Abstract
Candida albicans and Candida tropicalis are polymorphic fungi that develop antimicrobial-resistant biofilm communities that are characterized by multiple cell morphotypes. This study investigated cell type interconversion and drug and metal resistance as well as community organization in biofilms of these microorganisms that were exposed to metal ions. To study this, Candida biofilms were grown either in microtiter plates containing gradient arrays of metal ions or in the Calgary Biofilm Device for high-throughput susceptibility testing. Biofilm formation and antifungal resistance were evaluated by viable cell counts, tetrazolium salt reduction, light microscopy, and confocal laser scanning microscopy in conjunction with three-dimensional visualization. We discovered that subinhibitory concentrations of certain metal ions (CrO42−, Co2+, Cu2+, Ag+, Zn2+, Cd2+, Hg2+, Pb2+, AsO2−, and SeO32−) caused changes in biofilm structure by blocking or eliciting the transition between yeast and hyphal cell types. Four distinct biofilm community structure types were discerned from these data, which were designated “domed,” “layer cake,” “flat,” and “mycelial.” This study suggests that Candida biofilm populations may respond to metal ions to form cell-cell and solid-surface-attached assemblages with distinct patterns of cellular differentiation.
Biofilms are cell-cell or solid-surface-attached populations of microorganisms that are encased in a self-produced matrix of extracellular polymers. Biofilm formation is part of the ecological cycle for many yeasts including those from the genus Candida (10, 27). To date, more than 200 species of Candida have been identified, many of which are prevalent in rich soil and aquatic habitats that have been polluted with heavy metals (9, 22, 28). Candida albicans and Candida tropicalis are human opportunistic pathogens that are frequently isolated from these contaminated milieus. In fact, these two Candida spp. are known for high levels of resistance to many water-soluble metal ions, such as Hg2+, Pb2+, Cd2+, arsenate (AsO43−), and selenite (SeO32−) (3, 14, 25). Our research group has previously reported that C. tropicalis biofilms are up to 65 times more tolerant and/or resistant to heavy metal toxicity than the corresponding planktonic population (14). This suggests that biofilm formation may be a strategy for yeasts to survive exposure to these toxic inorganic ions.
C. albicans forms biofilms in a stepwise process that results in a polymer-entrenched arrangement of cells with budding yeast attached to the surface and tentacle-like chains of elongated hyphae, termed mycelia, on top (6, 13, 20). During our investigations of metal resistance (14), we have observed that metal exposure often affects the normal process of cellular differentiation that occurs during Candida biofilm development (J. J. Harrison, H. Ceri, and R. J. Turner, unpublished data). Cellular polymorphism in Candida populations may be significant as mature biofilms are more resistant to antifungal agents than those at an earlier stage of development (6). Furthermore, the polymorphic character of Candida spp. may play a role in pathogenic biofilm formation in some plants and animals, as hyphae may assist in the invasive penetration of physical barriers (27). There are a variety of environmental parameters that affect cellular differentiation in Candida spp.: these include temperature, nutrient status, CO2 levels, and pH as well as population density (23). The specific aim of this study was to examine how metal ions may affect cellular differentiation in C. albicans and C. tropicalis biofilms.
By using both light microscopy and confocal laser scanning microscopy (CLSM) in conjunction with three-dimensional (3D) visualization, this study examined the multicellular architecture of C. albicans and C. tropicalis biofilms before and after exposure to metal ions. This approach identified that many of the tested metal compounds functioned as environmental cues with the potential to block or to trigger a switch from yeast to hyphal cell morphotypes. At the level of the microbial community, metal exposure thus resulted in specific biofilm structure types. In other words, C. albicans and C. tropicalis differentiated in response to metal ions to form biofilms with distinct spatial arrangements of cells.
MATERIALS AND METHODS
Strains and growth media.
C. albicans 3153A (21) and C. tropicalis 99916 (14) were stored at −70°C in a Microbank (ProLab Diagnostics, Toronto, Canada) according to the manufacturer's directions. These yeasts were grown either in Trypticase soy broth (TSB; pH 7.2; EMD Chemicals Inc., Gibbstown, NJ) or in RPMI 1640 medium that was supplemented with l-glutamine (RPMI 1640 plus l-Glu; pH 7.2; Sigma-Aldrich, Oakville, Ontario, Canada) and 0.165 M 3-N-morpholinopropanesulfonic acid (MOPS; Sigma-Aldrich). Subcultures and viable cell counts were carried out by plating cells on Trypticase soy agar (EMD Chemicals Inc.) and incubating them at 35°C for 48 h. Serial dilutions were performed using 0.9% saline.
Stock solutions of antimicrobials and metal compounds.
Amphotericin B deoxycholate, sodium arsenite (NaAsO2), cadmium sulfate (CdSO4·8/3H2O), lead nitrate [Pb(NO3)2], sodium selenite (Na2SeO3), and silver nitrate (AgNO3) were purchased from Sigma Chemical Company, St. Louis, MO. Cupric sulfate (CuSO4·5H2O), potassium dichromate (K2Cr2O7), mercuric chloride (HgCl2), and zinc sulfate (ZnSO4·7H2O) were purchased from Fischer Scientific, Ottawa, ON, Canada. Cobalt chloride (CoCl2) was purchased from British Drug Houses Limited, Poole, England. All of these compounds were dissolved in double-distilled water and passaged through 0.22-μm syringe filters into sterile glass vials. Metal compounds and amphotericin B were stored at room temperature and −20°C, respectively. Working solutions of metals or amphotericin B were prepared by diluting the stock solutions with TSB or RPMI 1640 30 min prior to use.
Biofilm cultivation in microtiter plates.
The method of Ramage and Lopez-Ribot (26) was used to cultivate biofilms of C. albicans 3153A and C. tropicalis 99916 in flat-bottomed, 96-well Nunc microtiter plates (VWR International Ltd., Mississauga, Ontario, Canada). Nunc cell culture plates are made from polystyrene that is chemically modified to introduce charged functional groups to the polymer. This plastic is a suitable substratum for both C. albicans and C. tropicalis biofilm growth. In this approach, serial twofold dilutions of metal cations and oxyanions were made along the rows of wells in microtiter plates using RPMI 1640 medium. For each metal ion, 10 concentrations were examined in quadruplicate, leaving the first and last well of each row to serve as sterility and growth controls, respectively. In this regard, biofilms were grown in challenge plates prepared in a fashion similar to that for plates used for antifungal susceptibility testing (8).
