Abstract
Viruses on organic aggregates such as transparent exopolymeric particles (TEP) are not well investigated. The number of TEP-attached viruses was assessed along trophic gradients in the southwestern lagoon of New Caledonia by determining the fraction of viruses removed after magnetic isolation of TEP. In order to isolate TEP magnetically, TEP were formed in the presence of magnetic beads from submicrometer precursors collected along the trophic gradients. The mixed aggregates of TEP-beads-viruses were removed from solution with a magnetic field. The percentage of viruses associated with newly formed TEP averaged 8% (range, 3 to 13%) for most of the stations but was higher (ca. 30%) in one bay characterized by the low renewal rate of its water mass. The number of viruses (N) attached to TEP varied as a function of TEP size (d [in micrometers]) according to the formulas N = 100d1.60 and N = 230d1.75, respectively, for TEP occurring in water masses with short (i.e., <40 days) and long (i.e., >40 days) residence times. These two relationships imply that viral abundance decreases with TEP size, and they indicate that water residence time influences viral density and virus-bacterium interactions within aggregates. Our data suggest that the fraction of viruses attached to TEP is highest in areas characterized by a low renewal rate of the water mass and can constitute at times a significant fraction of total virus abundance. Due to the small distance between viruses and hosts on TEP, these particles may be hot spots for viral infection.
Aggregates, which represent one end of the organic matter size continuum (38), provide physicochemical heterogeneity in the seemingly homogeneous environment of the water column (2, 6, 37) and play the role of unique microcosms for microbes (13, 36). These hot spots of bacterial activity influence biogeochemical cycles (14, 15, 16, 27, 37). Also, it is well known that organic aggregates can harbor a specific bacterial community (1, 11, 26, 33, 35). The most recently detected organic aggregates are transparent exopolymeric particles (TEP) (4). They are formed by coagulation of the reactive fraction of dissolved organic matter, exist along a size continuum from >1 kDa to hundreds of micrometers, and constitute the organic glue promoting the formation of marine snow aggregates (30). TEP are always colonized by bacteria (4, 23, 31), which may modify their structure and reactivity. While there is evidence that organic aggregates also contain viruses, which may alter the current picture regarding distribution of viruses in the sea and modify their role in the microbial loop (9, 32), no detailed studies have been performed.
While the physicochemical properties of aggregates may regulate the dynamics of the attached viral community, there are various ways that viruses may in turn influence organic aggregates (34). Lysis products may act as glue to facilitate aggregation, and cell wall fragments may even serve as nuclei for aggregate formation. Indeed, the addition of viruses to rolling table incubations increased the size and stability of algal flocs (32). A similar process may occur within aggregates, where lysis could contribute to the stickiness of particles. However, it is also possible that enzymes released during lysis, including virus-borne lysozymes produced to digest cell walls, contribute to the dissolution of aggregates and that viral lysis products are released from the aggregates. It is believed that viral lysis stimulates bacterial activity, including enzymatic activity (12, 25). If this holds true, the viral loop (viral lysis-dissolved organic matter-bacteria [7]) or shunt (39) may also increase aggregate dissolution either from outside or from within aggregates. Finally, it remains unknown whether organic aggregates are a sink or a source for viruses.
A recent study of organic matter reactivity along eutrophic gradients in the lagoon of New Caledonia revealed that the transfer efficiency of organic matter from the dissolved to the particulate phase via aggregation processes was reduced when the residence time of the water masses increased (24). Such a reduction in organic matter reactivity may also influence the distribution and fate of attached viruses by modifying the structural relationship between aggregates and attached viruses. In addition, a long residence time of the water mass may lead to an enhanced retention time of the aggregates in the water column (24) and thus to prolonged maturation of the TEP-bacterium-virus relationships.
In this study, we quantified the percentage of viruses associated with TEP along trophic gradients in the southwestern lagoon of New Caledonia and linked these findings to the residence time of the water masses.
MATERIALS AND METHODS
Sampling.
Seawater samples were collected during November and December 2004 with a Teflon pump at 5 m from 10 stations distributed along two sampling gradients in the southwestern lagoon of New Caledonia, ranging from two semienclosed bays in the city of Noumea (∼130,000 inhabitants) to a station outside the reef barrier (Fig. 1). Each sampling gradient was sampled twice (four transects; 22, 24, and 29 November and 1 December) for the determination of viral abundance and TEP concentration. The experiments aimed at determining the fractions of TEP-attached viruses were conducted at three stations for each transect (i.e., 22 November, stations M41, M10, and M05; 24 November, stations D22, D08, and D01; 29 November, stations N33, N12, and N04; 1 December, stations M41, M10, and M33). The southwestern lagoon of New Caledonia is an enclosed, relatively shallow site (∼20 m) surrounded by oligotrophic oceanic water. In contrast to the oligotrophy observed near the coral barrier, the near-shore environment is subject to terrestrial and, especially in the bays around the city of Noumea, to both industrial and urban inputs that increase the general productivity in these areas. Eutrophication in Grande Rade Bay is mainly of industrial origin, due to the close proximity of a large nickel smelter, while in Sainte Marie Bay eutrophication is mostly due to wastewater outfalls from the Sainte Marie area (i.e., urban origin). Conductivity-temperature-depth casts were used on each sampling occasion to describe the vertical stratification.
