Abstract
We report the effects of pine and oak litter on species composition and diversity of mycorrhizal fungi colonizing 2-year-old Pinus sylvestris L. seedlings grown in a bare-root nursery in Lithuania. A layer of pine or oak litter was placed on the surface of the nursery bed soil to mimic natural litter cover. Oak litter amendment appeared to be most favorable for seedling survival, with a 73% survival rate, in contrast to the untreated mineral bed soil (44%). The concentrations of total N, P, K, Ca, and Mg were higher in oak growth medium than in pine growth medium. Relative to the control (pH 6.1), the pH was lower in pine growth medium (5.8) and higher in oak growth medium (6.3). There were also twofold and threefold increases in the C content of growth medium with the addition of pine and oak litter, respectively. Among seven mycorrhizal morphotypes, eight different mycorrhizal taxa were identified: Suillus luteus, Suillus variegatus, Wilcoxina mikolae, a Tuber sp., a Tomentella sp., Cenococcum geophilum, Amphinema byssoides, and one unidentified ectomycorrhizal symbiont. Forest litter addition affected the relative abundance of mycorrhizal symbionts more than their overall representation. This was more pronounced for pine litter than for oak litter, with 40% and 25% increases in the abundance of suilloid mycorrhizae, respectively. Our findings provide preliminary evidence that changes in the supply of organic matter through litter manipulation may have far-reaching effects on the chemistry of soil, thus influencing the growth and survival of Scots pine seedlings and their mycorrhizal communities.
In the boreal zone, Scots pine (Pinus sylvestris L.) is widely planted for reforestation and afforestation of marginally economic agricultural land (25, 26). For example, in Lithuania 76.4 million seedlings are planted each year; approximately 20% of these are Scots pine (36). An important factor in the performance of outplanted conifers is the association of plant roots with ectomycorrhizal (ECM) fungi (7, 37). ECM fungi are essential for nutrient acquisition and plant protection against root pathogens and drought stress (51). Pinus species are dependent on symbiosis to develop optimally under natural conditions (40).
ECM fungi naturally established in nurseries are diverse, and their establishment depends on several factors, including host species relationships, sylvicultural practices, and nursery conditions (11, 35). Early differences in ECM colonization of tree seedlings may affect their performance after outplanting to forest sites (30). The application of forest litter to nursery-grown seedlings can be useful in enhancing ECM colonization and the field performance of outplanted seedlings (8, 47).
In forest nurseries, attempts have been made to use various germination media instead of mineral soils. The Dunemann system of nursery practice demonstrated that spruce needles are a good medium for raising conifer seedlings (24). A series of experiments following the Dunemann scheme showed that germination, growth, and survival are better in spruce litter than in mineral soils (27). However, this material is not always available in nursery practice, and other more accessible leaf litters are also applied.
The quantity, quality, and heterogeneity of soil organic matter likely have a significant influence on the structure of mycorrhizal fungal communities. Mycorrhizal fungi can, through the production of extracellular enzymes (46), use organic forms of soil nutrients. Also, some mycorrhizal fungi possess limited saprotrophic ability (9). There are in fact differences in the mycorrhizal fungi colonizing pine roots in artificially constructed mixtures of pine versus oak leaf litter (10, 31), and it has been suggested that this is due to differences in nutrient availability or the effect of leaf litter extracts on fungal growth (2). However, information about the effects of forest litter amendment on ECM colonization of P. sylvestris seedlings growing in nursery soil is scarce.
In this study, we measured the growth and development of Scots pine seedlings and assessed the impact of pine and oak litter on ECM colonization of the seedlings. Specifically, we addressed two questions: whether the addition of leaf litter to the growth medium improves the growth of Scots pine seedlings, and whether litter addition affects ECM colonization or community structure of Scots pine seedlings.
MATERIALS AND METHODS
Litter collection, site preparation, and seedling measurements.
In March 2004, forest litter was collected in healthy, natural pine and oak stands of similar ages. The pine stand was approximately 70 years old; the soil was characterized as Albic Arenosols (20). The forest type was classified as nemoral pine forest (6.2.2) (17) with Vaccinium myrtilis in the undergrowth layer. A humus-podzol horizon, 6 cm deep, was mixed with an 8-cm-deep alluvial horizon. The oak stand was 90 years old and represented the acidophilous oak forest type (6.4.1) (17), with soil characterized as Eutric Planosols (20). Litter (3 cm deep) on the top layer of the soil was mixed with a 7-cm-deep humus horizon and a 9-cm-deep alluvial horizon. Mineral nursery soil where experiments were run was used as the control.
In April 2004, the leaf litters were transferred to the nursery of Vilnius University Botanical Garden (54°41′N, 25°14′E) and spread in a 20-cm layer on a prepared bed. For the preparation of each growth medium, 40 kg/m2 of forest litter was used. The nursery test design was five complete blocks with five plots per control and pine and oak treatment, randomly allocated in each block.
The pine seeds originated from the local provenance of Labanoras (55°14′N, 25°44′E). A 5- by 5-cm sawing stencil was used. Seedlings, 1 and 2 years old, were manually maintained and were not fertilized. After 2 years of growth under nursery conditions, 25 seedlings per growth medium were randomly selected, and their stem heights and root collar diameters were measured. The seedlings were dried at 65 ± 2°C for 24 h, and dry mass was estimated.
Chemical analysis.
At the end of the experiment, the upper 15 cm of growth medium was collected from the controls and the pine and oak treatments. For each treatment, growth medium was sampled from five points of three randomly selected plots. The five samples from each plot were mixed and dried at 40°C. The foliar carbon content and nutrient composition were determined in three composite samples of the group of five seedlings per treatment. Soil samples and milled pine needles, approximately 2.5 g (dry weight) each, were digested in a mixture of spectrally pure concentrated HNO3 and HClO4 in a proportion of 4:1 (vol/vol), diluted with double-distilled water to yield 25 ml. The soil pH was determined using a soil suspension in 0.5 M KCl.
