Abstract
We present results from epifluorescence, differential interference contrast, and transmission electron microscopy showing that Xenorhabdus nematophila colonizes a receptacle in the anterior intestine of the infective juvenile (IJ) stage of Steinernema carpocapsae. This region is connected to the esophagus at the esophagointestinal junction. The process by which X. nematophila leaves this bacterial receptacle had not been analyzed previously. In this study we monitored the movement of green fluorescent protein-labeled bacteria during the release process. Our observations revealed that Xenorhabdus colonizes the distal region of the receptacle and that exposure to insect hemolymph stimulated forward movement of the bacteria to the esophagointestinal junction. Continued exposure to hemolymph caused a narrow passage in the distal receptacle to widen, allowing movement of Xenorhabdus down the intestine and out the anus. Efficient release of both the wild type and a nonmotile strain was evident in most of the IJs incubated in hemolymph, whereas only a few IJs incubated in nutrient-rich broth released bacterial cells. Incubation of IJs in hemolymph treated with agents that induce nematode paralysis dramatically inhibited the release process. These results suggest that bacterial motility is not required for movement out of the distal region of the receptacle and that hemolymph-induced esophageal pumping provides a force for the release of X. nematophila out of the receptacle and into the intestinal lumen.
Xenorhabdus nematophila is a motile gram-negative bacterium that engages in a species-specific mutualistic association with the entomopathogenic nematode Steinernema carpocapsae (2, 8, 9, 21). The bacteria are carried in the intestine of the only free-living stage of this nematode, the third-stage infective juvenile (IJ), in a structure generally referred to as the bacterial vesicle (4) located between the pharynx and the intestine. The IJs live in the soil until they invade a susceptible insect host, seeking the hemolymph. Once in the hemocoel, the IJs release the bacteria into the insect's hemolymph. The bacteria participate in overcoming the insect's defense system and killing the host (6, 20). The bacteria proliferate in the insect cadaver, reaching high cell densities, at which point they produce diverse antimicrobial compounds that suppress the growth of antagonistic microorganisms (1, 25). The bacteria themselves, as well as the macromolecular degradation that they stimulate, provide a nutrient base suitable for nematode growth and reproduction (3, 10). When nematode numbers become high and nutrients become limiting in the insect cadaver, nematode progeny reassociate with bacteria and differentiate into the colonized, nonfeeding IJ stage that emerges into the soil to forage for a new host.
Recent studies have shown that colonization of the so-called vesicle of S. carpocapsae by X. nematophila is initiated by one or a few cells followed by bacterial outgrowth (16). During early stages of colonization, X. nematophila appears to bind to a subcellular intravesicular structure that freely moves in the lumen of the vesicle (15). This vesicular environment provides amino acids and cofactors that support bacterial growth (17). Furthermore, it has also been demonstrated that levels of colonization are significantly higher in in vivo-grown IJs than in IJs grown on bacterial lawns, suggesting that insect hemolymph may provide additional nutrients for bacterial growth (12).
Poinar and Thomas (22) observed that Xenorhabdus bacterial cells are released into the hemocoel through the anus. It was further shown that bacterial release into the intestine might be initiated when the IJ penetrates the midgut region of the insect (23). While it is clear that the bacteria move down the intestine and exit the IJ via the anus, little is known about the initial process by which X. nematophila is released from the vesicle, here referred to as the bacterial receptacle. Additionally, the anatomical features of the connection between the bacterial receptacle and the intestine had not been studied previously. In this study we provide further details on the colonization phase and describe the early stages of the bacterial release process. Additionally, the anatomical features of the receptacle were examined to further elucidate the bacterial colonization and release processes.
MATERIALS AND METHODS
Bacterial strains, culture conditions, and plasmid construction.
X. nematophila strains and plasmids used in this study are presented in Table 1. Permanent stocks of bacterial strains and plasmids were maintained at −80°C in Luria-Bertani (LB) broth supplemented with 25% glycerol. X. nematophila and Escherichia coli were grown at 30°C in LB broth, and X. nematophila was grown in medium that had not been exposed to light, according to the method of Xu and Hurlbert (25). E. coli S17-1λ pir was used to conjugally transfer plasmids into X. nematophila strains according to the method of Forst and Tabatabai (11). When appropriate, medium was supplemented with chloramphenicol (25 μg ml−1), kanamycin (30 μg ml−1), and ampicillin (50 μg ml−1). A fliC mutant was used to create the fliC::GFP strain. Plasmid pECM21, which expresses Super-Glo green fluorescent protein (GFP) (16), was conjugally transferred using S17-1λ pir into the fliC strain, and exconjugates were selected for on ampicillin (50 μg ml−1) and kanamycin (30 μg ml−1).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description and relevant characteristics | Reference or source |
|---|---|---|
| X. nematophila strains | ||
| 19061 | ATCC 19061; wild type; Ampr | ATCC |
| HGB340 | GFP+ derivative of ATCC 19061; Kanr | 16 |
| fliC::GFP+ strain | Derivative of ΔfliC strain; Cmr Kanr | This study |
| Plasmid PECM21 | PaphA-Super-Glo GFP gene fragment containing a 614-bp chromosomal insert from ATCC 19061; Kanr | 16 |
Nematode propagation.
