Abstract
Transcriptomes and growth physiologies of the hyperthermophile Thermotoga maritima and an antibiotic-resistant spontaneous mutant were compared prior to and following exposure to chloramphenicol. While the wild-type response was similar to that of mesophilic bacteria, reduced susceptibility of the mutant was attributed to five mutations in 23S rRNA and phenotypic preconditioning to chloramphenicol.
Phylogenetic analysis of antibiotic resistance genes in bacteria suggests a process of exchange and continuous evolution in the microbial world (5, 6). Microbial antibiotic resistance studies have focused on mesophilic bacteria and not on microorganisms that inhabit biologically restrictive niches. Hydrothermal environments, arguably populated by the most primitive microorganisms, have been minimally impacted by human intervention and, as such, could provide useful insights into the development of antibiotic resistance mechanisms.
Chloramphenicol (CAM) is a natural competitive inhibitor of the peptidyl transfer reaction catalyzed by 23S rRNA (25). Genome-wide transcriptional responses to CAM and other translational inhibitors present surprisingly similar features, even among phylogenetically diverse groups of mesophilic bacteria (10, 36, 45, 57). This transcriptional response usually involves induction of genes related to translational machinery and purine biosynthesis and repression of amino acid biosynthesis and aminoacyl-tRNA synthases (10, 28, 45). CAM induces both bacteriostatic and bactericidal effects (58), including increased translation inaccuracy events in vivo (65), filamentation (61), cold shock response (19, 45, 68), transient oxidative response (2), polyamine production (2, 52, 53), and up-regulation of genes encoding ribosomal proteins (10, 19, 36, 45, 57). Resistance to this antibiotic is typically associated with modifications in 23S rRNA and cellular processes that reduce cytoplasmic CAM accumulation (11, 12, 29, 43, 47, 54).
Thermotoga maritima, an evolutionarily deeply branched marine hyperthermophilic bacterium, is intrinsically resistant to aminoglycosides (27, 37) but sensitive to CAM. Here, transcriptomes of wild-type (WT) T. maritima MSB8 and a CAM-resistant spontaneous mutant were examined to compare response mechanisms of the hyperthermophilic and mesophilic bacteria. T. maritima strains were grown anaerobically at 80°C on sea salts medium supplemented with cellobiose (10 mM) under an N2 headspace (51). Growth was monitored by cell density determination using epifluorescence (acridine orange) microscopy (30, 31).
The resistant mutant (RM) was selected by successive passages with increasing concentrations of CAM (5 μg/ml to 500 μg/ml) and isolated by serial dilution of the culture to extinction (7). The MIC was determined by monitoring the optical density at 600 nm and by direct cell counts. Antibiotic thermostability was tested using an Escherichia coli-based bioassay; minimal thermal deterioration of CAM under the experimental conditions tested was noted (48). The RM strain was found to have a MIC (1 mg/ml) significantly higher than that for the WT (25 μg/ml). Sequencing (23S rRNA) of the RM strain was performed by the Integrated Biotech Laboratories, University of Georgia, Athens.
For CAM challenge in batch culture, a 16-liter Microgen fermentor (New Brunswick Scientific, Edison, NJ) was used; 8 liters of sea salts medium was prepared by heating to 100°C for 20 min. Prior to inoculation (2%) at 80°C, the medium was reduced with 10% (wt/vol) sodium sulfide. The culture was agitated at 200 rpm and sparged continuously with N2 to maintain anaerobic conditions. WT and RM cultures were challenged during mid-exponential phase (2 × 107 cells/ml) with 100 μg/ml of CAM. Samples were taken immediately before the CAM addition and then 5 and 30 min after challenge.
Continuous cultivation of T. maritima was performed in a 2-liter flask at 80°C (51, 59). A 7-h seed batch culture preceded continuous operation (dilution rate of 0.42 h−1). Mechanical and biological steady-state conditions were attained by allowing at least 5 reactor volume changes, at which time cell densities stabilized at approximately 108 cells/ml (51, 59). CAM was added anaerobically, such that the culture was immediately exposed to 100 μg/liter; simultaneously, CAM was added to the feed to 100 μg/liter. Samples (350 ml) for transcriptional profiling were obtained from both batch and continuous cultures, as described elsewhere (22). Development of the cDNA microarray for T. maritima and the associated experimental and statistical methodology have been described elsewhere (13, 31, 51).
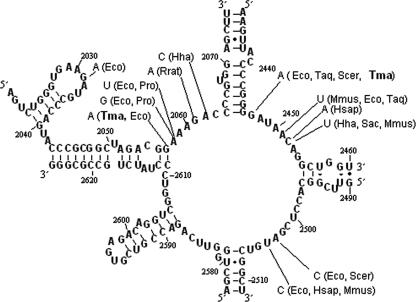
Based upon the genomic sequence of the T. maritima 23S rRNA (44), the RM was found to contain five mutations (Fig. 1 and Table 1). Mutations to nucleotide G2176 (E. coli 23S rRNA nomenclature used for reference) have been associated with resistance to translational inhibitors in other mesophilic bacteria (18, 21, 56). The mutation corresponding to G2568A was initially discovered in CAM-resistant Saccharomyces cerevisiae (17). When engineered into E. coli, the corresponding mutation, G2447A, was associated with resistance to CAM (64), while for reconstituted ribosomes from Thermus aquaticus, this mutation triggered a peptidyl-transferase activity rate reduction ranging from 30% to 73% (50). The third mutation in domain V, G2288, located at the F (final) site of tRNA transit through the ribosome, corresponds to an adenine present at the same location in E. coli (position 2169). This constitutes a novel, perhaps compensatory, mutation in T. maritima (3, 38, 39), Given the localization at the F site (34), the G2288A mutation most likely affects the final release of the nonaminoacylated tRNA to the cytoplasm, possibly tuning ribosome function during translation to minimize the loss of peptidyltransferase activity arising from the double mutation (G2176A and G2568A) at the peptidyltransferase center (PTC) (49, 69).
FIG. 1.
