ no. tested by CCIF (%)c | |||
| Pretreatment (61) | 39 (64) | 0 (0) | 2 (3) | 41 (67) | 21/25 (84) | 0 (0) | 27 (44) | 14/17 (82) |
| During treatment (75) | 29 (39) | 0 (0) | 3 (4) | 32 (43) | NA | 0 (0) | 41 (55) | NA |
| Posttreatment (28) | 14 (50) | 0 (0) | 0 (0) | 14 (50) | 4/14 (29) | 0 (0) | 12 (43) | 2/12 (17) |
| Total (164) | 82 (50) | 0 (0) | 5 (3) | 87 (53) | 25/39 (64) | 0 (0) | 80 (49) | 16/29 (55) |
NA, not applicable.
Seminested PCR was performed with primers GA75M/End-9.
A set of pretreatment samples and all posttreatment samples with a seminested PCR-positive result were tested by CCIF as indicated in the text.
Samples were positive by both ELISA and seminested PCR.
Effects of different candidate ESTs for swine waste management on the detection of animal enteric viruses by ELISA, RT-PCR, and/or seminested PCR.
PoSaV, RV-A, and RV-C were detected in pre- and posttreatment samples, but PoNoVs were detected only in pretreatment samples (Table 3 and 4). A total of 75 samples were collected at different steps during treatment. None of these samples were positive for PoNoV. However, 27/75 (36%) were positive for PoSaV by RT-PCR, ELISA, or both techniques. Thirty-two of 75 (43%) samples were also positive for RV-A by seminested PCR or both seminested PCR and ELISA. RV-C was detected in 41/75 (55%) samples by seminested PCR. Only 2 posttreatment samples of 28 (7%) were positive for PoSaV by RT-PCR, but RV-A and RV-C were detected in 14/28 (50%) and 12/28 (43%) posttreatment samples, respectively, by seminested PCR (Table 3 and 4).
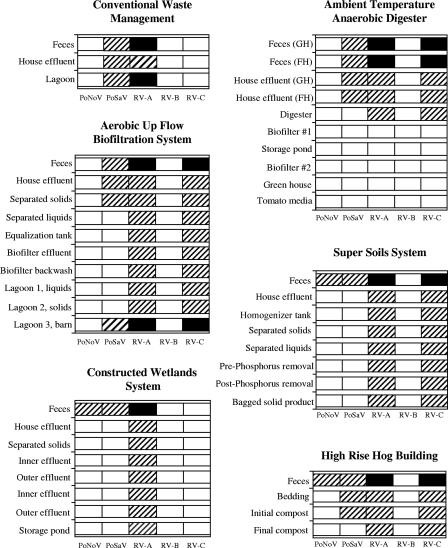
With respect to the applied technologies, PoSaV and RV-A were detected in feces (pretreatment samples) from control sites (CWM) and every site where a candidate EST was tested. However, PoNoV and RV-C were detected in pretreatment samples from the sites where the CWS, SSS, and HRHB (for PoNoV) or the AUFBS, SSS, ATAnD, and HRHB (for RV-C) were applied (Fig. 1). Among the samples collected during treatment, PoSaV was not detected in the CWS or SSS. With the ATAnD technology, PoSaV was no longer detected after house effluents were treated in the anaerobic digester. In the AUFBS, PoSaVs were detected in solids separated from the house effluent but not in liquids that were further processed. In the HRHB, PoSaV was detected in the initial compost samples but not the final compost samples. PoSaV was detected in lagoon samples (posttreatment) where CWM was used and in lagoon 3 (bypass) where the AUFBS was used but not in posttreatment samples from the five ESTs.
FIG. 1.
Detection of PoNoV, PoSaV, RV-A, RV-B, and RV-C in pre- and posttreatment samples as well as different steps following treatment in different candidate ESTs and CWM. □, negative; ▒ positive by RT-PCR, seminested PCR, and/or ELISA; ▪, positive by seminested PCR and CCIF.
