Abstract
Immobilization of uranium in groundwater can be achieved through microbial reduction of U(VI) to U(IV) upon electron donor addition. Microbial community structure was analyzed in ethanol-biostimulated and control sediments from a high-nitrate (>130 mM), low-pH, uranium-contaminated site in Oak Ridge, TN. Analysis of small subunit (SSU) rRNA gene clone libraries and polar lipid fatty acids from sediments revealed that biostimulation resulted in a general decrease in bacterial diversity. Specifically, biostimulation resulted in an increase in the proportion of Betaproteobacteria (10% of total clones in the control sediment versus 50 and 79% in biostimulated sediments) and a decrease in the proportion of Gammaproteobacteria and Acidobacteria. Clone libraries derived from dissimilatory nitrite reductase genes (nirK and nirS) were also dominated by clones related to Betaproteobacteria (98% and 85% of total nirK and nirS clones, respectively). Within the nirK libraries, one clone sequence made up 59 and 76% of sequences from biostimulated sediments but only made up 10% of the control nirK library. Phylogenetic analysis of SSU rRNA and nirK gene sequences from denitrifying pure cultures isolated from the site indicate that all belong to a Castellaniella species; nearly identical sequences also constituted the majority of biostimulated SSU rRNA and nirK clone libraries. Thus, by combining culture-independent with culture-dependent techniques, we were able to link SSU rRNA clone library information with nirK sequence data and conclude that a potentially novel Castellaniella species is important for in situ nitrate removal at this site.
Due to the Cold War legacy, uranium has become an important groundwater contaminant in the United States, thus mandating remediation by the U.S. Department of Energy (DOE). Soluble U(VI) can be biologically reduced to U(IV), which is insoluble, thus immobilizing the radionuclide and posing less of a threat to drinking water wells located near sources of contamination (24, 44). It has been suggested that bacteria capable of U(VI) reduction are ubiquitous in the environment (1), and recent field experiments have shown that the addition of electron donors (glucose, ethanol, or acetate) into injection wells will result in the stimulation of endogenous microorganisms in the subsurface to grow and reduce U(VI) (3, 12, 36, 54, 60, 64).
Microbial communities stimulated to reduce U(VI) via electron donor addition have been studied using both in situ and microcosm experiments. Members of the Geobacteraceae family have been stimulated during uranium reduction in contaminated sediments from Shiprock, NM (33), Rifle, CO (3, 12), and Oak Ridge, TN (51, 54). From studies done with sediment from Oak Ridge, Anaeromyxobacter was also stimulated under metal-reducing conditions (51, 55). In other studies, sulfate-reducing bacteria have been linked to uranium reduction (1, 13, 49, 52, 61). Of these, two studies have also found Clostridium to be associated with U(VI) reduction (52, 61), and another found that Pseudomonas was also stimulated upon uranium removal in high-salinity sediment (49).
At the DOE Field Research Center (FRC) in Oak Ridge, TN, where groundwater contains >130 mM nitrate and micromolar concentrations of uranium, addition of a biodegradable electron donor results in denitrification as the primary terminal electron-accepting process (36). Because nitrate serves as a more energetically favorable electron acceptor, uranium reduction has been shown to occur only after nitrate has been depleted to low levels (17, 23, 36, 48, 60). Thus, at sites such as the FRC, denitrifying bacteria are likely to play a critical role in uranium bioremediation. A recent phylogenetic survey of sediment from the FRC revealed several potential nitrate-reducing bacteria (2), but it remains unclear what species are involved in nitrate removal upon biostimulation.
The goal of this study was to characterize changes in the in situ microbial community structure of uranium- and nitrate-contaminated subsurface sediments upon stimulation with ethanol and to identify denitrifying bacteria that may be important during the in situ removal of nitrate. While other molecular studies have identified mainly sulfate and metal reducers in uranium-contaminated sediments, it was hypothesized in this study that electron donor addition to high-nitrate subsurface sediments cocontaminated with low levels of uranium would result mainly in the stimulation of denitrifying bacteria. Because denitrification is not a phylogenetically conserved function, numerous methods were used to analyze the microbial community structure of biostimulated and control sediments, including functional gene (nirK and nirS) clone libraries, small subunit (SSU) rRNA gene clone libraries, polar lipid fatty acid (PLFA) analysis, and cultivation of nitrate-reducing bacteria from FRC sediments. Results of this study show that biostimulation of high-nitrate subsurface sediments with ethanol results in a decrease in bacterial diversity and enriches for members of the class Betaproteobacteria, namely, members of the newly described genus Castellaniella (formerly Alcaligenes defragrans), which are capable of nitrate reduction.
MATERIALS AND METHODS
Field site description.
The field site in this study is the DOE's Environmental Remediation Sciences Program FRC, which is located near the western edge of the Y-12 national security complex at the Oak Ridge Reservation (Oak Ridge, TN). The source of the contamination plume in the shallow unconfined aquifer at the FRC comes from the former S-3 waste disposal ponds. These ponds received acidic (pH, <2) liquid waste containing nitric acid, uranium, technetium, other dissolved metals, and organic contaminants from 1951 to 1983; the ponds were neutralized in 1984 and capped in 1988. Several monitoring wells have been installed within the Area 1 field plot (just south of the former S-3 ponds), and groundwater within Area 1 has been described as acidic (pH ranging from 3.0 to 6.8), with high concentrations of nitrate (up to 168 mM), U(VI) (up to 5.8 μM), Tc(VII) (up to 12,000 pCi/liter), and <1 mM sulfate (36). Table 1 shows nitrate, uranium, and pH data from four monitoring wells before push-pull experiments began. Other contaminants in Area 1 include aluminum, nickel, tetrachloroethylene, and other chlorinated hydrocarbons. A more detailed description of the site as well as groundwater and sediment geochemical data can be found at the URL http://www.esd.ornl.gov/nabirfrc/index.html.
TABLE 1.
Summary of initial groundwater chemistry, push-pull test results, and sediment core characteristics
| Groundwater well (corresponding sediment core) | Initial groundwater chemistryc
|
Push-pull test resultb (C/C0)d
|
Sediment core data (following biostimulation)e
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NO3− (mM) | U(VI) (μM) | pH | NO3− | EtOH | U(VI) | NO3− (mM) | NO2− (mM) | Biomass (106 cells/g) | % U as U(IV) | |
| FW028 (FB064) | 167.2 | 2.22 | 4.4 | 0.327 | 0.358 | 15.2 | 126.5 | 17.7 | 204 | 67.1 |
| FW034 (FB067) | 0.769 | 0.475 | 6.79 | 0.640 | 0.528 | 0.924 | 39.77 | 14.8 | 18.5 | 75.7 |
| FW016 (FB066)a | 11.4 | 2.58 | 3.92 | 2.612 | 9.98 | 5.58 | 4.6 | |||
| FW021b | 142.3 | 5.80 | 3.05 | |||||||
FW016 served as a control well.
FW021 groundwater was used for the injection solution for push-pull tests in FW028 and FW034.
Data publicly available at http://public.ornl.gov/nabirfrc/frcsite3.cfm.
These data represent analyte concentrations (C) on the first date of the extraction phase (on or near the date of sediment core sampling) compared to initial concentrations in the injection solution (C0) and have been adjusted to account for loss due to dilution and dispersion, as determined by loss of bromide.
Sediment core FB064 was obtained 5 days after the injection phase began and 3 days prior to the start of the extraction phase for well FW028; FB067 was sampled 31 days after the injection phase began and on the same day of the start of the extraction phase.
In situ biostimulation of subsurface sediments.
Single-well, push-pull tests were done in wells FW028 and FW034 in Area 1 as previously described (36, 65, 66). Test solutions for push-pull tests were prepared using high-nitrate (>130 mM) Area 1 groundwater (from well FW021) amended with 300 mM ethanol, 50 to 100 mM sodium bicarbonate, and 1.25 mM Br− as a conservative tracer. Sediment cores were sampled adjacent to wells FW028 and FW034 (cores FB064 and FB067, respectively) approximately 1 week after injection of test solutions; the injection phase lasted only a few hours for FW028 but lasted approximately 3 weeks for FW034, due to differences in well flow characteristics due to past push-pull experiments. Thus, FB064 and FB067 were sampled 5 and 31 days, respectively, after the beginning of the injection phase. One sediment core (FB066) was also taken adjacent to an Area 1 donor control well FW016, which has never been biostimulated in push-pull tests. Sediment sampling and handling procedures followed those previously described (66) in order to keep sediment material anoxic. Core sizes were all approximately 1 meter in length and were sampled from the following depths below the surface: 6.1 to 7.0 m, 3.4 to 4.3 m, and 3.0 to 4.0 m for cores FB064, FB067, and FB066, respectively. Intact subsections of cores, approximately 10 cm in length, were frozen at −80°C and were later shipped on dry ice to the University of Oklahoma for molecular analysis. A subsection of another core from borehole FB064, taken from 5.2 to 5.7 m below the surface, was stored at 4°C and shipped to the University of Oklahoma on ice for enrichment and isolation of denitrifying bacteria.
Enrichment and isolation of denitrifying pure cultures.
Medium for enrichment of dissimilatory nitrate-reducing microorganisms was prepared anaerobically (5) with the following components (per liter): 10 ml vitamin solution (47), 5 ml metals solution (47), 0.1 g NaCl, 0.1 g NH4Cl, 10 mg KCl, 3 mg KH2PO4, 40 mg MgCl2·6H2O, 40 mg CaCl2·2H2O, 11.9 g HEPES, 11.7 g morpholineethanesulfonic acid (MES), and 8.5 g NaNO3. The pH of the medium was adjusted to either 4.5 or 7.5 using HCl or NaOH and dispensed into serum tubes under an N2 headspace. Ethanol was added from a sterile, anoxic stock solution to reach a final concentration of 100 mM.