Point mutations identified in the nucleotide sequence of 23S rRNA of a CAM-resistant mutant of Thermotoga maritima (Tma). Mutation sites are referenced to E. coli (Eco). Note that G2288A in T. maritima corresponds to an adenine at E. coli location 2169. Mutations identified in the 23S rRNA PTC loop related to CAM resistance are shown in Table 1 for T. maritima and other organisms/organelles: G2032A in E. coli (16); G2057A in E. coli (18) and T. maritima (this work); A2058G and A2058U in Propionibacterium acnes (Pro) (54) and E. coli (16); G2061A in Rattus rattus (Rrat) (35); A2062C in Halobacterium halobium (Hha) (40); G2447A in E. coli (64), S. cerevisiae (Scer) (17), Thermus aquaticus (Taq) (50), and T. maritima (this work); A2451U in Mus musculus (Mmus) (33), E. coli (64), and T. aquaticus (50); C2542A in Homo sapiens (Hsap) (9); C2542U in H. halobium (40), Sulfolobus acidocaldarius (Sac) (1), and M. musculus (60); A2053C in S. cerevisiae (17) and E. coli (70); and U2054C in E. coli (70) and H. sapiens (9, 33).
TABLE 1.
Mutations in the 23S rRNA PTC loop related to CAM resistance
| Mutation location in T. maritima | Mutation location in E. coli | 23S rRNA domain | Previously noted in other organism(s) (reference) |
|---|---|---|---|
| 2980_2981 (insG) | 2862_2863 (insG) | VI | No |
| G2568A | G2447A | V | Yes: E. coli (64), T. aquaticus (50), S. cerevisiae (17) |
| G2288A | A2169 | V | No |
| G2176A | G2057A | V | Yes: E. coli (18) |
| G907U | G830U | II | No |
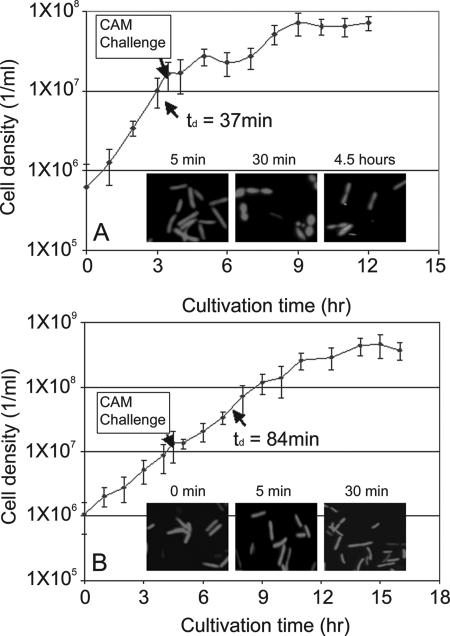
CAM addition to WT batch cultures impeded growth for about 3 h, after which time growth resumed briefly, albeit at a reduced rate (Fig. 2A). In contrast, after a slight pause following CAM addition, the RM strain continued to grow at the same rate as before antibiotic challenge, which was about half that of the WT (64 min versus a 37-min doubling time [td]) (Fig. 2B). Morphological changes have been reported for mesophilic bacteria upon exposure to antibiotics (15, 23, 26, 55, 61). Here, within 30 min of CAM exposure, the WT was coccoidal, whereas the RM retained the characteristic T. maritima rod-shaped morphology. CAM challenge of the WT continuous culture led to washout but had no noticeable effect on the RM culture.
FIG. 2.
CAM challenge of WT T. maritima (A) and the RM (B). The mutant strain grew more slowly than the WT prior to CAM challenge but showed no morphological changes after challenge compared to the response of the WT culture; WT morphology returned to the prechallenge state within several hours.
Differences between RM and WT transcriptomes prior to CAM challenge.
Different transcriptomes have been observed for wild-type bacteria and corresponding vancomycin-resistant mutants, even in the absence of the selective agent (42). Similar characteristics were observed here for T. maritima prior to CAM exposure (RM00 versus WT00) (Table 2). In continuous culture, only 19 open reading frames (ORFs) were differentially regulated twofold or more in the RM strain relative to the WT, compared to 131 ORFs in batch culture. In batch culture, 32 ribosomal structural proteins (2/3 of the identifiable T. maritima ribosomal proteins) were represented within this group (44). Other translation-associated genes up-regulated in the RM strain prior to challenge in batch culture included elongation factor (TM1502, TM1503, and TM1590), methionine aminopeptidase (TM1478), and SecY (TM1480) genes. Polyamine synthesis proteins (TM0654 to TM0656) and cold shock proteins CspC (TM1683) and CspL (TM1874) were also up-regulated prior to challenge, suggesting a preconditioning of the RM to offset the deleterious effect of CAM on RNA stability, protein translation, and oxidative stress.
TABLE 2.
Summary of differentially transcribed ORFs for Thermotoga maritima chloramphenicol challenge
| Culture | No. of ORFs with ≥2-fold change
|
Supplemental material source for detailed listing of ORFs | ||
|---|---|---|---|---|
| Up- regulated | Down-regulated | Total | ||
| Batch | ||||
| WT05 vs WT00 | 139 | 135 | 274 | Table S4 |
| WT30 vs WT05 | 7 | 29 | 36 | Table S6 |
| WT30 vs WT00 | 166 | 186 | 352 | |
| RM05 vs RM00 | 32 | 28 | 60 | Table S8 |
| RM30 vs RM05 | 279 | 241 | 520 | Table S10 |
| RM30 vs RM00 | 216 | 184 | 400 | |
| RM00 vs WT00 | 75 | 56 | 131 | Table S2 |
| RM05 vs WT05 | 218 | 212 | 430 | Table S12 |
| RM30 vs WT30 | 151 | 97 | 227 | Table S14 |
| Continuous | ||||
| WT05 vs WT00 | 125 | 131 | 256 | Table S3 |
| WT30 vs WT05 | 32 | 47 | 79 | Table S5 |
| WT30 vs WT00 | 81 | 102 | 183 | |
| RM05 vs RM00 | 7 | 1 | 8 | Table S7 |
| RM30 vs RM05 | 0 | 5 | 5 | Table S9 |
| RM30 vs RM00 | 23 | 7 | 30 | |
| RM00 vs WT00 | 7 | 12 | 19 | Table S1 |
| RM05 vs WT05 | 136 | 135 | 271 | Table S11 |
| RM30 vs WT30 | 74 | 80 | 154 | Table S13 |
Response of the WT to CAM challenge.