To inoculate these microtiter plates, C. albicans and C. tropicalis were streaked out twice on Trypticase soy agar and an inoculum was prepared by suspending colonies from the second agar subculture into 0.9% saline to match a 1.0 McFarland standard. Five microliters of the inoculating suspension was added to each well of the microtiter plates, and this resulted in a starting cell number of approximately 1 × 105 CFU ml−1. The inoculated plates were then placed on a gyratory shaker at 100 rpm, 35°C, and 95% relative humidity for 48 h. MICs were determined to assess the growth inhibition of C. albicans and C. tropicalis by metal ions by using a method adapted from the work of Fothergill and McGough (8). Here, this was accomplished by reading the optical density at 650 nm (OD650) of these growth plates using a Thermomax microtiter plate reader with Softmax Pro data analysis software (Molecular Devices, Sunnyvale, CA) after 48 h of incubation.
Examination of planktonic cells.
In a series of additional assays, C. albicans and C. tropicalis were grown in RPMI 1640 plus l-glutamine plus 0.165 M MOPS in microtiter plates for 48 h at 35°C on a gyratory shaker (in a process identical to that used to grow biofilms), except that plates were shaken at 175 rpm. One-hundred-microliter aliquots of planktonic cell suspensions were removed from the wells and transferred into new microtiter plates. Planktonic cells were then collected by centrifugation (1,100 × g for 5 min) and directly examined in microtiter plate wells by light microscopy (described below).
Biofilm cultivation in the CBD.
The method of Ceri et al. (5) was used to grow C. tropicalis 99916 biofilms in the Calgary Biofilm Device (CBD) (Innovotech, Edmonton, Alberta, Canada). This device consists of a lid with 96 pegs that may be fitted into a standard 96-well microtiter plate. The CBD is made of polystyrene and is thus a hydrophobic surface. Here, each device was modified by coating the pegs with l-lysine to facilitate the growth of C. tropicalis (13, 14). The CBD, when used as supplied by the manufacturer or when modified with l-lysine, did not support the growth of C. albicans (data not shown). To inoculate these devices, a 1.0-McFarland-standard inoculum was prepared as described above. This standard inoculum was then diluted 30-fold in TSB to attain a starting viable cell count of roughly 1 × 105 CFU ml−1. One hundred fifty microliters of this inoculum was transferred into each well of a 96-well microtiter plate. The dried, l-lysine-coated peg lids were then inserted into the microtiter plates containing this inoculum. These devices were placed on a gyratory shaker at 100 rpm for the desired incubation time at 35°C and 95% relative humidity.
Following this initial period of incubation, CBD biofilms were rinsed once with 0.9% saline (by placing the lid in a microtiter plate containing 200 μl of saline in each well) to remove loosely adherent planktonic cells. Four pegs were broken from each device after it had been rinsed, and these pegs were used to determine biofilm viable cell counts. Each of these pegs was placed into 200 μl of 0.9% saline and then disrupted using an ultrasonic cleaner on the setting “high” for a period of 5 min (Aquasonic model 250 HT; VWR Scientific, Mississauga, Canada). The disrupted biofilm cells were serially diluted 10-fold and then plated onto agar for viable cell counting.
In an additional set of experiments, CBD biofilms were grown for the initial 48-h period and then placed into microtiter plates with fresh medium containing one of the following: TSB, TSB plus 2.0 mM SeO32−, or TSB plus 0.25 mM CrO42−. In other words, SeO32− and CrO42− were added at subinhibitory concentrations that were otherwise sufficient to shift the predominant cell morphotype in C. tropicalis biofilms (see Results). The biofilms were then returned to the gyratory shaker (at 100 rpm) and incubated for an additional 24 h at 35°C and 95% relative humidity. Viable cell counts for these 72-h biofilms were then determined as outlined above.
Metal and antifungal susceptibility testing of C. tropicalis biofilms using the CBD.
High-throughput antibiotic and metal susceptibility testing of microbial biofilms using the CBD was performed as previously described (12, 15). In brief, serial twofold dilutions of metals were made from working solutions (in TSB) along the rows of wells in a 96-well microtiter plate. For each metal, 10 concentrations were examined in quadruplicate, leaving the first and last well of each row to serve as a sterility and growth control, respectively. Each well had a final volume of 200 μl, which was sufficient to completely immerse the biofilms. Following an initial growth period of 48 h, Candida biofilms were rinsed with 0.9% saline and then inserted into these challenge plates. During exposure, these plates were placed in an incubator at 35°C and 95% relative humidity. MICs were determined for the CBD model system by reading the OD650 of the challenge plates after an additional 48 h at 35°C (5). Where appropriate and after rinsing of the pegs, biofilm viable cell counts were obtained as described above.
Tetrazolium reduction assays.
Metal- or antifungal-exposed CBD biofilms were rinsed twice with 0.9% saline and then inserted into microtiter plates that contained the following in each well: 150 μl of phosphate-buffered saline (pH 7.2), 25 μl of TSB, and 25 μl of CellTiter 96 AQueous One solution (Promega Corporation, Madison, WI). AQueous One solution contains the tetrazolium salt 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS), which is presumably reduced to a colored formazan product by NADH and NADPH from metabolically active cells (4). These plates were wrapped in tinfoil and incubated at 35°C for 120 min. Biofilm metabolic activity was assessed by reading the OD490 of these plates on a microtiter plate reader as described above.
Light microscopy.
C. albicans and C. tropicalis biofilms cultivated in the bottoms of microtiter plate wells were examined using an inverted light microscope (Wild of Canada Limited, Ottawa, Ontario, Canada). Alternatively, an upright light microscope was used to examine pellicle formation (Wild of Canada Limited). Pictures were captured using a DCM130 1.3-megapixel digital video eyepiece camera with ScopePhoto 2.0 image software (TrueVision Microscopes Incorporated, St. Louis, MO). Images were contrast and brightness enhanced using Adobe Photoshop 7.0 (Adobe Systems Inc., San Jose, CA).
CLSM.
Biofilms were rinsed once with 0.9% saline and then fluorescently stained either with the Live/Dead BacLight Kit (Molecular Probes, Burlington, Ontario, Canada) or with Syto-9 and tetramethyl rhodamine isothiocyanate-conjugated concanavalin A (TRITC-ConA; Molecular Probes). These staining and fixing techniques were carried out according to the methods of Harrison et al. (13). Briefly, the Live/Dead BacLight kit contains two nucleic acid intercalators, Syto-9 (green emission, membrane permeant) and propidium iodide (red emission, membrane impermeant). Using this system, live cells stain green and dead cells stain orange-red. This has been previously shown to correlate well with viable cell counts of a calibrated suspension of Candida albicans (18). TRITC-ConA is a lectin with high affinity for glucose, glucosamine, and mannose sugars present in the cell wall and extracellular matrix of C. albicans and C. tropicalis (1). In cases where biofilm extracellular polysaccharides were stained with TRITC-ConA, biofilms were fixed with 5% glutaraldehyde.