Carbon and nitrogen contents of the 2-year-old Scots pine seedling needles were measured using a 4010 elemental combustion system. The remaining macroelements, aluminum, and microelements (Fe, Mn, Cu, and Zn) were measured by atomic absorption spectroscopy (Varian 220 FS) with atomization in an air-acetylene flame. The accuracy of the analyses was checked against standard reference material, namely, pine needles (SRM 1575) and tomato leaves (SRM 1573a) (National Institute of Standards and Technology [http://ts.nist.gov/MeasurementServices/ReferenceMaterials/ARCHIVED_CERTIFICATES/archived_certificates.htm]).
Analysis of mycorrhizal community structure.
At the time of harvest, 25 seedlings per treatment were taken for mycorrhizal assessment (five seedlings from one randomly selected plot of each block). The growth medium was washed off and overall mycorrhizal development was examined, as described elsewhere (49). On average, approximately 250 root tips were counted per seedling.
ECM morphotypes were described based on macroscopic observations (color of the mantle, presence of rhizomorphs, and extramatrical hyphae and cystidia) and referred to a database used by workers in the Laboratory of Mycorrhizal Research at the Institute of Dendrology, Polish Academy of Sciences (29, 49, 53). Live roots were identified on the basis of their turgid appearance. The numbers of live mycorrhizae of each morphotype and dead fine roots were recorded. The relative abundance of each morphotype (number of root tips of each morphotype/total number of fine roots) was calculated for each sample.
Selected samples of morphotypes were stored in 2% cetyltrimethylammonium bromide buffer at room temperature for further analysis. DNA was extracted from one root tip per sample using the miniprep method (22). Fungal symbionts were identified using PCR amplification of the internal transcribed spacer (ITS) with primers ITS-1 and ITS-4 (56). The PCR reagents and their final concentrations were 20 mM Tris-HCl (pH 8.4), 50 mM KCl, 2.5 mM MgCl2, 0.05% W-1 (QIAGEN), 200 mM ultrapure dATP, dCTP, dGTP, and dTTP (QIAGEN), 0.2 mM of the two primers (IBB PAN, Warsaw, Poland), and 1.75 units Taq DNA polymerase (QIAGEN). The PCR amplification sequence consisted of a first step at 94°C for 10 min followed by 35 cycles of 40 s at 92°C, 40 s at 57°C, and 80 s at 72°C using a T3 thermocycler (Biometra). ITS products (10 μl) were mixed with 10 μl reaction mixture containing 5 units of HinfI, MboI, or TaqI enzyme (EurX, Gdansk, Poland) and incubated for 1 to 2 h at 37°C (HinfI and MboI) or 65°C (TaqI). The amplified products and the restriction fragments were electrophoresed on 1.5% and 2% high-resolution agarose gels (Prona), respectively, stained with ethidium bromide, and photographed under UV light using a Polaroid or charge-coupled device camera. A 100-bp Gene Ruler DNA ladder (Fermentas) was used as a size standard.
The sizes of the restriction fragments were determined using Taxotron software (SAS Pasteur Institute, Paris, France) and compared with a data bank maintained by the Laboratory of Mycorrhizal Research at the Institute of Dendrology. One or two samples of each unique restriction fragment length polymorphism (RFLP) pattern were sequenced (with the exception of the Cenococcum type). Sequencing was performed with a CEQ 20000XL automatic sequencer using primers ITS1 and ITS4 and a Beckman Coulter DTCS Quick Start chemistry kit. ITS-PCR products were sequenced in the forward and reverse directions and merged to a contig by using BioEdit and ClustalW software. Consensus sequences were constructed, with manual editing of ambiguous readings, and compared to published sequences in the GenBank or UNITE database (34) using the BLAST tool. Species-level identification of mycorrhizae was defined as the sharing of >98% ITS region sequence identity with the reference sequence.
Statistical analysis.
Soil nutrients, chemical composition of seedlings, growth parameters, ratio of live to dead mycorrhizae, and species richness were analyzed by two-way analysis of variance (ANOVA), with litter treatment and block as the main factors, followed by Tukey's honestly significant difference (HSD) test, with a significance level of a P value of <0.05. No homogeneity of variance was found, and therefore differences between litter treatments in relative abundance of morphotypes were tested with the Kruskal-Wallis and Mann-Whitney U tests. Computations were performed using the statistical software package Statistica 5.5.
RESULTS
The properties of all nursery growth media are summarized in Table 1. Tukey's HSD test revealed significant differences between leaf litter treatments and control soil in nutrient concentrations, pH, and carbon content. The pHKCl was highest for oak litter, intermediate for the control soil, and lowest for pine litter. The P and K concentrations were significantly lower in pine litter than control soil. The concentrations of soil N, Ca, Mg, and C and the C/N ratio were highest in oak litter, intermediate in pine litter, and lowest in the control soil. The block effect was not significant (two-way ANOVA, P = 0.37).
TABLE 1.
Nutrient composition and pH of growth media at harvest of P. sylvestris seedlingsa
| Treatment | Ntotal (%) | Concn (mg kg−1) of:
|
C (%) | C/N | pHKCl | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| NH4+ | NO3− | P | K+ | Ca2+ | Mg2+ | |||||
| None (control) | 0.1 ± 0.002 c | 5.1 ± 0.58 a | 9.9 ± 0.18 a | 16.1 ± 0.35 a | 51.0 ± 0.49 a | 64.0 ± 0.2 c | 20.9 ± 0.35 c | 1.2 ± 0.02 c | 11.6 ± 0.03 c | 6.1 ± 0.03 b |
| Pine litter | 0.2 ± 0.004 b | 5.0 ± 0.75 a | 10.0 ± 0.58 a | 10.1 ± 0.78 b | 39.0 ± 0.87 b | 76.1 ± 0.64 b | 23.0 ± 0.35 b | 3.0 ± 0.06 b | 14.7 ± 0.01 b | 5.8 ± 0.02 c |
| Oak litter | 0.25 ± 0.01 a (0.0001) | 5.1 ± 0.64 a (0.997) | 9.1 ± 0.67 a (0.443) | 15.1 ± 0.52 a (0.001) | 49.2 ± 0.55 a (0.0001) | 93.1 ± 0.78 a (0.0001) | 25.1 ± 0.64 a (0.0021) | 4.5 ± 0.12 a (0.0001) | 17.8 ± 0.04 a (0.0001) | 6.3 ± 0.03 a (0.0001) |
Values are means ± standard errors (n = 3). Within a column, values with different letters are significantly different (P < 0.05; Tukey's test). P values are given in parentheses.