The results obtained for RV-A and RV-C differed from those for PoSaV and PoNoV. With ATAnD technology, RV-A and RV-C were detected in effluents from the anaerobic digester, but they were not detected after the first of two biofilters (biofilter no. 1) was used. RV-A was detected in posttreatment samples from the CWM site (lagoon samples) and also in posttreatment samples from the CWS site (storage pond) by seminested PCR. In the sites where both RV-A and RV-C were detected, results were generally similar. In the AUFBS, both viruses were detected by seminested PCR in samples collected from lagoons 1, 2, and 3 at the end of the treatment. In the SSS, samples from the bagged final product as well as the liquid obtained after phosphorus removal were positive for both viruses. Both viruses were also detected in the final compost produced after the HRHB treatment.
Detection of RV-A and RV-C infectious viral particles by CCIF in pre- and posttreatment samples.
A representative group of 25/41 pretreatment samples positive by seminested PCR for RV-A were tested by CCIF. Infectious RV-A particles were detected in 21/25 (84%) tested samples (Table 4). Positive samples belonged to the five ESTs tested and control sites (Fig. 1). For RV-C, a subset of 17/27 pretreatment samples positive by seminested PCR were tested. Fourteen of 17 (82%) samples were positive by CCIF and belonged to the sites at which the AUFBS, SSS, ATAnD, and HRHB were used (Table 4; Fig. 1).
All posttreatment samples positive for RV-A or RV-C by seminested PCR were assayed by CCIF. For RV-A, 4/14 (29%) posttreatment samples positive by seminested PCR were also positive by CCIF. For RV-C, 2/12 (17%) posttreatment samples positive by seminested PCR were also positive by CCIF. All samples positive for RV-A and RV-C by CCIF belonged to the control sites (CWM) and lagoon 3 (bypass; AUFBS) (Table 4; Fig. 1).
Challenge of Gn pigs with posttreatment samples.
To assess infectivity of posttreatment samples positive by seminested PCR but negative by CCIF, two Gn pigs were successively inoculated with H-125 (posttreatment sample positive for RV-C by seminested PCR but negative by CCIF) and sample H-134 (posttreatment sample positive for RV-A by seminested PCR but negative by CCIF). The H-125 sample did not cause diarrhea (score 0 to 1), and RV-C RNA was detected only by seminested PCR at PID1. At PID 7 and in the absence of diarrhea, Gn pigs were inoculated with H-134. The H-134 induced no diarrhea (score 0 to 1), and RV-A RNA was detected only by seminested PCR 1 day after inoculation. Seroconversion to RV-A and/or RV-C was not detected (data not shown).
DISCUSSION
Land application of agricultural waste occurs worldwide, and pathogens present in manure can affect soil and water quality, as well as the health of animals and humans working in livestock production. Different environmental factors affect the fate and transport of pathogens from waste into soil and water (12). Several technologies to reduce the impact of animal waste in the environment have been developed. In this study, we assessed the presence of five different enteric viruses after treatment of swine waste by five different candidate ESTs and in a CWM operation (control).
PoSaVs and RV-A were detected in fecal samples collected from pigs in each site under study. However, PoNoV was detected only at sites where the CWS, SSS, and HRHB were tested, allowing us to evaluate only these systems for PoNoV. Similarly, RV-C was detected only at sites where the AUFBS, ATAnD, SSS, and HRHB were evaluated. No differences related to seasonality or production size were detected (Fig. 1).
Animal enteric caliciviruses cause gastroenteritis in calves and pigs (19, 38) but have also been isolated from healthy pigs (40, 42). The PoSaV has emerged as an important pathogen associated with diarrhea and subclinical infections among pigs of all ages. Their prevalence in feces varies between 21 to 100%, in samples collected from pigs with diarrhea (90 to 100%) and clinically normal pigs (21 to 83%) (42). In our study, the PoSaVs were detected in 97% of pretreatment samples by RT-PCR, ELISA, or both techniques, regardless of the type of swine production where the technology was applied. PoNoVs were detected only in 3- to 9-month-old asymptomatic pigs in Japan, Europe, and United States, but their prevalence (0.5 to 20%) was much lower than that of PoSaV (33, 37, 43). In our study, PoNoVs were detected only in 20% of pretreatment samples. In contrast to PoSaVs, PoNoVs have only been detected in clinically healthy finisher pigs, suggesting that, as previously observed for human norovirus infections, asymptomatic shedding of PoNoV occurs in adults contributing to virus persistence in the field (28). Similar results were observed in our study, in which PoNoVs were detected only in fecal samples from sites with finishing pigs (the CWS, SSS, and HRHB) but not in samples collected in farrowing/gestation houses where the ATAnD technology was applied. However, PoNoVs were not detected in samples from the sites using the AUFBS and CWM that also contained finishing pigs, which is in agreement with previous field studies that indicate that the prevalence of PoNoVs in the field is not as widespread as that of PoSaV.