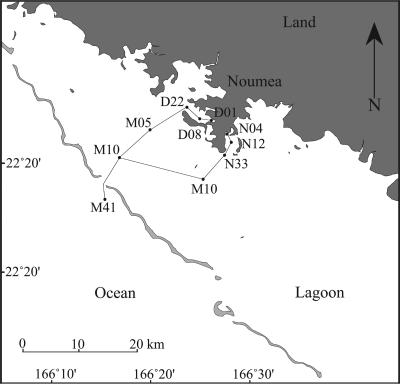
FIG. 1.
Map of the study area, with positions of the sampling stations. Stations D and N are in Grande Rade Bay and Sainte Marie Bay, respectively. (Modified from reference 24 with permission of the publisher.)
Residence time of water masses.
The parameter used to measure the “residence time” of the water mass is the local e-flushing time (LeFT) (in days). The LeFT is defined as the time required for a tracer mass contained within the control volume (station) to be reduced by a factor, 1/e, by waters coming from outside the lagoon; thus, it describes the replacement efficiency of water masses in the study area (19). The shorter the LeFT, the faster the water masses at the location will be definitely replaced and thus renewed. The annual average LeFT at the different stations was calculated from a hydrodynamic model that took into account topographic constraints, average wind conditions, and tidal cycles (19). During the sampling period, wind conditions were similar to those used as input parameters in the hydrodynamic model (i.e., well-established trade winds of about 8 to 10 m s−1). The LeFT was 0 at the offshore station (input parameter), and it was calculated for each station to range from 0.4 to 5.6 days in the lagoon stations, 12 to 17 days in Sainte Marie Bay, and 40 to 47 days in Grande Rade Bay (24).
Production of mixed aggregates of TEP-magnetic beads.
Seawater samples were filtered at a low and constant vacuum pressure (<15 kPa) through a 47-mm-diameter GF/C Whatman filter (nominal pore size, 1.2 μm) in order to isolate TEP precursors in the filtrate and to remove large particles. Two liters of filtrate was placed in 4-liter polycarbonate bottles (one per station). Superparamagnetic functionalized microspheres (PCM) were added to the bottles to obtain a final concentration of 2.5 × 105 PCM ml−1, in order to reach the optimum bead “demand” for the observed range of TEP volume concentration (<10 ppm [24]) (22). PCM (Dynabead MyOne carboxylic acid; Dynal Biotech) of 1 μm in diameter are composed of highly cross-linked polystyrene with evenly distributed magnetic material (26% iron content) and a large surface area (10 m2 g−1). The beads are coated with a hydrophilic layer of glycidyl ether, concealing the iron oxide inside them, and carboxylic acid groups are on the surface of the beads. The beads are colloidally stable in the absence of a magnetic field, and total separation is achieved when they are exposed to a magnetic field. The volume of PCM solution (Vi) to add to the filtrate in order to reach a final concentration of 2.5 × 105 PCM ml−1 was calculated with the formula Vi = Cf·Vf/Ci, where Ci is the initial concentration of microspheres and Vf is the volume of filtrate (2 liters). The initial concentration, Ci, was calculated with the formula Ci = 6W(1012)/ρπd3, where W is the mass of PCM per milliliter of solution (10 mg ml−1 for 10% solid volume, according to the manufacturer); ρ is the density of PCM, in grams per milliliter (1.8 g cm−3); and d is the bead diameter, in micrometers. In order to avoid clumping prior to the TEP-PCM formation process, the bead addition was diluted in 20 ml of 0.2-μm-filtered seawater and sonicated for 5 min. The bottles were placed on a horizontal culture shaker, and the solutions containing the TEP colloidal precursors and the PCM were agitated for 5 h at 150 rpm in order to form mixed TEP-PCM aggregates.
Magnetic removal of TEP and TEP-attached viruses.
TEP and TEP-attached viruses were removed from solution by use of the magnetic properties of the PCM, as follows. A 250-ml tissue culture flask was placed between two magnets (MPC-384; Dynal Biotech) held together by a rubber band. Then, 250-ml seawater samples from the bottles were dispensed into the tissue culture flasks (one per station) and left for 20 min inside the magnetic field for complete separation of the PCM from solution. Samples for TEP and virus determinations were collected in the initial solution before magnetic separation, defined as fraction A, and in the middle of the flask after complete magnetic separation while the magnetic field was still operating, defined as fraction C. Fraction B corresponds to the magnetically removed TEP and attached viruses (22). The TEP size spectra and virus concentrations were determined for fractions A and C. The TEP size spectra and virus concentrations retained in the magnetic field (fraction B) were obtained by calculating the difference between fractions A and C.
TEP determinations.