There were significant differences between control soil and the leaf litter variants in seedling height, needle dry weight, total weight, and survival rate (Table 2), without significant influence of block (two-way ANOVA, P = 0.38). The height and total dry weight of the seedlings were significantly greater for pine litter than control soil. The dry weight of needles was significantly higher for seedlings grown in oak litter than those in control soil. The survival rate of seedlings was highest for oak litter, intermediate for pine litter, and lowest for control soil.
TABLE 2.
Growth and survival of P. sylvestris seedlings after 2 years of growth in a nursery with untreated soil and soil amended with pine litter or oak littera
| Treatment | Seedling ht (cm) | Root collar diam (mm) | Dry wt (g) of:
|
Ratio of aboveground/belowground wt | Survival rate (%) of seedlings | |||
|---|---|---|---|---|---|---|---|---|
| Stem | Needles | Roots | Total seedling | |||||
| None (control) | 18.6 ± 0.62 b | 3.7 ± 0.12 a | 0.80 ± 0.06 a | 1.57 ± 0.13 b | 0.49 ± 0.05 a | 2.85 ± 0.20 b | 5.4 ± 0.46 a | 43.6 ± 0.05 c |
| Pine litter | 21.3 ± 0.68 a | 3.8 ± 0.13 a | 1.05 ± 0.08 a | 2.15 ± 0.17 ab | 0.69 ± 0.08 a | 3.89 ± 0.30 a | 5.3 ± 0.52 a | 64.0 ± 0.63 b |
| Oak litter | 18.5 ± 0.75 b (0.009) | 3.8 ± 0.07 a (0.875) | 0.94 ± 0.08 a (0.055) | 2.18 ± 0.16 a (0.009) | 0.66 ± 0.08 a (0.116) | 3.77 ± 0.28 ab (0.015) | 5.1 ± 0.30 a (0.0895) | 73.2 ± 0.89 a (0.0001) |
Values are means ± standard errors (n = 25). Within a column, values with different letters are significantly different (P < 0.05; Tukey's test). P values are given in parentheses.
Tukey's HSD test showed significant differences in the concentrations of C, K, Ca, Mn, and Al between seedlings grown in leaf litter and control soil (Table 3). Both the C concentration and the Al concentration were significantly lower for seedlings grown in the oak litter than the control soil, whereas the K concentration was significantly higher in seedlings grown in the oak litter than in seedlings grown in the control soil. The Ca concentrations in seedlings grown in pine and oak litter were significantly lower than the concentrations in seedlings grown in the control soil. The block effect on nutrient composition was not significant (two-way ANOVA, P = 0.27).
TABLE 3.
Foliar nutrient composition of P. sylvestris seedlings after 2 years of growth in a nursery with untreated soil and soil amended with pine litter or oak littera
| Treatment | Concn
|
C/N | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C (%) | N (%) | P (%) | K (%) | Ca (%) | Mg (%) | Fe (ppm) | Mn (ppm) | Cu (ppm) | Zn (ppm) | Al (ppm) | ||
| None (control) | 47.3 ± 0.32 a | 1.7 ± 0.08 a | 0.2 ± 0.01 a | 0.7 ± 0.02 b | 0.5 ± 0.01 a | 0.1 ± 0.004 a | 94.7 ± 23.50 a | 75.5 ± 12.01 ab | 5.0 ± 0.40 a | 66.8 ± 0.67 a | 257.2 ± 13.41 a | 27.5 ± 1.00 a |
| Pine litter | 47.5 ± 0.32 a | 1.7 ± 0.05 a | 0.2 ± 0.01 a | 0.8 ± 0.06 ab | 0.3 ± 0.03 c | 0.1 ± 0.01 a | 113.0 ± 12.01 a | 58.1 ± 3.38 b | 5.1 ± 0.45 a | 56.9 ± 3.78 a | 185.2 ± 18.31 ab | 26.1 ± 0.54 a |
| Oak litter | 44.3 ± 0.55 b (0.003) | 1.8 ± 0.10 a (0.873) | 0.2 ± 0.01 a (0.166) | 1.0 ± 0.04 a (0.008) | 0.4 ± 0.01 b (0.003) | 0.1 ± 0.01 a (0.268) | 214.0 ± 48.21 a (0.075) | 97.4 ± 7.36 a (0.044) | 6.3 ± 0.08 a (0.074) | 99.8 ± 20.96 a (0.107) | 169.7 ± 17.67 b (0.021) | 25.7 ± 1.37 a (0.487) |
Values are means ± standard errors (n = 9). Within a column, values with different letters are significantly different (P < 0.05; Tukey's test). P values are given in parentheses.