Steinernema carpocapsae (strain All) IJs were reared in vitro on lipid agar plates seeded with lawns of GFP-labeled X. nematophila. Plates were incubated at 30°C for 24 h before the addition of the nematodes. Approximately 1,000 IJs were surface sterilized with 0.5% bleach for 1 min and washed six times with sterile water before inoculation. After 14 days of incubation, a new generation of IJs was recovered by placing the agar plates in modified White traps according to procedures described previously (14). Manduca sexta fourth-instar larvae were considered for in vivo rearing of S. carpocapsae nematodes. Dead insects were placed in White traps, and IJs were harvested upon emergence according to procedures described by Kaya and Stock (14).
Morphology and ultrastructure of S. carpocapsae bacterial receptacle.
Rearing of IJs considered for morphological and ultrastructural observation of the bacterial receptacle followed procedures described by Flores-Lara et al. (8). A total of 40 IJs were examined for differential interference contrast (DIC) and confocal microscopy observations. The following measurements were considered: receptacle length (ReL), measured at the longest point and parallel or subparallel to the nematode longitudinal body axis; receptacle width, measured at the widest point and perpendicular to ReL; proximal receptacle length (RpL), measured from the receptacle most-anterior narrowing to the base of the basal bulb (including the esophagointestinal junction [EIJ]); proximal receptacle width, the widest portion measured perpendicular to RpL. Measurements of the bacterial receptacle were made with an Olympus BX51 microscope equipped with DIC optics and a digital image system (Olympus Microsuite).
To obtain a three-dimensional view of the bacteria in the vesicle, confocal series were obtained for unstimulated IJs and IJs exposed to hemolymph for 6 h and 24 h. IJs used for this study were colonized with GFP-labeled bacteria. An LSCM Leica TCS SP2 microscope (Heidelberg, Germany) with a 488-nm excitation line of a 20-mW argon ion laser was used, and the images were taken through an HCX PL apo 63× water-immersion objective with a 1.2 water correction. A series of optical Z sections, taken every 0.1 μm through the nematode, were taken over an area of 512 by 512 pixels at a scan speed of 400 Hz, and the photomultiplier tube settings were optimized throughout the studies using the glow over/under look up table overlay. Images were recorded on a personal computer using LCS Leica Confocal software (version 2.6) and processed for publication using MetaMorph version 5.0 and Adobe Photoshop 7.0 (Adobe Systems, Mountain View, CA).
The ultrastructure of the bacterial receptacle was studied with transmission electron microscopy (TEM) following procedures described by McClure and Stowel (18). Briefly, IJs that had emerged from White traps were rinsed in sterile distilled water three times and fixed overnight in glutaraldehyde (3% in 0.05 M sodium cacodylate buffer, pH 7.2) in individual chambers. IJs were postfixed in 2% aqueous osmium tetroxide for 1 h and then rinsed three times (10 min each wash) with distilled water. A dehydration process followed, consisting of placement of IJs in a graded series of ethanol (10 to 100% [vol/vol]) for 10 min each. Nematodes were cleared with propylene oxide (two times, 30 min each) and then infiltrated in a mixture of 50% Eponate resin (Ted Pella, Inc., Redding, CA) and 50% propylene oxide for 24 h. After two transfers (24 h each) in undiluted resin, IJs were embedded in undiluted Eponate 12 resin. IJ sectioning was done with a diamond knife in an LKB 800 ultramicrotome. Sections (70 nm thick) were collected on naked 200-mesh copper grids for electron microscopy. Grids were stained with saturated aqueous uranyl acetate at room temperature for 20 min followed by lead citrate at room temperature for 2 to 4 min. Grid-mounted sections were examined and photographed at 80 kV with a JEOL JEM-100cx electron microscope.
Quantification of colonizing bacteria.
Quantification of X. nematophila CFU per IJ was performed by grinding single nematodes according to the method of Goetsch et al. (12). IJs were propagated in M. sexta larvae, and an individual surface-sterilized IJ was resuspended in 100 μl of LB broth and homogenized for 2 min with a sterile tip on a motor-driven polypropylene pestle (Kontes). The homogenate (100 μl) was plated onto LB agar supplemented with ampicillin (50 μg ml−1), and after overnight incubation at 30°C colony numbers were determined. One hundred individual IJs were assessed by this method to determine the distribution of colonization within the nematode population.
To assess bacterial replication within the nematode, population grinding was carried out by suspending 1,000 surface-sterilized IJs in 400 μl of hemolymph treated with 5 mM glutathione (see below) and incubated for the times indicated. IJs were harvested, washed two times in LB broth, resuspended, homogenized, and plated as described above. Population grinding was repeated three times, and samples were plated in duplicate.
Bacterial release.
Release of X. nematophila was observed by incubating 1,000 surface-sterilized IJs in 400 μl of glutathione-treated hemolymph, LB broth, and water for the times indicated. The hemolymph was obtained by making a lesion alongside the horn of fourth-instar larvae of M. sexta. Extracted hemolymph was collected on ice and treated with 5 mM glutathione (Sigma) to prevent melanization (19). The hemolymph suspension was centrifuged at 10,000 rpm for 10 min at 4°C, and the supernatant was removed and used for these studies. To quantify the CFU released in each condition, IJs were allowed to completely settle on the bottom of the centrifuge tube. An aliquot of 50 μl of the supernatant was spread onto LB agar plates. These experiments were repeated four times, and sample assays were performed in duplicate. Results are reported as CFU/50 μl. Release was also monitored by epifluorescence microscopy as described below. To determine the percentage of IJs (within the observed IJ population) that were in the process of release (bacteria in intestinal lumen and rectum), 50 IJs containing GFP-labeled bacteria were used for epifluorescence microscopic observation at the time points previously indicated.
