Abstract
This study aimed to characterize the bacterium-destroying properties of a gliding arc plasma device during electric discharges and also under temporal postdischarge conditions (i.e., when the discharge was switched off). This phenomenon was reported for the first time in the literature in the case of the plasma destruction of microorganisms. When cells of a model bacterium, Hafnia alvei, were exposed to electric discharges, followed or not followed by temporal postdischarges, the survival curves exhibited a shoulder and then log-linear decay. These destruction kinetics were modeled using GinaFiT, a freeware tool to assess microbial survival curves, and adjustment parameters were determined. The efficiency of postdischarge treatments was clearly affected by the discharge time (t*); both the shoulder length and the inactivation rate kmax were linearly modified as a function of t*. Nevertheless, all conditions tested (t* ranging from 2 to 5 min) made it possible to achieve an abatement of at least 7 decimal logarithm units. Postdischarge treatment was also efficient against bacteria not subjected to direct discharge, and the disinfecting properties of “plasma-activated water” were dependent on the treatment time for the solution. Water treated with plasma for 2 min achieved a 3.7-decimal-logarithm-unit reduction in 20 min after application to cells, and abatement greater than 7 decimal logarithm units resulted from the same contact time with water activated with plasma for 10 min. These disinfecting properties were maintained during storage of activated water for 30 min. After that, they declined as the storage time increased.
The microbial contamination of liquids and surfaces is a major source of problems in numerous settings (food industry, hospitals). Various decontamination methods are available, and some of them are widely employed, including heat treatment, chemical disinfection with solutions or gases, filtration, and irradiation. Most conventional methods (autoclaving and dry heat) are associated with some degree of damage to the material or medium. Another method, gas sterilization using ethylene oxide and compatible gaseous compounds, allows low-temperature disinfection, but ethylene oxide has recently been considered to be both mutagenic and carcinogenic (26). Thus, the limitations of conventional techniques have motivated the search for alternative methods. Among the emerging techniques for decontamination, electric discharges appear to be a promising alternative (11, 20), as they are usual sources of plasma gases.
The gliding electric discharge (“glidarc”) device in humid air is an efficient and inexpensive source of nonthermal plasma which operates close to atmospheric pressure (it does not require a vacuum) and at room temperature. This plasma exhibits characteristics of both thermal and nonthermal plasma so that its interactions with matter are those of quenched plasma (16). With humid air as the working gas, °OH and NO° were identified by emission spectroscopy measurements as the principal radical species present in the glidarc plasma plume (2), and they are probably precursors of other active species. Such species endow the medium with very high, enhanced chemical reactivity. As a result, the gliding discharge device was first studied successfully for the treatment of gases (5) and liquids (24) to abate chemical pollutants. More recently, it was also tested successfully for decontamination of microorganisms (12, 22), which represents one of the most interesting applications of atmospheric pressure plasmas in recent years.
This work dealt with inactivation of bacterial suspensions using gliding arc plasma. In aqueous solution, a so-called temporal postdischarge phenomenon was recently highlighted in a chemical pollutant abatement setting. This phenomenon can simply be defined as the overall reactions that continue after the discharge is switched off, and it probably involves the presence of long-lived reactive species. The postdischarge phenomenon may be of great value in terms of environmental and industrial applications because the target continues to be treated without any contribution of energy. Costs are thus reduced. Although this phenomenon has been observed in the oxidation of inorganic aqueous solutions (7) and in plasma chemical nucleophilic substitution reactions (6), no attempts to use plasma temporal postdischarge disinfection have been reported previously, as far as we know. In this study, as a first step toward full evaluation of the viability of glidarc technology for industrial microbial decontamination, after characterizing the destruction of bacterial cells by classical gliding electric discharges, we focused on the study of temporal postdischarge treatment. Postdischarge efficiency was evaluated for cells that were present or not present in the fluid exposed to the discharge. In the latter case, treatment consisted of disinfection by plasma-activated water, the properties of which were studied. The entire study was conducted with Hafnia alvei, which was selected as a bacterial model. This gram-negative bacterium belongs to the family Enterobacteriaceae. It has been found in a number of foods, such as milk products and meats, and in humans it may be associated with several intestinal disorders, including gastroenteritis (31).
MATERIALS AND METHODS
Strain, cultures, and the preparation of suspensions.