Up-regulation of ribosomal proteins, transcription factors, and cold shock proteins has been implicated in the response of mesophilic bacteria to translational inhibitors (10, 36, 45, 57), and similar features were observed here for T. maritima (Tables 3 and 4). The WT response to CAM was immediate, with 274 and 256 ORFs differentially transcribed in batch and continuous cultures, respectively, within 5 min following CAM challenge (Table 2). Fewer ORFs responded during the 5- to 30-min period (36 for batch culture and 79 for continuous culture).
TABLE 3.
Batch culture transcriptional response of selected ORFs before and after exposure to CAM in the T. maritima MSB8 WT and RM strains
| Function and GeneID no. | Change (fold) of ORF before/after exposure to CAM
|
Annotation | ||||
|---|---|---|---|---|---|---|
| Before exposure (RM vs WT) | After exposure for:
|
|||||
| WT
|
RM
|
|||||
| 5 min | 30 min | 5 min | 30 min | |||
| Ribosomal proteins | ||||||
| TM0150 | NCb | 2.2 | 2.0 | NC | NC | Ribosomal protein L32 |
| TM0451 | NC | 2.3 | 2.1 | NC | NC | Ribosomal protein L33 |
| TM0452 | NC | NC | 2.2 | NC | NC | Pre-protein translocase SecE |
| TM1451 | NC | 5.2 | 6.4 | NC | 3.7 | RNA polymerase sigma A factor |
| TM1453-1458a | NC | 3.3 | 2.3 | NC | 2.4 | Ribosomal proteins |
| TM1480 | 2.6 | NC | NC | NC | NC | Pre-protein translocase SecY |
| TM1471-1487a | 2.1 | 1.6 | NC | NC | 2.4 | Ribosomal proteins |
| TM1489-1505a | 3.5 | 2.5 | 2.1 | NC | 1.9 | Ribosomal proteins |
| TM1565 | NC | 2.0 | 2.7 | 2.8 | NC | Signal recognition particle protein |
| TM1568 | 1.8 | 2.5 | 2.0 | 2.0 | NC | 16S rRNA processing protein |
| TM1569 | NC | 2.0 | 1.7 | 1.9 | NC | tRNA guanine-N1 methyltransferase |
| TM1590-1593a | 3.6 | 2.6 | 1.9 | 2.2 | 2.4 | Ribosomal proteins/translation factor |
| TM1627 | 2.0 | 2.3 | NC | 1.8 | 2.1 | General stress protein Ctc |
| Translation factors | ||||||
| TM1502 | 3.5 | 1.8 | 2.5 | NC | 2.7 | Translation elongation factor Tu |
| TM1503 | 2.6 | 1.6 | 2.0 | 1.9 | NC | Translation elongation factor G |
| TM1590 | 3.8 | 2.9 | 1.8 | 1.7 | 3.0 | Translation initiation factor IF-3 |
| Polyamines/oxidative response | ||||||
| TM0654-0659a | 2.0 | 3.9 | 6.6 | NC | 1.6 | Polyamine biosynthesis, rubrerythrin, neelaredoxin, rubredoxin |
| Defense mechanism | ||||||
| TM0119 | 1.9 | NC | NC | NC | 2.1 | Acetamidase, putative |
| TM0120 | 1.8 | NC | 2.2 | NC | 5.2 | Oxidoreductase, putative |
| ATP synthase | ||||||
| TM1609-1616a | NC | 2.7↓c | 3.2↓ | NC | 2.3↓ | F1 ATP synthase subunits |
| Purine biosynthesis | ||||||
| TM1245-1251a | 2.5↓ | 2.9↓ | 2.9↓ | NC | NC | Purine biosynthetic pathway |
| Amino acid biosynthesis | ||||||
| TM0545-0555a | NC | 2.5↓ | 2.8↓ | NC | ↓2.1 | Thr, Val, Leu, Ile biosynthesis |
| Sugar utilization | ||||||
| TM1068 | NC | 2.1 | 1.9 | NC | NC | α-Glucosidase |
| TM1195 | NC | 2.1 | 2.0 | NC | NC | β-Galactosidase |
| TM1219-1223a | NC | 2.2↓ | 2.0↓ | 1.8↓ | 2.3↓ | β-Linked sugars utilization operon |
| TM1835 | NC | 2.0 | 2.9 | NC | 2.5 | Cyclomaltodextrinase, putative |
| TM1840 | NC | 2.8 | 3.3 | NC | 2.9 | α-Amylase |
| TM1845 | NC | 2.2 | 2.2 | NC | NC | Pullulanase |
| DNA repair | ||||||
| TM0266 | NC | 7.1 | 2.4 | 2.0 | NC | DNA-binding protein, HU |
| TM0480 | NC | 2.0 | 2.4 | NC | NC | Exonuclease ABC, subunit A |
| TM0604 | NC | 4.1 | 2.7 | 2.2 | NC | DNA-binding protein |
| Cold shock proteins/chaperones | ||||||
| TM0198 | NC | 2.1 | 2.5 | NC | 1.9 | Clp protease, ATPase subunit |
| TM0505 | 3.3 | 3.9 | NC | 2.5 | NC | GroES |
| TM0506 | 4.6 | 2.0 | NC | 2.1 | 1.6 | GroEL |
| TM0571 | NC | 3.2 | 3.9 | NC | NC | Heat shock serine protease |
| TM1683 | 2.8 | 4.9 | 4.1 | NC | 2.2 | Cold shock protein |
| TM1874 | 3.1 | 3.4 | 5.8 | NC | 5.8 | Cold shock protein |
| tmRNA | ||||||
| TM0504 | 2.3 | 2.5 | 1.6 | 2.3 | 4.7 | tmRNA/signaling peptide |
For operons, change (fold) is reported as the arithmetic average for all ORFs in the locus.
NC, change was ≤1.5-fold.
↓, down-regulation.
TABLE 4.