To assess whether nematode activity, esophageal pumping, and/or bacterial motility was required for release of bacteria, hemolymph was treated with levamisole (2.0 mM) or ivermectin (100 μg/ml with 1% dimethyl sulfoxide), which causes nematode paralysis and esophageal (i.e., by the basal bulb) pumping, respectively. Cocultures of nematodes on fliC::GFP bacterial lawns were passaged through M. sexta as previously described. The fliC::GFP mutant strain is nonmotile and was used to determine if bacterial motility was required for release. The number of CFU/50 μl and the percentage of IJs releasing bacteria were assessed over an 8-h period with observations taken every 2 h. All experiments were repeated twice with duplicate samples except for treatment with ivermectin, which was performed one time.
Epifluorescence microscopy examination of X. nematophila colonization and release processes.
Microscopic observations of X. nematophila colonization and release processes were carried out by applying a small suspension of nematodes to a 2% agarose pad containing 5 mM levamisole (Sigma) supported by a glass slide. Epifluorescence microscopic observations were performed on a Leica upright compound microscope (Heidelberg, Germany) equipped with a 50-W mercury burner at ×630 magnification, and microscopy of GFP-labeled bacteria was performed using a fluorescein isothiocyanate filter. Images were recorded digitally using a Spot RT color digital camera (Diagnostics Instruments, Inc.). Images were acquired with Spot RT software version 3.2 and processed for publication using MetaMorph (version 5.0) and Adobe Photoshop 7.0 (Adobe Systems, Mountain View, CA).
RESULTS
Morphology and ultrastructure of the bacterial receptacle of S. carpocapsae.
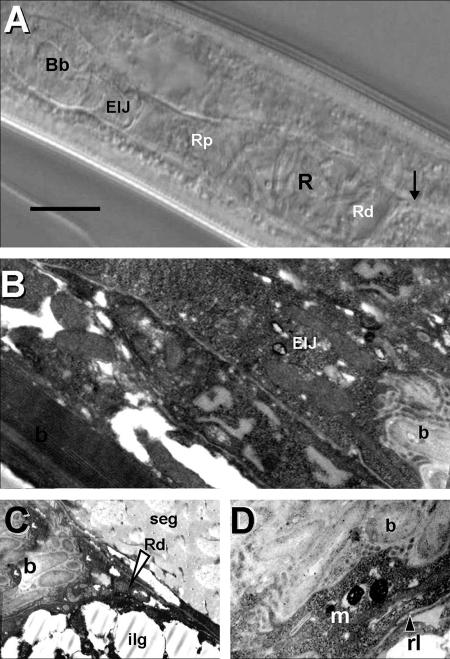
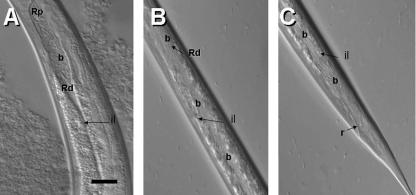
The site of colonization of S. carpocapsae had been generally described as a vesicle. Detailed observation by DIC microscopy suggests that this structure, here referred to as the bacterial receptacle, is a distended region of the anterior portion of the intestine (Fig. 1A and B). X. nematophila colonizes the distal region of this receptacle (Rd). The intestine is collapsed posterior to the bacterial receptacle (Fig. 1A and D). The proximal region of the receptacle remains open and is directly connected to the region of the basal bulb of the esophagus near the EIJ (Fig. 1A and D). This anterior connection is not a “specialized duct” but an extension of the anterior intestine that apparently expands or contracts based on the load of bacterial cells. DIC morphometric studies confirmed this observation, indicating significant differences (P < 0.05) in the length of the receptacle in colonized and uncolonized IJs (Table 2).
FIG. 1.
DIC and TEM images of the bacterial receptacle of S. carpocapsae. (A) DIC image of colonized IJ receptacle (R) showing proximal (Rp) and distal (Rd) ends. The arrow indicates the connection between the distal receptacle and the intestine. The anterior end of the nematode is oriented to the left side of the panel. Bb, basal bulb. (B) TEM image of anterior portion of bacterial receptacle in connection with the EIJ. b, bacterial cells. (C) Posterior portion of bacterial receptacle showing end (arrowhead), Rd, and bacterial cells (b). ilg, intestinal lipid globule; seg, secretory-excretory gland. (D) Close-up of bacterial receptacle showing bacterial cells (b), receptacle lining (rl), and amorphous matrix (m). Bar, 15 μm.
TABLE 2.
Morphometric data for the bacterial receptacle and proximal receptacle in colonized and uncolonized S. carpocapsae IJs
| Colonization status | Range (mean ± SD) (μm)a
|
|||||
|---|---|---|---|---|---|---|
| TBL | MBW | ReL | ReW | RpL | RpW | |
| Colonized | 543-721 (640 ± 47) | 24-37 (31 ± 3) | 13-36 (24 ± 6) | 4.5-15 (9.5 ± 2.5) | 8-31 (16 ± 10) | 1-5.5 (3.5 ± 0.5) |
| Uncolonized | 443-663 (594 ± 48) | 20-36 (29 ± 3.5) | 8-17 (13 ± 2.5) | 5-12 (7 ± 2) | 5-10 (6.5 ± 2) | 1-4 (2 ± 1) |
TBL, total IJ body length; MBW, maximum IJ body width; ReW, receptacle width; RpW, proximal receptacle width.