The TEP size spectra were determined for samples collected in situ and during the magnetic isolation procedure, from 5- and 10-ml samples, respectively, and filtered with 0.2-μm polycarbonate filters (31). TEP retained on each filter were stained with Alcian blue and, because TEP were not directly visible on the filter, were transferred to a microscope slide (4). For each slide, TEP size spectra were determined by counting and sizing of TEP at two successive magnifications (×400 and ×250) with a compound light microscope. Ten images were taken per slide and for each magnification, and the TEP size spectra were compiled by combining the size distributions obtained at each magnification. The equivalent spherical diameter (ESD) of each TEP was calculated by measuring its cross-sectional area with an image analysis system (ImagePro Plus; MediaCybernetics). Counts were combined and classified according to ESD. TEP size distributions were described with a power relation of the type dN/d(d) = kdδ, where d is the ESD and dN is the number of TEP per unit volume in the size range d to [d + d(d)]. The constant k depends on the concentration of particles, and the spectral slope, δ, describes the size distribution; when δ increases, the fraction of large particles increases. Both constants were estimated from regressions of log[dN/d(d)] versus log(d). TEP recovery after prefiltration through GF/C filters was assessed by comparing the TEP carbon (TEP-C) concentration before and after filtration. The TEP-C concentration was estimated from in situ TEP size spectra with the TEP size versus TEP-C content relationship (21).
Viral counts.
Water samples were preserved with glutaraldehyde (0.5% final concentration) for 30 min at 4°C and then flash frozen in liquid nitrogen and stored at −80°C until analysis. Samples were diluted 100-fold in Tris-EDTA. Viruses were stained with SYBR green and counted by flow cytometry (8).
TEP size versus number of attached viruses.
Assuming that the retention of viruses in the magnetic field is solely due to virus attachment to TEP, we estimated the relationship between TEP and attached viruses as follows. The number of TEP-attached viruses varies as a function of TEP structure and can be fitted to a power law relationship, N = adb, where N is the number of viruses attached per TEP, d is the ESD, and a and b are constants for a given sample. Summed over all particle sizes along the TEP size spectra, the total TEP-attached virus concentration (C) is given by the relationship C = a∑i·ni·dib, where ni is the concentration of TEP in size class i. a and b were estimated by the least-squares method, i.e., by finding the values of a and b for which the equation SSD = ∑j(Cj − a∑i·nij·dib)2 has minimal value (SSD is the sum of standard deviations, Cj is the concentration of TEP-attached viruses in sample j, and nij is the concentration of size class i TEP in sample j). The estimation of a and b permits the determination of the virus density of a given TEP as a function of its size. These constants were determined individually for each station.
Statistics.
Relationships between parameters were determined by correlation and regression analysis. Analysis of variance (ANOVA) and Tukey-Kramer HSD Posthoc tests were used to assess differences in parameters between environments. A probability (P) of <0.05 was considered significant. Data were log transformed before analysis when necessary to meet the requirements of normal distribution.
RESULTS
Characterization of sampling sites.
Physical and chemical characterizations of the sampling stations on the sampling dates can be found elsewhere (24). Briefly, salinity and temperature varied only slightly between sampling sites and dates, and no stratification of the water column was observed within the barrier reef. Nutrient (nitrate, nitrite, and phosphate) concentrations were highest at the heads of the bays and gradually decreased toward the mouths of the bays, the distant parts of the lagoon, and the open ocean. All sampling stations were characterized by inorganic nitrogen limitation. The TEP volume concentration varied between 0.1 ppm at the ocean station and 6.6 ppm at the heads of the bays, and the TEP size spectra were characterized by an increase of the fraction of large particles toward the heads of the bays. The distribution of chlorophyll a (Chl a) largely follows that of the nutrients, with maximum concentrations at the heads of the bays (1.68 ± 0.63 μg Chl a liter−1 [mean ± standard deviation]), rapidly decreasing outside the bays to a minimum at the ocean station (0.19 ± 0.09 μg Chl a liter−1).
Virus abundance.
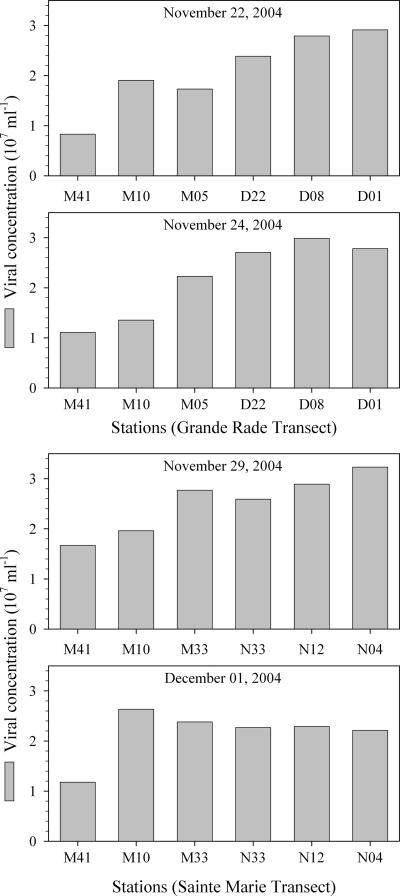
Virus abundances ranged from 8 × 106 ml−1 at the ocean station to 32 × 106 ml−1 at the head of Sainte Marie Bay (Fig. 2). In the transects from 21, 24, and 29 November, the greatest virus abundance was found in the middle or at the head of the bays, whereas in transect 4 (from 1 December), virus abundances were similar for all lagoon and bay stations. When stations were divided into offshore, lagoon, and bay environments, ANOVA showed that virus abundances in all environments were significantly different, with the highest values in the bays and lowest values offshore.
FIG. 2.
Virus abundances along the offshore-to-onshore transects (no replicate counts per station were performed).