RVs are ubiquitous in the environment. RV-A, RV-B, and RV-C infect pigs, and both group A and non-group A RVs can be detected in samples from the same farm. Disease is influenced by pig age, management conditions, and practices (46). Although disease is more frequently diagnosed in 1- to 5-week-old pigs, RV can also cause diarrhea in feeder pigs (23). In our study, RV-A and RV-C were detected in 67% and 44% of pretreatment (feces) samples, respectively (Table 4). RV-B was not detected in our study. This could be because of the absence of the virus, less shedding (3), or a test with low sensitivity (compared with the seminested PCRs applied for RV-A and RV-C). Whereas one or all of these factors could be involved, the fact that all RVs have similar stabilities suggests that the effects of these candidate ESTs on the detection of RV-B (if the virus is present) will be similar to their effects on the detection of RV-A and RV-C.
Results differed when different molecular techniques (RT-PCR or seminested PCR) and classical techniques, such as ELISA, were used for detection, and this could be explained by differences in the target and sensitivity of each test. Regarding the targets, RT-PCR (or seminested PCR) detects RNA (free or partially surrounded or surrounded by a protein core), whereas an ELISA detects complete virus or viral proteins (assuming that antigenic epitopes are intact). In addition, the sensitivity of the RT-PCR and ELISA methods for PoSaV were determined based on 10-fold serial dilution of a cell-cultured PoSaV Cowden strain, ranging from 101 to 107 50% tissue culture infective doses (TCID50)/ml. The smallest amount of PoSaV particles detected by ELISA was 105, whereas RT-PCR detected RNA in the sample containing 104 TCID50/ml (data not shown). Similarly, for RV-A, the sensitivities of RT-PCR, seminested PCR, and ELISA were determined based on 10-fold serial dilutions of the cell-cultured RV-A Wa strain, ranging from 101 to 108 fluorescent focus-forming units (FFU)/ml by CCIF. The smallest detectable amount of viral RV-A by ELISA was 104 FFU/ml, whereas the lowest positive signal by RT-PCR was from the sample containing 103 FFU/ml, and the seminested PCR increased this sensitivity by 100 times (101 FFU/ml). Thus, an RT-PCR/seminested PCR-positive and ELISA-negative sample could have been the consequence of partially or totally destroyed particles or, more likely, low virus concentrations. On the other hand, an RT-PCR-negative ELISA-positive sample could be a consequence of mutations in the sequence targeted by RT-PCR primers that do not result in a change at the epitope (protein level) detected by ELISA. At the present, RT-PCR is the most widely used assay to detect viral enteric pathogens in clinical and environmental samples (8, 24).
The five technologies evaluated for treatment of animal waste showed different results. Whereas each one of the candidate ESTs was able to decrease the PoSaV and/or PoNoV concentration to levels that were not detectable by a sensitive assay such as RT-PCR, not all of the ESTs were effective for RV-A and RV-C detection. In fact, posttreatment samples from the AUFBS (lagoons 1 and 2), CWS, SSS, and HRHB were negative for PoSaV and PoNoV, but aside from these CWS samples (in which RV-C was not initially detected), they were all positive for RV-A and RV-C. The differences observed could occur because the seminested PCR (RV-A and RV-C) has been shown to be more sensitive than RT-PCR (PoSaV and PoNoV), as indicated above, or they could be due to an actual decrease in concentrations of enteric caliciviruses but not of RV. The latter possibility is corroborated by other studies that indicate that RVs are some of the nonenveloped viruses that are most resistant to inactivation in different animal wastes (27). Based on these findings, the ATAnD system was the only technology able to completely decrease RV concentrations to undetectable levels as measured by highly sensitive molecular techniques. Posttreatment samples from the CWM site (control) and AUFBS lagoon 3 (which receives manure directly from the barn, without treatment) were positive by molecular techniques for those virus initially detected at each site.