Continuous culture transcriptional response of selected ORFs before and after exposure to CAM in the T. maritima MSB8 WT and RM strains
| Function and GeneID no. | Change (fold) of ORF before/after exposure to CAM
|
Annotation | ||||
|---|---|---|---|---|---|---|
| Before exposure (RM vs WT) | After exposure for:
|
|||||
| WT
|
TM
|
|||||
| 5 min | 30 min | 5 min | 30 min | |||
| Ribosomal proteins | ||||||
| TM0150 | NCb | 1.8 | 1.6 | NC | NC | Ribosomal protein L32 |
| TM0451 | NC | 1.7 | NC | NC | NC | Ribosomal protein L33 |
| TM0452 | NC | 1.6 | 1.7 | NC | NC | Pre-protein translocase SecE |
| TM1451 | NC | 3.7 | 2.8 | NC | NC | RNA polymerase sigma A factor |
| TM1453-1458a | NC | 3.9 | 2.7 | NC | NC | Ribosomal proteins |
| TM1480 | NC | 1.8 | NC | NC | NC | Pre-protein translocase SecY |
| TM1471-1487a | NC | 1.8 | NC | NC | NC | Ribosomal proteins |
| TM1489-1505a | NC | NC | 2.1 | NC | NC | Ribosomal proteins |
| TM1565 | NC | 4.3 | 4.4 | NC | NC | Signal recognition particle protein |
| TM1568 | NC | 3.2 | 2.3 | NC | NC | 16S rRNA processing protein |
| TM1569 | NC | 2.3 | 2.0 | NC | NC | tRNA guanine-N1 methyltransferase |
| TM1590-1593a | NC | 3.8 | 3.0 | NC | NC | Ribosomal proteins/translation factor |
| TM1627 | NC | 3.5 | 3.2 | NC | NC | General stress protein Ctc |
| Translation factors | ||||||
| TM1502 | 1.7 | NC | 2.2 | 1.7 | 2.1 | Translation elongation factor Tu |
| TM1503 | NC | NC | 2.4 | NC | NC | Translation elongation factor G |
| TM1590 | NC | 4.1 | 2.6 | NC | NC | Translation initiation factor IF-3 |
| Polyamines/oxidative response | ||||||
| TM0654-0659a | NC | 3.7 | 5.1 | NC | NC | Polyamine biosynthesis, rubrerythrin, neelaredoxin, rubredoxin |
| Defense mechanism | ||||||
| TM0119 | NC | NC | 1.6 | NC | 1.6 | Acetamidase, putative |
| TM0120 | NC | NC | NC | NC | NC | Oxidoreductase, putative |
| ATP synthase | ||||||
| TM1609-1616a | NC | 2.1↓c | NC | NC | NC | F1 ATP synthase subunits |
| Purine biosynthesis | ||||||
| TM1245-1251a | NC | 1.6↓ | NC | NC | NC | Purine biosynthetic pathway |
| Amino acid biosynthesis | ||||||
| TM0545-0555a | NC | 2.3↓ | NC | NC | NC | Thr, Val, Leu, Ile biosynthesis |
| Sugar utilization | ||||||
| TM1068 | NC | 1.6 | NC | NC | NC | α-Glucosidase |
| TM1195 | NC | 1.7↓ | NC | NC | NC | β-Galactosidase |
| TM1219-1223a | NC | 3.7↓ | 3.6↓ | NC | NC | β-Linked sugar utilization operon |
| TM1835 | NC | 4.2↓ | 3.9↓ | NC | NC | Cyclomaltodextrinase, putative |
| TM1840 | NC | NC | 2.2↓ | 1.6 | 1.7 | α-Amylase |
| TM1845 | NC | NC | NC | NC | NC | Pullulanase |
| DNA repair | ||||||
| TM0266 | NC | 3.1 | 1.6 | NC | NC | DNA-binding protein, HU |
| TM0480 | NC | NC | NC | NC | NC | Exonuclease ABC, subunit A |
| TM0604 | NC | 5.8 | 3.4 | NC | NC | DNA-binding protein |
| Cold shock proteins/chaperones | ||||||
| TM0198 | NC | 2.6 | 1.8 | NC | NC | Clp protease, ATPase subunit |
| TM0505 | NC | 3.4 | 1.6 | 1.8 | 2.8 | GroES |
| TM0506 | NC | 4.5 | NC | 2.1 | 3.3 | GroEL |
| TM0571 | 1.8 | 2.9 | 1.6 | NC | NC | Heat shock serine protease |
| TM1683 | NC | 2.7 | NC | 1.6↓ | 3.0↓ | Cold shock protein |
| TM1874 | NC | 3.1 | 2.8 | NC | NC | Cold shock protein |
| tmRNA | ||||||
| TM0504 | NC | NC | NC | NC | NC | tmRNA/signaling peptide |
For operons, change (fold) is reported as the arithmetic average for all ORFs in the locus.
NC, change was ≤1.5-fold.
↓, down-regulation.
In bacteria, polyamines are associated with a variety of functions, including osmoregulation, response to pH, oxidative stress, growth rate, and induction of death in late stationary phase (4, 14, 32, 66, 73). In hyperthermophiles, polyamines stabilize nucleic acids at high temperatures (63) and are essential components of in vitro protein synthesis systems (37, 67). In T. maritima MSB8, a direct correlation between the presence of polyamines and response to high temperatures has been reported (74). Here, TM0654 (spermidine synthase) and TM0655 (S-adenosylmethionine decarboxylase) were up-regulated in the WT upon exposure to CAM (see Tables 3 and 4). Divergently transcribed from the spermidine synthase genes is a putative operon, comprised of TM0657 (rubrerythrin), TM0658 (neelaredoxin), and TM0659 (rubredoxin). TM0657 to TM0659 were up-regulated upon exposure to CAM, especially in continuous culture. TM0657 is related to the Pyrococcus furiosus rubrerythrin in a mix-branched phylogenetic tree of bacterial/archaeal rubrerythrins (71). The functional role of the P. furiosus homologs to TM0657 to TM0659 has been recently demonstrated in vivo in E. coli (24); in addition to NAD(P)H rubredoxin oxidoreductase, these genes are required to complete the detoxification pathway for reactive oxygen species in anaerobic microbes (24, 71). It is noteworthy that the antimicrobial effect of CAM is not always associated with direct binding to the PTC (8, 46). For example, despite structural differences between the archaeal and bacterial ribosomes (72), methanogens are sensitive to CAM due to the presence of the aryl nitro group in this antibiotic, a moiety that under anaerobic conditions acts as an oxidizing agent (8, 46). Thus, a direct oxidative effect of CAM on T. maritima ribosomes cannot be ruled out.