TEP size versus number of attached viruses.
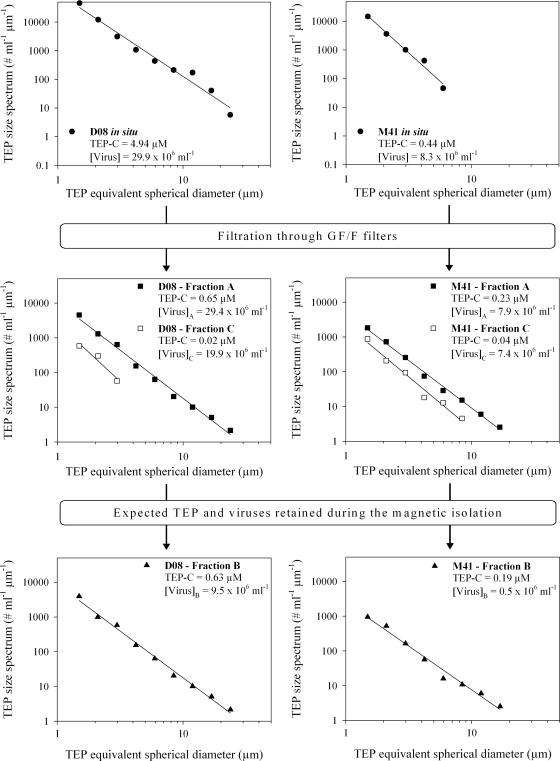
The effects of recovery of TEP after prefiltration and after magnetic isolation were assessed. Examples of size distributions of TEP are shown for one station in Grande Rade Bay (D08) and the offshore station (M41) (Fig. 3). In the bay stations, the expected TEP-C after prefiltration ranged from 5 to 25% of the in situ data, whereas it ranged from 34 to 100% in the lagoon and offshore stations. This suggests that prefiltration removed a significant fraction of TEP in some environments. Shaking after 1.2-μm filtration resulted in a slight shift toward higher size classes compared to the in situ data. Magnetic separation removed the majority of the TEP from the 1.2-μm-filtered water (average, 91%). In the open ocean station, 84% of the estimated TEP-C was removed, compared to 91% in Sainte Marie Bay and 95% in Grande Rade Bay and in the lagoon stations.
FIG. 3.
Examples of the evolution of TEP size spectra, TEP-C concentrations, and virus abundances during the magnetic isolation procedure. Examples are given for the offshore station (M41) and for one middle-bay station (D08).
Viral abundance before the magnetic removal of beads averaged 99% (range, 82 to 115%) of in situ abundances, with no significant differences between environments. The percentage of viruses on TEP, as estimated from the difference in viral abundance before and after magnetic TEP removal, ranged from 3 to 13% in most stations, with an average of 8%, except for the middle and head of Grande Rade Bay (ca. 30%).
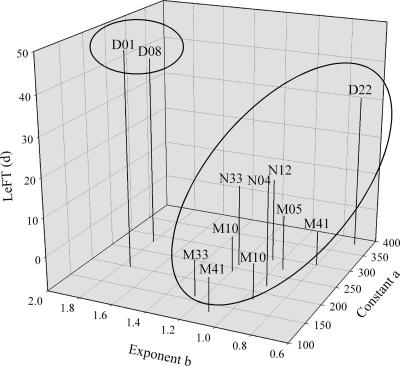
The number of viruses attached to TEP increased with the TEP size, and virus numbers scaled with the TEP diameter were raised to an average exponent, b, of ∼1.14 (from 0.70 to 1.80) (Fig. 4). Estimates of a and b by the least-squares method for each station showed that the results cluster in two groups: one group for which b is >1.5, consisting of samples collected in the middle and the head of Grande Rade Bay (D08 and D01), and one group for which b is <1.5, consisting of all other stations. The first group is characterized by the samples collected in water masses with very long residence times (i.e., LeFT > 40 days). The constants a and b were calculated by the least-squares method for each of the groups. The viral density (N [in numbers per cubic micrometer]) within a given TEP with diameter d (in micrometers) occurring in water masses with a long residence time (LeFT > 40 days [stations D01 and D08]) is given by the equation
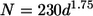 |
(1) |
and the viral density within a given TEP with diameter d occurring in water masses with a short residence time (LeFT < 40 days [all stations except D01 and D08]) is given by the equation
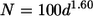 |
(2) |
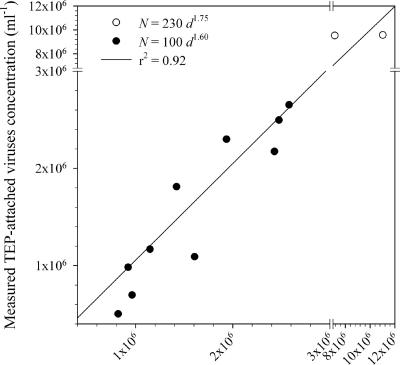
These two relationships indicate that the volume-specific number of TEP-attached viruses decreases when the TEP size increases. As an example, a TEP with a diameter of 1 μm has viral densities of 440 virus particles μm−3 and 190 virus particles μm−3 according to equations 1 and 2, respectively, while a TEP with a diameter of 100 μm has viral densities of 1.4 virus particles μm−3 and 0.3 virus particles μm−3 according to equations 1 and 2, respectively. Comparisons between the numbers of TEP-attached viruses expected from the above relationships and the measured numbers of TEP-attached viruses (i.e., viruses removed during TEP magnetic isolation) showed that these models explain 92% of the measured virus removal (Fig. 5).