H. alvei CIP 5731 was purchased from Institut Pasteur (France) and stored frozen in cryotubes at −20°C in glycerol. The microbial strain was cultured on Trypticase soy broth (TSB) (bioMérieux, Marcy l'Etoile, France) at 30°C without shaking. After two subcultures (1 ml in 9 ml TSB) at intervals of 8 and 24 h, 100 ml TSB was inoculated with 1 ml of a microbial suspension and incubated for 17 h. The bacteria were then in early stationary growth phase. After culture, microbial cells were harvested by centrifugation (4°C, 7,000 × g, 10 min), washed twice, and resuspended in 1.5 × 10−1 M NaCl to obtain concentrated suspensions (A600, 1.1) and diluted suspensions (A600, 0.08). Both types of suspensions were utilized for inactivation studies.
Gliding discharge treatments.
The plasma device utilized was a nonthermal quenched plasma device of the gliding arc type operating at atmospheric pressure with humid air as the plasma gas. The design of the device and the procedure for gas discharge have previously been described (22). Under operating conditions, the feed gas was provided by an air compressor. The air supplied (550 liters h−1) was bubbled through a water-filled Duran flask in order to saturate it with water vapor. The gap between the plasma source electrodes was kept constant at 3.5 mm. The distance between the electrode tips and the top of the liquid target was 13 cm. The reactor was fitted with a circulating water jacket to prevent any thermal effect, so that the temperature of the treated suspensions remained below 30°C. Valves and filters were adjusted to the reactor in order to allow sampling during plasma treatment, when necessary. For each series of experiments, 200-ml portions of stirred liquid samples (1.5 × 10−1 M NaCl or diluted suspensions) were exposed to the plasma discharge. The treatment time under plasma discharge was designated t*.
Temporal postdischarge treatments.
For temporal postdischarge treatments, diluted bacterial suspensions were initially treated for various t* with the glidarc reactor, as described above. Once the discharge was stopped, the bacterial suspension was left at the ambient temperature for various postdischarge times (tp) before sampling and determination of surviving cells.
During other experiments, 1.5 × 10−1 M NaCl solutions without bacterial cells were treated with the glidarc reactor for various t* and then referred to as “activated water.” Concentrated bacterial suspensions (12 ml) were diluted in activated water (188 ml) immediately after plasma treatment or after a period of storage. Thereafter, the suspensions were left at the ambient temperature for a variety of postdischarge periods (referred to as “contact time” [tc]) before samples were removed and survivors were counted.
Evaluation of surviving cells.
Before and after treatment (discharge and/or postdischarge), the numbers of surviving cells were determined by duplicate plate counting on Trypticase soy agar (bioMérieux). In order to prevent any destruction after sampling, the lethal effect of active species generated by glidarc plasma discharge was stopped with a universal neutralizing solution (3 g liter−1 l-α-phosphatidylcholine, 30 g liter−1 Tween 80, 5 g liter−1 sodium thiosulfate, 1 g liter−1 l-histidine, 30 g liter−1 saponine), as recommended in the NF EN 1040 European standard (1) with respect to disinfection. For this purpose, 1 ml of the suspension to be analyzed was diluted in 9 ml of neutralizing solution at each time point. Thereafter, classic 1/10 dilution in 1.5 × 10−1 M NaCl was performed prior to plating. As described in the European standard, control experiments were performed to verify the inactivity of the neutralization procedure with microbial cells and its efficiency against active species generated by glidarc plasma discharge. The number of cultivable bacteria (expressed as CFU) was counted after 2 days of incubation at 30°C. The limit of detection under our experimental conditions was 5 CFU ml−1. Each determination of the kinetics of destruction was performed with at least three independently grown cultures. The data presented below are the means of decimal logarithm CFU ml−1 values ± standard deviations.
Modeling of bacterial destruction.
GinaFiT, a freeware add-in for Microsoft Excel (9), was utilized to model inactivation kinetics. This tool enables testing of nine different types of microbial survival models, and the choice of the best fit depends on five statistical measures (i.e., sum of squared errors, mean sum of squared errors and its root, R2, and adjusted R2). During this study, the different models were applied to the mean values obtained from experimental data. The “shoulder + log-linear” inactivation model exhibited the best fit with our experimental data and was thus considered. It took account of a latency time before a decrease in the population size. The kinetics of destruction were expressed as follows (9):
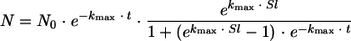 |
where N is the cell concentration (CFU ml−1) after a treatment time t (min), N0 is the initial cell concentration (CFU ml−1), kmax is the maximum inactivation rate (min−1), and Sl is the shoulder length (min) (i.e., the length of the lag phase).