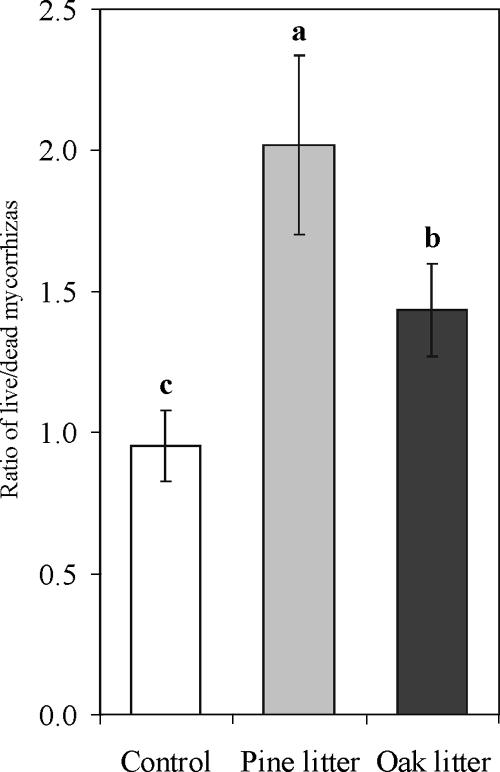
Under all variant growth conditions in our experiment, the degree of mycorrhizal colonization was very high and neared 100%. The ratio of live to dead mycorrhizae was significantly higher (two-way ANOVA, P < 0.005) for the litter treatments than for the control (Fig. 1). The block effect was not significant (two-way ANOVA, P = 0.44). Mycorrhizal roots from the seedlings grown in nursery soil with forest litter amendments were classified into eight morphotypes (Table 4). Comparison of the RFLP patterns against our reference databank (based on sporocarps and mycorrhizae) revealed the following ECM species: Suillus luteus and Suillus variegatus (both identified within the suilloid type), Wilcoxina mikolae, a Tuber species, Cenococcum geophillum, and Amphinema byssoides. Two morphotypes (orange and dark brown) with distinct RFLP patterns did not match any records in the databank. The suilloid/wilcoxina type was characterized by double colonization with S. luteus and W. mikolae. RFLP identification of suilloid, wilcoxina/suilloid, wilcoxina, and amphinema types was confirmed by ITS sequence analysis at the species level. The tuber morphotype was identified as a species of the Tuber genus (92% similarity to Tuber borchii DQ402505), and the dark brown morphotype was identified as a Tomentella species (96% similarity to Tomentella ellisii DQ068971). The orange type remained unidentified and on the basis of BLAST analysis was designated as “uncultured basidiomycete” (90% similarity to AY969603).
FIG. 1.
Ratio of live to dead mycorrhizae on P. sylvestris seedlings after 2 years of growth in a nursery with untreated soil and soil amended with pine litter or oak litter. Each bar shows the mean for 25 replicates ± standard error. Letters indicate significant differences between growth media at a P value of <0.05 (Tukey's test).
TABLE 4.
Description of mycorrhizal morphotypes
| Morphotype | Description | Identification | Best BLAST match(es) (% similarity) |
|---|---|---|---|
| Suilloid | Dichotomously branched; coralloid to subtuberculate and clusters; white, pink, or light brown; thick mantle; abundant; loose or fluffy extramatrical mycelium; white to brown strands present, ectomycorrhizal | Suillus luteusa,b | AJ272416: Suillus luteus (100) |
| Suillus variegatusa,b | AY898622: Suillus variegatus (100) | ||
| Suilloid/ wilcoxina | Repeatedly and dichotomously branched; very long; white to pink; thick mantle at the top (similar to suilloid morphotype); brown at the base; smooth; shiny mantle; ectomycorrhizal; characterized as double colonization with Suillus and Wilcoxina species | Suillus luteus/Wilcoxina mikolaea,b | AJ272416: Suillus luteus (100); DQ069000: Wilcoxina mikolae (100) |
| Wilcoxina | Single or dichotomously branched; fairly long; light to dark brown; pale tip; thin, reticulate, and shiny mantle; extramatrical hyphae and strands absent; ectendomycorrhizal | Wilcoxina mikolaea,b | DQ069000: Wilcoxina mikolae (100) |
| Tuber | Single or dichotomously branched, dark yellow to orange, club-shaped mycorrhizae with a smooth mantle texture and small cystidium emanating from the mantle, extramatrical mycelium and strands absent, ectomycorrhizal | Tuber sp.a,b | DQ402505: Tuber borchii (92) |
| Orange | Dichotomously or irregular branched, orange, smooth and thick mantle, milky opalescent, extramatrical mycelium and strands absent, ectomycorrhizal | Basidiomycetesb | AY969603: uncultured basidiomycete isolate (90) |
| Dark brown | Simply, dichotomously, or irregularly branched; dark brown to black; thick mantle; loose and thin dark brown and gray extramatrical mycelium; ectomycorrhizal | Tomentella sp.b | DQ068971: Tomentella ellisii (96) |
| Cenococcum | Simply or dichotomously branched, black, mantle, angular stellate arrangement with smooth, stiff hyphae radiating from mantle, ectomycorrhizal | Cenococcum geophilluma,c | Not sequenced |
| Amphinema | Simply or dichotomously branched, orange mantle with silvering, patched mycelium on the surface, very abundant, orange, loose extramatrical mycelium, ectomycorrhizal | Amphinema byssoidesa,b | AF291288: Amphinema byssoides (99) |
Identification by RFLP pattern.
Identification by BLAST.
Identification by morphology.
In total, six morphotypes were found in control soil, five in pine litter, and eight in oak litter. Differences between average species richness in controls and in pine and oak treatment variants (4.2, 2.8, and 5.7, respectively) were statistically significant (two-way ANOVA, P < 0.0001). The block effect was not significant (two-way ANOVA, P = 0.44). Four morphotypes were common to all soil variants (suilloid type, suilloid/wilcoxina type, wilcoxina type, and orange type). C. geophillum and the dark brown type occurred in control soil and oak litter, whereas the Tuber species was found in pine and oak litter. A. byssoides occurred only in oak litter. Regardless of the soil variant, the ECM species found most frequently on seedlings were from the suilloid group (S. luteus and S. variegatus) and W. mikolae.