Fluorescently labeled biofilms were placed in 2 drops of 0.9% saline on the surface of a glass coverslip. These pegs were examined using a Leica DM IRE2 spectral confocal and multiphoton microscope with a Leica TCS SP2 acoustic optical beam splitter (Leica Microsystems, Richmond Hill, Ontario, Canada). To eliminate artifacts associated with single or simultaneous dual wavelength excitation, samples were sequentially scanned, frame by frame, first at 488 nm (Syto-9) and then at 543 nm (propidium iodide or TRITC-ConA). Fluorescence emission was then sequentially collected in the green and red regions of the spectrum. A 63× water-immersion objective was used in all imaging experiments. Image capture and two-dimensional (2D) reconstruction of z-stacks were performed using Leica Confocal Software (Leica Microsystems).
3D visualization of microbial biofilms.
3D biofilm visualizations and computer animations were created using amira 3.1 (Mercury Computer Systems Inc., Chelmsford, MA) as previously reported by Harrison et al. (13). In this study, two methods were used to create 3D visualizations of microbial biofilms: (i) surface rendering, where 3D surfaces were created by interconnecting the boundary data points in CLSM image stacks, and (ii) volume rendering (or ray casting), where biomass was represented by direct visualization of the 3D volume data set. Both methods of 3D visualization allowed for dynamic display of the biofilm and enabled the examination of the biofilm from any arbitrarily selected viewpoint. This allowed for the detection of features not apparent from static 2D image stacks or from 3D images created using Leica Confocal Software.
RESULTS
Evaluation of the model systems used for studying Candida biofilm formation.
Although biofilm formation by C. albicans is well characterized, little is known about biofilm formation by C. tropicalis. In this study, we used two complementary approaches to address this problem. First, biofilm formation by C. albicans 3153A was examined side by side with that of C. tropicalis 99916 by using the microtiter plate growth method of Ramage and Lopez-Ribot (26). This method allowed for the direct examination of Candida biofilms by light microscopy. Biofilm formation by C. albicans 3153A and C. tropicalis 99916 in microtiter plates was similar in many regards to the stepwise developmental process described for C. albicans biofilm formation in the literature (6). However, mature C. tropicalis biofilms had fewer hyphae than similarly aged C. albicans biofilms (Fig. 1 and 2).
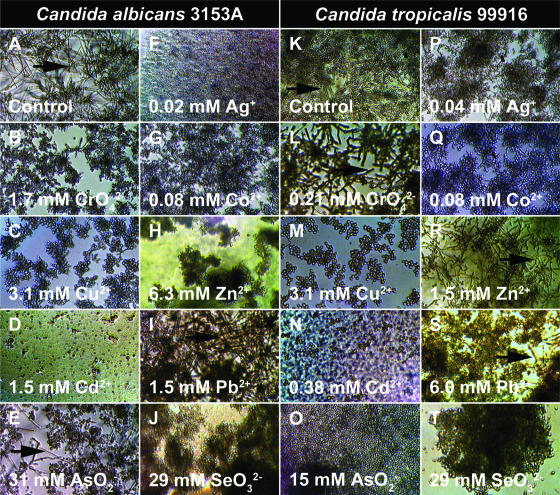
FIG. 1.
Metal ions may promote or inhibit cellular differentiation during biofilm growth of C. albicans 3153A and C. tropicalis 99916. Biofilms were grown in RPMI 1640 plus l-glutamine plus 0.165 M MOPS in microtiter plates for 48 h at 35°C on a gyratory shaker. (A) The untreated C. albicans 3153A biofilms consisted of yeast cells interspersed with many hyphae. (B to J) C. albicans 3153A biofilms were grown in medium containing the indicated metal ion, and with the exception of Pb2+, all of the metal ions inhibited hyphal formation. (K) The untreated C. tropicalis 99916 biofilms consisted of yeast cells intertwined with hyphae. In comparison to C. albicans, C. tropicalis produced fewer hyphae in the community. (L to T) C. tropicalis 99916 biofilms were grown in medium containing the indicated metal ion. For C. tropicalis grown in these conditions, CrO42− and Zn2+ triggered the formation of hyphal cells, whereas the remaining metal ions inhibited the formation of hyphal cells. For the sake of comparison, an inhibitory concentration of Cd2+ has been shown for C. albicans and C. tropicalis. These digital photos were captured at ×100 magnification, and the images were contrast and brightness enhanced using Adobe Photoshop 7.0. Arrows indicate the hyphal cells in the biofilm populations.
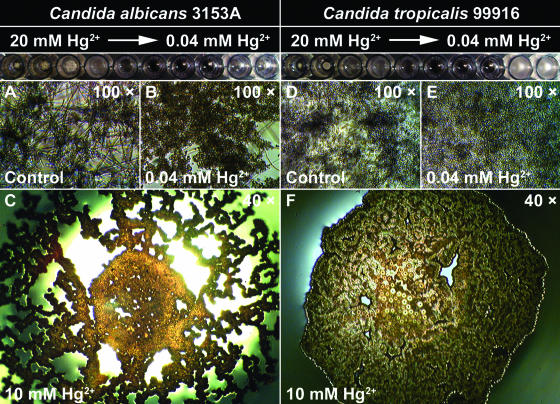
FIG. 2.
C. albicans 3153A and C. tropicalis 99916 undergo multiple shifts in growth rate and biofilm community structure when cultivated in a concentration gradient of divalent mercury cations (Hg2+). Biofilms were grown in RPMI 1640 plus l-glutamine plus 0.165 M MOPS in microtiter plates for 48 h at 35°C on a gyratory shaker. Pictured at the top of each column is a row from a microtiter plate that contained a log2 concentration gradient of Hg2+ (ranging from 20 mM to 0.04 mM). (A and D) Untreated C. albicans 3153A and C. tropicalis 99916 biofilms contained yeast cells interspersed with hyphae. (B and E) C. albicans 3153A or C. tropicalis 99916 biofilms cultivated in as little as 0.04 mM Hg2+ consisted of yeast cells only. (C and F) As the concentration of Hg2+ increased, C. albicans 3153A and C. tropicalis 99916 gradually abandoned solid-surface attachment, and at ≥10 mM Hg2+, these microorganisms formed a pellicle of yeast cells at the air-liquid interface.
The curved polystyrene pegs in the CBD are detachable; therefore, the peg surfaces were modified to facilitate yeast surface adhesion and, most importantly, were removed as required to facilitate CLSM. Note that the CBD, either used as supplied by the manufacturer or modified in a way that supported biofilm growth for C. tropicalis, could not be used to cultivate C. albicans biofilms (data not shown). The CBD is made of polystyrene, a hydrophobic substratum that may interfere with C. albicans surface adhesion (19). However, biofilm formation by C. tropicalis in the CBD followed a stepwise progression similar to that observed in microtiter plates. An advantage of the CBD is the easy transfer of biofilms to subsequent microtiter plates, and therefore, this system was additionally used for high-throughput biofilm susceptibility testing of C. tropicalis.