Anaerobic nitrate-reducing enrichment cultures were set up in an anaerobic glovebag by adding 1 g of homogenized biostimulated sediment from borehole FB064 to 10 ml nitrate-reducing liquid medium at both pH 4.5 and 7.5. Headspace of enrichment cultures was exchanged three times with N2 and incubated in the dark at room temperature. Upon observable growth and removal of nitrate, enrichments were serially diluted and plated onto solid nitrate-reducing media both with and without ethanol at either pH 4.5 or 7.5, depending on the pH of the enrichment culture. Nitrate-reducing solid medium had the same composition as the liquid media except it contained 1.5% agar and 1.7 g/liter NaNO3. After autoclaving, the medium was dispensed into plates and dried overnight. Plates were placed in an anaerobic glovebag (Coy Instruments) overnight. Subsequently, a piece of sterile filter paper was placed in the lid of each petri dish and saturated with 500 μl of a sterile, anoxic 1 M ethanol solution. All plates were incubated at room temperature in an anaerobic glovebag. Colonies from plates containing ethanol that differed in morphology from colonies on ethanol-free plates were further reisolated and transferred to nitrate-reducing liquid medium at pH 4.5 or 7.5. In total, 24 colonies were obtained from pH 7.5 enrichment cultures and 22 from pH 4.5 enrichment cultures.
DNA extraction.
DNA was extracted from frozen soil cores from boreholes FB064, FB067, and FB066 (from depths of 6.4, 4.6, and 3.6 m below the surface, respectively) using the FastDNA SPIN kit for soil (QBiogene, Irvine, CA), which involves a silica and ceramic bead-beating method to achieve cell lysis. Manufacturer's instructions were followed, except nuclease-free water was used as the eluent. In order to increase DNA yield and to account for heterogeneity of the cores, 10 DNA extractions using 0.3 g sediment were done from each core. The 10 DNA samples were then pooled and concentrated by using a Centrivap at 45°C. DNA samples were stored at −20°C.
DNA was extracted from pure cultures by boiling late-log-phase washed cells for 5 minutes; samples were centrifuged to remove cell debris, and supernatants were transferred to clean, sterile 1.5-ml microcentrifuge tubes and stored at −20°C for use as DNA template for PCRs.
PCR, cloning, and sequencing.
Partial SSU rRNA genes from sediment community DNA and denitrifying isolates were amplified using 2 μl of DNA template in a 50-μl PCR mixture (<100 ng/μl, final concentration) containing the following components: 1× PCR buffer (Invitrogen Corp., Carlsbad, CA), 2.5 mM MgCl2, 100 μM each deoxynucleoside triphosphate, 10 pmol/ml each primer (uni8f and eubac805r) (19), and 1.5 U of Platinum Taq DNA polymerase (Invitrogen). Amplification of partial SSU rRNA genes was carried out in a GeneAmp PCR system 9700 (Applied Biosystems, Foster City, CA) using the following parameters: initial denaturation at 94°C for 5 min; 35 cycles of 95°C for 30 s, 50°C for 60 s, and 72°C for 90 s; and a final extension step at 72°C for 20 min. Near-complete SSU rRNA genes of two denitrifying isolates (4.5A2 and 7.5A2) were amplified in the same manner, only using universal primers 27F and 1492R and an annealing temperature of 45°C.
Amplification of nirK and nirS genes from sediment community DNA and denitrifying isolates used the same PCR mixture as described above, except that primer concentrations were 12.5 pmol/ml, nirK primers were nirK1F and nirK5R, and nirS primers were nirS1F and nirS6R (9). PCR parameters were as follows: 94°C for 5 min; 35 cycles of 94°C for 30 s, 54°C for 45 s, and 72°C for 45 s; and a final extension at 72°C for 20 min.
PCR products were cloned using the TOPO TA cloning kit (Invitrogen Corp., Carlsbad, CA) either directly from the PCR product or after a gel purification step using a commercially available kit (QBioGene). Sequencing of inserts was performed by the Advanced Center for Genome Technology at the University of Oklahoma (Norman) or the Oklahoma Medical Research Foundation (Oklahoma City, OK).
Phylogenetic analysis.
SSU rRNA gene sequences were aligned using ClustalX (62). Sequences with similarities of ≥97% were placed into the same operational taxonomic unit (OTU); also, sequences with ≥93% similarity were placed into the same genus-level taxonomic group (GLTG). Possible chimera within our libraries were identified using Bellerophon (34) and by manual inspection. Chimeric sequences made up approximately 10% of total sequences and were removed from further phylogenetic analyses. Initial phylogenetic placement of each SSU rRNA gene OTU was determined using the Ribosomal Database Project's Classifier program (14). Closely related sequences and sequences identified from this site in previous studies were downloaded from GenBank and aligned with our sequences using ClustalX; the multiple alignment was imported into PAUP 4.01b10 for final phylogenetic analysis. Evolutionary distance-based trees were generated using the neighbor-joining algorithm and Jukes-Cantor corrections. Bootstrap values were determined using 1,000 replicates.
The Shannon-Weiner diversity index, Simpson's dominance index, and species evenness were calculated as previously described (57). A limitation of these indices is that each OTU is considered equivalent, regardless of the degree of sequence divergence (46). To ameliorate this bias, diversity indices were calculated at both the OTU level as well as the GLTG level; also, average nucleotide divergence was calculated for each clone library (46). Calculations of percent coverage were done as described elsewhere (58) at both the OTU and GLTG levels.
A chi-square test for an r × k contingency table was done to determine whether the population distribution in biostimulated samples differed from the unstimulated sample. Rows (r) were phylum affiliation, and columns (k) were different samples (biostimulated and unstimulated). Expected frequencies for each phylum in each sample (E) were calculated by the equation E = (row total) × [(column total)/(grand total)]. A chi-squared value was determined by the equation χ2 = Σ(O − E)2/E (O = observed frequency). The critical χ2 value was chosen with nine degrees of freedom and with a P value of 0.05.
Phylogenetic analysis of nirK and nirS genes was done similarly to that of the SSU rRNA genes described above. Sequences were grouped into OTUs based on ≥98% nucleotide sequence similarity, and the closest relatives were identified and downloaded using BLAST. Other reference nirK and nirS sequences were downloaded from the Functional Gene Pipeline/Repository (http://flyingcloud.cme.msu.edu/fungene/). Neighbor-joining trees were constructed from translated amino acid sequences. Similarity values reported in the results are based on amino acid similarity.
PLFA extraction and analysis.
Lyophilized sediment from each core was extracted with the single-phase chloroform-methanol-buffer system (8), as later modified (67). The total lipid extract was fractionated into neutral lipids, glycolipids, and polar lipids by silicic acid column chromatography (29). PLFA analysis was conducted as previously described (56). Biomass (cells/g of sediment) was calculated from total PLFA/g of sediment using the conversion 2.5 × 104 cells per pmol PLFA (6). Shannon-Weiner diversity indices for sediment samples were also calculated based on PLFA (31).
Analytical methods.
Uranium speciation from sediment cores FB064, FB067, and FB066 was determined by sequential extractions of total U(VI) (soluble and solids associated) and U(IV) from triplicate 0.5-g sediment subsamples using sodium bicarbonate and nitric acid, respectively (18). Uranium from each extraction was measured by kinetic phosphorescence analysis (KPA-11; Chemcheck Instruments, Richland, WA). Nitrate and nitrite from nitrate-reducing enrichments and sediment-associated pore water were measured by ion chromatography (model DX500 fitted with an AS-4A column; Dionex Corp., Sunnyvale, CA). Push-pull groundwater analysis was done at Oregon State University as previously described (36).
Nucleotide sequence accession numbers.
SSU rRNA, nirK, and nirS sequences from this study were deposited with GenBank and can be retrieved with accession numbers EF175318 to EF175380 and EF177768 to EF177803.
RESULTS
Isolation and phylogenetic analysis of denitrifying pure cultures.
From nitrate-reducing enrichments using biostimulated sediment as the inoculum, all 46 pure cultures, once restreaked for purity, shared the same colony morphology on nitrate-reducing medium: colonies were convex, round, small (<1 mm in diameter), and white, with smooth margins. Upon inoculation into liquid medium at pH 4.5 and pH 7.5, all pure cultures were capable of growth (to a final optical density of approximately 0.4 at 600 nm) using nitrate and ethanol as the sole electron acceptor and donor, respectively; gas production indicated that the organisms coupled growth to denitrification rather than reduction of nitrate to nitrite or ammonium. Because of the similar colony morphologies and growth characteristics, 10 of the pure cultures were chosen at random for phylogenetic analysis; SSU rRNA gene sequences of these isolates were 97.6 to 100% similar to each other with an average similarity of 99.4%, suggesting these isolates belong to the same species within the family Alcaligenaceae and the class Betaproteobacteria.
Two strains, 4.5A2 and 7.5A2 (isolated at pH 4.5 and 7.5, respectively), which had 99.9% SSU rRNA gene sequence similarity, were chosen for further phylogenetic analysis. Isolates 4.5A2 and 7.5A2 were 99.4 and 99.7% similar to clone FB46-16, which was identified from biostimulated FRC sediments in a previous study (51). The closest cultured relative was Alcaligenes sp. strain AMS10, which was isolated from a polycyclic aromatic hydrocarbon-degrading consortium (GenBank accession no. AY635901). The closest validly described relatives belong to the genus Castellaniella, which consists of two described species, C. defragrans and C. denitrificans, both of which were previously identified as Alcaligenes defragrans (40). Isolates 4.5A2 and 7.5A2 were 98.3 and 98.5% similar to C. defragrans 54Pin, which was isolated from activated sludge on nitrate and α-pinene (25), and 98.4% similar to C. denitrificans TJ4, a phenol-degrading, denitrifying bacterium (4). Neighbor-joining analysis and bootstrap values supported that FRC isolates 4.5A2 and 7.5A2 may not belong to either of the previously described species of Castellaniella and could represent a novel species within the genus Castellaniella (Fig. 1). However, further physiological tests are needed to prove this.