However, none of the techniques mentioned earlier differentiate between infectious and noninfectious virus particles, which is very important in the assessment of the risk of disease transmission from environmental samples. The detection of RNA in a sample does not always indicate the presence of infectious virus. Although it is expected that free RNA will be degraded in the absence of a protective viral core protein, no information is available about the time required for this to occur. To assess infectivity, we tested a group of pretreatment and all posttreatment RV-A and/or RV-C seminested PCR-positive samples by CCIF. Pretreatment samples were positive by CCIF, indicating that infectious RV-A or RV-C particles were present before treatment. All posttreatment samples collected from each candidate EST site but not from the CWM site or AUFBS lagoon 3 (bypass) were negative by the CCIF infectivity assay for RV-A and RV-C, indicating the absence of a concentration of infectious particles detectable by this assay. However, there are two potential deficiencies in this assay for RV: first, the sensitivity of the test, and second, the fact that wild-type RVs initially may not replicate efficiently in the MA104 cells used for CCIF assay. Regarding the latter, because pretreatment samples coming from each site and posttreatment samples from control sites were positive by CCIF, these findings suggest that although replication may not be very efficient, it was adequate for CCIF detection, as long as the virus concentration was high enough based on the test detection limits.
To determine whether infectious particles were present at concentrations not detected by CCIF, we inoculated Gn pigs, which should be the most sensitive indication of virus infectivity. One- to five-day-old Gn pigs develop profuse watery, yellow-to-white flocculent diarrhea 1 to 2 days after inoculation with RV. The clinical signs last up to 7 days and resolve in 7 to 14 days. Seroconversion to IgM and IgG in serum is detectable at PID 7 and 21, respectively (46). Pigs who recovered from RV infection are protected from a second infection by homologous RV but not heterologous RV. Moreover, dual infection of calves with RV-A and RV-C has shown an increase in RV-C shedding (5). Under a natural scenario, RV diarrhea may be less severe than the experimental disease developed by Gn pigs because of the moderating presence of actively (by a previous infection) or passively (via colostrum or milk) acquired RV-specific antibodies (46). We inoculated Gn pigs with samples positive for RV-A and RV-C by seminested PCR but negative by CCIF and observed the pigs for clinical signs for 31 days postexposure. Neither diarrhea nor seroconversion to IgM or IgG in serum were detected. Virus shedding was detected only at PID 1 by seminested PCR, but it was not detected by ELISA. These results indicate that Gn pigs were not infected by posttreatment samples and suggest that they would not secrete infectious virus in the field.
Virus inactivation depends on many factors, such as environmental temperature, pH, and type of animal waste. Differences were also found in the various steps at which viruses were no longer detectable by using molecular techniques. In samples collected at different steps during the ATAnD process, PoSaV was not detected after anaerobic treatment, but RV-A and RV-C were detected at this step, and samples from the following step (use of biofilter no. 1) were negative. In the AUFBS, solids separated from house effluents were positive for both PoSaV and RVs, but the liquid portion remained positive only for RVs. These differences could be consequences of the physical properties of each virus, the sensitivity of the assay, or the fact that the needed inactivation time for liquid waste is greater than that for solid waste (27).
In summary, enteric viruses present in animal wastes can survive for long periods of time if proper treatment is not applied, and they may constitute a public health concern in regard to the quality of the environment surrounding swine production operations. This study presents, to our knowledge, the first evaluation of the impacts of different candidate ESTs on the detection of viruses in swine manure and suggests that although only the ATAnD can reduce virus concentrations to undetectable levels as evaluated by molecular techniques, all the technologies tested were effective in reducing virus infectivity when evaluated by a virus infectivity assay (CCIF) and Gn pig inoculation. However, these findings should also be evaluated in consideration of the ability of each technology to reduce the impacts of other factors (organic and inorganic residues, bacterial pathogens, and ammonia volatilization, etc.) on the environment.
Acknowledgments
We thank Lynn Worley-Davis and Huawei Sun for collecting the samples at NCSU and OSU, respectively, and Robert Dearth for organizing and storing them at OSU. We also thank the North Carolina State University Animal and Poultry Waste Management Center, where the AUFBS, CWS, ATAnD, and SSS technologies were tested, and the Ohio Agricultural Research and Development Center, where the HRHB technology was tested. Sequencing was performed at the Plant-Microbe Genomics Facility of The Ohio State University.