RESULTS
Destruction of bacteria under direct discharge.
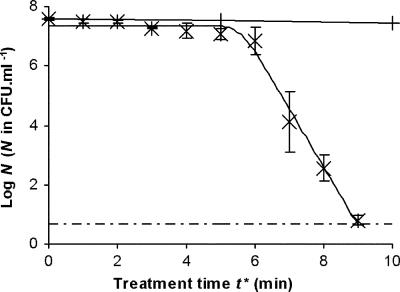
The effect of the glidarc decontamination technique on the cultivability of planktonic cells of H. alvei was first studied under direct discharge. In this setting, suspensions of bacterial cells were subjected to the electric discharge and reactions were neutralized immediately after sampling, as described in Materials and Methods. Under these conditions, the treatment time corresponded to the t*. As illustrated in Fig. 1, the kinetics of destruction of the bacterial concentration versus the treatment time presented two phases: an initial phase, during which the number of cultivable bacteria remained almost steady, followed by a rapid decrease. The reduction in the initial population was completed within less than 4 min, and after gliding discharge treatment for 10 min, no cultivable cells could be detected. The “shoulder + log-linear” GInaFiT model was applied to our experimental data and revealed satisfactory adjustment (R2 = 0.9902), showing that decay followed a first-order kinetic law with an inactivation rate (kmax) of 4.51 ± 0.30 min−1. The Sl was estimated to be 5.57 ± 0.17 min by this model. A control experiment was also carried out with nonionized gas, and the results showed that the gas had no biocidal effect unless it was activated by the electric discharge (Fig. 1).
FIG. 1.
Survival curve for H. alvei treated with gliding arc plasma discharge (×). The values are the means ± standard deviations for at least three experiments conducted with independently grown cultures. +, blank (treatment with un-ionized humid air); solid line, GInaFiT inactivation model; dashed and dotted line, detection threshold.
Destruction of bacteria during direct discharge followed by temporal postdischarge.
The effect of the glidarc decontamination technique on the cultivability of planktonic cells of H. alvei was also studied under temporal postdischarge conditions. In this case, bacterial cells suspended in the aqueous media were subjected to the electric discharge for t*. Then, after extinction of the discharge, the cells were left in the aqueous media without neutralization in order to reveal destruction by temporal postdischarge. Under these conditions, the treatment time was equal to the plasma discharge time (t*) plus the postdischarge time (tp). Neutralization was performed after the postdischarge time.
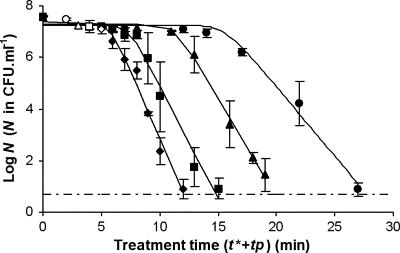
Figure 2 shows the survival curves for H. alvei planktonic cells subjected to temporal postdischarge for different periods of glidarc plasma treatment. All these curves presented the same profile, which is similar to that obtained with direct discharge, with an initial lag phase followed by decay of the cultivable population. For all the different curves, the “shoulder + log-linear” GInaFiT model fitted the data relatively satisfactorily (R2 > 0.9733). Inactivation constants, as well as the lengths of the shoulder phase, are shown in Table 1. All the conditions tested made it possible to achieve more than 7 decimal logarithm units of destruction, but the efficiency of postdischarge treatments was clearly affected by the discharge duration. Postdischarge treatment was more efficient after a longer t*, and no detectable cells (from the initial population containing 4 × 107 bacteria per ml) remained after 13 min for a t* of 5 min, after 16 min for a t* of 4 min, after 21 min for a t* of 3 min, and after 28 min for a t* of 2 min. As illustrated in Fig. 2 and Table 1, both the length of the lag phase and the inactivation rate depended on t*. More precisely, it could even be considered that there was a positive linear correlation between kmax and t* and a negative linear correlation between Sl and t* (Fig. 3). Within the domain studied, the kmax and the Sl could then be expressed as kmax = 0.37t* + 0.56 (R2 = 0.9776) and Sl = −3.36t* + 21.68 (R2 = 0.9654), respectively.