TEM confirmed the above observations. Figure 1B and C illustrate the continuous connection between the receptacle and the basal bulb. Higher-resolution TEM images showed that the bacterial cells are embedded in an amorphous matrix and that the lining of the receptacle is a smooth thin layer (average, 0.13 μm) that lacks microvilli (Fig. 1C and D).
Colonization of the receptacle.
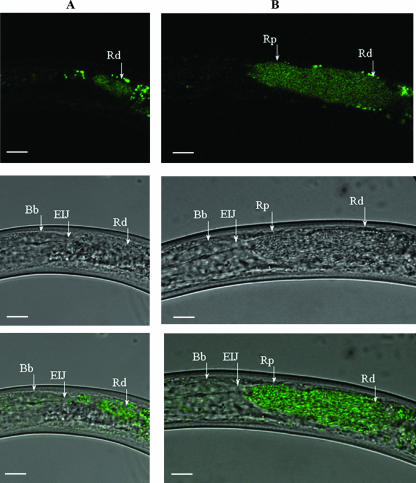
Colonization of the receptacle was studied using a strain of X. nematophila (HGB340) that constitutively expresses an enhanced isoform of GFP. HGB340 colonizes IJs as well as the parent strain does and, in addition, is readily detected inside IJs by epifluorescence microscopy (16). Figure 2A shows a confocal image of Xenorhabdus cells (green) over a DIC image in an unstimulated IJ. The bacteria colonize the distal region of the receptacle. After 24 h of incubation in hemolymph (Fig. 2B) the bacteria occupy the entire receptacle, up to but not beyond the EIJ. The average length of the area occupied by the bacteria (posterior portion of distal receptacle to EIJ) was 50.5 ± 1.4 μm (range, 40.3 to 59.8 μm) while the average width of the area occupied in the proximal receptacle region was 8.3 ± 0.5 μm (range, 4.7 to 13.5 μm).
FIG. 2.
Confocal image of X. nematophila cells (green) over DIC image. (A) Unstimulated IJ containing X. nematophila cells within the Rd. The anterior end of the nematode is oriented to the left side of the panels. Spots anterior and posterior to the receptacle are due to autofluorescent tissue of the nematode. (B) An IJ incubated in hemolymph for 24 h showing forward movement of X. nematophila from the Rd to the proximal receptacle (Rp). Top row, confocal images; middle row, DIC images; bottom row, overlay images. Bars, 10 μm.
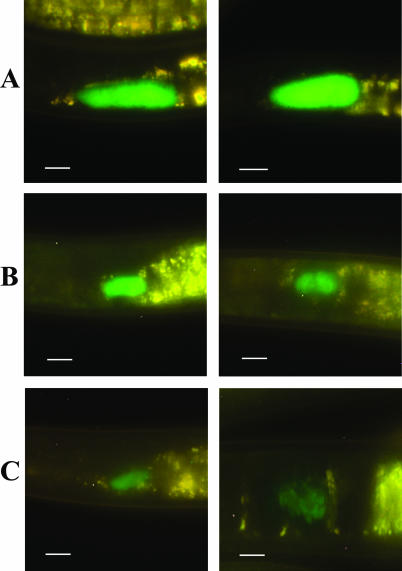
Examination of in vivo-reared IJs by epifluorescence microscopy revealed that the unstimulated nematode population was not uniformly colonized (Fig. 3). From a total of 308 IJs examined 39% were colonized at high levels (Fig. 3A), 48% were colonized at medium levels (Fig. 3B), 10% contained low levels of bacteria (Fig. 3C), and 3% remained uncolonized (data not shown).
FIG. 3.
Epifluorescent images of the level of colonization (high [A], medium [B], and low [C]) of the distal area of the receptacle in S. carpocapsae IJs. In all panels, the anterior end of the nematode is oriented to the left side of the panel. Left and right panels are images representative of the different levels of colonization. Bars, 10 μm.
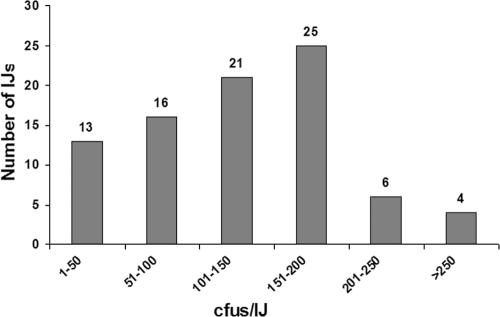
The range of colonization within a population of nematodes was independently examined by assessing the number of viable bacterial cells (CFU) in individual IJs. Figure 4 shows that 35% of the nematodes contained more than 151 CFU, 37% of the IJs contained between 150 and 51 CFU, and 13% of the nematodes contained 50 or fewer CFU while bacteria were not detected in the remaining IJs. These findings indicate that the level of colonization can vary considerably within a nematode population.
FIG. 4.
Analysis of colonization levels by single nematode grindings. One hundred individual nematodes were individually homogenized to release bacteria from the receptacle.
Analysis of initial phase of bacterial movement.