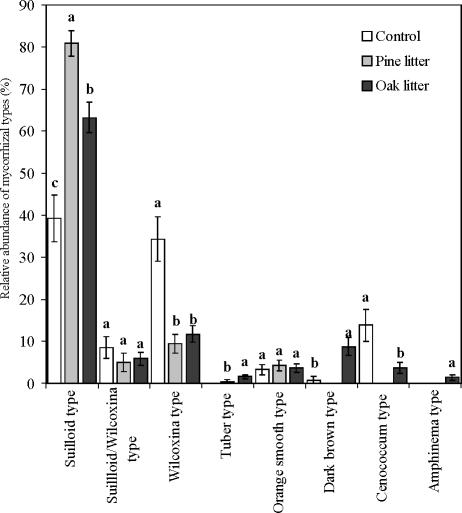
Significant differences in ECM abundance were found depending on the litter addition (Kruskal-Wallis, P < 0.005) (Fig. 2). The suilloid type was most abundant in pine litter, intermediate in oak litter, and least abundant in control soil. Conversely, the wilcoxina type was most abundant in control soil, whereas there was no difference in abundance between pine and oak litter. C. geophillum was present in significantly lower abundance in oak litter than in control soil, whereas the dark-brown type was more abundant in oak litter than in control soil. There were no differences between soil types in the abundance of suilloid/wilcoxina and orange types.
FIG. 2.
Variations in the proportions of mycorrhizal morphotypes colonizing P. sylvestris seedlings after 2 years of growth in a nursery with untreated soil and soil amended with pine litter and oak litter. Each bar shows the mean for 25 replicates ± standard error. Letters indicate significant differences between growth media at a P value of <0.05 (Mann-Whitney U test).
DISCUSSION
Plant litter can influence the patterns of growth and development of forest trees through a number of processes involving both the physical and chemical environment. Studies of the effects of litter removal outnumber studies of the effects of litter addition more than 2:1. There are even fewer studies addressing the influence of plant litter addition on seedling growth under nursery conditions (50). Depending on the litter type, a number of interacting factors may be present, including physical constraints on root growth, water relations, allelopathic chemical leaching from the litter, and nutrient availability (39). The amount of litter and the way the litter is introduced into the soil (as a layer deposited on the soil surface [12] or thoroughly mixed with the soil [31]) may also be of importance.
In our experimental system, a layer of pine or oak litter was placed on the surface of the nursery bed soil in order to mimic natural litter cover. There were significant differences in seedling growth and establishment in control soil versus the litter treatments. Oak litter appeared to be most favorable for seedling survival. With oak litter, the survival rate was more than 73%, whereas with the untreated control, only 44% of seedlings remained alive. Several physical and chemical factors may influence survival. Physical factors include humidity and temperature, which are affected by the depth of the litter layer. Field and greenhouse results show an increase in seedling establishment with a moderate litter layer but reduced emergence with a thick litter layer (18, 28). The litter layer acts as an interface between the soil surface and the atmosphere, providing the soil surface with a degree of protection from rain (5) and solar radiation (41) and buffering it against fluctuations in temperature (33, 45) and water content (23). In our study, a layer of humus (20 cm in the beginning of the experiment) effectively protected the growth medium against changes in air temperature and made seedlings more resistant to drought. It was especially effective in the oak litter treatment. Oak litter provides cooler and moister conditions (28), which might have been important during the dry and hot summers of 2004 and 2005, when the experiments were performed. An earlier study showed that litter addition improves the growth of dipterocarp seedlings in tropical rain forests (6).
As a major source of soil organic matter, litter often plays a role in determining the pH of the soil surface horizon. Compared to the pH in the control soil (untreated mineral bed soil, pH 6.1), the pH was lower in pine litter (5.8) and higher in oak litter (6.3). The increased pH in oak litter growth medium was probably related to the increased content of base cations (Ca, K, and Mg). The decreased pH in pine litter growth medium might be related to organic acids (including acetic acid, oxalic acid, humic acid, and tannic acid) liberated from litter. This is especially acute in soils under coniferous trees, such as pine, spruce, and fir trees, which return fewer base cations to the soil than do most deciduous trees (19).
In our experiment, the most pronounced effects of litter amendment were increases in the C/N ratio and C content. The C content was increased twofold in growth medium from the pine litter treatment and threefold in the growth medium of the oak litter treatment. These findings are consistent with the results of measurements obtained after a few years of litter addition (42, 45). Litter addition resulted also in higher total N, P, K, Ca, and Mg concentrations in oak growth medium than pine growth medium, and this is likely connected with the growth of seedlings and mycorrhizal development. Pine litter, but not oak litter, increased seedling height relative to the control (a 14% increase). Relative to the control, there were 36% and 32% increases in the dry weight of seedlings with pine and oak litter, respectively. This was primarily the result of better needle growth rather than enhanced root growth; root growth was also affected, but not significantly. Litter addition, particularly the addition of pine litter, also increased the ratio of live to dead mycorrhizae, suggesting increased root growth or decreased mortality in litter-treated plots. Soil moisture affects fine root biomass: a wet forest stand has more living than dead roots, and drought increases the mortality of fine roots (44).
The lack of a correlation between growth parameters and foliar nitrogen and phosphorus concentration led us to suppose that these were not growth-limiting factors. However, if the tissue concentrations of N and P are expressed as total needle accumulation (needle concentration times mass), uptake of N and P was significantly higher in plants from pine- and oak-amended soils than in plants grown in control soil. Some macroelements (K and Ca) and microelements (Fe, Mn, Cu, and Zn) also appeared to be more available in plants grown in soil amended with oak versus pine litter, but not all of the differences were statistically different.
Among the distinguished mycorrhizal morphotypes, eight different mycorrhizal taxa were identified: S. luteus, S. variegatus, W. mikolae, a Tuber species, a Tomentella species, C. geophilum, A. byssoides, and one ECM symbiont not identified to species level. One morphotype, designated the wilcoxina/suilloid type, consisted of two species (W. mikolae and S. luteus). Multispecies morphotypes are common in nursery conditions (M. Rudawska, personal communication) and reflect a succession of colonizations of Scots pine seedlings ranging from ectendomycorrhizal Wilcoxina to ECM suilloid fungi. The species diversity of mycorrhizal fungi observed in this nursery is consistent with the range described for Scots pine seedlings from bare-root forest nurseries (48, 29, 38).