This research was partly supported by USDA-IFAFS grant number 2001-52103-11302. Salaries and research support were provided by state and federal funds appropriated to the Ohio Agricultural Research and Development Center, The Ohio State University.
Footnotes
Published ahead of print on 29 June 2007.
REFERENCES
- 1.Azevedo, M. S., L. Yuan, K. I. Jeong, A. Gonzalez, T. V. Nguyen, S. Pouly, M. Gochnauer, W. Zhang, A. Azevedo, and L. J. Saif. 2005. Viremia and nasal and rectal shedding of rotavirus in gnotobiotic pigs inoculated with Wa human rotavirus. J. Virol. 79:5428-5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barreiros, M. A., A. A. Alfieri, A. F. Alfieri, K. C. Medici, and J. P. Leite. 2003. An outbreak of diarrhoea in one-week-old piglets caused by group A rotavirus genotypes P[7],G3 and P[7],G5. Vet. Res. Commun. 27:505-512. [DOI] [PubMed] [Google Scholar]
- 3.Bridger, J. C. 1980. Detection by electron microscopy of caliciviruses, astroviruses and rotavirus-like particles in the faeces of piglets with diarrhoea. Vet. Rec. 107:532-533. [PubMed] [Google Scholar]
- 4.Chang, K. O., A. V. Parwani, D. Smith, and L. J. Saif. 1997. Detection of group B rotaviruses in fecal samples from diarrheic calves and adult cows and characterization of their VP7 genes. J. Clin. Microbiol. 35:2107-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, K. O., P. R. Nielsen, L. A. Ward, and L. J. Saif. 1999. Dual infection of gnotobiotic calves with bovine strains of group A and porcine-like group C rotaviruses influences pathogenesis of the group C rotavirus. J. Virol. 73:9284-9293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole, D., L. Todd, and S. Wing. 2000. Concentrated swine feeding operations and public health: a review of occupational and community health effects. Environ. Health Perspect. 108:685-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dewey, C., S. Carman, T. Pasma, G. Josephson, and B. McEwen. 2003. Relationship between group A porcine rotavirus and management practices in swine herds in Ontario. Can. Vet. J. 44:649-653. [PMC free article] [PubMed] [Google Scholar]
- 8.Divizia, M., R. Gabrieli, A. Macaluso, B. Bagnato, L. Palombi, E. Buonomo, F. Cenko, L. Leno, S. Bino, A. Basha, and A. Pana. 2005. Nucleotide correlation between HAV isolates from human patients and environmental samples. J. Med. Virol. 75:8-12. [DOI] [PubMed] [Google Scholar]
- 9.Environmental Protection Agency. 12 February 2003, posting date. National pollutant discharge elimination system permit regulation and effluent limitation guidelines and standards for concentrated animal feeding operations (CAFOs). Environmental Protection Agency, Washington, DC. http://www.epa.gov/EPA-WATER/2003/February/Day-12/w3074.htm.