The connection between accumulation of mRNAs from ribosomal protein operons and challenge with translational inhibitors has been observed across all bacterial groups, including E. coli, Streptococcus pneumoniae, Mycobacterium tuberculosis, Haemophilus influenzae, and Bacillus subtilis (10, 19, 36, 45, 57). This response is usually accompanied by down-regulation of amino acid synthesis and up-regulation of transcription/translation factors (e.g., IF-3, GreA, EF-Tu, and NusA). In the case of the WT batch culture, this effect was quite clear. The response to CAM indicated accumulation of mRNAs for ribosomal protein operons and down-regulation of genes associated with the biosynthesis of amino acids. Down-regulation of FoF1 ATP synthase structural components was observed, as was down-regulation of the cellobiose uptake transporter (TM1219 to TM1223); cellobiose was the primary carbon and energy source used here. The purine salvage and de novo synthesis pathways in the WT were down-regulated within 5 min after CAM challenge, a response previously noted in B. subtilis (20). In the WT, CAM-induced genes encoding transfer-messenger RNA (tmRNA), DNA repair, and cold and heat shock proteins were up-regulated.
Response of the RM to CAM challenge.
Table 2 summarizes genome-wide differential transcription for the RM in batch and chemostat cultures at 5 and 30 min after CAM challenge. In continuous culture, the RM was relatively insensitive to CAM challenge at 5 min; only eight genes were differentially transcribed (seven up and one down). In fact, the overall RM response in continuous culture was limited, with only 30 ORFs responding (23 up and 7 down) over the 30-min period after CAM challenge. The insensitivity to CAM in the chemostat likely relates to the 10-fold-lower ratio of CAM to cells compared to batch culture.
At 5 min postchallenge in the RM batch culture, ribosomal and heat shock proteins responded. However, in contrast to the WT at 5 min, there was no response of cold shock genes (TM1683 and TM1874) and the overall transcriptional response was limited (60 ORFs: 32 up and 28 down). The impact of batch CAM challenge on the RM transcriptome was most pronounced between 5 and 30 min (520 ORFs: 279 up and 241 down). The basis for this delayed response is unknown but may reflect a difference in CAM affinity for its ribosomal targets and/or a mutation in cell wall permeability, as reported previously in Burkholderia cepacia and H. influenzae (11, 12, 54). Many of the differentially expressed transcripts in the RM are annotated as hypotheticals (see Table S10 in the supplemental material) and could not be assigned to any COG category or to a family of recognizable protein domains (41, 62).
Up-regulation of cold shock proteins was observed along with significant down-regulation of heat shock proteins, a reversal of the initial response. In the RM at 30 min, induction of the heat shock proteins DnaK, GroEL, and GroES was noted, similar to prechallenge. In mesophilic bacteria, chaperone overproduction can buffer negative fitness effects associated with deleterious mutations and this may be the case here (39).
Conclusions.
These results add to the limited information available on the bacterial resistome. Although the data reported here are for a nonpathogenic species from an unusual habitat, certain features in common with antibiotic response in mesophilic bacteria were noted. Increasingly stringent selective pressure facilitated the selection of a resistant strain that exhibited directly adaptive mutations (five were noted in 23S rRNA) and probably yet undefined compensatory ones, resulting in a phenotype that was preconditioned to antibiotic challenge. It was interesting that ORFs encoding many hypothetical proteins were triggered in both the WT and RM upon antibiotic exposure, indicating that there is still much to be learned about this phenomenon.
Supplementary Material
Acknowledgments
This work was supported in part by grants from the NASA Exobiology, DOE Energy Biosciences, and NSF Biotechnology Programs. M.R.J. acknowledges support from a Department of Education GAANN Fellowship, and SBC acknowledges support from an NIEHS Bioinformatics Traineeship.
We acknowledge helpful discussions with James Brown at NCSU.
Footnotes
Published ahead of print on 8 June 2007.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Aagaard, C., H. Phan, S. Trevisanato, and R. A. Garrett. 1994. A spontaneous point mutation in the single 23S rRNA gene of the thermophilic archaeon Sulfolobus acidocaldarius confers multiple drug resistance. J. Bacteriol. 176:7744-7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albesa, I., M. C. Becerra, P. C. Battan, and P. L. Paez. 2004. Oxidative stress involved in the antibacterial action of different antibiotics. Biochem. Biophys. Res. Commun. 317:605-609. [DOI] [PubMed] [Google Scholar]
- 3.Andersson, D. I., and B. R. Levin. 1999. The biological cost of antibiotic resistance. Curr. Opin. Microbiol. 2:489-493. [DOI] [PubMed] [Google Scholar]
- 4.Apirakaramwong, A., K. Kashiwagi, V. S. Raj, K. Sakata, Y. Kakinuma, A. Ishihama, and K. Igarashi. 1999. Involvement of ppGpp, ribosome modulation factor, and stationary phase-specific sigma factor sigma(S) in the decrease in cell viability caused by spermidine. Biochem. Biophys. Res. Commun. 264:643-647. [DOI] [PubMed] [Google Scholar]
- 5.Barlow, M., and B. G. Hall. 2002. Origin and evolution of the AmpC β-lactamases of Citrobacter freundii. Antimicrob. Agents Chemother. 46:1190-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barlow, M., and B. G. Hall. 2002. Phylogenetic analysis shows that the OXA beta-lactamase genes have been on plasmids for millions of years. J. Mol. Evol. 55:314-321. [DOI] [PubMed] [Google Scholar]
- 7.Baross, J. A. 1993. Isolation and cultivation of hyperthermophilic bacteria from marine and freshwater habitats, p. 21-30. In P. F. Kemp, B. F. Sherr, E. B. Sherr, and J. B. Cole (ed.), Handbook of methods in aquatic microbial ecology. Lewis Publishers, Boca Raton, FL.