Oak litter increases diversity of mycorrhizal fungi, as eight different species were found in this treatment in comparison to six for the control soil and five for the pine litter treatment. There is some evidence that growth and nutrient uptake of birch or pine seedlings increases with increasing ECM diversity on tree root systems (3, 4, 32). Higher ECM diversity is also important as a pool of genetic diversity which permits the species (both phytobiont and mycobiont) to react to environmental change. Thus, higher diversity of ECM symbionts may also ensure better protection against pathogens and consequently greater survival in nursery and after outplanting (55).
Suilloid, Wilcoxina, and Cenococcum mycorrhizal types were dominant on tested seedlings, irrespective of litter addition. A. byssoides and a Tuber sp. were found only in litter treatments. These taxa are generally found more often in nurseries with nutrient-rich soils (48, 49, 53), and both species are more abundant in fertilized plots than in unfertilized control plots (21). Appearance of these mycorrhizae on litter-amended seedlings may be a result of propagules introduced with the litter. It may also reflect the ability of leaf litter to create a new ecological niche wherein some species can utilize extra resources better than they can in mineral soil.
The addition of forest litter had a greater effect on the relative proportions of mycorrhizal symbionts than it did on the species diversity. Seedlings grown in untreated nursery soil were 40% colonized by W. mikolae, which exhibits predominantly stress-tolerant, ruderal colonization strategies (52) and is one of the most common symbionts of conifers grown in commercial nurseries (29, 38, 48, 49, 53, 54). Addition of litter reduced the abundance of this symbiont in favor of suilloid mycorrhizae, represented by S. luteus and S. variegatus. This phenomenon was particularly pronounced for pine litter; there was a 40% increase in the relative abundance of suilloid mycorrhizae colonizing seedlings grown in pine litter and a 25% increase for seedlings grown in oak litter. Thus, we conclude that suilloid mycorrhizae are better adapted to the conditions related to litter addition than W. mikolae. A previous study reported a similar litter-associated shift in the proportions of W. mikolae and Suillus granulatus mycorrhizae colonizing Pinus contorta seedlings regenerated after a stand-replacing fire (12). One may speculate that increased abundance of suilloid mycorrhizae after litter treatment affects seedling growth and survival. ECM associations enhance water uptake by their hosts (43), and this enhancement has been attributed in part to production of rhizomorphs and their function in water transport (16). Suilloid mycorrhizae are classified as a long-distance exploration type, with a thick mantle, abundant fan-shaped extramatrical hyphae, and highly differentiated hydrophobic rhizomorphs (1). These features may be attributed to better water supply and may have resulted in higher rates of growth and survival of seedlings in our experiment.
The increased percentage of suilloid mycorrhizae with the addition of pine and oak litter also implies their use of organic forms of litter nutrients through the production of extracellular enzymes (46). A number of Suillus species have been found to use organic N compounds (13). The mycelial systems of Suillus species have been shown to possess a wide range of enzyme activities that can degrade more recalcitrant plant compounds; this has been demonstrated both in pure cultures and in an intact mycelial system (13). There is some evidence that ectomycorrhiza formation by S. luteus increases the decomposition of organic substrates added to soil microcosms (14).
A few findings suggest that the addition of forest litter contributes to the control of mycorrhizal fungal communities (6, 10, 15, 31). Our finding that forest litter amendment influenced the relative proportions of individual mycorrhizal symbionts rather than the overall symbiont distribution is similar to another finding regarding P. contorta seedlings (12).
In conclusion, this study shows that changes in the supply of organic matter through litter manipulation may have far-reaching effects on soil chemistry, influencing the growth and survival of Scots pine seedlings and their mycorrhizal communities. Molecular methods enable precise determination of the composition of such communities and prediction of the relationships between different functional groups of fungi and the environmental variables of litter. Therefore, the potential exists for development of indicators of mycorrhizal diversity that could be incorporated into forest management in the future. However, to test the general applicability of relationships between fungi and litter variables, further surveys incorporating a broader range of litter types and tree species are required.
Acknowledgments
We are grateful to Halina Narożna and Barbara Werner (Institute of Dendrology, Polish Academy of Sciences) for technical assistance. We also thank three anonymous reviewers for comments on the manuscript.
This research was funded by grant T-06052 from the Lithuanian State Science and Studies Foundation.
Footnotes
Published ahead of print on 8 June 2007.
REFERENCES
- 1.Agerer, R. 2001. Exploration types of ectomycorrhizae. Mycorrhiza 11:107-114. [Google Scholar]
- 2.Baar, J., W. A. Ozinger, I. L. Sweers, and T. W. Kuyper. 1994. Stimulatory and inhibitory effects of needle and grass extracts on the growth of some ectomycorrhizal fungi. Soil Biol. Biochem. 26:1073-1079. [Google Scholar]
- 3.Baxter, J. W., and J. Dighton. 2001. Ectomycorrhizal diversity alters growth and nutrient acquisition of grey birch (Betula populifolia) seedlings in host-symbiont culture conditions. New Phytol. 152:139-149. [DOI] [PubMed] [Google Scholar]
- 4.Baxter, J. W., and J. Dighton. 2005. Diversity-functioning relationships in ectomycorrhizal fungal communities, p. 383-398. In J. Dighton, J. White and P. Oudemans (ed.), The fungal community: its organization and role in the ecosystem, 3rd ed. Marcel-Dekker, New York, NY.
- 5.Benkobi, L., M. J. Trlica, and J. L. Smith. 1993. Soil loss as affected by different combinations of surface litter and rock. J. Environ. Qual. 22:657-661. [Google Scholar]
- 6.Brearley, F. Q., M. C. Press, and J. D. Scholes. 2003. Nutrients obtained from leaf litter can improve the growth of dipterocarp seedlings. New Phytol. 160:101-110. [DOI] [PubMed] [Google Scholar]
- 7.Castellano, M. A. 1996. Outplanting performance of mycorrhizal inoculated seedlings, p. 223-291. In K. G. Mukerji (ed.), Concepts in mycorrhizal research. Kluwer, Dordrecht, The Netherlands.