To study the initial events of the release process, IJs were incubated in either hemolymph or a nutrient-rich medium (LB broth) and released bacteria were monitored by assessing CFU per 50 μl of the incubation medium. After 2 h of exposure to hemolymph, bacterial release was already detectable and the number of bacteria released progressively increased during the 8-h period of incubation (Table 3). In contrast, bacterial release was not detected in IJs incubated in nutrient-rich broth until 8 h, at which time very low numbers of CFU were recovered (Table 3). These findings suggest that nutrient signals alone are not sufficient to stimulate bacterial release. As expected, bacteria were not released from IJs incubated in water.
TABLE 3.
Release of X. nematophila cells from the receptacle of IJs incubated in various media
| Medium | Avg CFU/50 μl (SE) at time point:
|
||||
|---|---|---|---|---|---|
| 2 h | 4 h | 6 h | 8 h | 24 h | |
| Hemolymph | 20 (7) | 89 (24) | 308 (88) | 497 (117) | <106 |
| LB broth | 0 | 0 | 0 | 1 (0.6) | <104 |
| Water | 0 | 0 | 0 | 0 | 0 |
Analysis of the release process by epifluorescence microscopy showed that after 2 h of incubation in hemolymph approximately 7% of the nematode population contained bacteria moving through the intestine or being released out of the anus. By 4 h, 25% of the IJs were releasing bacteria. The increase in the number of IJs releasing bacteria during this early period correlated with the increase of CFU recovered from the hemolymph (Table 3). The number of IJs releasing bacteria continued to increase such that by 24 h nearly 90% of the IJs contained bacteria in their intestine and anal region. The level of colonization in most IJs observed over the 24-h incubation period was not significantly reduced. This indicates that only a portion of the bacterial population leaves the receptacle during the release process. Unlike the events that occurred during hemolymph stimulation, only 1 to 2% of the IJs incubated in LB broth for 24 h contained bacteria in their intestine.
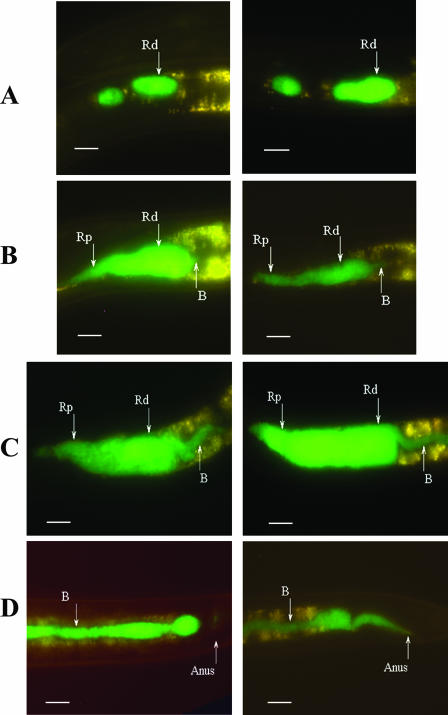
During early periods of incubation in hemolymph forward movement of small clusters of cells was frequently observed in the proximal region (Fig. 5A). The bacteria continued to move forward towards the basal bulb as the IJs were exposed to hemolymph for longer periods of time (Fig. 5B). Additionally, bacteria were also observed moving posteriorly through a narrow passage in the Rd (Fig. 5B and C). During prolonged exposure to hemolymph the bacteria were observed moving down the intestine and out the anus (Fig. 5D).
FIG. 5.
Epifluorescent images of movement of X. nematophila in the receptacle of the IJ exposed to hemolymph. (A) Clusters of bacteria in the distal and proximal receptacle region. (B) Forward movement of cells to the EIJ region and initial movement of bacteria out of the posterior region of the distal receptacle. (C) Bacterial cells moving posteriorly out of the distal receptacle. (D) Bacterial cells moving down the intestine and out the anus. In all panels the anterior end of the nematode is oriented to the left. Left and right panels are images representative of the movement of bacteria in hemolymph-stimulated IJs. B, bacteria. Bars, 10 μm.
A confocal microscopic analysis was performed with IJs incubated for 24 h in hemolymph to obtain a higher-resolution view of bacterial movement out of the receptacle (see file S1 in the supplemental material). This series showed that the proximal receptacle can be bent, due to nematode movement, and that the distal receptacle was distended and filled with bacteria. The funnel-shaped structure of the distal receptacle filled with bacteria (B) moving into the intestine can be seen as the series progresses from one side of the nematode to the other.
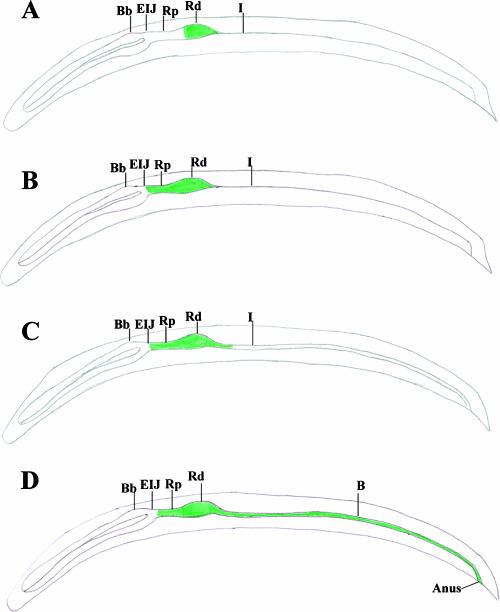
DIC microscopic analysis revealed that the narrow passage at the distal region of the receptacle widens and bacteria move into the intestine in IJs exposed to hemolymph (Fig. 6). It was also observed that during the early phase of the release process (i.e., after 3 h of hemolymph exposure) the bacterial cells are pulsed up and down the receptacle. This action presumably forces the widening or opening of the narrow posterior passage of the receptacle so that bacteria can move into the intestinal lumen. A schematic representation of the initial movement of bacteria forward to the esophagointestinal region and the widening of the posterior passage and subsequent movement of bacteria down the intestine is shown in Fig. 7.