- 10.Farkas, T., S. Nakajima, M. Sugieda, X. Deng, W. Zhong, and X. Jiang. 2005. Seroprevalence of noroviruses in swine. J. Clin. Microbiol. 43:657-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gabbay, Y. B., B. Jiang, C. S. Oliveira, J. D. Mascarenhas, J. P. Leite, R. I. Glass, and A. C. Linhares. 1999. An outbreak of group C rotavirus gastroenteritis among children attending a day-care centre in Belem, Brazil. J. Diarrhoeal Dis. Res. 17:69-74. [PubMed] [Google Scholar]
- 12.Gerba, C. P., and J. E. Smith, Jr. 2005. Sources of pathogenic microorganisms and their fate during land application of wastes. J. Environ. Qual. 34:42-48. [PubMed] [Google Scholar]
- 13.Geyer, A., T. Sebata, I. Peenze, and A. D. Steele. 1996. Group B and C porcine rotaviruses identified for the first time in South Africa. J. S. Afr. Vet. Assoc. 67:115-116. [PubMed] [Google Scholar]
- 14.Gouvea, V., R. I. Glass, P. Woods, K. Taniguchi, H. F. Clark, B. Forrester, and Z. Y. Fang. 1990. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J. Clin. Microbiol. 28:276-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gouvea, V., J. R. Allen, R. I. Glass, Z. Y. Fang, M. Bremont, J. Cohen, M. A. McCrae, L. J. Saif, P. Sinarachatanant, and E. O. Caul. 1991. Detection of group B and C rotaviruses by polymerase chain reaction. J. Clin. Microbiol. 29:519-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green, K. Y., R. M. Chanock, and A. Z. Kapikian. 2001. Human calicivirus, p. 841-874. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed., vol. 1. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 17.Grewal, S., S. Sreevatsan, and F. C. Michel, Jr. 2007. Persistence of Listeria and Salmonella during swine manure treatment. Compost Sci. Util. 15:53-62. [Google Scholar]
- 18.Guan, T. Y., and R. A. Holley. 2003. Pathogen survival in swine manure environments and transmission of human enteric illness—a review. J. Environ. Qual. 32:383-392. [DOI] [PubMed] [Google Scholar]
- 19.Guo, M., J. Hayes, K. O. Cho, A. V. Parwani, L. M. Lucas, and L. J. Saif. 2001. Comparative pathogenesis of tissue culture-adapted and wild-type Cowden porcine enteric calicivirus (PEC) in gnotobiotic pigs and induction of diarrhea by intravenous inoculation of wild-type PEC. J. Virol. 75:9239-9251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humenik, F. J., J. M. Rice, C. L. Baird, and R. Koelsch. 2004. Environmentally superior technologies for swine waste management. Water Sci. Technol. 49:15-21. [PubMed] [Google Scholar]
- 21.Janke, B. H., J. K. Nelson, D. A. Benfield, and E. A. Nelson. 1990. Relative prevalence of typical and atypical strains among rotaviruses from diarrheic pigs in conventional swine herds. J. Vet. Diagn. Investig. 2:308-311. [DOI] [PubMed] [Google Scholar]
- 22.Keener, H. M., D. L. Elwell, T. A. Menke, and R. R. Stowell. 2001. Design and performance of a high-rise hog facility manure drying bed. Appl. Eng. Agric. 17:703-709. [Google Scholar]
- 23.Kim, Y., K. O. Chang, B. Straw, and L. J. Saif. 1999. Characterization of group C rotaviruses associated with diarrhea outbreaks in feeder pigs. J. Clin. Microbiol. 37:1484-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lodder, W. J., and A. M. de Roda Husman. 2005. Presence of noroviruses and other enteric viruses in sewage and surface waters in The Netherlands. Appl. Environ. Microbiol. 71:1453-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer, R., E. Bohl, and E. Kohler. 1964. Procurement and maintenance of germ-free seine for microbiological investigations. Appl. Microbiol. 12:295-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parreno, V., D. C. Hodgins, L. de Arriba, S. Y. Kang, L. Yuan, L. A. Ward, T. L. To, and L. J. Saif. 1999. Serum and intestinal isotype antibody responses to Wa human rotavirus in gnotobiotic pigs are modulated by maternal antibodies. J. Gen. Virol. 80:1417-1428. [DOI] [PubMed] [Google Scholar]
- 27.Pesaro, F., I. Sorg, and A. Metzler. 1995. In situ inactivation of animal viruses and a coliphage in nonaerated liquid and semiliquid animal wastes. Appl. Environ. Microbiol. 61:92-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rockx, B., M. De Wit, H. Vennema, J. Vinje, E. De Bruin, Y. Van Duynhoven, and M. Koopmans. 2002. Natural history of human calicivirus infection: a prospective cohort study. Clin. Infect. Dis. 35:246-253. [DOI] [PubMed] [Google Scholar]
- 29.Saif, L. J., E. H. Bohl, K. W. Theil, R. F. Cross, and J. A. House. 1980. Rotavirus-like, calicivirus-like, and 23-nm virus-like particles associated with diarrhea in young pigs. J. Clin. Microbiol. 12:105-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saif, L. J., and B. Jiang. 1990. Non-group A rotaviruses of humans and animals, p. 339-371. In R. F. Ramig (ed.), Rotaviruses. Springer-Verlag, Berlin, Germany.