FIG. 2.
Survival curves for H. alvei subjected to temporal postdischarge time (tp) after different t*, including (⧫) 5 min, (▪) 4 min, (▴) 3 min, and (•) 2 min. For each curve, the open symbol indicates the beginning of postdischarge treatment. The values are means ± standard deviations for at least three experiments conducted with independently grown cultures. Solid lines, GInaFiT; dashed and dotted line, detection threshold.
TABLE 1.
“Shoulder + log-linear” model parameters for survival curves for H. alvei subjected to temporal postdischarge after different t*a
| t* (min) | kmax (min−1) (mean ± SD) | Sl (min) (mean ± SD) | Log10N0 (mean ± SD) | R2 |
|---|---|---|---|---|
| 2 | 1.24 ± 0.11 | 15.51 ± 0.81 | 7.27 ± 0.15 | 0.9888 |
| 3 | 1.77 ± 0.09 | 11.27 ± 0.33 | 7.29 ± 0.09 | 0.9976 |
| 4 | 2.00 ± 0.14 | 7.23 ± 0.43 | 7.27 ± 0.18 | 0.9841 |
| 5 | 2.39 ± 0.20 | 5.65 ± 0.47 | 7.37 ± 0.29 | 0.9733 |
The “shoulder + log-linear” model is described in Materials and Methods. The values for the different constants (kmax, Sl, and N0) were calculated automatically by GInaFiT (9). N0 was expressed in CFU ml−1.
FIG. 3.
Correlation between model temporal postdischarge kinetic parameters, including kmax (▪) and Sl (⧫), and t*.
Destruction of bacteria by activated water.
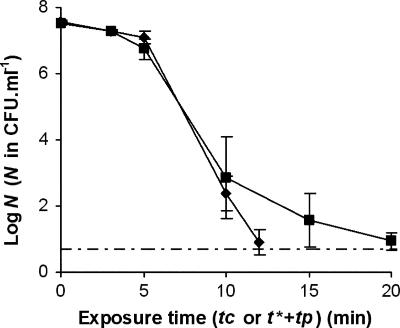
The efficiency of bacterial destruction by temporal postdischarge treatments was clearly demonstrated for bacteria exposed to electric discharge. The postdischarge behavior of bacteria without precontact with the discharge was then considered. For this purpose, as described in Materials and Methods, 1.5 × 10−1 M NaCl was exposed to the discharge for 5 min, and the bacteria were then immediately placed in contact with the plasma-treated solution (referred to as activated water) for a period called the contact time (tc) before neutralization. The survival curve obtained under these conditions for H. alvei cells is shown in Fig. 4 and compared to that obtained previously for exposure of bacteria to direct discharge for a t* of 5 min. Even if comparable, the destruction was slightly less efficient when cells were not subjected to the discharge, due to the existence of a tail in destruction kinetics or to log-linear destruction with a lower inactivation rate. Given the number of points in the log-linear destruction phase and the variability of bacterial concentrations obtained after 10 and 15 min of exposure to activated water, more precise analysis of the activated water inactivation curve was not performed. However, it can be pointed out that reductions of more than 5 decimal logarithm units were obtained in less than 15 min with activated water. This shows that exposure of bacteria to direct discharge was not a prerequisite for their destruction by plasma technology.
FIG. 4.
Kinetics of destruction of H. alvei cells exposed for a t* of 5 min to the glidarc plasma discharge and then to postdischarge (during tp; ⧫) or exposed to activated water for a contact time (tc; ▪). In the latter case, activation was achieved by exposure for 5 min of 1.5 × 10−1 M NaCl without cells to gliding arc plasma discharge. The values are means ± standard deviations for at least three experiments conducted with independently grown cultures. Dotted and dashed line, detection threshold. tc, contact time; tp, postdischarge time.
Characterization of the disinfecting properties of activated water.
Activated water was thus intrinsically efficient, and next characterization of its disinfecting properties was attempted. First, the effect of the t* used to treat the solutions on subsequent bacterial cell destruction by activated water was evaluated. Various t* were used with 1.5 × 10−1 M NaCl solutions just prior to mixing with bacteria, and surviving cells were counted after contact times of 10 and 20 min. All conditions tested resulted in significant reductions in the initial cultivable population (Table 2). However, no detectable cells were obtained only for a t* of 10 min and a contact time of 20 min. Table 2 shows that the disinfecting efficiency of activated water increased with the plasma electric discharge time, and it was again shown that the temporal postdischarge effect was controlled by the duration of the discharge.