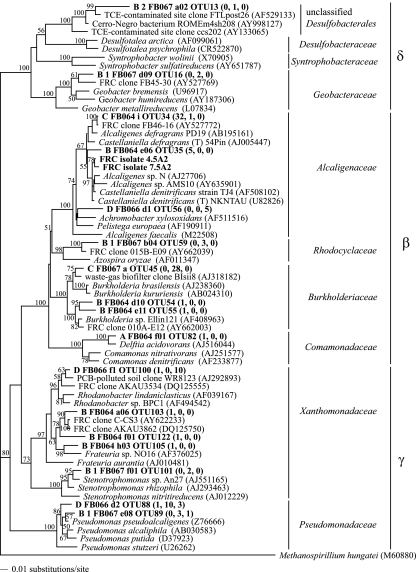
FIG. 1.
Distance phylogram based on near-full-length SSU rRNA gene sequences (approximately 1,490 bp) from FRC isolates (in bold), FRC sediment clone sequences (clone C FB064 I OTU34 was identified from the FRC biostimulated sediment in this study), and other members of Castellaniella as well as related organisms in the order Burkholderiales (accession numbers are shown in parentheses). Bootstrap values are based on 1,000 replicates and are shown for branches with bootstrap support of >50%.
While the nirS gene was not detected by PCR in any of the 10 isolates, all contained a nirK gene, which provides evidence that these strains are denitrifying bacteria. All nirK partial gene sequences from these isolates were 99.0 to 100% similar to each other, reaffirming that these isolates are likely different strains among the same species. Furthermore, translated amino acid sequences of NirK from isolates 4.5A2 and 7.5A2 were 100% identical to each other, 84.8% identical to NirK of a clone identified from acetate-fed activated sludge (clone NR2-819K1; GenBank accession no. BAD36891), and 81.8% identical to NirK from Alcaligenes sp. strain N, isolated from a denitrifying reactor (20).
In situ biostimulation of contaminated subsurface sediments and reduction of U(VI).
Push-pull tests were done with ethanol-amended, high-nitrate (142.3 mM) FW021 groundwater (neutralized with bicarbonate) in two wells, FW028 and FW034. Prior to biostimulation, the groundwater from FW028 contained high levels of nitrate (167.2 mM) and uranium (2.2 μM) and was more acidic than FW034, which contained <1 mM nitrate and 0.475 μM uranium (Table 1). The control well, FW016, was also acidic but contained 11.4 mM nitrate (Table 1). Following injection of ethanol-amended FW021 groundwater into FW028 and FW034, push-pull data showed nitrate and ethanol loss in both test wells by the time of sediment sampling and U(VI) accumulation in FW028, suggesting U(IV) oxidation may have occurred in this well (Table 1). However, analysis of uranium from bicarbonate- and nitric acid-extractable fractions from sediment cores showed that the majority of the uranium in both cores adjacent to ethanol-stimulated wells (FB064 and FB067, corresponding to wells FW028 and FW034, respectively) was reduced, whereas only 4.6% of the total uranium from the control core FB066 (adjacent to FW016) was reduced (Table 1), suggesting that the U in stimulated cores remained fairly reduced, compared to the control, which has never been biostimulated. Some of the U(IV) in biostimulated cores may have been due to previous push-pull tests performed in adjacent wells (36). Biomass estimates based on total PLFA from sediment cores following in situ biostimulation showed that FB064 and FB067 had approximately 37- and 3-fold higher biomass than the control core, FB066 (Table 1). Pore water nitrate concentrations from the three cores varied, which can be explained by the differences in initial nitrate concentrations of the three sites. Nitrite was present at high concentrations (≥10 mM) in all three (Table 1), indicating that nitrate reduction was not complete in these sediment cores.
Differences in bacterial community structure between ethanol-stimulated and unstimulated sediment samples. (i) Diversity statistics.
According to all diversity indices calculated from SSU rRNA gene clone library data (at both the OTU and GLTG levels), both biostimulated sediments, FB064 and FB067, were less diverse than the control sediment, FB066 (Table 2). The percent coverage was 64, 78, and 71% (at the OTU level) and 83, 83, and 80% (at the GLTG level) for sediment samples FB064, FB067, and FB066, respectively. There was a significant negative linear correlation between log biomass of the sediments and average nucleotide divergence (r = −0.999, P = 0.01), indicating that genetic diversity decreased with increasing biomass. Similarly, when diversity indices were calculated based on GLTGs, there were negative correlations between log biomass versus Shannon-Weiner diversity index (r = −0.992, P < 0.05) and log biomass versus evenness (r = −0.999, P = 0.01). In addition, there was a positive correlation between log biomass and Simpson's dominance index at both the OTU level (r = 0.977, P < 0.1) and the GLTG level (r = 0.993, P < 0.04), indicating that increasing biomass resulted in the selection of one dominant species or genus. Correlations between log biomass and diversity indices were more significant when using GLTGs rather than OTUs; this was due to the high number of OTUs in sample FB064 that belonged to the same GLTG. Taking all diversity indices into account, biostimulation may have led to an overall decrease in bacterial diversity and an increase in dominance of one species or genus. Past push-pull biostimulation experiments performed in injection wells FW028 and FW034 (36) may have also contributed to this effect.
TABLE 2.
Descriptive diversity statistics based on SSU rRNA gene clone library data from two ethanol-stimulated sediments and one control sediment
| Sample (condition) | No. of clones | No. of OTUsa | No. of GLTGsb | Avg. nucleotide diversity | Diversity index based on OTUs
|
Diversity index based on GLTGs
|
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Shannon-Weiner | Simpson's | Evenness | Shannon-Weiner | Simpson's | Evenness | |||||
| FB064 (stimulated) | 58 | 23 | 13 | 0.0994 | 2.010 | 0.3181 | 0.641 | 1.234 | 0.5333 | 0.481 |
| FB067 (stimulated) | 64 | 21 | 18 | 0.1518 | 2.173 | 0.2266 | 0.714 | 2.012 | 0.2427 | 0.696 |
| FB066 (control) | 51 | 21 | 16 | 0.1767 | 2.512 | 0.1272 | 0.825 | 2.222 | 0.1572 | 0.801 |
Similarity cutoff of 97% (OTUs).
Similarity cutoff of 93% (GLTGs).
(ii) Community composition.
The majority of clones from SSU rRNA gene clone libraries from the biostimulated sediment cores, FB064 and FB067, belonged to the beta, delta, and gamma subdivisions of Proteobacteria (88.5%); the remaining clones belonged to Bacteroidetes, Firmicutes, Actinobacteria, Acidobacteria, and candidate divisions TM7, ZB1, termite group I, and WD272_C2 (Table 3 and Fig. 2 and 3). In the SSU rRNA gene clone library from the unstimulated sediment core (FB066), Proteobacteria (beta and gamma subdivisions) made up only 56.9% of the total clones, while other clones were affiliated with Acidobacteria (27.5% of total clones), Bacteroidetes, Firmicutes, and candidate division WD272_C2 (Table 3 and Fig. 2 and 3). By performing chi-square tests based on r × k contingency tables of frequencies of each phylum, it was found that the community structures of the two biostimulated samples (FB064 and FB067) did not differ significantly (P > 0.2), whereas community structures of the biostimulated versus unstimulated samples were significantly different (P < 0.001). Thus, biostimulation of subsurface sediments with ethanol-amended groundwater significantly impacted the subsurface microbial community structure at the phylum/division level. Most noticeably, these differences may have been due to the frequencies of Proteobacteria and Acidobacteria OTUs in the biostimulated versus the control clone libraries (Table 3).
TABLE 3.
Summary of phylogenetic distributions of SSU rRNA clones from samples FB064, FB067, and FB066
| Phylum or candidate division | % of total clones
|
||
|---|---|---|---|
| FB064 (stimulated) | FB067 (stimulated) | FB066 (control) | |
| Proteobacteria | 93.1 | 84.4 | 56.9 |
| Betaproteobacteria | 79.3 | 50.0 | 9.8 |
| Deltaproteobacteria | 0.0 | 6.3 | 0.0 |
| Gammaproteobacteria | 12.1 | 26.6 | 47.1 |
| Unclassified | 1.7 | 1.6 | 0.0 |
| Bacteroidetes | 1.7 | 0.0 | 2.0 |
| Firmicutes | 3.4 | 3.1 | 2.0 |
| Actinobacteria | 0.0 | 1.6 | 2.0 |
| Acidobacteria | 0.0 | 1.6 | 27.5 |
| Candidate division WD272_C2 | 0.0 | 6.3 | 9.8 |
| Candidate division TM7 | 1.7 | 0.0 | 0.0 |
| Candidate division ZB1 | 0.0 | 1.6 | 0.0 |
| Termite group I | 0.0 | 1.6 | 0.0 |
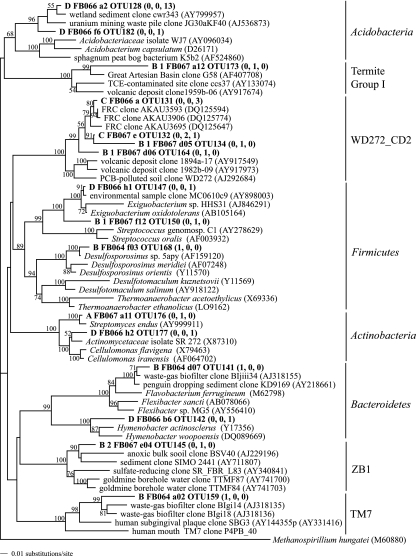
FIG. 2.