FIG. 4.
Estimates of the constants a and b for each station as a function of the residence time of the water masses. The constants a and b were estimated from the power law relationship N = adb, where N is the number of TEP-attached viruses and d is the ESD of the TEP.
FIG. 5.
Comparison between measured and expected TEP-attached viruses (based on the relationships N = 230d1.75 and N = 100d1.60 and the TEP size spectra removed during magnetic isolation).
Expected TEP-attached viruses.
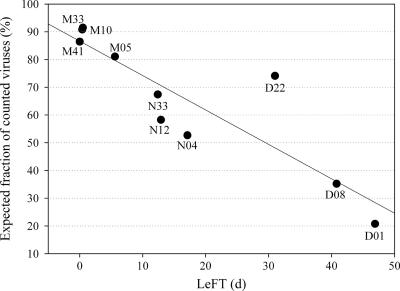
Using the in situ TEP size spectra (e.g., Fig. 3) and the relationships of TEP sizes versus numbers of attached viruses described above and assuming that all viruses were enumerated by flow cytometry (including the attached ones), we estimated the abundance of viruses on TEP in situ. The expected TEP-attached viral abundance ranged from 5 to 45% (average, 15%), compared to total viral abundance measured in situ, for stations with a LeFT of <10 days, from 30 to 102% (average, 62%) for stations with a LeFT of between 10 and 40 days, and from 160 to 410% (average, 282%) for stations with a LeFT of >40 days. Within the bays, this percentage increased from the mouth to the head in all transects. While the fraction of attached viruses is correlated to the TEP concentration, this fraction increased drastically for stations with a very low water mass renewal rate. Since the fraction of TEP-attached viruses cannot be higher than the total virus abundance, this suggests that viruses attached to aggregates were not enumerated by flow cytometry. When the virus density on TEP (as determined by magnetic removal) was compared to the expected virus density, more than 80% of the expected counts were actually found in the measurements from the open ocean and the lagoon stations, compared to ca. 50 to 75% at Sainte Marie Bay and the mouth of Grande Rade Bay and to ca. 20 to 35% in the rest of the Grande Rade Bay stations (Fig. 6).
FIG. 6.
Expected fraction of viruses counted by flow cytometry with the assumption that some TEP-attached viruses escape detection.
DISCUSSION
Critical evaluation of methods of quantifying viruses on aggregates.
There are two general ways to determine the relation between TEP size and the number of attached viruses. The most intuitive approach is to proceed as for TEP-bacterium determinations (22, 31), i.e., by staining both TEP and viruses in order to size individual TEP and enumerate the number of viruses attached to it. While viruses in marine snow aggregates have been studied by using embedding of aggregates followed by thin sectioning and transmission electronic microscopy (32), this approach is time-consuming and only possible for visible aggregates that can be embedded, i.e., not for TEP. Alternatively, the optical thin sectioning of laser scanning microscopy coupled with three-dimensional reconstruction of nucleic acid-stained particles might be useful for assessing viral densities on small aggregates. Although an accumulation of viruses can be seen by laser scanning microscopy (unpublished data) and epifluorescence microscopy (20), quantification of viruses and clear identification within organic aggregates is often not possible (20). Finally, due to the wide size differences between viruses (60 to 80 nm [40]) and TEP (from ca. 1 to >100 μm [4]), and because viruses are approximately an order of magnitude more abundant than bacteria, such an approach would be extremely time-consuming.
As a consequence of the problems outlined above, we followed an indirect approach without enumerating viruses inside TEP. Samples were prefiltered to isolate submicrometric TEP precursors from large particles. TEP reformed from precursors were selectively removed from solution after they had been associated with magnetic beads, and the magnetically labeled TEP were isolated with a magnetic field (22). Owing to their size, viruses are not affected by turbulence; thus, shear coagulation was not the origin of TEP-virus attachment during TEP formation from submicrometric precursors. Therefore, the observed relationships between TEP and viruses probably have roots in the association between TEP precursors and viruses. Assuming that the TEP-virus association remains steady along the TEP size spectra (i.e., from submicrometer sizes to the largest TEP), then the relationship determined in the laboratory should reflect that of naturally occurring TEP. However, the relationships between TEP sizes and numbers of attached viruses may underestimate the fraction of TEP-attached viruses for at least three reasons. First, not all TEP are removed during the magnetic isolation procedure. While likely a small faction for most environments, at the open water station 16% of the TEP, as estimated from the expected carbon content, were not removed. As most of the TEP not removed during magnetic isolation are small and thus contain a proportionally large number of viruses, this could result in a significant underestimation of the number of attached viruses. Second, a significant proportion of TEP was removed by prefiltration at some stations, and these TEP might have contained a significant amount of viruses.