TABLE 2.
Effects of the length of plasma activation of 1.5 × 10−1 M NaCl solutions on the temporal postdischarge destruction of H. alvei cells
| Contact time (min) | Log10N after contact with 1.5 × 10−1 M NaCl activated fora:
|
||
|---|---|---|---|
| 3 min | 5 min | 10 min | |
| 10 | 7.09 ± 0.08 | 2.84 ± 1.24 | 1.98 ± 0.18 |
| 20 | 3.86 ± 0.40 | 0.94 ± 0.25 | NDb |
N is the number of cultivable cells (in CFU ml−1). Log10 N0 was 7.58 ± 0.03 CFU ml−1. The values are the means ± standard deviations for at least three experiments conducted with independently grown cultures.
ND, not detectable (<5 CFU ml−1).
The influence of the length of storage of activated water was also examined. NaCl solutions (1.5 × 10−1 M) were treated with plasma discharge for a t* of 5 min and then stored at the ambient temperature for increasing periods of time from 5 min to 24 h before they were mixed with bacteria. H. alvei surviving cells were detected as described above after 10 and 20 min of contact time with stored activated water. The results obtained under these conditions are shown in Table 3 and can be compared with those obtained when activated water was not stored before use (Table 2). The disinfecting properties of activated water were not affected by storage for 30 min, but thereafter the efficiency decreased with the storage duration. Nevertheless, a residual disinfecting efficiency was sustained, even after 24 h of storage. Contact for 20 min with 24-h-old activated water resulted in a decimal logarithm reduction of approximately 2.5 U.
TABLE 3.
Effects of the resting time of activated water on the temporal postdischarge destruction of H. alvei cellsa
| Contact time (min) | Log10N after contact with activated water stored forb:
|
||||||
|---|---|---|---|---|---|---|---|
| 5 min | 15 min | 30 min | 1 h | 2 h | 5 h | 24 h | |
| 10 | 2.59 ± 0.14 | 2.35 ± 0.43 | 3.21 ± 0.48 | 6.20 ± 1.03 | 7.18 ± 0.08 | 7.29 ± 0.03 | 7.18 ± 0.27 |
| 20 | 2.13 ± 0.84 | 1.62 ± 0.40 | 2.14 ± 0.99 | 3.86 ± 0.33 | 3.30 ± 0.95 | 5.70 ± 0.45 | 4.97 ± 1.50 |
Activation of 1.5 × 10−1 M NaCl was achieved following exposure for 5 min to gliding arc plasma discharge.
Log10 N0 was 7.58 ± 0.03 CFU ml−1. Log10 N obtained after contact with nonstored activated water is shown in Table 2. The values are the means ± standard deviations for at least three experiments conducted with independently grown cultures.
DISCUSSION
The main goal of this work was to demonstrate the effectiveness of glidarc technology in microbial decontamination, especially by means of temporal postdischarge (i.e., after the electric discharge is switched off). It was shown previously that the glidarc technique, like other plasma techniques (14, 20), was able to destroy planktonic, adherent, and biofilm bacteria under direct discharge (12, 22). However, this study was the first investigation of bacterial destruction by temporal postdischarge, whatever the plasma technology considered. This temporal postdischarge has to be distinguished from the postdischarges or afterglows usually used for plasma treatment that are actually spatial postdischarges, inducing the destruction of microorganisms disposed of in the afterglow plume of an operating discharge (23, 32). The occurrence of such a phenomenon is of considerable economic interest in terms of its industrial and environmental applications, as the inactivation initiated by plasma discharge requires no extra energy supply to continue and has displayed efficiency which is comparable to that achieved during plasma discharges (Fig. 1 and 2). Current development of plasma devices is focused on decontamination of medical supplies and military equipment (30). Decontamination of food product surfaces (meats, vegetables) is also under study (10, 33). In all cases, careful attention has to be paid to product deterioration. As temporal postdischarge was shown to be efficient even when microorganisms were not exposed to the plasma plume (Fig. 3 and Tables 2 and 3), the products to be decontaminated do not need to be subjected to the discharge, which should limit their alteration by the treatment. This is interesting as the lethal effect of temporal postdischarge is sustained (at a lower degree of efficacy) for several hours after the plasma treatment ends. Plasma-activated water could thus be introduced as a sanitizer and potentially used for industrial cleaning in place. Nevertheless, as discussed below, this requires further characterization of active species.