- 8.Colinas, C., R. Molina, J. Trappe, and D. Perry. 1994. Ectomycorrhizas and rhizosphere microorganisms of seedlings of Pseudotsuga menziessi (Mirb) Franco planted on a degraded site and inoculated with forest soils pretreated with selective biocides. New Phytol. 127:529-537. [Google Scholar]
- 9.Colpaert, J., and K. Van Tichelen. 1996. Decomposition, nitrogen and phosphorous mineralization from beech leaf litter colonized by ectomycorrhizal or litter decomposing basidiomycetes. New Phytol. 134:123-132. [Google Scholar]
- 10.Conn, C., and J. Dighton. 2000. Litter quality influences on decomposition, ectomycorrhizal community structure and mycorrhizal root surface acid phosphatase activity. Soil Biol. Biochem. 32:489-496. [Google Scholar]
- 11.Croghan, C. F. 1984. Survey for mycorrhizal fungi in lake states tree nurseries. Mycologia 76:951-953. [Google Scholar]
- 12.Cullings, K. W., M. H. New, S. Makhija, and V. T. Parker. 2003. Litter addition effects on the ectomycorrhizal associates of a lodgepole pine (Pinus contorta) stand in Yellowstone National Park. Appl. Environ. Microbiol. 69:3772-3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dahlberg, A., and R. D. Finlay. 1999. Suillus, p. 33-55. In J. W. G. Cairney and S. M. Chambers (ed.), Ectomycorrhizal fungi: key genera in profile. Springer, Berlin, Germany.
- 14.Dighton, J., E. D. Thomas, and P. M. Latter. 1987. Interactions between tree roots, mycorrhizas, a saprotrophic fungus and the decomposition of organic substrates in a microcosm. Biol. Fert. Soils 4:145-150. [Google Scholar]
- 15.Dighton, J., A. S. M. Bonilla, R. A. Jimînes-Nûnez, and N. Martînez. 2000. Determinants of leaf litter patchiness in mixed species New Jersey pine barrens forest and its possible influence on soil and soil biota. Biol. Fertil. Soils 31:88-293. [Google Scholar]
- 16.Duddridge, J. A., A. Malibari, and D. J. Read. 1980. Structure and function of mycorrhizal rhizomorphs with special reference to their role in water transport. Nature 287:834-836. [Google Scholar]
- 17.European Environment Agency. 2007. European forest types. Categories and types for sustainable forest management reporting and policy. EEA technical report no. 9/2006, 2nd ed. European Environment Agency, Copenhagen, Denmark.
- 18.Facelli, J. M., R. Williams, S. Fricker, and B. Ladd. 1999. Establishment and growth of seedlings of Eucalyptus obliqua: interactive effects of litter, water, and pathogens. Austr. J. Ecol. 24:484-494. [Google Scholar]
- 19.Finzi, A. C., N. Van Breemen, and C. D. Canham. 1998. Canopy tree-soil interactions within temperate forests: tree species effects on soil pH and exchangeable cations. Ecol. Appl. 8:447-454. [Google Scholar]
- 20.Food and Agriculture Organization of the United Nations. 1998. World reference base for soil resources. International Soil Reference and Information Centre, Rome, Italy.
- 21.Fransson, P. M. A., A. F. S. Taylor, and R. D. Finlay. 2000. Effects of continuous optimal fertilization on belowground ectomycorrhizal community structure in a Norway spruce forest. Tree Physiol. 20:599-606. [DOI] [PubMed] [Google Scholar]
- 22.Gardes, M., and T. Bruns. 1996. Community structure of ectomycorrhizal fungi in a Pinus muricata forest: above- and belowground views. Can. J. Bot. 74:1572-1583. [Google Scholar]
- 23.Ginter, D. L., K. W. Mcleod, and C. Sherrod. 1979. Water stress in longleaf pine induced by litter removal. Forest Ecol. Manag. 2:13-20. [Google Scholar]
- 24.Grant, W. 1952. The Dunemann system of nursery practice. Quart. J. For. 46:247-250. [Google Scholar]
- 25.Haselwandter, K., and G. D. Bowen. 1996. Mycorrhizal relations in trees for agroforestry and land rehabilitation. For. Ecol. Manag. 81:1-17. [Google Scholar]
- 26.Hedlund, K., and D. Gormsen. 2002. Mycorrhizal colonization of plants in set-aside agricultural land. Appl. Soil Ecol. 19:71-78. [Google Scholar]
- 27.Hutt, P. A. 1956. The Dunemann nursery system. Q. J. For. 50:155-156. [Google Scholar]
- 28.Ibáñez, I., and E. W. Schupp. 2002. Effects of litter, soil surface conditions, and microhabitat on Cercocarpus ledifolius Nutt. Seedling emergence and establishment. J. Arid Environ. 52:209-221. [Google Scholar]
- 29.Iwański, M., M. Rudawska, and T. Leski. 2006. Mycorrhizal associations of nursery grown Scots pine (Pinus sylvestris L.) seedlings in Poland. Ann. For. Sci. 63:715-723. [Google Scholar]
- 30.Jones, M. D., D. M. Durall, and J. W. G. Cairney. 2003. Ectomycorrhizal fungal communities in young forest stands regenerating after clearcut logging. New Phytol. 157:399-422. [DOI] [PubMed] [Google Scholar]
- 31.Jonsson, L. M., J. Dighton, J. Lussenhop, and R. Koide. 2006. The effect of mixing ground leaf litters to soil on the development of pitch pine ectomycorrhizal and soil arthropod communities in natural soil microcosm systems. Soil Biol. Biochem. 38:134-144. [Google Scholar]
- 32.Jonsson, L., M.-C. Nilsson, D. Wardle, and O. Zackrisson. 2001. Context dependent effects of ectomycorrhizal species richness on tree seedling productivity. Oikos 93:353-364. [Google Scholar]
- 33.Judas, M. 1990. The development of earthworm populations following manipulation of the canopy leaf litter in a beech wood on limestone. Pedobiologia 34:247-255. [Google Scholar]
- 34.Kõljalg, U., K. H. Larsson, K. Abarenkov, R. H. Nilsson, I. J. Alexander, U. Eberhardt, S. Erland, K. Hoiland, R. Kjøller, E. Larsson, T. Pennanen, R. Sen, A. F. Taylor, L. Tedersoo, and T. Vrålstad. 2005. UNITE: a database providing web-based methods for the molecular identification of ectomycorrhizal fungi. New Phytol. 166:1063-1068. [DOI] [PubMed] [Google Scholar]
- 35.Le Tacon, F. 1992. Variations in field response of forest trees to nursery ectomycorrhizal inoculation in Europe, p. 119-134. In D. J. Read, D. H. Lewis, A. H. Fitter, and I. J. Alexander (ed.) Mycorrhizas in ecosystems. CAB International, Cambridge, United Kingdom.