FIG. 6.
DIC images showing bacterial release process. Shown is the opening of the bacterial receptacle into the intestine. Notice the expansion of the intestinal lumen (il) and the migration of the bacterial cells (b) into this lumen. The anterior end of the nematode is oriented to the top of the panel. Bar, 6 μm.
FIG. 7.
Schematic representation of bacteria moving both forward towards the basal bulb and rearward out of the distal receptacle through the narrow passage and into the intestine. (A) The Rd colonized by X. nematophila in the unstimulated IJ. (B) IJs exposed to hemolymph begin pharyngeal pumping, and forward movement of bacteria commences. (C) Widening of the narrow passage in the distal receptacle allowing bacteria to move into the intestine. (D) Movement of bacteria down the intestine and out the anus. Bb, basal bulb; I, intestine; B, bacteria.
Finally, we observed that bacterial replication (CFU per IJ) was not detectable during the first 6 h of exposure to hemolymph (data not shown). Since bacterial release began after the first 2 h of hemolymph exposure, these findings suggest that bacterial replication was not required for initial release of the bacteria. At 8 and 24 h the average CFU per IJ increased more than twofold and fourfold, respectively, relative to the unstimulated IJ population.
Nematode activity is required for movement out of the receptacle.
The question of whether bacterial release required nematode activity was assessed by incubating IJs in hemolymph treated with either levamisole or ivermectin, which causes nematode paralysis or cessation of esophageal pumping, respectively (5). Levamisole treatment suppressed both the recovery of CFU and the proportion of IJs that contained bacteria in their intestine (Table 4). Similar results were observed when IJs were incubated in the presence of ivermectin (data not shown). Thus, nematode activity in the form of esophageal pumping was required for movement of bacteria out of the receptacle. We also speculate that intestinal movement (i.e., peristalsis) is involved in the movement of the bacteria towards the rectum. Bacterial motility was not required for release of X. nematophila from IJs. Nematodes colonized with the fliC::GFP nonmotile strain released bacteria as efficiently as did IJs colonized with the wild-type strain (data not shown).
TABLE 4.
Release of bacteria from receptacle is dependent on nematode activity
| Condition | Avg CFU/50 μl (SE) | % Releaseda |
|---|---|---|
| Hemolymph | 504 (37) | 27 |
| Hemolymph plus 2 mM levamisoleb | 72 (50.5) | 4 |
Percent release is out of 50 IJs, the same IJs as those used for determining CFU/50 μl for treated and untreated hemolymph.
Similar results were obtained when hemolymph was treated with ivermectin (100 μg/ml with 1% dimethyl sulfoxide).
DISCUSSION
Colonization of S. carpocapsae was recently shown to be initiated by one or a few X. nematophila cells that enter the bacterial receptacle (16). Many of the nutrients required for outgrowth are present in the bacterial receptacle and acquired from insect hemolymph (12, 17). In addition, an unattached acellular mass referred to as the intravesicular structure, to which X. nematophila binds, was recently identified within the receptacle (15). While recent studies have provided new information concerning the colonization of S. carpocapsae, little was known about the range of colonization of individual IJs within a population and the process by which X. nematophila moves out of the receptacle into the intestine. Furthermore, the morphology and ultrastructure of the receptacle had not been reexamined since the early studies by Bird and Akhurst (4). The present study was designed to address these questions.
Results from this study suggest that X. nematophila colonizes an expanded receptacle region of the anterior intestine of S. carpocapsae rather than a vesicle as previously described. By definition, a vesicle is “a small sac or hollow organ in the body, especially one containing fluid.” According to our observations this definition does not apply to the structure that harbors Xenorhabdus symbionts. In contrast, the receptacle is open at its proximal end by a stretched tube-like connection that leads to the EIJ. The distal portion of the receptacle is the region that is colonized by bacteria. Eventually, this distal portion opens up to the intestinal lumen to release the bacterial cells. TEM observations showed that the bacterial cells are embedded in an amorphous matrix and the lining of the receptacle in S. carpocapsae is a smooth thin layer that lacks microvilli. The receptacle wall in this nematode species is regular and rather thin (ca. 0.14 μm). No microvilli were observed in any of the specimens studied. According to Bird and Akhurst (4) and Endo and Nickel (7), the ultrastructural features of the receptacle vary across nematode species. For instance, these authors reported that while the thickness of the vesicle walls of Steinernema glaseri and an unnamed Steinernema sp. strain (isolate Q1) were different, both possessed microvilli. In contrast, the receptacle of Steinernema feltiae (=Steinernema bibionis) had a thick wall but lacked microvilli. Further studies on the ultrastructural features of the bacterial receptacle among other Steinernema spp. are warranted given the diversity that has already been observed.