Distance phylogram of Proteobacteria partial SSU rRNA gene sequences (approximately 800 bp). Bootstrap values are based on 1,000 replicates and are shown for branches with bootstrap support of >50%. Selected OTUs from this study as well as FRC isolate sequences are in bold, and numbers in parentheses indicate the number of clones belonging to that OTU from sediments FB064, FB067, and FB066, respectively. Accession numbers of sequences from GenBank are in parentheses.
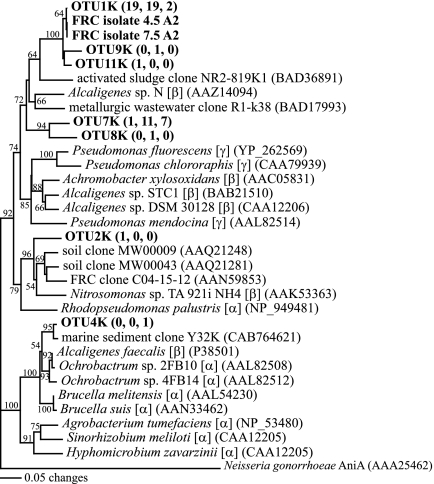
FIG. 3.
Distance phylogram of non-Proteobacteria partial SSU rRNA gene sequences (approximately 800 bp). Bootstrap values are based on 1,000 replicates and are shown for branches with bootstrap support of >50%. Selected OTUs from this study are in bold, and numbers in parentheses indicate the number of clones belonging to that OTU from sediments FB064, FB067, and FB066, respectively. Accession numbers of sequences from GenBank are in parentheses.
Biostimulation resulted in an increase in the proportion of Betaproteobacteria sequences in the SSU rRNA gene clone libraries (9.8% of total clones in FB066 compared to 79.3% and 50.0% in FB064 and FB067, respectively) (Table 3). As biomass of the samples increased (Table 1), so did the percent of clones that belong to Betaproteobacteria (Table 3). Of the Betaproteobacteria clones from the core with the highest biomass, FB064, 69.6% belonged to OTU 34 and 10.7% belonged to OTU 35. Both OTUs 34 and 35 grouped with members of the genus Castellaniella (Fig. 2) and were 100% and 97.6% similar to FRC isolate 7.5A2, respectively. Only one clone from FB067 belonged to OTU 34; rather, 87.5% of Betaproteobacteria clones from FB067 belonged to OTU 45, whose closest relative was clone BIsii8 (97.8% similarity), which was identified from an industrial waste gas biofilter (26). Its two closest cultured relatives were Burkholderia brasilensis, an N2-fixing bacterium (GenBank accession no. AJ238360), and Burkholderia kururiensis, a trichloroethylene-degrading bacterium isolated from a trichloroethylene-contaminated aquifer (72).
Unlike the effect observed on the class Betaproteobacteria, biostimulation resulted in a decrease in the proportion of Gammaproteobacteria sequences in the SSU rRNA gene clone libraries (Table 3). In the control clone library (FB066), 47.1% of total clones were affiliated with Gammaproteobacteria, and of these, the majority (70.8%) belonged to the family Xanthomonadaceae, while others were affiliated with Pseudomonadaceae. The dominant Gammaproteobacteria OTU from the control FB066 (OTU 100) belonged to the genus Rhodanobacter and was closely related to other sequences identified from unstimulated contaminated sites, including groundwater from the FRC (Fig. 2).
Similarly, biostimulated sediments contained a decreased proportion of Acidobacteria clones compared to the control sediment (Table 3). The dominant OTU from the control sediment sample FB066 (OTU 128) belonged to Acidobacteria and clustered with other environmental Acidobacteria clones (Fig. 3); however, only one Acidobacteria-affiliated sequence was detected in the biostimulated libraries (Table 3).
(iii) Novel bacterial diversity identified in SSU rRNA gene clone libraries.
From the three SSU rRNA gene clone libraries generated in this study, 7.5% of all clones belonged to divisions with no cultivated representatives. Three clones belonged to candidate divisions TM7, termite group I, and ZB1 (Table 3; Fig. 3). Nine clones from FB066 and FB067 (belonging to five OTUs) clustered with each other and with other clones, belonging to the candidate division WD272_C2, from the FRC (Fig. 3). The closest non-FRC relatives of these clones came from volcanic ash and polychlorinated biphenyl-polluted soil; bootstrap values from Fig. 3 support that these clones likely belong to the same division as these novel FRC sequences. This candidate division, based on Hugenholtz taxonomy (16), may represent either a novel division or a novel lineage within the Firmicutes (Fig. 3).
(iv) PLFA analysis of sediment samples.
In accordance with clone library data, PLFA data (Table 4) showed that community structure was more diverse and evenly distributed in the unstimulated sample (FB066) compared to the two biostimulated sediment samples (FB064 and FB067). Shannon-Weiner (H) indices calculated from PLFA data further confirm that the unstimulated sediment was less diverse (H = 2.774) than the stimulated sediments, FB064 (H = 1.908) and FB067 (H = 2.461). As with clone library data, there was a significant negative linear correlation between log biomass and Shannon-Weiner diversity index based on PLFA data (r = −0.992, P < 0.05).
TABLE 4.
PLFA analysis of samples FB064, FB067, and FB066a
| PLFA group and name | % of total PLFA
|
||
|---|---|---|---|
| FB064 (stimulated) | FB067 (stimulated) | FB066 (control) | |
| Total normal saturates | 27.30 | 34.39 | 28.92 |
| 14:0 | 1.02 | 1.34 | 0.00 |
| 15:0 | 0.18 | 0.00 | 0.00 |
| 16:0 | 25.70 | 26.37 | 20.33 |
| 17:0 | 0.10 | 0.52 | 0.70 |
| 18:0 | 0.30 | 6.16 | 7.08 |
| 20:0 | 0.00 | 0.00 | 0.33 |
| 22:0 | 0.00 | 0.00 | 0.48 |
| Total mid-chain branched saturates | 0.38 | 0.00 | 12.07 |
| i10me16 | 0.16 | 0.00 | 1.27 |
| 10Me16:0 | 0.18 | 0.00 | 3.84 |
| 12me16:0 | 0.04 | 0.00 | 0.65 |
| i10me17:0 | 0.00 | 0.00 | 4.90 |
| 10Me18:0 | 0.00 | 0.00 | 1.40 |
| Total terminal branched saturates | 3.67 | 11.18 | 22.59 |
| i14:0 | 0.12 | 0.17 | 0.00 |
| i15:0 | 1.09 | 4.00 | 5.69 |
| a15:0 | 1.05 | 1.45 | 4.44 |
| i16:0 | 0.59 | 0.73 | 3.30 |
| i17:0 | 0.66 | 3.35 | 6.51 |
| a17:0 | 0.15 | 1.49 | 2.65 |
| Total branched monounsaturates | 1.54 | 6.05 | 7.66 |
| br16:1a | 0.02 | 0.00 | 0.00 |
| br16:1b | 0.04 | 0.00 | 0.00 |
| i17:1a | 0.41 | 1.48 | 1.97 |
| i17:1b | 0.00 | 0.00 | 0.00 |
| br18:1 | 0.98 | 4.57 | 5.69 |
| br19:1 | 0.09 | 0.00 | 0.00 |
| Total monounsaturates | 66.96 | 47.07 | 28.76 |
| 16:1w9c | 0.07 | 0.33 | 0.00 |
| 16:1w7c | 10.21 | 4.56 | 1.83 |
| 16:1w7t | 0.56 | 1.81 | 0.00 |
| 16:1w5c | 0.22 | 0.76 | 0.00 |
| cy17:0 | 31.79 | 7.40 | 4.96 |
| 17:1 | 0.17 | 0.00 | 0.00 |
| 18:1w9c | 0.11 | 14.34 | 9.66 |
| 18:1w7c | 7.44 | 8.25 | 5.79 |
| 18:1w7t | 0.59 | 3.87 | 2.44 |
| 18:1w5c | 0.22 | 0.00 | 0.00 |
| cy19:0 | 15.52 | 5.75 | 4.09 |
| 19:1 | 0.07 | 0.00 | 0.00 |
| Total polysaturates | 0.00 | 1.31 | 0.00 |
Data shown in bold represent the dominant PLFAs from described species among the genus Castellaniella (40).
As the biomass of the samples increased (Table 1), so did the percentage of monounsaturates (Table 4), which are generally indicative of gram-negative bacteria (68). Furthermore, biostimulated samples contained a smaller percentage of terminal branched saturates compared to the control (Table 4). Terminal branched saturates are generally indicative of gram-positive bacteria; however, other microorganisms may contain these fatty acids as well (68).
Table 4 shows that the dominant PLFAs from the genus Castellaniella (C16:0, C16:1ω7c, C17:0 cyclo, and C18:1ω7c) (40) were higher in the biostimulated samples than in the control. Although other microorganisms can contain these particular PLFAs, it is likely that some or most of these fatty acids that increased with biomass were derived from Castellaniella species, given that species of this genus were dominant in biostimulated clone libraries.
Denitrifying community composition based on nirK and nirS clone libraries.