The kinetics of destruction were studied under various conditions, including direct discharge followed or not followed by temporal postdischarge. In both cases, the kinetics of destruction fitted well with the “shoulder + log-linear” GInaFiT model (Fig. 1 and 2). In previous studies, the kinetics of plasma treatment were often polyphasic (14, 20). Biphasic curves with a lag phase were previously observed during the destruction of planktonic cells by direct discharges using the same type of plasma equipment (22), as well as with other types of discharges (17), and the reported values for shoulder lengths and inactivation rates were comparable to those obtained during the present study. For example, Maeda et al. (17) obtained a lag phase of 14 min and an inactivation rate of 1 min−1. Our study showed that these two parameters were contrastingly linearly modified with discharge time t*. This situation can be compared with the results of thermal destruction, where biphasic curves have also been observed, and both shoulder lengths and inactivation rates have been clearly related to the destruction temperature by some authors (4, 8). Thus, the quantity of energy injected (as heat or electricity) is a key parameter in bacterial inactivation, as it is for chemical compound abatement using glidarc techniques (13). The energy provided to the discharge was used to create active (and/or lethal) species. In this context, we can assume that the lag phase was a manifestation of accumulated damage which became irreparable (and thus lethal) above a critical level. However, from results shown in Fig. 4, it can be deduced that this level depended not only on the amount of active species but also on the contact time between cells. This corresponds to the CT parameter (concentration × contact time) frequently employed in the case of disinfection with classical chemical compounds (25).
The course of the kinetics of destruction of microorganisms by any lethal agent can be related to the rate of the reaction between the agent and the microorganism under study and also to the variable resistance of individual cells in a given population (25, 27). For example, the fact that a small fraction of any population can survive treatments that kill the majority of that population has frequently been reported (which leads to a survival curve with a tail, as shown in Fig. 4), and intrapopulation diversity has been postulated to be a mechanism to ensure survival following exposure to stress (3). Moreover, in the specific case of plasma treatment, polyphasic curves could result from the successive actions of several lethal agents that depend on the plasma device (15, 19). In the present study, the pH of H. alvei suspensions fell from 6.1 to 3.6 with only 1 min of treatment, and HNO3 was detected in the suspended fluids. But acidification alone cannot explain the cell mortality observed during discharge and postdischarge treatments, as immersion for 30 min of an H. alvei suspension in acidified NaCl (HNO3, pH 3.6) induced a reduction in the number of cultivable cells which was less than 2 decimal logarithm units (data not shown). Glidarc destruction is probably also based on the chemical processes of oxidation. As parallel experiments conducted in NaCl solutions and deionized water produced the same level of destruction (data not shown), plasma treatment could not be suspected to induce the formation of chlorine active species, which are potent oxidizers used as sanitizers (18). On the other hand, various free radicals (e.g., °OH and NO°) are formed in the plasma plume (2). These radicals are highly active in the inactivation of microorganisms (20, 29) and possibly involved in direct discharge treatments. Through recombination and collisions, these labile free radicals may give rise to new longer-life-span species that would be responsible for the lethal effects of temporal postdischarge. In agreement, H2O2 (a molecule derived from °OH) was proposed to be the principal agent for oxidation in the temporal postdischarge of chemical compounds (7), and NO° was oxidized to HNO2 and subsequently to HNO3, both of which were detected in plasma-treated fluids (data not shown). The evolution over time of discharge-generated active species led to evolution of activated water efficiency (Table 2). Together with lesions induced directly by plasma discharges on bacterial cells (due to, for example, an electric field, as in the case of destruction by a pulsed electric field [28]), the evolution of active species was also probably responsible for the difference in efficiency observed when the cells were in the fluid or not in the fluid during the discharge (Fig. 4).
To summarize, for discharge alone or under in postdischarge conditions, the efficiency probably results from a combination of different effects, principally of a chemical nature. We plan to pursue our studies to determine the implications for different chemical active species, particularly in the setting of the lethal postdischarge effect of glidarc. Even though preliminary studies have shown that the glidarc treatment of water does not generate genotoxic compounds (21), the production of potential toxic by-products also needs to be carefully examined before any practical applications can be proposed, especially in the food and water industries.