- 8.Beckler, G. S., L. A. Hook, and J. N. Reeve. 1984. Chloramphenicol acetyltransferase should not provide methanogens with resistance to chloramphenicol. Appl. Environ. Microbiol. 47:868-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blanc, H., C. W. Adams, and D. C. Wallace. 1981. Different nucleotide changes in the large ribosomal-RNA gene of the mitochondrial-DNA confer chloramphenicol resistance on 2 human cell-lines. Nucleic Acids Res. 9:5785-5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boshoff, H. I., T. G. Myers, B. R. Copp, M. R. McNeil, M. A. Wilson, and C. E. Barry III. 2004. The transcriptional responses of Mycobacterium tuberculosis to inhibitors of metabolism: novel insights into drug mechanisms of action. J. Biol. Chem. 279:40174-40184. [DOI] [PubMed] [Google Scholar]
- 11.Burns, J. L., L. A. Hedin, and D. M. Lien. 1989. Chloramphenicol resistance in Pseudomonas cepacia because of decreased permeability. Antimicrob. Agents Chemother. 33:136-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burns, J. L., P. M. Mendelman, J. Levy, T. L. Stull, and A. L. Smith. 1985. A permeability barrier as a mechanism of chloramphenicol resistance in Haemophilus influenzae. Antimicrob. Agents Chemother. 27:46-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chhabra, S. R., K. R. Shockley, S. B. Conners, K. L. Scott, R. D. Wolfinger, and R. M. Kelly. 2003. Carbohydrate-induced differential gene expression patterns in the hyperthermophilic bacterium Thermotoga maritima. J. Biol. Chem. 278:7540-7552. [DOI] [PubMed] [Google Scholar]
- 14.Cohen, S. S. 1998. Guide to the polyamines, 2nd ed. Oxford University Press, Oxford, United Kingdom.
- 15.Cooper, M. A., J. M. Andrews, and R. Wise. 1991. Bactericidal activity of sparfloxacin and ciprofloxacin under anaerobic conditions. J. Antimicrob. Chemother. 28:399-405. [DOI] [PubMed] [Google Scholar]
- 16.Douthwaite, S. 1992. Functional interactions within 23S rRNA involving the peptidyltransferase center. J. Bacteriol. 174:1333-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dujon, B. 1980. Sequence of the intron and flanking exons of the mitochondrial 21S rRNA gene of yeast strains having different alleles at the omega and rib-1 loci. Cell 20:185-197. [DOI] [PubMed] [Google Scholar]
- 18.Ettayebi, M., S. M. Prasad, and E. A. Morgan. 1985. Chloramphenicol-erythromycin resistance mutations in a 23S rRNA gene of Escherichia coli. J. Bacteriol. 162:551-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evers, S., K. Di Padova, M. Meyer, H. Langen, M. Fountoulakis, W. Keck, and C. P. Gray. 2001. Mechanism-related changes in the gene transcription and protein synthesis patterns of Haemophilus influenzae after treatment with transcriptional and translational inhibitors. Proteomics 1:522-544. [DOI] [PubMed] [Google Scholar]
- 20.Eymann, C., G. Homuth, C. Scharf, and M. Hecker. 2002. Bacillus subtilis functional genomics: global characterization of the stringent response by proteome and transcriptome analysis. J. Bacteriol. 184:2500-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furneri, P. M., G. Rappazzo, M. P. Musumarra, G. Tempera, and L. S. Roccasalva. 2000. Genetic basis of natural resistance to erythromycin in Mycoplasma hominis. J. Antimicrob. Chemother. 45:547-548. [DOI] [PubMed] [Google Scholar]
- 22.Gao, J., M. W. Bauer, K. R. Shockley, M. A. Pysz, and R. M. Kelly. 2003. Growth of hyperthermophilic archaeon Pyrococcus furiosus on chitin involves two family 18 chitinases. Appl. Environ. Microbiol. 69:3119-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gould, I. M., and F. M. MacKenzie. 1997. The response of Enterobacteriaceae to beta-lactam antibiotics round forms, filaments and the root of all evil. J. Antimicrob. Chemother. 40:495-499. [DOI] [PubMed] [Google Scholar]
- 24.Grunden, A. M., F. E. Jenney, Jr., K. Ma, M. Ji, M. V. Weinberg, and M. W. Adams. 2005. In vitro reconstitution of an NADPH-dependent superoxide reduction pathway from Pyrococcus furiosus. Appl. Environ. Microbiol. 71:1522-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansen, J. L., P. B. Moore, and T. A. Steitz. 2003. Structures of five antibiotics bound at the peptidyl transferase center of the large ribosomal subunit. J. Mol. Biol. 330:1061-1075. [DOI] [PubMed] [Google Scholar]
- 26.Horii, T., M. Kobayashi, K. Sato, S. Ichiyama, and M. Ohta. 1998. An in-vitro study of carbapenem-induced morphological changes and endotoxin release in clinical isolates of gram-negative bacilli. J. Antimicrob. Chemother. 41:435-442. [DOI] [PubMed] [Google Scholar]
- 27.Huber, R., H. Langworthy, H. Konig, M. Thomm, C. Woese, B. Steyr, and K. O. Stetter. 1986. Thermotoga maritima sp nov. represents a new genus of unique extremely thermophilic eubacteria growing up to 90°C. Arch. Microbiol. 144:324-333. [Google Scholar]
- 28.Hutter, B., C. Schaab, S. Albrecht, M. Borgmann, N. A. Brunner, C. Freiberg, K. Ziegelbauer, C. O. Rock, I. Ivanov, and H. Loferer. 2004. Prediction of mechanisms of action of antibacterial compounds by gene expression profiling. Antimicrob. Agents Chemother. 48:2838-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Izard, T. 2001. Structural basis for chloramphenicol tolerance in Streptomyces venezuelae by chloramphenicol phosphotransferase activity. Protein Sci. 10:1508-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson, M. R., S. B. Conners, C. I. Montero, C. J. Chou, K. R. Shockley, and R. M. Kelly. 2006. The Thermotoga maritima phenotype is impacted by syntrophic interaction with Methanococcus jannaschii in hyperthermophilic coculture. Appl. Environ. Microbiol. 72:811-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson, M. R., C. I. Montero, S. B. Conners, K. R. Shockley, S. L. Bridger, and R. M. Kelly. 2005. Population density-dependent regulation of exopolysaccharide formation in the hyperthermophilic bacterium Thermotoga maritima. Mol. Microbiol. 55:664-674. [DOI] [PubMed] [Google Scholar]
- 32.Jung, I. L., and I. G. Kim. 2003. Polyamines and glutamate decarboxylase-based acid resistance in Escherichia coli. J. Biol. Chem. 278:22846-22852. [DOI] [PubMed] [Google Scholar]
- 33.Kearsey, S. E., and I. W. Craig. 1981. Altered ribosomal RNA genes in mitochondria from mammalian cells with chloramphenicol resistance. Nature 290:607-608. [DOI] [PubMed] [Google Scholar]
- 34.Kirillov, S. V., J. Wower, S. S. Hixson, and R. A. Zimmermann. 2002. Transit of tRNA through the Escherichia coli ribosome: cross-linking of the 3′ end of tRNA to ribosomal proteins at the P and E sites. FEBS Lett. 514:60-66. [DOI] [PubMed] [Google Scholar]
- 35.Koike, K., M. Taira, Y. Kuchino, K. Yaginuma, T. Sekiguchi, and M. Kobayashi. 1983. Nucleo-mitochondrial interactions, p. 372-387. In R. J. Schweyen, K. Wolf, and F. Kauderwictz (ed.), Mitochondria. Walter de Gruyter, Berlin, Germany.