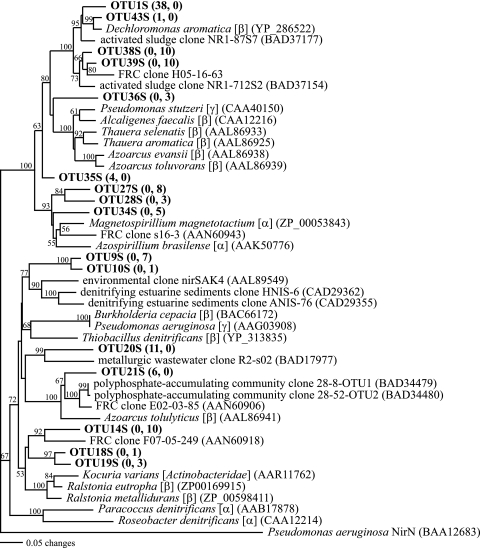
From the three nirK clone libraries, 67 clones were sequenced and 10 OTUs were identified. From all three nirK libraries, 98.5% of clones had closest cultured relatives that are Betaproteobacteria (Table 5). Ethanol stimulation resulted in an increase in proportion of total sequences within nirK clone libraries that belong to OTU1K (Table 5; Fig. 4). Clones belonging to OTU1K made up 76 and 59.4% of total clones from libraries derived from biostimulated cores FB064 and FB067, respectively, but only 20% of the total clones from the control clone library from FB066. Also, OTU1K was 100% similar to the nirK sequences from isolate 4.5A2 and 7.5A2, indicating that these genes may belong to the same Castellaniella species dominant in nitrate-reducing enrichments and in SSU rRNA gene clone libraries from biostimulated sediment (Fig. 4), although it is possible some of these genes belong to other species, as horizontal transfer of nirK genes within a site has previously been implicated (32). Seventy percent of clones from the control nirK clone library from FB066 belonged to OTU7K, whose closest relative was the nirK gene product from Alcaligenes sp. strain DSM30128 (81.7% similarity). Amino acid sequences derived from OTU1K and OTU7K, however, were only 77.8% similar to each other.
TABLE 5.
Summary of distributions of nirK OTUs from samples FB064, FB067, and FB066 and nirS OTUs from samples FB064 and FB067
| OTU group and name | No. of clones per OTU
|
Closest cultured relative(s)b | % Similarityc | ||
|---|---|---|---|---|---|
| FB064 (stimulated) | FB067 (stimulated) | FB066a (control) | |||
| nirK OTUs | |||||
| 1K | 19 | 19 | 2 | FRC isolates 4.5A2 and 7.5A2 | 100.0 |
| 2K | 1 | 0 | 0 | Nitrosomonas sp. strain TA92liNH4 | 84.8 |
| 4K | 0 | 0 | 1 | Ochromobactrum sp. strain 4FB14 | 93.5 |
| 7K | 1 | 11 | 7 | Alcaligenes sp. strain DSM 30128 | 81.7 |
| 8K | 0 | 1 | 0 | FRC isolate 4.5A2 | 84.7 |
| 9K | 0 | 1 | 0 | FRC isolate 4.5A2 | 94.2 |
| 10K | 1 | 0 | 0 | FRC isolate 4.5A2 | 92.5 |
| 11K | 1 | 0 | 0 | FRC isolate 4.5A2 | 98.0 |
| 12K | 1 | 0 | 0 | FRC isolate 4.5A2 | 87.5 |
| 13K | 1 | 0 | 0 | FRC isolate 4.5A2 | 100.0 |
| nirS OTUs | |||||
| 1S | 38 | 0 | Dechloromonas aromatica | 90.5 | |
| 9S | 0 | 7 | Thiobacillus denitrificans | 75.9 | |
| 10S | 0 | 1 | Ralstonia eutropha | 75.1 | |
| 11S | 0 | 1 | Magnetospirillum magnetotacticum | 85.1 | |
| 14S | 0 | 10 | Ralstonia metallidurans | 78.5 | |
| 18S | 0 | 1 | Ralstonia eutropha | 82.2 | |
| 19S | 0 | 3 | Ralstonia eutropha | 82.7 | |
| 20S | 11 | 0 | Ralstonia metallidurans | 74.1 | |
| 21S | 6 | 0 | Azoarcus tolulyticus | 81.5 | |
| 22S | 1 | 0 | Azoarcus tolulyticus | 84.3 | |
| 23S | 1 | 0 | Azoarcus tolulyticus | 94.6 | |
| 24S | 1 | 0 | Azoarcus tolulyticus | 87.0 | |
| 25S | 1 | 0 | Azoarcus tolulyticus | 86.6 | |
| 26S | 0 | 2 | Ralstonia metallidurans | 84.4 | |
| 27S | 0 | 8 | Magnetospirillum magnetotacticum | 86.6 | |
| 28S | 0 | 3 | Magnetospirillum magnetotacticum | 82.8 | |
| 34S | 0 | 5 | Magnetospirillum magnetotacticum | 85.2 | |
| 35S | 4 | 0 | Magnetospirillum magnetotacticum | 87.9 | |
| 36S | 0 | 3 | Thauera aromatica | 80.4 | |
| Pseudomonas stutzeri | 80.4 | ||||
| 37S | 2 | 0 | Ralstonia metallidurans | 74.7 | |
| 38S | 0 | 10 | Dechloromonas aromatica | 88.8 | |
| 39S | 0 | 10 | Dechloromonas aromatica | 87.8 | |
| 41S | 0 | 3 | Dechloromonas aromatica | 89.8 | |
| 42S | 0 | 1 | Dechloromonas aromatica | 91.9 | |
| 43S | 1 | 0 | Dechloromonas aromatica | 96.6 | |
| 44S | 0 | 2 | Dechloromonas aromatica | 88.8 | |
An amplified PCR product using nirS primers could not be obtained from this sample.
GenBank accession numbers for closest cultured relatives are located next to corresponding genus and species names in Fig. 4 and 5.
Similarity values are based on pair-wise distance values from multiple alignment files using translated amino acid sequences.
FIG. 4.
Distance phylogram of partial nirK gene product sequences. Bootstrap values are based on 1,000 replicates and are shown for branches with bootstrap support of >50%. Selected OTUs from this study are in bold, and numbers in parentheses indicate the number of clones belonging to that OTU from sediments FB064, FB067, and FB066, respectively. Accession numbers of sequences downloaded from GenBank are in parentheses.
Clone libraries from nirS genes were constructed from biostimulated samples FB064 and FB067 but not from the control core, FB066, since nirS PCR product could not be obtained from this sample. While the overwhelming majority of nirK clones seemed to belong to Castellaniella, nirS clone libraries were more diverse than nirK clone libraries (Table 5); although the reason for this difference in diversity is unknown, the inverse relationship between nirS and nirK diversity in groundwater at the FRC has previously been observed (69). From the nirS clone libraries constructed from the two biostimulated samples, FB064 and FB067, 136 clones were sequenced and 26 OTUs were identified. In accordance with nirK libraries, the majority of clones from the nirS libraries had closest cultured relatives that are Betaproteobacteria (84.6% of total clones); these clones, however, were related to families other than Alcaligenaceae (Table 5). The dominant OTU from FB064 was OTU1S (57.6% of total clones), which was closely related to the nirS gene product from the anaerobic benzene-degrading Dechloromonas aromatica (90.5% similarity) (Fig. 5). OTU20S made up 16.7% of the nirS clone library from FB064 (Table 5), and its closest relative was clone R2-s02 (77.6% similarity), identified from a metallurgic wastewater treatment system (71) (Fig. 5). NirS sequences from FB067 were more diverse (Table 5), and the most abundant OTUs clustered with D. aromatica (OTUs 38S and 39S), Ralstonia metallidurans and R. eutropha (OTUs 14S, 18S, and 19S), and Magnetospirillum magnetotacticum (OTUs 27S, 28S, and 34S) (Fig. 5).
FIG. 5.
Distance phylogram of partial nirS gene product sequences. Bootstrap values are based on 1,000 replicates and are shown for branches with bootstrap support of >50%. Selected OTUs from this study are in bold, and numbers in parentheses indicate the number of clones belonging to that OTU from sediments FB064 and FB067, respectively. Accession numbers of sequences downloaded from GenBank are in parentheses.
DISCUSSION
By using a combination of PLFA analysis, SSU rRNA and functional gene (nirK and nirS) clone libraries, and a cultivation approach, we were able to examine the effect of biostimulation on microbial community structure and identify and isolate a microorganism that likely plays a role in nitrate removal in an acidic aquifer cocontaminated with nitrate and uranium. The use of PCR and cloning methods for microbial community analysis is qualitative or “semiquantitative,” due to several well-recognized limitations (30). In this study, PCR and cloning biases may have affected the frequency in which some OTUs and GLTGs in clone libraries were detected. Also, the limited number of clones analyzed may have led to underestimated levels of diversity and detection of only the most abundant species and genera. The percent coverage in each library ranged from 64 to 78% at the OTU level and 80 to 83% at the GLTG level. The use of PLFA analysis, however, as a quantitative measure helped demonstrate the inverse relationship between biomass and diversity, while the cultivation approach confirmed the dominance of Castellaniella in sediment from FB064 and its ability to grow on ethanol and nitrate. However, variations in numbers of specific organisms or groups were only semiquantitative, as they were based on clone library data; a quantitative approach, such as real-time PCR or fluorescent in situ hybridization using group-specific primers/probes, would help determine whether the numbers of organisms within these samples were different.