Acknowledgments
We thank Laurent Guillier for introducing us to the GInaFiT add-in for Microsoft Excel and for stimulating discussions on the analysis of destruction kinetics.
Georges Kamgang-Youbi's thesis received financial support from the “Service de la Coopération et d'Action Culturelle (SCAC)” at the French Embassy in Cameroon via an EGIDE scholarship.
Footnotes
Published ahead of print on 8 June 2007.
REFERENCES
- 1.Anonymous. 1997. Chemical disinfectants and antiseptics. Basic bactericidal activity. Test method and requirements (phase 1). European Standard EN1040. AFNOR, Paris, France.
- 2.Benstaali, B., P. Boubert, B. G. Chéron, A. Addou, and J. L. Brisset. 2002. Density and rotational temperatures measurements of the NO° and °OH radicals produced by a gliding arc in humid air and their interaction with aqueous solutions. Plasma Chem. Plasma Proc. 22:553-571. [Google Scholar]
- 3.Booth, I. R. 2002. Stress and the single cell: intrapopulation diversity is a mechanism to ensure survival upon exposure to stress. Int. J. Food Microbiol. 78:19-30. [DOI] [PubMed] [Google Scholar]
- 4.Bréand, S., G. Fardel, J. P. Flandrois, L. Rosso, and R. Tomassone. 1998. Model of the influence of time and mild temperature on Listeria monocytogenes nonlinear survival curves. Int. J. Food Microbiol. 40:185-195. [DOI] [PubMed] [Google Scholar]
- 5.Czernichowski, A. 1994. Gliding arc. Applications to engineering and environment control. Pure Appl. Chem. 66:1301-1310. [Google Scholar]
- 6.Doubla, A., and J. L. Brisset. 2006. Post-discharge kinetics associated with a plasmachemical nucleophilic substitution and application to the analysis of plasma activated CO. J. Appl. Electrochem. 36:77-85. [Google Scholar]
- 7.Doubla, A., F. Abdelmalek, K. Khélifa, A. Addou, and J. L. Brisset. 2003. Post-discharge plasma-chemical oxidation of Iron(II) complexes. J. Appl. Electrochem. 33:73-77. [Google Scholar]
- 8.Garzaroli, C., B. Zanini, and C. Peri. 1996. Comparison of thermal death models for Enterococcus faecium in Bologna sausage. Ann. Microbiol. Enzymol. 46:97-108. [Google Scholar]
- 9.Geeraerd, A. H., V. P. Valdramidis, and J. F. Van Impe. 2005. GInaFiT, a freeware tool to assess non-log-linear microbial survivor curves. Int. J. Food Microbiol. 102:95-105. [DOI] [PubMed] [Google Scholar]
- 10.Guan, D., and D. G. Hoover. 2005. Emerging decontamination techniques for meat, p. 388-417. In J. Sofos (ed.), Improving the safety of fresh meat. CRC Press, Boca Raton, FL.
- 11.Hnatiuc, E. 2002. Procédés basés sur les décharges électriques, p. 219-291. In E. Hnatiuc (ed.), Procédés électriques de mesure et de traitement des polluants. Tech & Doc, Paris, France.
- 12.Kamgang, J. O., R. Briandet, J. M. Herry, J. L. Brisset, and M. Naïtali. Destruction of planktonic, adherent and biofilm cells of Staphylococcus epidermidis using a gliding discharge in humid air. J. Appl. Microbiol., in press. [DOI] [PubMed]
- 13.Laguardia, L., E. Vassallo, F. Cappitelli, E. Mesto, A. Cremona, C. Sorlini, and G. Bonizzoni. 2005. Investigation of the effects of plasma treatments on biodeteriorated ancient paper. Appl. Surf. Sci. 252:1159-1166. [Google Scholar]
- 14.Laroussi, M. 2005. Low temperature plasma-based sterilization: overview and state-of-the-art. Plasma Process. Polym. 2:391-400. [Google Scholar]
- 15.Laroussi, M., and F. Leipold. 2004. Evaluation of the roles of reactive species, heat and UV radiation in the inactivation of bacterial cells by air plasma at atmospheric pressure. Int. J. Mass Spectrom. 233:81-86. [Google Scholar]
- 16.Lesueur, H., A. Czernichowski, and J. Chapelle. November 1988. Dispositif de génération de plasma basse température par formation de décharges électriques glissantes. French patent 2,639,172.