- 36.Lin, J. T., M. B. Connelly, C. Amolo, S. Otani, and D. S. Yaver. 2005. Global transcriptional response of Bacillus subtilis to treatment with subinhibitory concentrations of antibiotics that inhibit protein synthesis. Antimicrob. Agents Chemother. 49:1915-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Londei, P., S. Altamura, R. Huber, K. O. Stetter, and P. Cammarano. 1988. Ribosomes of the extremely thermophilic eubacterium Thermotoga maritima are uniquely insensitive to the miscoding-inducing action of aminoglycoside antibiotics. J. Bacteriol. 170:4353-4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maisnier-Patin, S., and D. I. Andersson. 2004. Adaptation to the deleterious effects of antimicrobial drug resistance mutations by compensatory evolution. Res. Microbiol. 155:360-369. [DOI] [PubMed] [Google Scholar]
- 39.Maisnier-Patin, S., J. R. Roth, A. Fredriksson, T. Nystrom, O. G. Berg, and D. I. Andersson. 2005. Genomic buffering mitigates the effects of deleterious mutations in bacteria. Nat. Genet. 37:1376-1379. [DOI] [PubMed] [Google Scholar]
- 40.Mankin, A. S., and R. A. Garrett. 1991. Chloramphenicol resistance mutations in the single 23S rRNA gene of the archaeon Halobacterium halobium. J. Bacteriol. 173:3559-3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marchler-Bauer, A., J. B. Anderson, P. F. Cherukuri, C. DeWeese-Scott, L. Y. Geer, M. Gwadz, S. He, D. I. Hurwitz, J. D. Jackson, Z. Ke, C. J. Lanczycki, C. A. Liebert, C. Liu, F. Lu, G. H. Marchler, M. Mullokandov, B. A. Shoemaker, V. Simonyan, J. S. Song, P. A. Thiessen, R. A. Yamashita, J. J. Yin, D. Zhang, and S. H. Bryant. 2005. CDD: a Conserved Domain Database for protein classification. Nucleic Acids Res. 33:D192-D196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mongodin, E., J. Finan, M. W. Climo, A. Rosato, S. Gill, and G. L. Archer. 2003. Microarray transcription analysis of clinical Staphylococcus aureus isolates resistant to vancomycin. J. Bacteriol. 185:4638-4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mosher, R. H., N. P. Ranade, H. Schrempf, and L. C. Vining. 1990. Chloramphenicol resistance in Streptomyces: cloning and characterization of a chloramphenicol hydrolase gene from Streptomyces venezuelae. J. Gen. Microbiol. 136:293-301. [DOI] [PubMed] [Google Scholar]
- 44.Nelson, K. E., R. A. Clayton, S. R. Gill, M. L. Gwinn, R. J. Dodson, D. H. Haft, E. K. Hickey, J. D. Peterson, W. C. Nelson, K. A. Ketchum, L. McDonald, T. R. Utterback, J. A. Malek, K. D. Linher, M. M. Garrett, A. M. Stewart, M. D. Cotton, M. S. Pratt, C. A. Phillips, D. Richardson, J. Heidelberg, G. G. Sutton, R. D. Fleischmann, J. A. Eisen, O. White, S. L. Salzberg, H. O. Smith, J. C. Venter, and C. M. Fraser. 1999. Evidence for lateral gene transfer between Archaea and bacteria from genome sequence of Thermotoga maritima. Nature 399:323-329. [DOI] [PubMed] [Google Scholar]
- 45.Ng, W.-L., K. M. Kazmierczak, G. T. Robertson, R. Gilmour, and M. E. Winkler. 2003. Transcriptional regulation and signature patterns revealed by microarray analyses of Streptococcus pneumoniae R6 challenged with sublethal concentrations of translation inhibitors. J. Bacteriol. 185:359-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O'Brien, R. W., and J. G. Morris. 1971. The ferredoxin-dependent reduction of chloramphenicol by Clostridium acetobutylicum. J. Gen. Microbiol. 67:265-271. [DOI] [PubMed] [Google Scholar]
- 47.Okusu, H., D. Ma, and H. Nikaido. 1996. AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple-antibiotic-resistance (Mar) mutants. J. Bacteriol. 178:306-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peteranderl, R., E. B. Shotts, Jr., and J. Wiegel. 1990. Stability of antibiotics under growth conditions for thermophilic anaerobes. Appl. Environ. Microbiol. 56:1981-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pfister, P., N. Corti, S. Hobbie, C. Bruell, R. Zarivach, A. Yonath, and E. C. Bottger. 2005. 23S rRNA base pair 2057-2611 determines ketolide susceptibility and fitness cost of the macrolide resistance mutation 2058A→G. Proc. Natl. Acad. Sci. USA 102:5180-5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Polacek, N., M. Gaynor, A. Yassin, and A. S. Mankin. 2001. Ribosomal peptidyl transferase can withstand mutations at the putative catalytic nucleotide. Nature 411:498-501. [DOI] [PubMed] [Google Scholar]
- 51.Pysz, M. A., S. B. Conners, C. I. Montero, K. R. Shockley, M. R. Johnson, D. E. Ward, and R. M. Kelly. 2004. Transcriptional analysis of biofilm formation processes in the anaerobic, hyperthermophilic bacterium Thermotoga maritima. Appl. Environ. Microbiol. 70:6098-6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raina, A., and S. S. Cohen. 1966. Polyamines and RNA synthesis in a polyauxotrophic strain of E. coli. Proc. Natl. Acad. Sci. USA 55:1587-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raina, A., M. Jansen, and S. S. Cohen. 1967. Polyamines and the accumulation of ribonucleic acid in some polyauxotrophic strains of Escherichia coli. J. Bacteriol. 94:1684-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rajyaguru, J. M., and M. J. Muszynski. 1997. Association of resistance to trimethoprim/sulphamethoxazole, chloramphenicol and quinolones with changes in major outer membrane proteins and lipopolysaccharide in Burkholderia cepacia. J. Antimicrob. Chemother. 40:803-809. [DOI] [PubMed] [Google Scholar]