Several studies have documented impacts of radionuclide, heavy metal, and hydrocarbon contamination on microbial community structure, and the general consensus is that pollution decreases microbial diversity (22, 28, 39, 43, 45, 57). Two previous studies done on microbial community structures of pristine versus contaminated areas of the aquifer at the FRC have found that contamination resulted in a decrease in microbial diversity and selected for Betaproteobacteria species related to or belonging to Azoarcus (22) and Alcaligenaceae (57). Furthermore, Betaproteobacteria were found to be abundant in other contaminated environments, including polychlorinated biphenyl-contaminated soil (50), a waste gas biofilter (26, 27), metal- and petroleum-contaminated soil (39), heavy metal-amended soil microcosms (45), and metallurgic wastewater (70). Similarly, our results show that Betaproteobacteria SSU rRNA clones, primarily those affiliated with Alcaligenaceae and Burkholderiaceae, are present in contaminated sediment samples from the FRC (Fig. 2). Also, the majority of nirK and nirS clones in this study shared similarity to nirK and nirS gene products from cultured Betaproteobacteria belonging to the families Alcaligenaceae and Burkholderiaceae as well as Rhodocyclaceae (Table 5; Fig. 4 and 5), suggesting that several of the Betaproteobacteria genera detected in SSU clone libraries may also be capable of denitrification at this site. In a recent phylogenetic survey of bacterial populations from FRC sediment, SSU rRNA clones belonging to Alcaligenaceae and Burkholderiaceae were found to be dominant as well as metabolically active (2). These results, along with the results of this study, suggest that the enrichment of Betaproteobacteria in sediments observed in this study could be due to growth of Betaproteobacteria already widespread and/or active in the aquifer prior to biostimulation that have adapted to the groundwater contaminants at the FRC, which include nitrate, heavy metals, radionuclides, and hydrocarbons.
While our SSU rRNA gene clone libraries showed an abundance of Betaproteobacteria clones in biostimulated sediments, multiple lines of evidence suggest the dominance of a Castellaniella species in biostimulated sediments and its role in nitrate removal in situ. While several studies have proven successful in using molecular approaches to identify bacteria important in bioremediation (12, 33, 61), very few studies have both identified and isolated microorganisms responsible for in situ bioremediation. In one study, organisms were cultivated that had been identified by DGGE from 2,4-dichlorophenoxyacetic acid-degrading enrichments; these isolates were capable of 2,4-dichlorophenoxyacetic acid degradation, suggesting their importance in bioremediation in contaminated environments (42). Another study used stable isotope probing of RNA to show that Azoarcus was involved in benzene degradation in groundwater incubations under denitrifying conditions and further isolated organisms belonging to the same phylotype, showing that they could oxidize benzene to CO2 (41). These two studies, however, do not prove the importance of the isolated organisms for in situ bioremediation. In a different study, however, stable isotope probing was used to identify in situ naphthalene degraders; one dominant clone was identified, and an isolate matching this clone (belonging to the genus Polaromonas) was cultivated and shown to also contain a naphthalene dioxygenase gene also detected in the site sediment (38). Similarly, in this study, isolates belonging to the genus Castellaniella were cultivated that matched dominant clones from both SSU rRNA gene and nirK clone libraries generated from biostimulated sediment where nitrate reduction was occurring. Furthermore, PLFA analysis from sediment samples showed an increase in fatty acids common to the genus Castellaniella were associated with biomass increase. Both Castellaniella sp. strains 4.5A2 and 7.5A2 contained nirK and were capable of growth on nitrate as the sole electron acceptor and producing gaseous end product, indicating these organisms are capable of denitrification; if the Castellaniella organisms identified in situ through SSU rRNA and nirK clone libraries shared similar physiology to these isolates, then Castellaniella might play an active role in denitrification at this site upon biostimulation with ethanol. Along with the Polaromonas study (38), this paper shows a relationship between microbial community structure and function through the isolation of a microorganism dominant in clone libraries while also using functional gene sequences to suggest that the microorganism is involved in the process of interest in situ.
The Castellaniella species identified in this study may represent a novel species (Fig. 1). Other Castellaniella organisms have been isolated from activated sludge and are capable of denitrification coupled to the oxidation of monoterpenes (25), taurine (15), and phenol (4). Furthermore, other Alcaligenaceae isolates have been implicated in the degradation of xenobiotic compounds (10) as well as in nitrate removal systems (53). FRC Castellaniella isolates 4.5A2 and 7.5A2 are pH tolerant and were isolated at both low and neutral pHs; thus, they may have been able to out-compete other denitrifiers for nitrate in the acidic groundwater found in Area 1.
A similar molecular ecology study at the FRC found that electron donor addition resulted in an increase in Gammaproteobacteria, such as Geobacter and Anaeromyxobacter, in contaminated FRC Area 1 sediments (51). However, push-pull tests in those experiments were done with low-nitrate groundwater from well GW835 (36), and samples were taken at the end of the extraction phase. Those experiments point to an important role for Fe(III)-reducing bacteria during biostimulation. In this study, groundwater wells were injected with high-nitrate (>130 mM) groundwater from FW021, and sediment samples were taken 1 week after injection of ethanol-amended groundwater (at the beginning of the extraction phase), at which point denitrification was likely occurring (Table 1). The differences in nitrate concentrations of the injection solutions as well as the time at which sediment samples were taken could reflect the differences in community compositions based on SSU rRNA gene clone libraries. Since several terminal electron-accepting processes sequentially occur during biostimulation (36), it is likely that the results from our study provide a snapshot of the microbial community structure during the denitrification phase, while the previous study (51) provides a snapshot of the microbial community structure when geochemical conditions were more reduced. This would reflect observations of other studies that shifts in microbial community structure occur during different stages of bioremediation processes (35, 37, 73).
In this study descriptive diversity statistics are provided to describe the effect of biostimulation on in situ diversity of microbial populations. A recent study has shown that bioremediation in a fluidized bed reactor treating nitrate- and uranium-contaminated groundwater resulted in an initial decrease in bacterial diversity followed by an increase in diversity (35). In accordance with this finding, other molecular studies have also shown that biostimulation of hydrocarbon-contaminated sediments results in an initial decrease in species diversity followed by an increase in diversity (37, 59). Our results also support that biostimulation resulted in a decrease in bacterial diversity; however, it is possible that biodiversity could later increase, as observed in the above studies. The effects of fluctuations in species diversity on ecosystem function (in this case, nitrate and uranium removal from groundwater at the FRC) are unclear. While many ecological studies have linked species richness or high species diversity in natural systems or microcosms with an increase in ecosystem function and/or stability (7, 11, 63), few studies have examined the effect of bacterial species diversity on ecosystem function in engineered systems, where often one substrate is available for consumption, as opposed to natural ecosystems, where increased species richness might aid in a more productive consumption of all available resources. For example, in glucose-fed methanogenic bioreactors, it was found that a bioreactor with lower bacterial diversity, or more “flexible” microbial communities, was more functionally stable than a more species-rich bioreactor (21). Similarly, at the FRC, the desired ecosystem function (i.e., nitrate and uranium reduction) may likely be unaffected by lower diversity when a simple substrate such as ethanol is used as an electron donor.
Acknowledgments
We thank Dave Watson and Maryanna Bogle for field sampling and providing sediment core material, Melora Park for assistance with groundwater data analysis, and Mandy Michalsen for helpful discussions.
This work was supported by the Office of Biological and Environmental Research of the Office of Science, U.S. Department of Energy, Environmental Remediation Sciences Program (FG03-02ER63443, DE-FC02-96ER62278, and FG02-00ER62986).
Footnotes
Published ahead of print on 8 June 2007.