The fraction of attached viruses on naturally occurring TEP was estimated from in situ TEP size spectra (24) and from TEP size-versus-viral-density relationships (this study). Several factors might have influenced these data. For example, although the measured virus abundance on TEP was quite well predicted by the model (r2 = 0.92), we cannot be sure that this relationship also holds true for larger aggregates. However, it must be mentioned that the TEP size distribution after 1.2-μm filtration in water and after shaking was similar to that found under in situ conditions. Thus, while some uncertainty remains, the presented technique should be able to reveal primary trends.
Fraction of TEP-attached viruses: a neglected but significant reservoir.
One of the reasons for investigating the contribution of TEP-attached viruses to the total viral community is uncertainty about the detection of viruses on large aggregates by flow cytometry; i.e., the side scatter of the aggregates should remove viruses from their typical detection frame. The difficulty in detecting aggregate-attached viruses by flow cytometry is illustrated by the finding that virus abundances did not differ before and after 1.2-μm filtration despite the high turbidity in the bays. Furthermore, some aggregates, including TEP, are probably disrupted when injected into the flow cytometer, which may contribute to the strong deviations sometimes observed between viral counts with epifluorescence microscopy and with flow cytometry in turbid environments (unpublished data). Assuming that flow cytometry does not allow the enumeration of TEP-attached viruses (i.e., the viral abundance represents only free viruses), one can estimate the expected abundance of TEP-attached viruses from in situ TEP size spectra and the relationships describing the viral density on TEP. This exercise suggests that virus abundance can be underestimated by up to 80% in the head of Grande Rade Bay and by up to 20% at the lagoon and offshore stations (Fig. 6). This indicates that our flow cytometry- and magnetic bead-based approach to estimating total viral abundance and viral abundance on TEP works well in oligotrophic aggregate-poor environments, such as the open ocean and the lagoons, whereas in aggregate-rich environments there is a need for the development of better detection methods for viruses on TEP and other aggregates. If we add the attachment of viruses to non-TEP aggregates, the actual virus abundance in aquatic environments may be more severely underestimated than that revealed by consideration of only the TEP pool.
Interaction between virus and aggregates: ecological interpretations.
There are almost no data on total virus abundance and viral infection on aggregates. One study from the Mediterranean Sea suggested that the virus/bacterium ratio in algal flocs is similar to that in the environment (32), and another study suggested that the infection frequencies of bacteria on aggregates are similar to those in ambient water (34). Our study showed that viral density decreased with increasing TEP size. Estimated viral density was ca. 1 × 1010 to 3 × 1010 ml−1 for TEP with a diameter of 10 μm and 10 × 1010 to 30 × 1010 ml−1 for TEP with a diameter of 2 μm. A similar tendency of lower virus density on aggregates of <5 μm than on aggregates of >20 μm in equivalent spherical diameter was also found in river and floodplain waters by epifluorescence microscopy (20). In addition, the estimated viral density on TEP was highest in Grande Rade Bay, where the residence time of the water mass was longest. It has been suggested that the long residence time of the water mass in Grande Rade Bay was responsible for a prolonged retention of TEP in the water column via a reduction of TEP buoyancy, caused by an alteration in TEP sticking properties (24). Such a prolonged retention time in the water column may explain the higher viral density in TEP. In the above-cited study on river systems, water age did not affect the abundance of viruses on aggregates, whereas aggregate quality had an influence (20). Thus, the observed effect of water residence time in our study might have also been due to changed aggregate quality; indeed, it was observed for the lagoon of Noumea that the aggregate quality changed with water residence time (24).
Among the differences in virus-host interactions between ambient water and aggregates are the potentially reduced virus diffusion on aggregates due to the organic matrix and the much shorter distance between viruses and hosts. For example, the density of viruses was ca. 3 to 4 orders of magnitude higher on TEP (estimated from the conservative magnetic bead approach) than in ambient water. It is also well known that the host density can be higher on aggregates (27). However, no data on encounter rates are available for hosts, since the restriction of diffusion on aggregates has not been quantified. While details of virus-host interactions on aggregates remain unknown, the high viral density suggests that TEP constitute an infection site for bacteria and thus play a role in the control of bacterial diversity and production. Overall, our experimental data and in situ estimates indicate that (i) the fraction of viruses attached to TEP is more important in bays with a low water mass renewal rate, (ii) viral density decreases with TEP size, and (iii) the fraction of TEP-attached viruses can be significant.
Fate of TEP-attached viruses.
While in bays TEP tend to remain suspended in the water column, thus favoring the installation of a recycling ecosystem, they may sink and promote the vertical export of matter in lagoons and offshore waters (24). It has been shown before that sediments can be storage places for viruses and increase their survival (18). For the offshore environment, where the water depth rapidly drops to >3,000 m in the New Caledonian Trough, this would mean a removal of organic carbon to the deep sea as part of the biological pump. Also, sinking TEP may not only transport phage-host systems to the deep sea and deep-sea sediments but also provide for a substantial supply of genetic information. Recently, it has been shown that extracellular DNA plays a crucial role in deep-sea sediments (10), and viruses transported by TEP may contribute to that. It has been suggested that vertical transport fuels the deep sea with viruses and can explain the high abundances found in this environment (17, 29). Transport via sinking TEP would be such a mechanism.