Growth inhibition of C. albicans and C. tropicalis by metal ions.
Candida spp. are known for high levels of resistance to metal ions. A logical starting point for this study was to determine metal ion MICs. Our research group has previously determined the susceptibility of planktonic C. tropicalis 99916 to metal ions using a broth microdilution assay in rich medium (14). Here, two alternative and nonstandard approaches were used to determine growth inhibition of C. albicans and C. tropicalis. This was carried out to enable a direct comparison between MICs and the concentrations of metal ions affecting cellular differentiation in biofilm communities.
First, MIC determinations were made from the Ramage and Lopez-Ribot (26) microtiter plate assays used to grow C. albicans and C. tropicalis biofilms in minimal medium (RPMI 1640 plus l-glutamine plus 0.165 M MOPS). In other words, planktonic growth inhibition was assessed from the same microtiter plates (with a serial, twofold dilution gradient of metal ion concentrations) that were used to grow biofilms from a planktonic inoculum. These susceptibility data are presented in Table 1, and each MIC is reported as the median and range of four to eight independent replicates.
TABLE 1.
Minimum concentrations of metal ions that inhibit growth or that affect the cellular differentiation of C. albicans 3153A and C. tropicalis 99916 during the initial stages of biofilm developmenta
| Organism and metal ion | MIC (mM)b | Minimum concn blocking or enhancing the yeast-hyphal cell type transition (mM)b | Predominant cell typec |
|---|---|---|---|
| C. albicans 3153A | |||
| CrO42− | 6.8 (6.8-14) | 0.85 (0.43-1.7) | Yeast |
| Co2+ | 0.70 (0.31-0.78) | 0.03 (0.02-0.04) | Yeast |
| Cu2+ | 3.1 (3.1-6.3) | 1.6 (1.6-3.1) | Yeast |
| Ag+ | 0.06 (0.04-0.08) | 0.01 (0.01-0.02) | Yeast |
| Zn2+ | 15 | 1.4 (0.9-1.9) | Hyphae |
| Cd2+ | NDd | ND | Yeast |
| Hg2+ | 0.16 | 0.04 | Yeast |
| Pb2+ | >24.1 | No change | |
| AsO2− | 55 | 41 (14-55) | Yeast |
| SeO32− | 58 (29-58) | 15 (7.3-15) | Yeast |
| C. tropicalis 99916 | |||
| CrO42− | 1.7 | 0.21 | Hyphae |
| Co2+ | 0.69 (0.31-0.75) | 0.02 | Yeast |
| Cu2+ | 6.3 | 3.1 | Yeast |
| Ag+ | 0.06 (0.05-0.08) | 0.08 (0.04-0.08) | Yeast |
| Zn2+ | 6.3 (6.3-13) | 1.8 (0.45-3.1) | Hyphae |
| Cd2+ | ND | ND | Yeast |
| Hg2+ | 0.15 (0.15-0.31) | 0.04 | Yeast |
| Pb2+ | >24.1 | 6.0 (3.0-12) | Yeast |
| AsO2− | 62 | 10 (6.8-13.6) | Yeast |
| SeO32− | 29 | 7.3 (7.3-14) | Yeast |
Biofilms were grown in the presence of metal ions in microtiter plates using RPMI 1640 medium.
Values are expressed as the median of four to eight replicates with the range (if applicable) indicated in parentheses.
Cell morphotype to which the population shifted after exposure to the indicated metal ion concentration.
ND, not determined due to the formation precipitates.
The second approach used here was to determine MICs from the CBD challenge plates. In this model system, cells shed from the surface of biofilms (at an intermediate stage of biofilm development) served as the inoculum for the planktonic susceptibility assay. In other words, planktonic cells were derived from biofilms that may have continued growing, developing, and/or shedding during a 24-h metal exposure. These susceptibility data are presented in Table 2, and each value is reported as the median and range of four to eight independent replicates.
TABLE 2.
Minimum concentrations of metal ions that inhibit growth or that affect the cellular differentiation of C. tropicalis 99916 at an intermediate stage of biofilm developmenta
| Metal ion | MIC (mM)b | Minimum concn blocking or enhancing the yeast-hyphal cell type transition (mM)b | Predominant cell typec |
|---|---|---|---|
| CrO42− | 4.4 (4.4-18) | 0.27 (0.27-2.2) | Hyphae |
| Co2+ | 4.2 (2.2-4.3) | ≤0.53 | Yeast |
| Cu2+ | 48 (31-64) | 0.49 (≤0.49-0.49) | Yeast |
| Ag+ | 0.59 | ≤0.59 | Yeast |
| Zn2+ | NDd | ≤0.96 | Yeast |
| Cd2+ | 4.5 (1.8-7.2) | 1.1 | Yeast |
| Hg2+ | 0.46 (0.31-0.62) | ≤0.08 | Yeast |
| Pb2+ | 40 | 2.4 | Yeast |
| AsO2− | 29 (19-39) | ≤1.2 | Yeast |
| SeO32− | 63 | ≤2.0 | Yeast |
Biofilms were grown for 48 h in the CBD using TSB medium and were then exposed to metal ions for an additional 24 h.
Values are expressed as the median of four to eight replicates with the range (if applicable) indicated in parentheses.
Cell morphotype to which the population shifted after exposure to the indicated metal ion concentration.
ND, not determined due to the formation of precipitates.
Sub-MIC concentrations of metal ions influence cellular differentiation in C. albicans and C. tropicalis biofilms.
To determine if metal ions affect cellular differentiation in C. albicans and C. tropicalis, individual wells in the microtiter plates used to grow biofilms were systematically examined using light microscopy. Cell morphology in biofilm communities was qualitatively ranked on a five-point scale (all ranks were relative to the growth control; 1, a large shift towards the hyphal cell type; 2, a small shift towards the hyphal cell type; 3, approximately the same proportions of yeast and hyphal cells as those in the growth control; 4, a small shift towards the yeast cell type; 5, a large shift towards the yeast cell type). The minimum concentration blocking or enhancing the yeast-hyphal cell morphotype transition was defined as the lowest concentration resulting in a rank score of ≤2 or ≥4. These data are presented in Table 1; each value represents the median and range of four to eight independent replicates, and the predominant cell type at that concentration is indicated.
Under these growth conditions, almost all of the metal ions that were examined blocked or triggered the transition between yeast and hyphal cell types in Candida biofilms. Light microscopy images of these biofilms were captured using a digital camera, and representative pictures are shown in Fig. 1. In most instances, metal ions (Co2+, Cu2+, Ag+, Cd2+, Hg2+, Pb2+, AsO2−, and SeO32−) inhibited hyphal cell type formation in biofilms of C. albicans and C. tropicalis. Note that in this study, we used an incubation temperature of 35°C for biofilm cultivation. Since temperature may affect cellular differentiation in Candida spp., we repeated a portion of these assays at 37°C. Metal ions affected cellular differentiation in similar fashions at these two temperatures (data not shown).