Our findings raise the question of why the distal, but not the proximal, portion of the receptacle is colonized. One possibility may relate to the compartmentalization of nutrients. Many of the nutrients required for growth appear to be present in the receptacle (17). Nutrient concentration for bacterial growth may be sufficient in the distal part of the receptacle but insufficient in the proximal region. Alternatively, specific sites of attachment may exist in the distal but not the proximal region. X. nematophila was shown to adhere to an intravesicular structure (15) which, if localized to the distal region, could provide initial sites of attachment. Whether the intravesicular structure preferentially localizes to the distal region of the receptacle remains to be determined. Another possibility is that the pumping action of the basal bulb forces Xenorhabdus cells to move to the distal region that in the IJ apparently forms a cul-de-sac where bacteria could begin to multiply. These possibilities are not mutually exclusive, and all may play a role in the compartmental colonization of the receptacle of S. carpocapsae.
It was shown previously in IJs exposed to hemolymph that X. nematophila migrated through the intestine and exited out the anus of S. carpocapsae (22, 23). However, the process by which the bacteria moved out of the receptacle and into the intestine remained unknown. We show that, after 2 h of hemolymph exposure of the IJs, bacteria move forward out of the distal portion of the receptacle, in a pulsatile movement that continues for several hours (i.e., 2 to 3 h) until the bacteria are released through a narrow passage in the Rd and move into the intestine (Fig. 7). Small clusters of bacterial cells were frequently seen in the proximal region due to this movement. The up-down flux of bacteria in the receptacle may force the posterior connection of the receptacle to open, releasing the bacteria into the intestinal lumen. During the release process the bacteria were also pulsed forward to the EIJ region. Bacterial multiplication was not apparent until 8 h after exposure to hemolymph, while bacterial release was evident by 2 h. Furthermore, levamisole and ivermectin suppressed release, indicating that nematode activity, in particular esophageal pumping, was necessary for movement of bacteria out of the receptacle. Additionally, we show that bacterial motility was not required for release. We speculate that a combination of esophageal pumping followed by intestinal expansion and peristalsis is involved in the bacterial release process.
While hemolymph was known to stimulate the release of X. nematophila from S. carpocapsae (15), the component or combination of components that induced basal bulb pumping had not been addressed previously. We found that a nutrient-rich medium (LB broth) was ineffective at stimulating bacterial release, suggesting that common nutrients such as amino acids, sugars, and vitamins do not induce pharyngeal pumping in S. carpocapsae. Arthropod hemolymph was shown to stimulate the release of the sister taxon, Photorhabdus luminescens, from its mutualistic nematode host Heterorhabditis bacteriophora (5). Photorhabdus spp. do not colonize a receptacle-like structure but rather inhabit the anterior (cardias) region of the nematode intestine and, unlike Xenorhabdus, are released by regurgitation via the nematode mouth. Regurgitation was stimulated by a low-molecular-weight, heat- and protease-stable component(s) of arthropod hemolymph. Whether a similar compound stimulates the pharyngeal pumping of S. carpocapsae is presently being investigated.
To our knowledge the present study is the first in which the range of colonization has been assessed in S. carpocapsae. We show that more than 85% of the IJ population was colonized at medium to high levels as measured by epifluorescence analysis while few (<5%) remained uncolonized. These findings indicate that X. nematophila is highly proficient in colonizing its nematode host. In contrast, other steinernematid species are colonized poorly, such that a majority of individual IJs in the population remain uncolonized (4, 24). The relatively high level of colonization may play a role in the ability of S. carpocapsae to outcompete other steinernematid species that are poorly colonized (24). S. carpocapsae has a wide biogeographic distribution in temperate regions (13). Thus, the relatively high rate at which X. nematophila colonizes its nematode host may be one of the numerous factors involved in the seemingly ubiquitous distribution of S. carpocapsae.
Supplementary Material
Acknowledgments
We thank Heather Owen (University of Wisconsin—Milwaukee) for training and assistance in microscopy and K. Plichta (University of Arizona) for assistance with DIC image capture and morphometric analysis. We thank Eric Martens (Washington University, St. Louis, MO) and Heidi Goodrich-Blair (University of Wisconsin—Madison) for providing suggestions for the release experiments and bacterial strains and plasmids used in this study. We also thank Jane Witten (University of Wisconsin—Milwaukee) for providing Manduca sexta larvae and Dong-Jin Kim (University of Wisconsin—Milwaukee) for providing the fliC strain. We express our gratitude to Charles Wimpee and members of the Forst laboratory for suggestions and critical reading of the manuscript.
This research was funded in part by NSF-IOB Program grants to S.F. (NSF no. 0416747) and S.P.S. (NSF no. 0146644).
Footnotes
Published ahead of print on 25 May 2007.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Akhurst, R. J. 1982. Antibiotic activity of Xenorhabdus spp., bacteria symbiotically associated with insect pathogenic nematodes of the families Heterorhabditidae and Steinernematidae. J. Gen. Microbiol. 128:3061-3065. [DOI] [PubMed] [Google Scholar]
- 2.Akhurst, R. J. 1983. Neoaplectana species: specificity of association with bacteria of the genus Xenorhabdus. Exp. Parasitol. 55:258-263. [DOI] [PubMed] [Google Scholar]
- 3.Akhurst, R. J., and N. E. Boemare. 1990. Biology and taxonomy of Xenorhabdus, p. 75-90. In R. Gaugler and H. K. Kaya (ed.), Entomopathogenic nematodes in biological control. CRC Press, Boca Raton, FL.