A radically opposing pathway for TEP-attached viruses may be via the upward vertical transport of positively buoyant organic aggregates. It has been suggested that under certain circumstances, TEP may serve as an upward lift for TEP-attached particles (24). Because the eruption of rising bubbles through the surface microlayer leads to the formation of marine aerosols that contain viruses (5), the TEP-surface microlayer-aerosol pathway may represent a significant vector of transport for viruses across the air-sea interface and a dispersal mechanism for those microorganisms. This could be significant in areas such as New Caledonia, where the trade winds are active for ca. 85% of the year.
Acknowledgments
We thank E. Rochelle-Newall for constructive comments during preparation of the manuscript. Thanks are due to the crew of the R/V Coris for assistance during sampling.
This research was supported by the French National Research Agency (ANR-ECCO program), by the French Ministry of Overseas Territories (Ministère de l'Outre-Mer [MOM]), and by the French Research Institute for Development (IRD).
Footnotes
Published ahead of print on 22 June 2007.
REFERENCES
- 1.Acinas, S. G., J. Anton, and F. Rodriguez-Valera. 1999. Diversity of free-living and attached bacteria in offshore western Mediterranean waters as depicted by analysis of genes encoding 16S rRNA. Appl. Environ. Microbiol. 65:514-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alldredge, A., and Y. Cohen. 1987. Can microscale patches persist in the sea? Microelectrode study of marine snow, fecal pellets. Science 235:689-691. [DOI] [PubMed] [Google Scholar]
- 3.Reference deleted.
- 4.Alldredge, A. L., U. Passow, and B. E. Logan. 1993. The abundance and significance of a class of large, transparent organic particles in the ocean. Deep-Sea Res. 40:1131-1140. [Google Scholar]
- 5.Aller, J. Y., M. R. Kuznetsova, C. J. Jahns, and P. F. Kemp. 2005. The sea surface microlayer as a source of viral and bacterial enrichment in marine aerosols. J. Aerosol Sci. 36:801-812. [Google Scholar]
- 6.Blackburn, N., and T. Fenchel. 1999. Influence of bacteria, diffusion and shear on micro-scale nutrient patches, and implications for bacterial chemotaxis. Mar. Ecol. Prog. Ser. 189:1-7. [Google Scholar]
- 7.Bratbak, G., F. Thingstad, and M. Heldal. 1994. Viruses and the microbial loop. Microb. Ecol. 28:209-221. [DOI] [PubMed] [Google Scholar]
- 8.Brussaard, C. P. D., D. Marie, and G. Bratbak. 2000. Flow cytometry detection of viruses. J. Virol. Methods 85:175-182. [DOI] [PubMed] [Google Scholar]
- 9.Brussaard, C. P. D., X. Mari, J. D. L. Van Bleijswijk, and M. J. W. Veldhuis. 2005. A mesocosm study of Phaeocystis globosa (Prymnesiophyceae) population dynamics. II. Significance for the microbial community. Harmful Algae 4:875-893. [Google Scholar]
- 10.Danovaro, R., C. Corinaldesi, A. Dell'Anno, M. Fabiano, and C. Corselli. 2005. Viruses, prokaryotes and DNA in the sediments of a deep-hypersaline anoxic basin (DHAB) of the Mediterranean Sea. Environ. Microbiol. 7:586-592. [DOI] [PubMed] [Google Scholar]
- 11.DeLong, E. F., D. G. Franks, and A. L. Alldredge. 1993. Phylogenetic diversity of aggregate-attached vs. free-living marine bacterial assemblages. Limnol. Oceanogr. 38:924-934. [Google Scholar]
- 12.Fuhrman, J. A. 1999. Marine viruses and their biogeochemical and ecological effects. Nature 399:541-548. [DOI] [PubMed] [Google Scholar]
- 13.Grossart, H.-P., T. Kiørboe, K. W. Tang, M. Allgaier, E. M. Yam, and H. Ploug. 2006. Interactions between marine snow and heterotrophic bacteria: aggregate formation and microbial dynamics. Aquat. Microb. Ecol. 42:19-26. [Google Scholar]
- 14.Grossart, H.-P., and M. Simon. 1993. Limnetic macroscopic organic aggregates (lake snow): occurrence, characteristics, and microbial dynamics in Lake Constance. Limnol. Oceanogr. 38:532-546. [Google Scholar]
- 15.Grossart, H.-P., and M. Simon. 1998. Bacterial colonization and microbial decomposition of limnetic organic aggregates (lake snow). Aquat. Microb. Ecol. 15:127-140. [Google Scholar]
- 16.Grossart, H.-P., T. Berman, M. Simon, and K. Pohlmann. 1998. Occurrence and microbial dynamics of macroscopic organic aggregates (lake snow) in Lake Kinneret, Israel, in fall. Aquat. Microb. Ecol. 14:59-67. [Google Scholar]
- 17.Hara, S., I. Koike, K. Terauchi, H. Kamiya, and E. Tanoue. 1996. Abundance of viruses in deep oceanic waters. Mar. Ecol. Prog. Ser. 145:269-277. [Google Scholar]
- 18.Hewson, I., J. M. O'Neil, J. A. Fuhrman, and W. C. Dennison. 2001. Virus-like particle distribution and abundance in sediments and overlying waters along eutrophication gradients in two subtropical estuaries. Limnol. Oceanogr. 46:1734-1746. [Google Scholar]
- 19.Jouon, A., P. Douillet, S. Ouillon, and P. Fraunié. 2006. Calculations of hydrodynamic time parameters in a semi-opened coastal zone using a 3D hydrodynamic model. Cont. Shelf Res. 26:1395-1415. [Google Scholar]
- 20.Luef, B., F. Aspetsberger, T. Hein, F. Huber, and P. Peduzzi. Impact of hydrology on free-living and particle-associated microorganisms in a river floodplain system (Danube, Austria). Freshwater Biol., in press.