It is worth noting that C. albicans and C. tropicalis did not respond in the same way to all metal ions; for example, CrO42− triggered the transition to the hyphal cell morphotype in C. tropicalis 99916 biofilms (Fig. 1L), whereas C. albicans 3153a biofilms exposed to this heavy metal oxyanion were mostly yeast cells (Fig. 1B). A similar situation was observed for Zn2+ (Fig. 1H and R). In the cases of Co2+, Ag+, and Hg2+, concentrations in the micromolar range were sufficient to affect the yeast-hyphal cell type transition (Table 1). The response of C. albicans and C. tropicalis to Hg2+ was particularly interesting, as fungal growth was inhibited at an intermediate range of concentrations (0.16 to 0.64 mM) but resumed above a threshold concentration (1.24 mM) (Fig. 2). Furthermore, the location of biofilm formation in the microtiter plate wells changed with Hg2+ concentration. At Hg2+ concentrations of ≤0.08 mM, the majority of biofilm growth was surface adherent (Fig. 2B and E), whereas at higher concentrations (≥2.5 mM), C. albicans and C. tropicalis abandoned surface-adherent growth to form pellicles at the air-liquid interface (Fig. 2C and F).
We performed a similar, systematic light microscopy analysis for C. tropicalis 99916 biofilms cultivated in the CBD. In this model system, biofilms were grown for an initial 48-h period (corresponding to an intermediate stage of biofilm development) and then were transferred into challenge plates containing metal ions. These data are summarized in Table 2; each value represents the median and range of four to eight independent replicates, and the predominant cell type at that concentration is indicated.
Similar to the microtiter plate assay system, most of the metal ions examined (Co2+, Cu2+, Ag+, Zn2+, Cd2+, Hg2+, Pb2+, AsO2−, and SeO32−) suppressed hyphal formation at an intermediate stage of C. tropicalis biofilm development. Hg2+ was the most toxic ion examined in this model system, and it suppressed hyphal formation at a concentration of 0.08 mM. As a sole exception among the compounds tested, 0.27 mM CrO42− triggered hyphal cell formation. When C. tropicalis biofilm formation in microtiter plates (RPMI 1640 plus l-glutamine plus 0.165 M MOPS) was compared to that in the CBD (TSB), metal ions had a similar effect on cellular differentiation, with the exception of Zn2+.
In every case, the shift in the predominant cell morphotype of C. albicans 3153A and C. tropicalis 99916 biofilms occurred at concentrations of metal ions that were lower than the corresponding MICs (Tables 1 and 2). Note that metal ion concentrations lower than the MIC may reduce the population growth rate substantially; however, these data indicate that metal ions may suppress or enhance the interconversion of yeast and hyphal cell morphotypes without completely arresting population growth.
Metal ions may enhance or suppress cellular differentiation during planktonic growth of C. albicans and C. tropicalis.
We selected a subset of metal ions and examined their effect on the cellular dimorphism of planktonic cells (data not shown). Untreated C. albicans 3153A planktonic cell populations were composed of yeast cells and hyphae; however, when cells were grown in media containing metal ions (Co2+, Zn2+, Hg2+, Pb2+, and SeO32−), the formation of hyphal cells was suppressed (data not shown). Similarly, untreated C. tropicalis 99916 planktonic cell populations had yeast and hyphal cells; however, many metal ions (Cu2+, Hg2+, AsO2−, and SeO32−) inhibited hyphal formation by planktonic C. tropicalis. Similar to biofilms, Zn2+ triggered the formation of C. tropicalis 99916 hyphal cells (data not shown). In summary, the changes in planktonic cellular dimorphism were similar to those reported for biofilms. It is noteworthy that in many instances planktonic cells did not grow at concentrations of metal ions where biofilms otherwise formed at the bottom of microtiter plates. This observation of biofilm metal resistance fits well with a previous report from our research group (14).
Metal ions alter the normal course of C. tropicalis biofilm development and influence 3D community organization.
The development, structure, and survival of metal-exposed C. tropicalis 99916 biofilms (cultivated in the CBD) were further investigated by CLSM, by 3D visualization, and by viable cell counting. CLSM imaging was performed in triplicate for at least one subinhibitory and at least one fungistatic concentration for each metal ion, of which a representative example is illustrated in Fig. 3 and 4. The reported mean viable cell counts and standard deviations were based on three to eight independent replicates each.
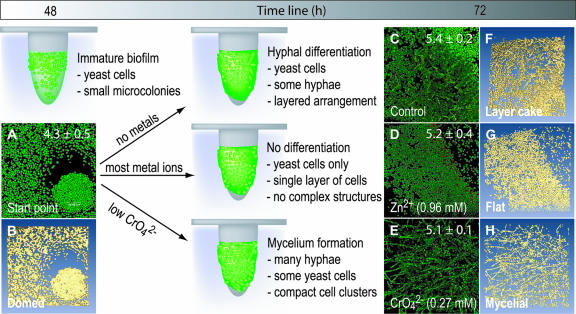
FIG. 3.
C. tropicalis 99916 forms biofilm communities with characteristic 3D structure that may be influenced by metal ions. Here, the heavy metal ions Zn2+ and CrO42− influenced the maturation of C. tropicalis communities at an intermediate stage of biofilm development. In these experiments, C. tropicalis was grown on pegs in the CBD and was then exposed to Zn2+ and CrO42− for 24 h. The exposed biofilms were stained with the Live/Dead BacLight kit, imaged by CLSM, and then visualized using amira 3.1. The mean and standard deviation of biofilm cell densities were evaluated by viable cell counting (log10 CFU peg−1), and this is indicated where appropriate. (A and B) The 2D average projection and isosurface rendering of a C. tropicalis biofilm grown for 48 h on the CBD. This “domed” biofilm structure type was named for the formation of small microcolonies of yeast cells in surface-adherent communities. (C and F) The 2D average projection and isosurface rendering of an untreated C. tropicalis biofilm grown for a total of 72 h. This structure type was named “layer cake” for the biphasic arrangement of yeast and hyphal cells in the community. (D and G) The 2D average projection and isosurface rendering of a C. tropicalis biofilm exposed to 0.96 mM Zn2+ for 24 h. These “flat” biofilms had few hyphae and lacked microcolony structures. (E and H) The 2D average projection and isosurface rendering of a C. tropicalis biofilm exposed to 0.27 mM CrO42− for 24 h. “Mycelial” biofilms consisted of masses of hyphal cells attached to the polystyrene surface with few yeast cells remaining in the community. Each panel represents an area of 238 by 238 μm. Green cells are alive, and red cells are dead.