- 36.Lithuanian State Forest Management Institute. 2001. Lithuanian statistical yearbook of forestry. Lithuanian State Forest Management Institut, Kaunas, Lithuania.
- 37.Marx, D. H. 1991. The practical significance of ectomycorrhizae in forest establishment, p. 54-90. In Ecophysiology of ectomycorrhizae of forest trees. Marcus Wallenberg Foundation Symposium, Proc. 7. Marcus Wallenberg Foundation, Stockholm, Sweden.
- 38.Menkis, A., R. Vasiliauskas, A. F. S. Taylor, J. Stenlid, and R. Finlay. 2005. Fungal communities in mycorrhizal roots of conifer seedlings in forest nurseries under different cultivation systems, assessed by morphotyping, direct sequencing and mycelial isolation. Mycorrhiza 16:33-41. [DOI] [PubMed] [Google Scholar]
- 39.Michelsen, A., I. K. Schmidt, S. Jonasson, J. Dighton, H. E. Jones, and T. V. Callaghan. 1995. Inhibition of growth, and effects on nutrient uptake of arctic graminoids by leaf extracts—allelopathy or resource competition between plants and microbes? Oecologia 103:407-418. [DOI] [PubMed] [Google Scholar]
- 40.Molina, R., H. Massicote, and J. M. Trappe. 1992. Specificity phenomena in mycorrhizal symbioses: community-ecological consequences and practical implications, p. 357-423. In A. M. F. Routledge (ed.), Mycorrhizal functioning: an integrative plant-fungal process. Chapman and Hall, Inc., New York, NY.
- 41.Ogee, J., and Y. Brunet. 2002. A forest floor model for heat and moisture including a litter layer. J. Hydrol. 255:212-233. [Google Scholar]
- 42.Park, J. H., and E. Matzner. 2003. Controls on the release of dissolved organic carbon and nitrogen from a deciduous forest floor investigated by manipulations of aboveground litter inputs and water flux. Biogeochemistry 66:265-286. [Google Scholar]
- 43.Parke, J. L. 1983. The role of ectomycorrhizas in drought tolerance of Douglas-fir seedlings. New Phytol. 95:83-95. [Google Scholar]
- 44.Persson, H. 1983. The distribution and productivity of fine roots in boreal forests. Plant Soil 71:87-101. [Google Scholar]
- 45.Ponge, J. F., P. Arpin, and G. Vannier. 1993. Collembolan response to experimental perturbations of litter supply in a temperate forest ecosystem. Eur. J. Soil Sci. 29:141-153. [Google Scholar]
- 46.Read, D. J., J. R. Leake, and J. Perez-Moreno. 2004. Mycorrhizal fungi as drivers of ecosystem processes in heathland and boreal forest biomes. Can. J. Bot. 82:1243-1263. [Google Scholar]
- 47.Roldán, A., I. Querejeta, J. Albaladejo, and V. Castillo. 1996. Survival and growth of Pinus halepensis Miller seedlings in a semi-arid environment after forest soil transfer, terracing and organic amendments. Ann. Sci. For. 53:1099-1112. [Google Scholar]
- 48.Rudawska, M., T. Leski, and R. Gornowicz. 2001. Mycorrhizal status of Pinus sylvestris L. nursery stock in Poland as influenced by nitrogen fertilization. Dendrobiology 46:49-58. [Google Scholar]
- 49.Rudawska, M., T. Leski, L. K. Trocha, and R. Gornowicz. 2006. Ectomycorrhizal status of Norway spruce seedlings from bare-root forest nurseries. For. Ecol. Manag. 236:375-384. [Google Scholar]
- 50.Sayer, E. J. 2006. Using experimental manipulation to assess the roles of leaf litter in the functioning of forest ecosystems. Biol. Rev. 80:1-31. [DOI] [PubMed] [Google Scholar]
- 51.Smith, S. E., and D. J. Read. 1997. Mycorrhizal symbiosis. Academic Press, London, United Kingdom.
- 52.Taylor, D. L., and T. D. Bruns. 1999. Community structure of ectomycorrhizal fungi in a Pinus muricata forest: minimal overlap between the mature forest and resistant propagule communities. Mol. Ecol. 8:1837-1850. [DOI] [PubMed] [Google Scholar]
- 53.Trocha, L. K., M. Rudawska, T. Leski, and M. Dabert. 2006. Genetic diversity of naturally established ectomycorrhizal fungi on Norway spruce seedlings under nursery conditions. Microb. Ecol. 52:418-425. [DOI] [PubMed] [Google Scholar]
- 54.Ursic, M., and R. L. Peterson. 1997. Morphological and anatomical characterization of ectomycorrhizas and ectendomycorrhizas on Pinus strobus seedlings in a southern Ontario nursery. Can. J. Bot. 75:2057-2072. [Google Scholar]
- 55.Whipps, J. M. 2004. Prospects and limitations for mycorrhizas in biocontrol of root pathogens. Can. J. Bot. 82:1198-1227. [Google Scholar]
- 56.White, T. J., T. Bruns, S. Lee, and J. Taylor. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p. 315-322. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (ed.), PCR protocols, a guide to methods and applications. Academic Press, San Diego, CA.