- 17.Maeda, Y., N. Igura, M. Shimoda, and I. Hayakawa. 2003. Bactericidal effect of atmospheric gas plasma on Escherichia coli K12. Int. J. Food Sci. Technol. 38:889-892. [Google Scholar]
- 18.Maris, P. 1995. Modes of action of disinfectants. Rev. Sci. Tech. Off. Int. Epizoot. 14:47-55. [DOI] [PubMed] [Google Scholar]
- 19.Moisan, M., J. Barbeau, M. C. Crevier, J. Pelletier, N. Philip, and B. Saoudi. 2002. Plasma sterilization. Methods and mechanisms. Pure Appl. Chem. 74:349-358. [Google Scholar]
- 20.Moisan, M., J. Barbeau, S. Moreau, J. Pelletier, M. Tabrizian, and L'H. Yahia. 2001. Low-temperature sterilization using gas plasmas: a review of the experiments and an analysis of the inactivation mechanisms. Int. J. Pharm. 226:1-21. [DOI] [PubMed] [Google Scholar]
- 21.Moreau, M. 2006. Décontamination bactérienne par décharge électrique de type arc rampant dans l'air humide, à température ambiante et à pression atmosphérique: application à la destruction de bactéries du genre Erwinia. Ph.D. thesis. University of Rouen, Rouen, France.
- 22.Moreau, M., M. G. J. Feuilloley, N. Orange, and J. L. Brisset. 2005. Lethal effect of the gliding arc discharges on Erwinia spp. J. Appl. Microbiol. 98:1039-1046. [DOI] [PubMed] [Google Scholar]
- 23.Moreau, S., M. Moisan, M. Tabrizian, J. Barbeau, J. Pelletier, A. Ricard, and L'H. Yahia. 2000. Using the flowing afterglow of plasma to inactivate Bacillus subtilis spores: influence of the operating conditions. J. Appl. Phys. 88:1166-1174. [Google Scholar]
- 24.Moussa, D., and J. L. Brisset. 2003. Disposal of spent tributylphosphate by glidarc arc plasma. J. Hazard. Mater. B 102:189-200. [DOI] [PubMed] [Google Scholar]
- 25.Najm, I. 2006. An alternative interpretation of disinfection kinetics. J. Am. Water Works Assoc. 98:93-101. [Google Scholar]
- 26.Ohkawa, H., T. Akitsu, M. Tsuji, H. Kimura, M. Kogoma, and K. Fukushima. 2006. Pulse-modulated, high-frequency plasma sterilization at atmospheric pressure. Surf. Coat. Technol. 200:5829-5835. [Google Scholar]
- 27.Peleg, M. 2000. Microbial survival curves—the reality of flat “shoulders” and absolute thermal death times. Food Res. Int. 33:531-538. [Google Scholar]
- 28.Picart, L., and J. C. Cheftel. 2003. Pulsed electric field, p. 360-427. In P. Zeuthen and L. Bøgh-Sørensen (ed.), Food preservation techniques. CRC Press, Boca Raton, FL.
- 29.Purevdorj, D., N. Igura, O. Ariyada, and I. Hayakawa. 2003. Effect of feed gas composition of gas discharge plasmas on Bacillus pumilus spore mortality. Lett. Appl. Microbiol. 37:31-34. [DOI] [PubMed] [Google Scholar]
- 30.Roth, J. R. 2005. Potential industrial applications of the One Atmosphere Uniform Glow discharge plasma (OAUGDP) operating in ambient air. Phys. Plasma 12:57103. [Google Scholar]
- 31.Stock, I., M. Rahman, K. J. Sherwood, and B. Wiedemann. 2005. Natural antimicrobial susceptibility patterns and biochemical identification of Escherichia albertii and Hafnia alvei strains. Diagn. Microbiol. Infect. Dis. 51:151-163. [DOI] [PubMed] [Google Scholar]
- 32.Villeger, S., S. Cousty, A. Ricard, and M. Sixou. 2003. Sterilization of E. coli bacterium in a flowing N2-O2 post-discharge reactor. J. Phys. D. 36:L60-L62. [Google Scholar]
- 33.Vleugels, M., G. Shama, X. T. Deng, E. Greenacre, T. Brocklehurst, and M. G. Kong. 2005. Atmospheric plasma inactivation of biofilm-forming bacteria for food safety control. IEE Trans. Plasma Sci. 33:824-828. [Google Scholar]