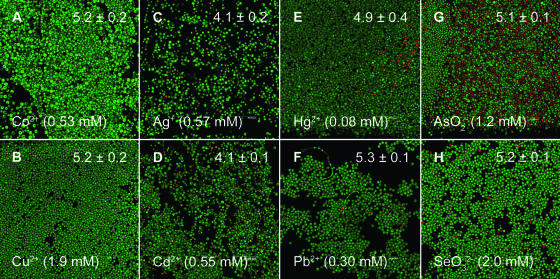
FIG. 4.
Many metal ions (Co2+, Cu2+, Ag+, Cd2+, Hg2+, Pb2+, AsO2−, and SeO32−) inhibited hyphal formation by C. tropicalis 99916 at an intermediate stage of biofilm development. In every case, treating biofilms with these compounds resulted in the “flat” biofilm community structure type. Biofilm populations were grown, imaged, and enumerated as described in the legend to Fig. 1. Each panel represents an area of 238 by 238 μm. Green cells are alive, and red cells are dead.
After 48 h of incubation in the CBD (the start point for these experiments), C. tropicalis formed a surface-adherent layer of yeast cells that was up to 20 μm thick. Growth was greatest near the air-liquid-surface interface at the top of the peg, and in some regions, yeast cells were organized into circular formations (Fig. 3A). After the initial 48-h incubation period, the growth medium was changed and the biofilms were returned to the incubator for an additional 24 h (with or without shaking to generate shear and flow forces). Similar processes of cellular differentiation occurred under both static and dynamic shear conditions; therefore, we initially chose to focus on static exposure conditions.
In the untreated controls, C. tropicalis biofilms continued to grow and to differentiate; hyphae and pseudohyphae were prominent near the air-liquid-surface interface (Fig. 3C). Biomass was heterogeneously distributed across the peg surface, and biofilms were up to 80 μm thick in some regions. Single-cell layers of yeast covered most of the remaining peg surface. Correlative with viable cell counts, Live/Dead staining qualitatively revealed that the vast majority (>95%) of C. tropicalis cells were viable. In contrast to growth controls, concentrations lower than 2 mM of Zn2+ (Fig. 3D), Co2+, Cu2+, Ag+, Cd2+, Pb2+, arsenite (AsO2−), and selenite (SeO32−), as well as 0.08 mM Hg2+, suppressed hyphal formation (Fig. 4). Strikingly, biofilms exposed to CrO42− produced the opposite result, triggering the formation of mycelia (Fig. 3F).
Collectively, four biofilm structure types were discerned from the data presented here, and an example of each was analyzed using 3D visualization; we have designated these as (i) “domed” (Fig. 3B), (ii) “layer cake” (Fig. 3D), (iii) “flat” (Fig. 3F), and (iv) “mycelial” (Fig. 3G). To summarize, the domed structure resulted from an extended, initial incubation of C. tropicalis on a shaker, where it formed mushroom-cap-shaped microcolonies composed entirely of yeast cells (Fig. 3B). Layer cake describes the archetypal mature Candida biofilm structure that had a basal layer of yeast cells with hyphae protruding into the bulk medium. This occurred after spent medium from biofilm cultures was replaced with fresh medium following the initial 48-h incubation period and biofilms were returned to the incubator (Fig. 3D). The flat structure type resulted from the addition of metal cations, AsO2− and SeO32−, to the growth medium (Fig. 3F). Finally, the mycelial structure type resulted from exposure of Candida biofilms to low levels of CrO42− (Fig. 3G). An isosurface-rendered computer animation of each Live/Dead-stained biofilm structure type has been provided elsewhere (see videos S1 to S4 in the supplemental material).
Metal ion exposure may alter the resistance of C. tropicalis biofilms to antimicrobial agents.
Previous studies have indicated that the multidrug resistance of C. albicans biofilms arises during community maturation, a process that is linked to cellular differentiation (6). Since metal ions may alter the normal process of Candida biofilm development, we specifically hypothesized that growing biofilms in subinhibitory concentrations of metals may alter the antifungal resistance of the surface-adherent community. In this set of experiments, C. tropicalis 99916 biofilms were grown in the CBD for an initial 48 h and then the growth medium was replaced with one of the following: TSB, TSB plus 2.0 mM SeO32−, or TSB plus 0.25 mM CrO42−. This approach was taken to produce C. tropicalis biofilm communities with the layer cake, flat, and mycelial structure types, respectively.
To rigorously investigate the process of cellular differentiation in these biofilm communities (under dynamic, as opposed to static, shear and flow force conditions), we stained these biofilms using Syto-9 and TRITC-ConA (to label cellular biomass and extracellular polysaccharides, respectively). Each CLSM imaging experiment was performed in triplicate, and a representative of each was analyzed by volume rendering. These additional data, illustrated in Fig. 5, indicate that in all instances, the C. tropicalis biofilm cells were encapsulated in a layer of extracellular polysaccharides and that the process of cellular differentiation mirrored that described for static exposure conditions.
FIG. 5.
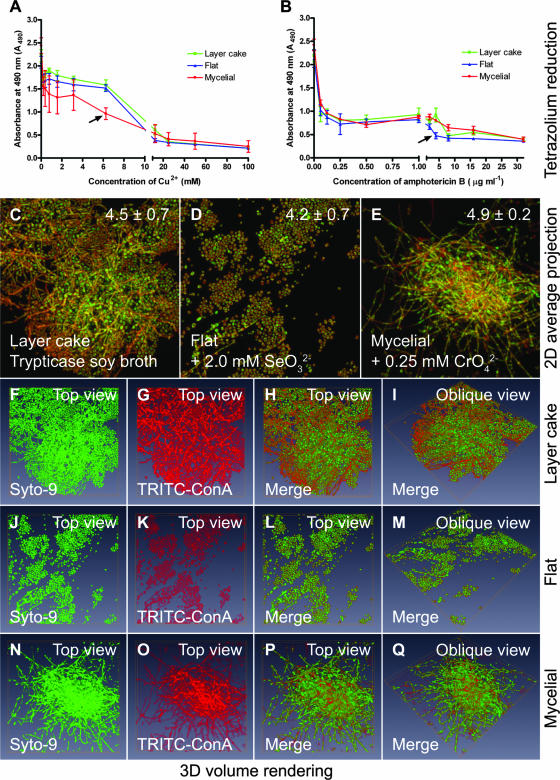
Cultivation of C. tropicalis 99916 biofilms in SeO32− and CrO42− decreases the resistance of the fungal community to amphotericin B and Cu2+, respectively. In these experiments, C. tropicalis biofilms were grown on pegs in the CBD for 48 h in TSB and then transferred into fresh medium containing no additive, SeO32−, or CrO42− for an additional 24 h. Biofilms cultivated in this manner were either exposed to antifungals (Cu2+ and amphotericin B) or fixed and then stained with Syto-9 and TRITC-ConA (for CLSM and 3D visualization). The mean and standard deviation of biofilm cell densities were evaluated by viable cell counting (log10 CFU peg−1), and this is indicated where appropriate. (A) Reduction of MTS, a tetrazolium salt, by C. tropicalis “layer cake,” “flat,” and “mycelial” biofilms after a 24-h exposure to Cu2+. (B) Reduction of MTS by C. tropicalis “layer cake,” “flat,” and “mycelial” biofilms after a 24-h exposure to amphotericin B. (C to E) The 2D average projections of CLSM image stacks for biofilms grown on the CBD pegs. (F to Q) Volume rendering of the Syto-9- and TRITC-ConA-labeled 3D volume data sets extrapolated from the image z-stacks used to create the images of the “layer cake,” “flat,” and “mycelial” biofilms in panels C to E. Each panel represents an area of 238 by 238 μm. Green fluorescence corresponds to cellular biomass, whereas red fluorescence corresponds to extracellular polymers.