- 4.Bird, A. F., and R. J. Akhurst. 1983. The nature of the intestinal vesicle in nematodes of the family Steinernematidae. Int. J. Parasitol. 16:511-518. [Google Scholar]
- 5.Ciche, A. T., and J. C. Ensign. 2003. For the insect pathogen Photorhabdus luminescens, which end of the nematode is out? Appl. Environ. Microbiol. 69:1890-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunphy, G. B., and J. M. Webster. 1991. Antihemocytic surface components of Xenorhabdus nematophilus var. dutki and their modification by serum of nonimmune larvae of Galleria mellonella. J. Invertebr. Pathol. 58:40-51. [Google Scholar]
- 7.Endo, B. Y., and W. R. Nickle. 1995. Ultrastructure of anterior and mid-regions of infective juveniles of Steinernema feltiae. Fundam. Appl. Nematol. 18:271-294. [Google Scholar]
- 8.Flores-Lara, Y., D. Renneckar, S. Forst, H. Goodrich-Blair, and P. Stock. 2007. Influence of nematode age and culture conditions on morphological and physiological parameters in the bacterial vesicle of Steinernema carpocapsae (Nematoda: Steinernematidae). J. Invertebr. Pathol. 95:110-118. [DOI] [PubMed] [Google Scholar]
- 9.Forst, S., and D. Clark. 2002. Bacteria-nematodes symbiosis, p. 57-77. In R. Gaugler (ed.), Entomopathogenic nematology. CABI Publishing, London, United Kingdom.
- 10.Forst, S., B. Dowds, N. Boemare, and E. Stackenbrandt. 1997. Xenorhabdus spp. and Photorhabdus spp.: bugs that kill bugs. Annu. Rev. Microbiol. 51:47-72. [DOI] [PubMed] [Google Scholar]
- 11.Forst, S. A., and N. Tabatabai. 1997. Role of the histidine kinase, EnvZ, in the production of outer membrane proteins in the symbiotic-pathogenic bacterium, Xenorhabdus nematophilus. Appl. Environ. Microbiol. 63:962-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goetsch, M., H. Owen, B. Goldman, and S. Forst. 2006. Analysis of the PixA inclusion body protein of Xenorhabdus nematophila. J. Bacteriol. 188:2706-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hominick, W. M. 2002. Biogeography, p. 115-143. In R. Gaugler (ed.), Entomopathogenic nematology. CABI Publishing, London, United Kingdom.
- 14.Kaya, H. K., and S. P. Stock. 1997. Techniques in insect nematology, p. 281-324. In L. A. Lacey (ed.), Manual of techniques in insect pathology. Academic Press, San Diego, CA.
- 15.Martens, E. C., and H. Goodrich-Blair. 2005. The Steinernema carpocapsae intestinal vesicle contains a subcellular structure with which Xenorhabdus nematophila associates during colonization initiation. Cell. Microbiol. 12:1723-1735. [DOI] [PubMed] [Google Scholar]
- 16.Martens, E. C., K. Heungens, and H. Goodrich-Blair. 2003. Early colonization events in the mutualistic association between Steinernema carpocapsae nematodes and Xenorhabdus nematophila bacteria. J. Bacteriol. 185:3147-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martens, E. C., F. M. Russell, and H. Goodrich-Blair. 2005. Analysis of Xenorhabdus nematophila metabolic mutants yields insight into stages of Steinernema carpocapsae nematode intestinal colonization. Mol. Microbiol. 1:28-45. [DOI] [PubMed] [Google Scholar]
- 18.McClure, M. J., and L. J. Stowel. 1978. A simple method of processing nematodes for electron microscopy. J. Nematol. 18:376-377. [PMC free article] [PubMed] [Google Scholar]
- 19.Orchard, S. S., and H. Goodrich-Blair. 2004. Identification and functional characterization of a Xenorhabdus nematophila oligopeptide permease. Appl. Environ. Microbiol. 70:5621-5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park, D., and S. Forst. 2006. Co-regulation of motility, exoenzymes, and antibiotic production by the EnvZ-OmpR-FlhDC-FliA pathway in Xenorhabdus nematophila. Mol. Microbiol. 6:1397-1412. [DOI] [PubMed] [Google Scholar]
- 21.Poinar, G. O., Jr., and G. M. Thomas. 1966. Significance of Achromobacter nematophilus Poinar and Thomas (Achromobacteraceae: Eubacteriales) in the development of the nematode, DD-136 (Neoaplectana spp. Steinernematidae). Parasitology 56:385-390. [DOI] [PubMed] [Google Scholar]
- 22.Poinar, G. O., and G. M. Thomas. 1967. The nature of Achromobacter nematophilus as an insect pathogen. J. Invertebr. Pathol. 9:510-514. [Google Scholar]
- 23.Sicard, M., K. Brugirard-Ricaud, S. Pages, A. Lanois, N. E. Boemare, M. Brehelin, and A. Givaudan. 2004. Stages of infection during the tripartite interaction between Xenorhabdus nematophila, its nematode vector, and insect hosts. Appl. Environ. Microbiol. 70:6473-6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sicard, M., J. Hinsinger, N. LeBrun, S. Pages, N. Boemare, and C. Moulia. 2006. Interspecific competition between entomopathogenic nematodes (Steinernema) is modified by their bacterial symbionts (Xenorhabdus). BMC Evol. Biol. 6:68-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu, J., and R. E. Hurlbert. 1990. Toxicity of irradiated media for Xenorhabdus spp. Appl. Environ. Microbiol. 56:815-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.