REFERENCES
- 1.Abdelouas, A., W. Lutze, W. Gong, E. H. Nuttall, B. A. Strietelmeier, and B. J. Travis. 2000. Biological reduction of uranium in groundwater and subsurface soil. Sci. Total Environ. 250:21-35. [DOI] [PubMed] [Google Scholar]
- 2.Akob, D. M., H. J. Mills, and J. E. Kostka. 2007. Metabolically active microbial communities in uranium-contaminated subsurface sediments. FEMS Microbiol. Ecol. 59:95-107. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, R. T., H. A. Vrionis, I. Ortiz-Bernad, C. T. Resch, P. E. Long, R. Dayvault, K. Karp, S. Marutzky, D. R. Metzler, A. Peacock, D. C. White, M. Lowe, and D. R. Lovley. 2003. Stimulating the in situ activity of Geobacter species to remove uranium from the groundwater of a uranium-contaminated aquifer. Appl. Environ. Microbiol. 69:5884-5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baek, S. H., K. H. Kim, C. R. Yin, C. O. Jeon, W. T. Im, K. K. Kim, and S. T. Lee. 2003. Isolation and characterization of bacteria capable of degrading phenol and reducing nitrate under low-oxygen conditions. Curr. Microbiol. 47:462-466. [DOI] [PubMed] [Google Scholar]
- 5.Balch, W. E., and R. S. Wolfe. 1976. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressurized atmosphere. Appl. Environ. Microbiol. 32:781-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balkwill, D. L., F. R. Leach, J. T. Wilson, J. F. McNabb, and D. C. White. 1988. Equivalence of microbial biomass measures based on membrane lipid and cell wall components, adenosine triphosphate, and direct counts in subsurface aquifer sediments. Microb. Ecol. 16:73-84. [DOI] [PubMed] [Google Scholar]
- 7.Bell, T., J. A. Newman, B. W. Silverman, S. L. Turner, and A. K. Lilley. 2005. The contribution of species richness and composition to bacterial services. Nature 436:1157-1160. [DOI] [PubMed] [Google Scholar]
- 8.Bligh, E. G., and W. J. Dryer. 1954. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911-917. [DOI] [PubMed] [Google Scholar]
- 9.Braker, G., J. Zhou, L. Wu, A. H. Devol, and J. M. Tiedje. 2000. Nitrite reductase genes (nirK and nirS) as functional markers to investigate diversity of denitrifying bacteria in Pacific northwest marine sediment communities. Appl. Environ. Microbiol. 66:2096-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Busse, H. J., T. el-Banna, H. Oyaizu, and G. Auling. 1992. Identification of xenobiotic-degrading isolates from the beta subclass of the Proteobacteria by a polyphasic approach including 16S rRNA partial sequencing. Int. J. Syst. Bacteriol. 42:19-26. [DOI] [PubMed] [Google Scholar]
- 11.Cardinale, B. J., M. A. Palmer, and S. L. Collins. 2002. Species diversity enhances ecosystem functioning through interspecific facilitation. Nature 415:426-429. [DOI] [PubMed] [Google Scholar]
- 12.Chang, Y. J., P. E. Long, R. Geyer, A. D. Peacock, C. T. Resch, K. Sublette, S. Pfiffner, A. Smithgall, R. T. Anderson, H. A. Vrionis, J. R. Stephen, R. Dayvault, I. Ortiz-Bernad, D. R. Lovley, and D. C. White. 2005. Microbial incorporation of 13C-labeled acetate at the field scale: detection of microbes responsible for reduction of U(VI). Environ. Sci. Technol. 39:9039-9048. [DOI] [PubMed] [Google Scholar]
- 13.Chang, Y. J., A. D. Peacock, P. E. Long, J. R. Stephen, J. P. McKinley, S. J. Macnaughton, A. K. Hussain, A. M. Saxton, and D. C. White. 2001. Diversity and characterization of sulfate-reducing bacteria in groundwater at a uranium mill tailings site. Appl. Environ. Microbiol. 67:3149-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cole, J. R., B. Chai, R. J. Farris, Q. Wang, S. A. Kulam, D. M. McGarrell, G. M. Garrity, and J. M. Tiedje. 2005. The Ribosomal Database Project (RDP-II): sequences and tools for high-throughput rRNA analysis. Nucleic Acids Res. 33:D294-D296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denger, K., H. Laue, and A. M. Cook. 1997. Anaerobic taurine oxidation: a novel reaction by a nitrate-reducing Alcaligenes sp. Microbiology 143:1919-1924. [DOI] [PubMed] [Google Scholar]
- 16.DeSantis, T. Z., P. Hugenholtz, N. Larsen, M. Rojas, E. L. Brodie, K. Keller, T. Huber, D. Dalevi, P. Hu, and G. L. Andersen. 2006. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 72:5069-5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elias, D. A., L. R. Krumholz, D. Wong, P. E. Long, and J. M. Suflita. 2003. Characterization of microbial activities and U reduction in a shallow aquifer contaminated by uranium mill tailings. Microb. Ecol. 46:83-91. [DOI] [PubMed] [Google Scholar]
- 18.Elias, D. A., J. M. Senko, and L. R. Krumholz. 2003. A procedure for quantitation of total oxidized uranium for bioremediation studies. J Microbiol. Methods 53:343-353. [DOI] [PubMed] [Google Scholar]
- 19.Elshahed, M. S., J. M. Senko, F. Z. Najar, S. M. Kenton, B. A. Roe, T. A. Dewers, J. R. Spear, and L. R. Krumholz. 2003. Bacterial diversity and sulfur cycling in a mesophilic sulfide-rich spring. Appl. Environ. Microbiol. 69:5609-5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Etchebehere, C., I. Errazquin, E. Barrandeguy, P. Dabert, R. Moletta, and L. Muxi. 2001. Evaluation of the denitrifying microbiota of anoxic reactors. FEMS Microbiol. Ecol. 35:259-265. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez, A. S., S. A. Hashsham, S. L. Dollhopf, L. Raskin, O. Glagoleva, F. B. Dazzo, R. F. Hickey, C. S. Criddle, and J. M. Tiedje. 2000. Flexible community structure correlates with stable community function in methanogenic bioreactor communities perturbed by glucose. Appl. Environ. Microbiol. 66:4058-4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fields, M. W., T. Yan, S. K. Rhee, S. L. Carroll, P. M. Jardine, D. B. Watson, C. S. Criddle, and J. Zhou. 2005. Impacts on microbial communities and cultivable isolates from groundwater contaminated with high levels of nitric acid-uranium waste. FEMS Microbiol. Ecol. 53:417-428. [DOI] [PubMed] [Google Scholar]
- 23.Finneran, K. T., M. E. Housewright, and D. R. Lovley. 2002. Multiple influences of nitrate on uranium solubility during bioremediation of uranium-contaminated subsurface sediments. Environ. Microbiol. 4:510-516. [DOI] [PubMed] [Google Scholar]
- 24.Finneran, K. T. A., T. Robert, K. P. Nevin, and D. R. Lovley. 2002. Potential for bioremediation of uranium-contaminated aquifers with microbial U(VI) reduction. Soil Sediment Contam. 11:339-357. [Google Scholar]
- 25.Foss, S., U. Heyen, and J. Harder. 1998. Alcaligenes defragrans sp. nov., description of four strains isolated on alkenoic monoterpenes ((+)-menthene, alpha-pinene, 2-carene, and alpha-phellandrene) and nitrate. Syst. Appl. Microbiol. 21:237-244. [DOI] [PubMed] [Google Scholar]
- 26.Friedrich, U., K. Prior, K. Altendorf, and A. Lipski. 2002. High bacterial diversity of a waste gas-degrading community in an industrial biofilter as shown by a 16S rDNA clone library. Environ. Microbiol. 4:721-734. [DOI] [PubMed] [Google Scholar]
- 27.Friedrich, U., H. Van Langenhove, K. Altendorf, and A. Lipski. 2003. Microbial community and physicochemical analysis of an industrial waste gas biofilter and design of 16S rRNA-targeting oligonucleotide probes. Environ. Microbiol. 5:183-201. [DOI] [PubMed] [Google Scholar]
- 28.Frostegard, A., A. Tunlid, and E. Baath. 1993. Phospholipid fatty acid composition, biomass, and activity of microbial communities from two soil types experimentally exposed to different heavy metals. Appl. Environ. Microbiol. 59:3605-3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guckert, J. B., C. P. Antworth, P. D. Nichols, and D. C. White. 1985. Phospholipid, ester-linked fatty acid profiles as reproducible assays for changes in prokaryotic community structure of estuarine sediment. FEMS Microb. Ecol. 31:147-158. [Google Scholar]
- 30.Head, I. M., J. R. Saunders, and R. W. Pickup. 1998. Microbial evolution, diversity, and ecology: a decade of ribosomal RNA analysis of uncultivated microorganisms. Microb. Ecol. 35:1-21. [DOI] [PubMed] [Google Scholar]
- 31.Hedrick, D. B., A. Peacock, J. R. Stephen, S. J. Macnaughton, J. Bruggemann, and D. C. White. 2000. Measuring soil microbial community diversity using polar lipid fatty acid and denaturing gradient gel electrophoresis data. J. Microbiol. Methods 41:235-248. [DOI] [PubMed] [Google Scholar]
- 32.Heylen, K., D. Gevers, B. Vanparys, L. Wittebolle, J. Geets, N. Boon, and P. De Vos. 2006. The incidence of nirS and nirK and their genetic heterogeneity in cultivated denitrifiers. Environ. Microbiol. 8:2012-2021. [DOI] [PubMed] [Google Scholar]
- 33.Holmes, D. E., K. T. Finneran, R. A. O'Neil, and D. R. Lovley. 2002. Enrichment of members of the family Geobacteraceae associated with stimulation of dissimilatory metal reduction in uranium-contaminated aquifer sediments. Appl. Environ. Microbiol. 68:2300-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huber, T., G. Faulkner, and P. Hugenholtz. 2004. Bellerophon: a program to detect chimeric sequences in multiple sequence alignments. Bioinformatics 20:2317-2319. [DOI] [PubMed] [Google Scholar]
- 35.Hwang, C., W. M. Wu, T. J. Gentry, J. Carley, S. L. Carroll, C. Schadt, D. Watson, P. M. Jardine, J. Zhou, R. F. Hickey, C. S. Criddle, and M. W. Fields. 2005. Changes in bacterial community structure correlate with initial operating conditions of a field-scale denitrifying fluidized bed reactor. Appl. Microbiol. Biotechnol. 71:748-760. [DOI] [PubMed] [Google Scholar]
- 36.Istok, J. D., J. M. Senko, L. R. Krumholz, D. Watson, M. A. Bogle, A. Peacock, Y. J. Chang, and D. C. White. 2004. In situ bioreduction of technetium and uranium in a nitrate-contaminated aquifer. Environ. Sci. Technol. 38:468-475. [DOI] [PubMed] [Google Scholar]