Lastly, biofilms cultured under these conditions were exposed to Cu2+ and amphotericin B. The metabolic capacity of the exposed biofilms, as a measure of community resistance, was evaluated by reduction of MTS, a tetrazolium salt (Fig. 5). Although neither directly correlative nor conclusive, the loss of phenotypic variation in C. tropicalis 99916 biofilms induced by CrO42− and SeO32− was associated with a decrease in the biofilm resistance to Cu2+ and amphotericin B, respectively. Similar results were obtained using an Alamar Blue viability assay (data not shown). These data suggest that metal ion exposure may in some instances change the process of community maturation that gives rise to antifungal resistance.
DISCUSSION
The data in this report indicate that metal ions may suppress or enhance cellular differentiation in C. tropicalis biofilms, thereby giving rise to multicellular aggregates with spatially distinct distributions of cell morphotypes. This evidence implies either that metal ions may function as regulators (i.e., signals) or that subinhibitory concentrations of metal ions may induce stress that interferes with the transition of yeast to hyphal cell morphotypes. There is some evidence to support the direct interaction of certain metal ions with a C. albicans transcription factor, CaMac1 (17); however, the latter explanation fits well with the observed microbicidal synergy between metal ions and antifungals (either amphotericin B or Cu2+).
There are three logical precedents for undertaking this study. (i) In an ecological context, certain yeasts have the potential to overwhelm some bacterial species normally present in sites that have been polluted with metals (9). In principle, this may occur because bacterial biofilms are killed time-dependently by comparatively low concentrations of metal ions (11, 16), whereas biofilms of yeasts, such as Candida spp., may continue growing (14). This is an important concept, as a standard way of distinguishing C. albicans and C. tropicalis from bacteria and other Candida species is through the use of bismuth sulfite glucose glycine yeast agar, which employs a bismuth salt as a selective agent (24). (ii) The heavy metals Cu2+ and Zn2+ inhibit the transition between yeast and hyphal cell morphotypes in planktonic suspensions of C. albicans (2, 29). Yamaguchi (30) identified that the planktonic hyphal to yeast cell shift is optimal when 9 μM Zn2+ is added to a minimal medium depleted of trace metals. (iii) Biofilm formation by Candida spp. is a process that normally involves differentiation, and thus, community maturation (i.e., cellular polymorphism) concomitantly arises with drug resistance (6). This cumulative evidence led us to hypothesize that metal ions may influence cellular differentiation, community structure, and antifungal resistance of mature biofilms.
Biofilm formation by yeasts is best characterized for C. albicans. In this microorganism, biofilm formation is a stepwise developmental process that proceeds through three stages: (i) an “early” phase characterized by adhesion of blastospores (yeast cells) to the surface, (ii) an “intermediate” phase where yeast cells have proliferated to cover a large surface area and have begun to produce extracellular polymers, and (iii) a “maturation” phase. Mature C. albicans biofilms are matrix entrenched and arranged into layers, with yeast cells attached to the surface and hyphae on top (6, 20). The data in Fig. 1 to 4 indicate that metal ions may act at the intermediate stage of C. albicans and C. tropicalis biofilm development to redirect the final pattern of cellular differentiation in the solid-surface-attached community.
It is unlikely that the counter ions of metal salts—such as Na+, K+, Cl−, NO3−, or SO42−—influenced the observed morphological changes in C. albicans and C. tropicalis biofilms. For example, nitrate (NO3−) salts of both Pb2+ and Ag+ were used in this study. In the case of Ag+, morphological changes occurred in C. albicans biofilms around 0.04 mM, whereas for Pb2+ there was no observed change in cell morphology at concentrations of 24 mM. Further, Na+, K+, Cl−, NO3−, and SO42− are present in the rich and minimal medium formulations used to grow the biofilms in control and test groups.
The interaction of C. albicans and C. tropicalis with metal ions may represent a fundamental biotic-abiotic interaction. Since biofilm maturation involves the emergence of drug resistance in parallel with multiple cell morphotypes (6), metals may alter biofilm susceptibility to natural or synthetic antimicrobial agents. Furthermore, metal ions may be important regulators affecting a cell type switch that is coregulated with a transition from commensalism to virulence, suppressing the disease process of an otherwise infectious fungal biofilm (7). At the very least, the effect of metal ions on the differentiation of Candida spp. may be a preliminary biological indicator of metal pollution in the environment.
Supplementary Material
Acknowledgments
This work has been supported through discovery grants from the Natural Sciences and Engineering Research Council (NSERC) of Canada to R.J.T., H.C., and Y.H. NSERC has also provided a Canada Graduate Scholarship (CGSD) to J.J.H., who was additionally supported by a Ph.D. studentship from the Alberta Heritage Foundation for Medical Research (AHFMR). Corporate funding has been provided by Innovotech. The Informatics Circle of Research Excellence (iCORE) has provided a Graduate Scholarship award for J.Y. R.M. was funded by the Westaim Chair for Biofilm Research. M.R. has been funded by a postdoctoral fellowship from the Iranian Ministry of Health and Medical Education through the Faculty of Pharmacy at Shahid Beheshti University of Medical Sciences. CLSM was made possible through a Canadian Foundation for Innovation (CFI) Bone and Joint Disease Network grant to H.C. The Alberta Innovation and Science Research Investments Program (ISRIP) has also supported this project through a grant to R.J.T., H.C., and R.M.