- 31.Smith, J. E., Jr., P. D. Millner, W. Jakubowski, N. Goldstein, and R. Rynk (ed.). 2005. Contemporary perspectives on infectious disease agents in sewage sludge and manure. The JG Press, Emmaus, PA.
- 32.Spencer, J. L., and J. Guan. 2004. Public health implications related to spread of pathogens in manure from livestock and poultry operations. Methods Mol. Biol. 268:503-515. [DOI] [PubMed] [Google Scholar]
- 33.Sugieda, M., H. Nagaoka, Y. Kakishima, T. Ohshita, S. Nakamura, and S. Nakajima. 1998. Detection of Norwalk-like virus genes in the caecum contents of pigs. Arch. Virol. 143:1215-1221. [DOI] [PubMed] [Google Scholar]
- 34.Tauxe, R. V. 2002. Emerging foodborne pathogens. Int. J. Food Microbiol. 78:31-41. [DOI] [PubMed] [Google Scholar]
- 35.Terrett, L. A., L. J. Saif, K. W. Theil, and E. M. Kohler. 1987. Physicochemical characterization of porcine pararotavirus and detection of virus and viral antibodies using cell culture immunofluorescence. J. Clin. Microbiol. 25:268-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thu, K. M. 2002. Public health concerns for neighbors of large-scale swine production operations. J. Agric. Saf. Health 8:175-184. [DOI] [PubMed] [Google Scholar]
- 37.van der Poel, W. H., J. Vinje, R. van der Heide, M. I. Herrera, A. Vivo, and M. P. Koopmans. 2000. Norwalk-like calicivirus genes in farm animals. Emerg. Infect. Dis. 6:36-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Poel, W. H., R. van der Heide, F. Verschoor, H. Gelderblom, J. Vinje, and M. P. Koopmans. 2003. Epidemiology of Norwalk-like virus infections in cattle in The Netherlands. Vet. Microbiol. 92:297-309. [DOI] [PubMed] [Google Scholar]
- 39.Vanotti, M. B., P. D. Millner, P. G. Hunt, and A. Q. Ellison. 2005. Removal of pathogen and indicator microorganisms from liquid swine manure in multi-step biological and chemical treatment. Bioresour. Technol. 96:209-214. [DOI] [PubMed] [Google Scholar]
- 40.Wang, Q. H., M. G. Han, S. Cheetham, M. Souza, J. A. Funk, and L. J. Saif. 2005. Porcine noroviruses related to human noroviruses. Emerg. Infect. Dis. 11:1874-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang, Q. H., M. G. Han, J. A. Funk, G. Bowman, D. A. Janies, and L. J. Saif. 2005. Genetic diversity and recombination of porcine sapoviruses. J. Clin. Microbiol. 43:5963-5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang, Q. H., K. O. Chang, M. G. Han, S. Sreevatsan, and L. J. Saif. 2006. Development of a new microwell hybridization assay and an internal control RNA for the detection of porcine noroviruses and sapoviruses by reverse transcription-PCR. J. Virol. Methods 132:135-145. [DOI] [PubMed] [Google Scholar]
- 43.Wang, Q. H., M. Souza, J. A. Funk, W. Zhang, and L. J. Saif. 2006. Prevalence of noroviruses and sapoviruses in swine of various ages determined by reverse transcription-PCR and microwell hybridization assays. J. Clin. Microbiol. 44:2057-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Will, L. A., P. S. Paul, T. A. Proescholdt, S. N. Aktar, K. P. Flaming, B. H. Janke, J. Sacks, Y. S. Lyoo, H. T. Hill, L. J. Hoffman, et al. 1994. Evaluation of rotavirus infection and diarrhea in Iowa commercial pigs based on an epidemiologic study of a population represented by diagnostic laboratory cases. J. Vet. Diagn. Investig. 6:416-422. [DOI] [PubMed] [Google Scholar]
- 45.Woode, G. N. 1978. Epizootiology of bovine rotavirus infection. Vet. Rec. 103:44-46. [DOI] [PubMed] [Google Scholar]
- 46.Yuan, L., G. W. Stevenson, and L. J. Saif. 2006. Rotavirus and reovirus, p. 435-454. In B. E. Straw, J. J. Zimmerman, S. D'Allaire, and D. J. Taylor (ed.), Diseases of swine, 9th ed. Blackwell Publishing Professional, Ames, IA.