- 21.Mari, X. 1999. Carbon content and C:N ratio of transparent exopolymeric particles (TEP) produced by bubbling exudates of diatoms. Mar. Ecol. Prog. Ser. 183:59-71. [Google Scholar]
- 22.Mari, X., and H. G. Dam. 2004. Production, concentration, and isolation of transparent exopolymeric particles using paramagnetic functionalized microspheres. Limnol. Oceanogr. Methods 2:13-24. [Google Scholar]
- 23.Mari, X., and T. Kiørboe. 1996. Abundance, size distribution and bacterial colonization of transparent exopolymeric particles (TEP) during spring in the Kattegat. J. Plankton Res. 18:969-986. [Google Scholar]
- 24.Mari, X., E. Rochelle-Newall, J.-P. Torréton, O. Pringault, A. Jouon, and C. Migon. 2007. Water residence time: a regulatory factor of the DOM to POM transfer efficiency. Limnol. Oceanogr. 52:808-819. [Google Scholar]
- 25.Middelboe, M., N. O. G. Jorgensen, and N. Kroer. 1996. Effects of viruses on nutrient turnover and growth efficiency of noninfected marine bacterioplankton. Appl. Environ. Microbiol. 62:1991-1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moeseneder, M. M., C. Winter, and G. J. Herndl. 2001. Horizontal and vertical complexity of attached and free-living bacteria of the eastern Mediterranean Sea, determined by 16S rDNA and 16S rRNA fingerprints. Limnol. Oceanogr. 46:95-107. [Google Scholar]
- 27.Muller-Niklas, G., S. Schuster, E. Kaltenbock, and G. J. Herndl. 1994. Organic content and bacterial metabolism in amorphous aggregations of the northern Adriatic Sea. Limnol. Oceanogr. 39:58-68. [Google Scholar]
- 28.Reference deleted.
- 29.Parada, V., E. Sintes, H. M. van Haken, M. G. Weinbauer, and G. J. Herndl. 11 May 2007. Viral abundance, decay and diversity in the meso- and bathypelagic waters of the North Atlantic. Appl. Environ. Microbiol. doi: 10.1128/AEM.00029-07. [DOI] [PMC free article] [PubMed]
- 30.Passow, U. 2002. Transparent exopolymer particles (TEP) in aquatic environments. Prog. Oceanogr. 55:287-333. [Google Scholar]
- 31.Passow, U., and A. L. Alldredge. 1994. Distribution, size and bacterial colonization of transparent exopolymer particles (TEP) in the ocean. Mar. Ecol. Prog. Ser. 113:185-198. [Google Scholar]
- 32.Peduzzi, P., and M. Weinbauer. 1993. Effect of concentrating the virus-rich 2-200 nm size fraction of seawater on the formation of algal flocs (marine snow). Limnol. Oceanogr. 38:1562-1565. [Google Scholar]
- 33.Phillips, C. J., Z. Smith, T. M. Embley, and J. I. Prosser. 1999. Phylogenetic differences between particle-associated and planktonic ammonia-oxidizing bacteria of the β subdivision of the class Proteobacteria in the northwestern Mediterranean Sea. Appl. Environ. Microbiol. 65:779-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Proctor, L. M., and J. A. Fuhrman. 1991. Roles of viral-infection in organic particulate-flux. Mar. Ecol. Prog. Ser. 69:133-142. [Google Scholar]
- 35.Rath, J., K. Y. Wu, G. J. Herndl, and E. F. DeLong. 1998. High phylogenetic diversity in a marine-snow-associated bacterial assemblage. Aquat. Microb. Ecol. 14:261-269. [Google Scholar]
- 36.Simon, M., H.-P. Grossart, B. Schweitzer, and H. Ploug. 2002. Microbial ecology of organic aggregates in aquatic ecosystems. Aquat. Microb. Ecol. 28:175-211. [Google Scholar]
- 37.Smith, D. C., M. Simon, A. L. Alldredge, and F. Azam. 1992. Intense hydrolytic enzyme activity on marine aggregates and implications for rapid particle dissolution. Nature 359:139-141. [Google Scholar]
- 38.Verdugo, P., A. L. Alldredge, F. Azam, D. L. Kirchman, U. Passow, and P. H. Santschi. 2004. The oceanic gel phase; a bridge in the DOM-POM continuum. Mar. Chem. 92:67-85. [Google Scholar]
- 39.Wilhelm, S. W., and C. A. Suttle. 1999. Viruses and nutrient cycles in the sea—viruses play critical roles in the structure and function of aquatic food webs. Bioscience 49:781-788. [Google Scholar]
- 40.Wommack, K. E., and R. R. Colwell. 2000. Virioplankton: viruses in aquatic ecosystems. Microbiol. Mol. Biol. Rev. 64:69-114. [DOI] [PMC free article] [PubMed] [Google Scholar]