Footnotes
Published ahead of print on 8 June 2007.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Al-Fattani, M. A., and J. L. Douglas. 2006. Biofilm matrix of Candida albicans and Candida tropicalis: chemical composition and role in drug resistance. J. Med. Microbiol. 55:999-1008. [DOI] [PubMed] [Google Scholar]
- 2.Bedell, G. W., and D. R. Soll. 1979. Effects of low concentrations of zinc on the growth and dimorphism of Candida albicans: evidence for zinc-resistant and -sensitive pathways for mycelium formation. Infect. Immun. 26:348-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berdicevsky, I., D. Lea, D. Marzbach, and S. Yannai. 1993. Susceptibility of different yeast species to environmental toxic metals. Environ. Pollut. 80:41-44. [DOI] [PubMed] [Google Scholar]
- 4.Berridge, M. V., and A. S. Tan. 1993. Characterization of the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch. Biochem. Biophys. 303:474-482. [DOI] [PubMed] [Google Scholar]
- 5.Ceri, H., M. E. Olson, C. Stremick, R. R. Read, D. W. Morck, and A. G. Buret. 1999. The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities in bacterial biofilms. J. Clin. Microbiol. 37:1771-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandra, J., D. M. Kuhn, P. K. Mukherjee, L. L. Hoyer, T. McCormick, and M. J. Ghannoum. 2001. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J. Bacteriol. 183:5385-5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Douglas, J. L. 2003. Candida biofilms and their role in infection. Trends Microbiol. 11:30-36. [DOI] [PubMed] [Google Scholar]
- 8.Fothergill, A. W., and D. A. McGough. 1995. In vitro antifungal susceptibility testing of yeasts, p. 5.15.1-5.15.16. In H. D. Isenberg and J. Hindler (ed.), Clinical microbiology procedures handbook, vol. 1. ASM Press, Washington, DC. [Google Scholar]
- 9.Hagler, A. N., and L. C. Mendocs-Hageler. 1981. Yeasts from marine and estuarine waters with different levels of pollution in the State of Rio de Janeiro, Brazil. Appl. Environ. Microbiol. 416:173-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall-Stoodley, L., J. W. Costerton, and P. Stoodley. 2004. Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol. 2:95-108. [DOI] [PubMed] [Google Scholar]
- 11.Harrison, J. J., H. Ceri, N. J. Roper, E. A. Badry, K. M. Sproule, and R. J. Turner. 2005. Persister cells mediate tolerance to metal oxyanions in Escherichia coli. Microbiology 151:3181-3195. [DOI] [PubMed] [Google Scholar]
- 12.Harrison, J. J., H. Ceri, C. Stremick, and R. J. Turner. 2004. Biofilm susceptibility to metal toxicity. Environ. Microbiol. 6:1220-1227. [DOI] [PubMed] [Google Scholar]
- 13.Harrison, J. J., H. Ceri, J. Yerly, C. A. Stremick, Y. Hu, R. Martinuzzi, and R. J. Turner. 2006. The use of microscopy and three-dimensional visualization to evaluate the structure of microbial biofilms cultivated in the Calgary Biofilm Device. Biol. Proced. Online 8:194-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrison, J. J., M. Rabiei, R. J. Turner, E. A. Badry, K. M. Sproule, and H. Ceri. 2006. Metal resistance in Candida biofilms. FEMS Microbiol. Ecol. 55:479-491. [DOI] [PubMed] [Google Scholar]
- 15.Harrison, J. J., R. J. Turner, and H. Ceri. 2005. High-throughput metal susceptibility testing of microbial biofilms. BMC Microbiol. 5:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison, J. J., R. J. Turner, and H. Ceri. 2005. Persister cells, the biofilm matrix and tolerance to metal cations in biofilm and planktonic Pseudomonas aeruginosa. Environ. Microbiol. 7:981-994. [DOI] [PubMed] [Google Scholar]
- 17.Huang, G. H., X. Y. Nie, and J. Y. Chen. 2006. CaMac1, a Candida albicans copper ion-sensing transcription factor, promotes filamentous and invasive growth in Saccharomyces cerevisiae. Acta Biochim. Biophys. Sin. (Shanghai) 38:213-217. [DOI] [PubMed] [Google Scholar]
- 18.Jin, Y., T. Zhang, Y. H. Samaranayake, H. H. P. Fang, H. K. Yip, and L. P. Samaranayake. 2005. The use of probes and stains for improved assessment of cell viability and extracellular polymeric substances in Candida albicans biofilms. Mycopathologia 159:353-360. [DOI] [PubMed] [Google Scholar]
- 19.Krom, B. P., J. B. Cohen, G. E. McElhaney-Feser, and R. L. Cihlar. 2007. Optimized candidal biofilm microtiter assay. J. Microbiol. Methods 68:421-423. [DOI] [PubMed] [Google Scholar]
- 20.Kuhn, D. M., J. Chandra, P. K. Mukherjee, and M. A. Ghannoum. 2002. Comparison of biofilms formed by Candida albicans and Candida parapsilosis on bioprosthetic surfaces. Infect. Immun. 70:878-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lafleur, M. D., C. A. Kumamoto, and K. Lewis. 2006. Candida albicans biofilms produce antifungal tolerant persister cells. Antimicrob. Agents Chemother. 50:3839-3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez-Archilla, A. I., A. E. Gonzalez, M. C. Terron, and R. Amils. 2004. Ecological study of the fungal populations of the acidic Tinto River in southwestern Spain. Can. J. Microbiol. 50:923-934. [DOI] [PubMed] [Google Scholar]
- 23.Nickerson, K. W., A. L. Atkin, and J. M. Hornby. 2006. Quorum sensing in dimorphic fungi: farnesol and beyond. Appl. Environ. Microbiol. 72:3805-3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nickerson, W. J. 1953. Reduction of inorganic substances by yeasts. I. Extracellular reduction of sulfite by species of Candida. J. Infect. Dis. 93:43-56. [DOI] [PubMed] [Google Scholar]
- 25.Podgorskii, V. S., T. P. Kasatkina, and O. G. Lozovaia. 2004. Yeasts—biosorbents of heavy metals. Mikrobiol. Zh. 1:91-103. [PubMed] [Google Scholar]
- 26.Ramage, G., and J. L. Lopez-Ribot. 2005. Techniques for antifungal susceptibility testing of Candida albicans biofilms. Methods Mol. Med. 118:71-79. [DOI] [PubMed] [Google Scholar]
- 27.Ramage, G., S. P. Saville, P. D. Thomas, and J. L. Lopez-Ribot. 2005. Candida biofilms: an update. Eukaryot. Cell 4:633-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suihko, M. L., and E. S. Hoekstra. 1999. Fungi present in some recycled fibre pulps and paperboards. Nord. Pulp Paper Res. J. 14:199-203. [Google Scholar]
- 29.Vaughn, V. J., and E. D. Weinberg. 1978. Candida albicans dimorphism and virulence: role of copper. Mycopathologia 64:39-42. [DOI] [PubMed] [Google Scholar]
- 30.Yamaguchi, H. 1975. Control of dimorphism in Candida albicans by zinc: effect on cell morphology and composition. J. Gen. Microbiol. 86:370-372. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.