- 55.Rajyaguru, J. M., D. S. Torres, E. Abel, M. C. Richardson, and M. J. Muszynski. 1998. Application of X-ray micrography and imaging to study the effect of gentamicin on Pseudomonas aeruginosa. J. Antimicrob. Chemother. 41:557-561. [DOI] [PubMed] [Google Scholar]
- 56.Ross, J. I., E. A. Eady, J. H. Cove, C. E. Jones, A. H. Ratyal, Y. W. Miller, S. Vyakrnam, and W. J. Cunliffe. 1997. Clinical resistance to erythromycin and clindamycin in cutaneous propionibacteria isolated from acne patients is associated with mutations in 23S rRNA. Antimicrob. Agents Chemother. 41:1162-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sabina, J., N. Dover, L. J. Templeton, D. R. Smulski, D. Söll, and R. A. LaRossa. 2003. Interfering with different steps of protein synthesis explored by transcriptional profiling of Escherichia coli K-12. J. Bacteriol. 185:6158-6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sat, B., R. Hazan, T. Fisher, H. Khaner, G. Glaser, and H. Engelberg-Kulka. 2001. Programmed cell death in Escherichia coli: some antibiotics can trigger mazEF lethality. J. Bacteriol. 183:2041-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shockley, K. R., K. L. Scott, M. A. Pysz, S. B. Conners, M. R. Johnson, C. I. Montero, R. D. Wolfinger, and R. M. Kelly. 2005. Genome-wide transcriptional variation within and between steady states for continuous growth of the hyperthermophile Thermotoga maritima. Appl. Environ. Microbiol. 71:5572-5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Slott, E. F., Jr., R. O. Shade, and R. A. Lansman. 1983. Sequence analysis of mitochondrial DNA in a mouse cell line resistant to chloramphenicol and oligomycin. Mol. Cell. Biol. 3:1694-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steel, C., Q. Wan, and X. H. Xu. 2004. Single live cell imaging of chromosomes in chloramphenicol-induced filamentous Pseudomonas aeruginosa. Biochemistry 43:175-182. [DOI] [PubMed] [Google Scholar]
- 62.Tatusov, R. L., N. D. Fedorova, J. D. Jackson, A. R. Jacobs, B. Kiryutin, E. V. Koonin, D. M. Krylov, R. Mazumder, S. L. Mekhedov, A. N. Nikolskaya, B. S. Rao, S. Smirnov, A. V. Sverdlov, S. Vasudevan, Y. I. Wolf, J. J. Yin, and D. A. Natale. 2003. The COG database: an updated version includes eukaryotes. BMC Bioinformatics 4:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Terui, Y., M. Ohnuma, K. Hiraga, E. Kawashima, and T. Oshima. 2005. Stabilization of nucleic acids by unusual polyamines produced by an extreme thermophile, Thermus thermophilus. Biochem. J. 388:427-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thompson, J., D. F. Kim, M. O'Connor, K. R. Lieberman, M. A. Bayfield, S. T. Gregory, R. Green, H. F. Noller, and A. E. Dahlberg. 2001. Analysis of mutations at residues A2451 and G2447 of 23S rRNA in the peptidyltransferase active site of the 50S ribosomal subunit. Proc. Natl. Acad. Sci. USA 98:9002-9007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thompson, J., M. O'Connor, J. A. Mills, and A. E. Dahlberg. 2002. The protein synthesis inhibitors, oxazolidinones and chloramphenicol, cause extensive translational inaccuracy in vivo. J. Mol. Biol. 322:273-279. [DOI] [PubMed] [Google Scholar]
- 66.Tkachenko, A. G., and L. Y. Nesterova. 2003. Polyamines as modulators of gene expression under oxidative stress in Escherichia coli. Biochemistry (Moscow) 68:850-856. [DOI] [PubMed] [Google Scholar]
- 67.Uzawa, T., A. Yamagishi, T. Ueda, N. Chikazumi, K. Watanabe, and T. Oshima. 1993. Effects of polyamines on a continuous cell-free protein synthesis system of an extreme thermophile, Thermus thermophilus. J. Biochem. (Tokyo) 114:732-734. [DOI] [PubMed] [Google Scholar]
- 68.VanBogelen, R. A., and F. C. Neidhardt. 1990. Ribosomes as sensors of heat and cold shock in Escherichia coli. Proc. Natl. Acad. Sci. USA 87:5589-5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vester, B., and S. Douthwaite. 2001. Macrolide resistance conferred by base substitutions in 23S rRNA. Antimicrob. Agents Chemother. 45:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vester, B., and R. A. Garrett. 1988. The importance of highly conserved nucleotides in the binding region of chloramphenicol at the peptidyl transfer centre of Escherichia coli 23S ribosomal RNA. EMBO J. 7:3577-3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weinberg, M. V., F. E. Jenney, Jr., X. Cui, and M. W. W. Adams. 2004. Rubrerythrin from the hyperthermophilic archaeon Pyrococcus furiosus is a rubredoxin-dependent, iron-containing peroxidase. J. Bacteriol. 186:7888-7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yonath, A. 2005. Antibiotics targeting ribosomes: resistance, selectivity, synergism and cellular regulation. Annu. Rev. Biochem. 74:649-679. [DOI] [PubMed] [Google Scholar]
- 73.Yoshida, M., K. Kashiwagi, A. Shigemasa, S. Taniguchi, K. Yamamoto, H. Makinoshima, A. Ishihama, and K. Igarashi. 2004. A unifying model for the role of polyamines in bacterial cell growth, the polyamine modulon. J. Biol. Chem. 279:46008-46013. [DOI] [PubMed] [Google Scholar]
- 74.Zellner, G., and H. Kneifel. 1993. Caldopentamine and caldohexamine in cells of Thermotoga species, a possible adaptation to the growth at high temperatures. Arch. Microbiol. 159:472-476. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.