- 37.Iwamoto, T., K. Tani, K. Nakamura, Y. Suzuki, M. Kitagawa, M. Eguchi, and M. Nasu. 2000. Monitoring impact of in situ biostimulation treatment on groundwater bacterial community by DGGE. FEMS Microbiol. Ecol. 32:129-141. [DOI] [PubMed] [Google Scholar]
- 38.Jeon, C. O., W. Park, P. Padmanabhan, C. DeRito, J. R. Snape, and E. L. Madsen. 2003. Discovery of a bacterium, with distinctive dioxygenase, that is responsible for in situ biodegradation in contaminated sediment. Proc. Natl. Acad. Sci. USA 100:13591-13596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joynt, J., M. Bischoff, R. Turco, A. Konopka, and C. H. Nakatsu. 2006. Microbial community analysis of soils contaminated with lead, chromium and petroleum hydrocarbons. Microb. Ecol. 51:209-219. [DOI] [PubMed] [Google Scholar]
- 40.Kampfer, P., K. Denger, A. M. Cook, S. T. Lee, U. Jackel, E. B. Denner, and H. J. Busse. 2006. Castellaniella gen. nov., to accommodate the phylogenetic lineage of Alcaligenes defragrans, and proposal of Castellaniella defragrans gen. nov., comb. nov. and Castellaniella denitrificans sp. nov. Int. J. Syst. Evol. Microbiol. 56:815-819. [DOI] [PubMed] [Google Scholar]
- 41.Kasai, Y., Y. Takahata, M. Manefield, and K. Watanabe. 2006. RNA-based stable isotope probing and isolation of anaerobic benzene-degrading bacteria from gasoline-contaminated groundwater. Appl. Environ. Microbiol. 72:3586-3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee, T. H., S. Kurata, C. H. Nakatsu, and Y. Kamagata. 2005. Molecular analysis of bacterial community based on 16S rDNA and functional genes in activated sludge enriched with 2,4-dichlorophenoxyacetic acid (2,4-D) under different cultural conditions. Microb. Ecol. 49:151-162. [DOI] [PubMed] [Google Scholar]
- 43.Liao, M., C. L. Chen, and C. Y. Huang. 2005. Effect of heavy metals on soil microbial activity and diversity in a reclaimed mining wasteland of red soil area. J. Environ. Sci. (China) 17:832-837. [PubMed] [Google Scholar]
- 44.Lovley, D. R., and E. J. P. Phillips. 1992. Bioremediation of uranium contamination with enzymatic uranium reduction. Environ. Sci. Technol. 26:2228-2234. [Google Scholar]
- 45.Macnaughton, S., J. R. Stephen, Y. J. Chang, A. Peacock, C. A. Flemming, K. T. Leung, and D. C. White. 1999. Characterization of metal-resistant soil eubacteria by polymerase chain reaction-denaturing gradient gel electrophoresis with isolation of resistant strains. Can. J. Microbiol. 45:116-124. [DOI] [PubMed] [Google Scholar]
- 46.Martin, A. P. 2002. Phylogenetic approaches for describing and comparing the diversity of microbial communities. Appl. Environ. Microbiol. 68:3673-3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McInerney, M. J., M. P. Bryant, and N. Pfenning. 1979. Anaerobic bacterium that degrades fatty acids in syntrophic association with methanogens. Arch. Microbiol. 122:129-135. [DOI] [PubMed] [Google Scholar]
- 48.Michalsen, M. M., B. A. Goodman, S. D. Kelly, K. M. Kemner, J. P. McKinley, J. W. Stucki, and J. D. Istok. 2006. Uranium and technetium bio-immobilization in intermediate-scale physical models of an in situ bio-barrier. Environ. Sci. Technol. 40:7048-7053. [DOI] [PubMed] [Google Scholar]
- 49.Nevin, K. P., K. T. Finneran, and D. R. Lovley. 2003. Microorganisms associated with uranium bioremediation in a high-salinity subsurface sediment. Appl. Environ. Microbiol. 69:3672-3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nogales, B., E. R. Moore, E. Llobet-Brossa, R. Rossello-Mora, R. Amann, and K. N. Timmis. 2001. Combined use of 16S ribosomal DNA and 16S rRNA to study the bacterial community of polychlorinated biphenyl-polluted soil. Appl. Environ. Microbiol. 67:1874-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.North, N. N., S. L. Dollhopf, L. Petrie, J. D. Istok, D. L. Balkwill, and J. E. Kostka. 2004. Change in bacterial community structure during in situ biostimulation of subsurface sediment cocontaminated with uranium and nitrate. Appl. Environ. Microbiol. 70:4911-4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nyman, J. L., T. L. Marsh, M. A. Ginder-Vogel, M. Gentile, S. Fendorf, and C. Criddle. 2006. Heterogeneous response to biostimulation for U(VI) reduction in replicated sediment microcosms. Biodegradation 17:303-316. [DOI] [PubMed] [Google Scholar]
- 53.Ozeki, S., I. Baba, N. Takaya, and H. Shoun. 2001. A novel C1-using denitrifier alcaligenes sp. STC1 and its genes for copper-containing nitrite reductase and azurin. Biosci. Biotechnol. Biochem. 65:1206-1210. [DOI] [PubMed] [Google Scholar]
- 54.Peacock, A. D., Y. J. Chang, J. D. Istok, L. Krumholz, R. Geyer, B. Kinsall, D. Watson, K. L. Sublette, and D. C. White. 2004. Utilization of microbial biofilms as monitors of bioremediation. Microb. Ecol. 47:284-292. [DOI] [PubMed] [Google Scholar]
- 55.Petrie, L., N. N. North, S. L. Dollhopf, D. L. Balkwill, and J. E. Kostka. 2003. Enumeration and characterization of iron(III)-reducing microbial communities from acidic subsurface sediments contaminated with uranium(VI). Appl. Environ. Microbiol. 69:7467-7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pinkart, H. C., D. B. Ringelberg, Y. M. Piceno, S. J. MacNaughton, and D. C. White. 2002. Biochemical approaches to biomass measurements and community structure analysis, p. 101-103. In C. J. Hurst, R. L. Crawford, G. R. Knudsen, M. J. McInerney, and L. D. Stetzenbach (ed.), Manual of environmental microbiology, 2nd ed. ASM Press, Washington, DC.
- 57.Reardon, C. L., D. E. Cummings, L. M. Petzke, B. L. Kinsall, D. B. Watson, B. M. Peyton, and G. G. Geesey. 2004. Composition and diversity of microbial communities recovered from surrogate minerals incubated in an acidic uranium-contaminated aquifer. Appl. Environ. Microbiol. 70:6037-6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rogers, D. R., C. M. Santelli, and K. J. Edwards. 2003. Geomicrobiology of deep-sea deposits: estimating community diversity from low-temperature seafloor rocks and minerals. Geobiology 1:109-117. [Google Scholar]
- 59.Roling, W. F., M. G. Milner, D. M. Jones, K. Lee, F. Daniel, R. J. Swannell, and I. M. Head. 2002. Robust hydrocarbon degradation and dynamics of bacterial communities during nutrient-enhanced oil spill bioremediation. Appl. Environ. Microbiol. 68:5537-5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Senko, J. M., J. D. Istok, J. M. Suflita, and L. R. Krumholz. 2002. In-situ evidence for uranium immobilization and remobilization. Environ. Sci. Technol. 36:1491-1496. [DOI] [PubMed] [Google Scholar]
- 61.Suzuki, Y., S. D. Kelly, K. M. Kemner, and J. F. Banfield. 2003. Microbial populations stimulated for hexavalent uranium reduction in uranium mine sediment. Appl. Environ. Microbiol. 69:1337-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tilman, D., P. B. Reich, and J. M. Knops. 2006. Biodiversity and ecosystem stability in a decade-long grassland experiment. Nature 441:629-632. [DOI] [PubMed] [Google Scholar]
- 64.Vrionis, H. A., R. T. Anderson, I. Ortiz-Bernad, K. R. O'Neill, C. T. Resch, A. D. Peacock, R. Dayvault, D. C. White, P. E. Long, and D. R. Lovley. 2005. Microbiological and geochemical heterogeneity in an in situ uranium bioremediation field site. Appl. Environ. Microbiol. 71:6308-6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reference deleted.
- 66.Watson, D. B., G. R. Moline, P. M. Jardine, S. K. Holladay, T. L. Melhorn, B. Gu, A. V. Palumbo, B. P. Spalding, C. C. Brandt, and W. E. Doll. 2001, posting date. Natural and Accelerated Bioremediation Research (NABIR) Field Research Center (FRC) site characterization plan. http://public.ornl.gov/nabirfrc/other/scp.pdf.
- 67.White, D., W. M. Davis, J. S. Nickels, J. D. King, and R. J. Bobbie. 1979. Determination of the sedimentary microbial biomass by extractable lipid phosphate. Oecologia 40:51-62. [DOI] [PubMed] [Google Scholar]
- 68.White, D. C., J. O. Stair, and D. B. Ringelberg. 1996. Quantitative comparisons of in situ microbial biodiversity by signature biomarker analysis. J. Ind. Microbiol. 17:185-196. [Google Scholar]
- 69.Yan, T., M. W. Fields, L. Wu, Y. Zu, J. M. Tiedje, and J. Zhou. 2003. Molecular diversity and characterization of nitrite reductase gene fragments (nirK and nirS) from nitrate- and uranium-contaminated groundwater. Environ. Microbiol. 5:13-24. [DOI] [PubMed] [Google Scholar]
- 70.Yoshie, S., N. Noda, T. Miyano, S. Tsuneda, A. Hirata, and Y. Inamori. 2002. Characterization of microbial community in nitrogen removal process of metallurgic wastewater by PCR-DGGE. Water Sci. Technol. 46:93-98. [PubMed] [Google Scholar]
- 71.Yoshie, S., N. Noda, S. Tsuneda, A. Hirata, and Y. Inamori. 2004. Salinity decreases nitrite reductase gene diversity in denitrifying bacteria of wastewater treatment systems. Appl. Environ. Microbiol. 70:3152-3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang, H., S. Hanada, T. Shigematsu, K. Shibuya, Y. Kamagata, T. Kanagawa, and R. Kurane. 2000. Burkholderia kururiensis sp. nov., a trichloroethylene (TCE)-degrading bacterium isolated from an aquifer polluted with TCE. Int. J. Syst. Evol. Microbiol. 50:743-749. [DOI] [PubMed] [Google Scholar]
- 73.Zucchi, M., L. Angiolini, S. Borin, L. Brusetti, N. Dietrich, C. Gigliotti, P. Barbieri, C. Sorlini, and D. Daffonchio. 2003. Response of bacterial community during bioremediation of an oil-polluted soil. J. Appl. Microbiol. 94:248-257. [DOI] [PubMed] [Google Scholar]